Abstract
Background
There is a 3-fold redundancy in the Genetic Code; most amino acids are encoded by more than one codon. These synonymous codons are not used equally; there is a Codon Usage Bias (CUB). This article will provide novel information about the origin and evolution of this bias.
Results
Codon Usage Bias (CUB, defined here as deviation from equal usage of synonymous codons) was studied in 113 species. The average CUB was 29.3 ± 1.1% (S.E.M, n = 113) of the theoretical maximum and declined progressively with evolution and increasing genome complexity. A Pan-Genomic Codon Usage Frequency (CUF) Table was constructed to describe genome-wide relationships among codons. Significant correlations were found between the number of synonymous codons and (i) the frequency of the respective amino acids (ii) the size of CUB. Numerous, statistically highly significant, internal correlations were found among codons and the nucleic acids they comprise. These strong correlations made it possible to predict missing synonymous codons (wobble bases) reliably from the remaining codons or codon residues.
Conclusion
The results put the concept of "codon bias" into a novel perspective. The internal connectivity of codons indicates that all synonymous codons might be integrated parts of the Genetic Code with equal importance in maintaining its functional integrity.
Background
The genetic code is redundant: 20 amino acids plus start and stop signals are coded by 64 codons. This redundancy increases the resistance of genes to mutation: the third codon letters (wobble bases) can often be interchanged without affecting the primary sequence of the protein product. Nevertheless, wobble base usage is highly conserved in mRNA sequences (there is no or very little individual or intra-species variation) and, interestingly, some wobble mutations (though they are called silent mutations) are known to cause genetic disease with no change in the amino acid sequences [1].
However, the wobble bases are not randomly selected, as they might be if interchangeability were unrestricted. There is codon bias, i.e. codon usage is not equally distributed between the possible synonyms; some redundant codons are preferentially used. This bias is described in Codon Usage Frequency (CUF) Tables [2].
Many studies confirm the existence of codon bias and significant correlations have been found between codon bias and various biological parameters such as gene expression level [3-6] gene length [7-9], gene translation initiation signal [10], protein amino acid composition [11], protein structure [12,13], tRNA abundance [14-17], mutation frequency and pattern, [18,19] and GC composition [20-23].
These observations may not be universally valid because some statistically significant observations in one species are not reproduced in another. However, there is a strong expectation that codon bias, which is obviously well conserved in different species, reflects a general biological function because of the universal nature of the Genetic Code and the structure and function of nucleic acids and proteins.
The aim of this study is to investigate the possible origin of so-called "codon bias", measure it quantitatively and compare it among many species.
Materials and methods
Codon Usage Frequency (CUF) Tables were obtained for 113 different organisms from the Codon Usage Database (NCBI-GenBank, update: November 16, 2006 [24]). The organisms were selected from KEGG (Kyoto Encyclopedia of Genes and Genomes, [25]) and represented a wide variety of species from different evolutionary lines [Additional file 1].
To calculate Codon Usage Bias (CUB) numerically, I assumed that statistically equal usage of all available synonymous codons is the neutral "starting point" for the development of species-specific codon usages, and the CUB is the sum of the deviations from such random, equal usage.
The codons (i, 64) were divided into 21 subgroups (j, corresponding to the 20 amino acids and 1 stop signal). The number of occurrences of a codon was normalized and the frequencies of the codons (CUFij) in each fraction were calculated. The sum of CUFif in a fraction was always treated as 100% so the sum of all fractions was 2100%. ni is the number of synonymous codons in the jth fraction and nj = 64
CUFij is the frequency (%) of the ith codon in the jth fraction encoded by ni synonymous codons.
These fractional frequencies were compared to the random fractional frequencies (rCUFij), defined as the fractional frequency that a codon would have if all alternative codons were used randomly and equally.
| rCUF(1j) = rCUF(2j) = rCUF(n)j = rCUF(ij) = 100/ni (%) |
The sum of rCUF in a fraction is also 100% and in each fraction altogether is 2100%.
CUB is defined as the absolute difference between CUF and rCUF:-
More simply, CUB is the absolute number of fractional frequencies minus the number expected if usage of synonymous codons was uniform.
CUB may be used in some cases with its +/- orientation indicated. In these cases, positive values indicate over-utilization of a codon (e.g. dominant codons) while negative values indicate under-utilization (suppression).
CUBmin = 0 if CUFij = rCUFij and the Calculated Maximal Possible CUBmax is 2416.7%. This is the value when only one of all the possible synonymous codons is used (100% frequency) for every amino acid and for the stop signal.
Further explanation of the CUB calculation is given in [Additional file 2], together with an example. CUFij (%) is not to be confused with a "regular" codon frequency (CUFi), which indicates the frequency of a codon in the entire genome (all 21 fractions) and is usually given in the CUF Tables in #/1000 units.
The definition of CUB in this article is not directly comparable to other widely used definitions such as CUI.
Results
Quantitative evaluation of codon bias
CUB = 0% when all available synonymous codons are equally used. The maximal calculated bias, CUBmax = 100%, indicates that only one codon is used for each amino acid (and for the stop signal), while the remaining 43 codons are not used at all. I calculated CUB in 113 species and found that the average value is 29.3 +/- 1.1% (S.E.M, n = 113). There seems to be a modest but significant decrease in the bias during evolution: bacteria and archeoata have the highest bias while vertebrates have the lowest. Eukaryotes have significantly lower CUB than prokaryotes. Humans have the lowest value (18.9%) (Figure 1).
Figure 1.
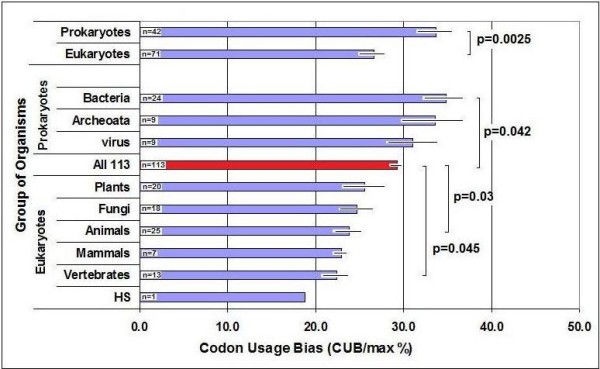
Codon Usage Bias (CUB) in Some Organisms. Mean +/- S.E.M, n: number of species in the group.
There is a slight negative correlation between the size of the codon- and gene-pool of an organism and its CUB (p < 0.01, n = 113, not shown). The size and complexity of both genome and proteome increase with evolution, while the CUB decreases. A larger codon pool seems to utilize more codon variation, which leads to lower differences between the usage frequencies of synonymous codons.
Qualitative evaluation of CUB
Detailed analysis of different species reveals wide variations in CUB (Figure 2). There is a seemingly random variation in CUB between amino acids and different groups of organisms. However, a comparison of closely-related species with large codon pools shows very similar patterns. For example, all mammals have very similar CUB patterns.
Figure 2.
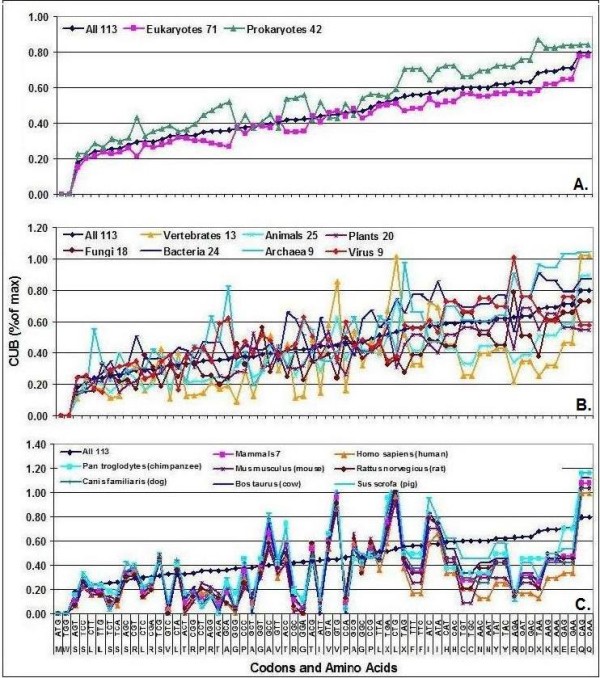
CUB Comparisons. Codon Usage Biases (CUB) were calculated in 113 species and sorted into subgroups. The mean CUBs of the 64 codons in the indicated subgroups are shown. (CUBmax = 100% for the 64 codons altogether). A: superdomains, B: kingdoms, C: some mammals.
Pan-genomic codon usage
I accumulated the CUF data from the 113 species into a single CUF Table (Table 1). This Table is intended to give a virtual representation of all organisms (Pan-Genome) and a numerical representation of the "universal" translation machinery. As many as 288 × E10 codons are represented in this collection. The distribution of CUB values in the Pan-Genomic CUF Table is illustrated in Figure 3. The transition from maximum-positive to maximum-negative values is smooth and there is no obvious or unambiguous border between the so-called dominant and prohibited codons. All possible codons are used.
Table 1.
Pan-Genomic CUF & CUB Table
| Am.Acid | Codon | Number | CUFi (#/1 k) | CUFij (% of fraction) | rCUF (% of fraction | CUBij (%) | |CUBij(%)| | Am.Acid | Codon | Number | CUFi (#/1 k) | CUFij (% of fraction) | rCUF (% of fraction | CUBij (%) | |CUBij(%)| |
| GLY | GGG | 3598776.0 | 12.5 | 19.0 | 25.0 | -6.0 | 6.0 | Trp | TGG | 3675912.0 | 12.7 | 100.0 | 100.0 | 0.0 | 0.0 |
| GLY | GGA | 5477754.0 | 19.0 | 28.9 | 25.0 | 3.9 | 3.9 | End | TGA | 308407.0 | 1.1 | 39.9 | 33.0 | 6.9 | 6.9 |
| GLY | GGT | 4451391.0 | 15.4 | 23.5 | 25.0 | -1.5 | 1.5 | Cys | TGT | 2509240.0 | 8.7 | 47.2 | 50.0 | -2.8 | 2.8 |
| GLY | GGC | 5445255.0 | 18.9 | 28.7 | 25.0 | 3.7 | 3.7 | Cys | TGC | 2810369.0 | 9.7 | 52.8 | 50.0 | 2.8 | 2.8 |
| Glu | GAG | 9756293.0 | 33.8 | 51.4 | 50.0 | 1.4 | 1.4 | End | TAG | 183171.0 | 0.6 | 23.7 | 33.0 | -9.3 | 9.3 |
| Glu | GAA | 9209632.0 | 31.9 | 48.6 | 50.0 | -1.4 | 1.4 | End | TAA | 281718.0 | 1.0 | 36.4 | 33.0 | 3.4 | 3.4 |
| Asp | GAT | 8195141.0 | 28.4 | 54.9 | 50.0 | 4.9 | 4.9 | Tyr | TAT | 4107194.0 | 14.2 | 48.6 | 50.0 | -1.4 | 1.4 |
| Asp | GAC | 6731842.0 | 23.3 | 45.1 | 50.0 | -4.9 | 4.9 | Tyr | TAC | 4337253.0 | 15.0 | 51.4 | 50.0 | 1.4 | 1.4 |
| Val | GTG | 6428801.0 | 22.3 | 35.3 | 25.0 | 10.3 | 10.3 | Leu | TTG | 4737403.0 | 16.4 | 17.5 | 16.7 | 0.8 | 0.8 |
| Val | GTA | 2695055.0 | 9.3 | 14.8 | 25.0 | -10.2 | 10.2 | Leu | TTA | 3136971.0 | 10.9 | 11.6 | 16.7 | -5.1 | 5.1 |
| Val | GTT | 4781792.0 | 16.6 | 26.3 | 25.0 | 1.3 | 1.3 | Phe | TTT | 5426287.0 | 18.8 | 48.0 | 50.0 | -2.0 | 2.0 |
| Val | GTC | 4280999.0 | 14.8 | 23.5 | 25.0 | -1.5 | 1.5 | Phe | TAC | 5873939.0 | 20.4 | 52.0 | 50.0 | 2.0 | 2.0 |
| Ala | GCG | 3487704.0 | 12.1 | 16.8 | 25.0 | -8.2 | 8.2 | Ser | TCG | 2485280.0 | 8.6 | 10.8 | 16.7 | -5.9 | 5.9 |
| Ala | GCA | 5031084.0 | 17.4 | 24.3 | 25.0 | -0.7 | 0.7 | Ser | TCA | 3962926.0 | 13.7 | 17.2 | 16.7 | 0.6 | 0.6 |
| Ala | GCT | 5779334.0 | 20.0 | 27.9 | 25.0 | 2.9 | 2.9 | Ser | TCT | 4499262.0 | 15.6 | 19.6 | 16.7 | 2.9 | 2.9 |
| Ala | GCC | 6432441.0 | 22.3 | 31.0 | 25.0 | 6.0 | 6.0 | Ser | TCC | 4191190.0 | 14.5 | 18.2 | 16.7 | 1.6 | 1.6 |
| Arg | AGG | 3071603.0 | 10.6 | 18.9 | 16.7 | 2.3 | 2.3 | Arg | CGG | 2379693.0 | 8.2 | 14.7 | 16.7 | -2.0 | 2.0 |
| Arg | AGA | 3953550.0 | 13.7 | 24.4 | 16.7 | 7.7 | 7.7 | Arg | CGA | 1898114.0 | 6.6 | 11.7 | 16.7 | -5.0 | 5.0 |
| Ser | AGT | 3473736.0 | 12.0 | 15.1 | 16.7 | -1.6 | 1.6 | Arg | CGT | 2071451.0 | 7.2 | 12.8 | 16.7 | -3.9 | 3.9 |
| Ser | AGC | 4391636.0 | 15.2 | 19.1 | 16.7 | 2.4 | 2.4 | Arg | CGC | 2842349.0 | 9.8 | 17.5 | 16.7 | 0.9 | 0.9 |
| Lys | AAG | 8869890.0 | 30.7 | 52.7 | 50.0 | 2.7 | 2.7 | Gin | CAG | 6974185.0 | 24.2 | 58.6 | 50.0 | 8.6 | 8.6 |
| Lys | AAA | 7946577.0 | 27.5 | 47.3 | 50.0 | -2.7 | 2.7 | Gin | CAA | 4922495.0 | 17.1 | 41.4 | 50.0 | -8.6 | 8.6 |
| Asn | AAT | 6514892.0 | 22.6 | 51.9 | 50.0 | 1.9 | 1.9 | His | CAT | 3408853.0 | 11.8 | 49.7 | 50.0 | -0.3 | 0.3 |
| Asn | AAC | 6036774.0 | 20.9 | 48.1 | 50.0 | -1.9 | 1.9 | His | CAC | 3453004.0 | 12.0 | 50.3 | 50.0 | 0.3 | 0.3 |
| Met | ATG | 6909100.0 | 23.9 | 100.0 | 100.0 | 0.0 | 0.0 | Leu | CTG | 7327412.0 | 25.4 | 27.1 | 16.7 | 10.4 | 10.4 |
| Ile | ATA | 3373624.0 | 11.7 | 22.2 | 33.0 | -10.8 | 10.8 | Leu | CTA | 2418342.0 | 8.4 | 8.9 | 16.7 | -7.7 | 7.7 |
| Ile | ATT | 5925942.0 | 20.5 | 39.0 | 33.0 | 6.0 | 6.0 | Leu | CTT | 4540618.0 | 15.7 | 16.8 | 16.7 | 0.1 | 0.1 |
| Ile | ATC | 5905801.0 | 20.5 | 38.8 | 33.0 | 5.8 | 5.8 | Leu | CTC | 4907197.0 | 17.0 | 18.1 | 16.7 | 1.5 | 1.5 |
| Thr | ACG | 2486009.0 | 8.6 | 15.9 | 25.0 | -9.1 | 9.1 | Pro | CCG | 2861706.0 | 9.9 | 18.9 | 25.0 | -6.1 | 6.1 |
| Thr | ACA | 4473401.0 | 15.5 | 28.6 | 25.0 | 3.6 | 3.6 | Pro | CCA | 4491106.0 | 15.6 | 29.7 | 25.0 | 4.7 | 4.7 |
| Thr | ACT | 4084032.0 | 14.2 | 26.1 | 25.0 | 1.1 | 1.1 | Pro | CCT | 4142534.0 | 14.4 | 27.4 | 25.0 | 2.4 | 2.4 |
| Thr | ACC | 4619047.0 | 16.0 | 29.5 | 25.0 | 4.5 | 4.5 | Pro | CCC | 3615638.0 | 12.5 | 23.9 | 25.0 | -1.1 | 1.1 |
| Summs | 288600157.0 | 1000.0 | 2100.0 | 2097.9 | 2.1 | 245.4 | |||||||||
| 10.14% of CUBmax | |||||||||||||||
Figure 3.
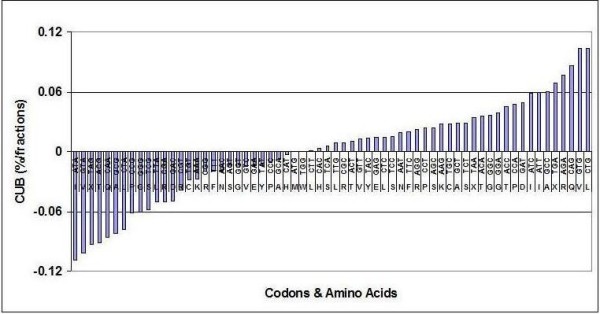
Distribution of Pan-Genomic CUB. CUB was taken from Pan-Genomic Codon Usage Table and sorted in ascending order.
There is a significant positive correlation between the number of synonymous codons (ni, #/amino acid) and the propensity of amino acids in the proteome (#/1000 amino acid residues). A similar correlation exists between synonymous codon frequency and CUB (Figure 4). These important correlations were discovered by analyzing the Pan-Genomic CUF Table (64 values) and were confirmed using individual data from all species (113 × 21 values).
Figure 4.
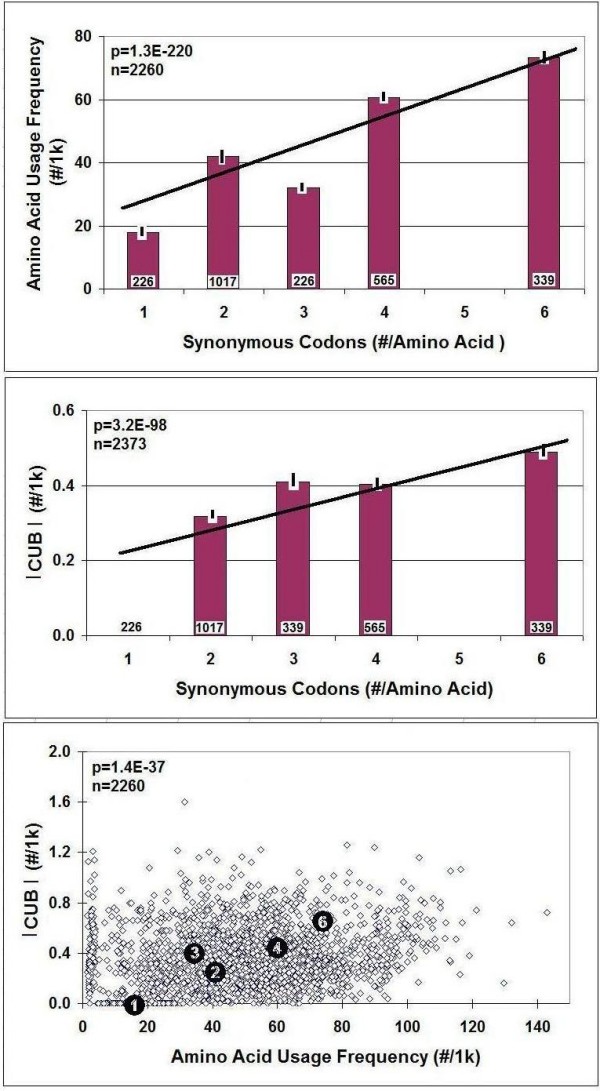
Correlations between Synonymous Codon Usage Frequency, Amino Acid Usage Frequency and Codon Usage Bias (CUB). The columns represent mean ± S.E.M., n is indicated within the columns. The significance of correlations is also included. Black circles indicate the positions of mean values and the numbers in the black circles indicate the number of synonymous codons/amino acid.
Another possible way to evaluate the possible phylogenetic relationships among CUBs in different species is to use the Pan-Genomic CUB Table as a common reference. I performed correlation analyses and compared the lists of species-specific CUB values to the list of mean CUB values in the Pan-Genomic CUB Table (64 × 113 comparisons), then used the significance of correlations as an indicator of CUB distances [Additional file 3].
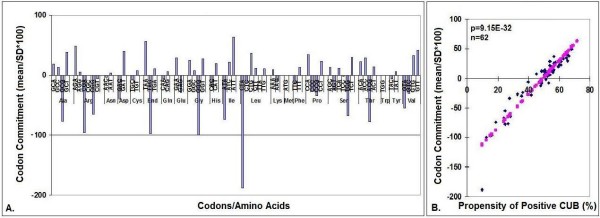
I found that the CUB of vertebrates is most similar (least distant) to the average CUB, while bacteria and viruses are most distant from it. This correlation analysis involves all codons and gives no information about the development of individual CUBs. I therefore compared the codon-specific CUB values in the 113 species to obtain a rough estimate of the stability of (commitment to) a CUB through evolution. The mean/SD of the 113 amino acid-specific CUB values gives a good estimate how this stability (Figure 5).
Figure 5.
Estimation of Codon Commitment. The mean ± SD values of CUB were calculated for the 64 codons (n = 113). The mean/SD*100 values were regarded as the measure of a codon's commitment to a given CUB through evolution. Very low (-) values indicate strong negative CUB (under-utilization of that codon) while the meaning of high (+) values is the opposite. The codon commitment value reflects the propensity towards over-utilized codons (positive CUB). A: individual values, B: correlation analyses.
Internal dynamics of codons
Correlations between individual CUB frequencies
When one of the synonymous codons is used more frequently than expected (positive CUB), another will be less frequently used (negative CUB). More generally, this means that codon usage changes in a subgroup of the 64 codons will be accompanied by changes in the opposite direction in the remaining codons.
I sorted the CUB values (64 × 113 = 7,232 listed in total) in the Pan-Genomic CUB Table according to their sizes and +/- directions [Additional file 4]. This sorting divided the 64 codons (c) into two subgroups (Ac and Bc) and the 113 species (s) into two additional groups (As and Bs). The Ac-As and Bc-Bs subgroups contained predominantly over-represented (positive CUB) codons and are located in the opposite diagonal corners of the Table. The Ac-Bs and Bc-As fields contained predominantly under-represented (negative CUB) codons and are located in the other opposite diagonal corners of the Table.
There is an internal inverse relationship between codons, which is valid and the same for all species. This inverse relationship is shown in a compressed and simplified form in Figure 6a, b.
Figure 6.
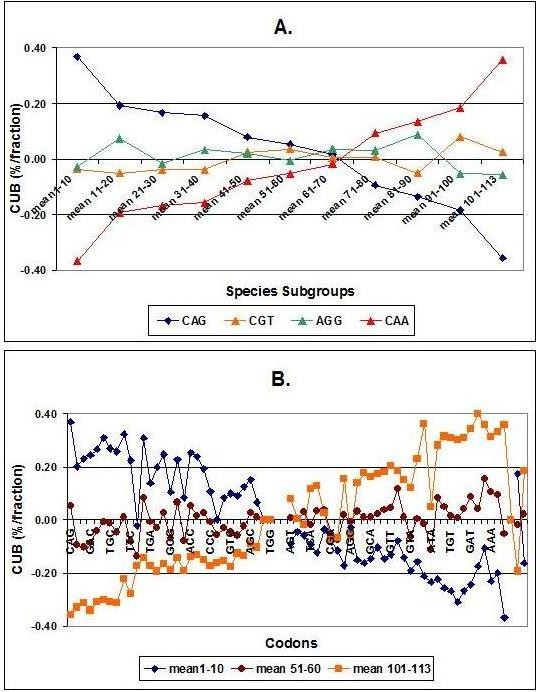
Species Dependent Internal Correlation between CUBs. Codon usage biases (CUBs) from 113 species were sorted as described in the text and divided into 11 consecutive subgroups. Each symbol represents the mean of CUB values from 10 different species. The values were sorted for species subgroups (A) and for codons (B). Only some representative samples are included (4 codons of total 64 and 3 groups of different species of total 11).
Negative correlations were expected between some subgroups of CUBs and others in the same species. Surprisingly, however, all codons and all species belong to only 2 clusters with highly correlated, opposite dynamics.
The above figures indicate that there is a close internal and inverse correlation between the CUBs of different codons. The magnitude and orientation of a CUB shows wide variation between species. Our collection of 113 species is too limited for any conclusion about the phylogenetic rules of development of CUB to be drawn, but the first impression is an absence of phylogenetic rules:
- about half the species under-utilize about half the codons, while the other half show the opposite behavior in respect of the remaining codons.
- It is difficult to find a correlation between CUB and taxon boundaries. All mammals (in the table) show a homogenous CUB pattern, while other taxa are much more diverse.
- Most codons show a wide pangenomic variation in CUB, but some vary much less than others (Figure 5). Some codons (TAG, GGG, CGA, CTA) are under-utilized by more than 80% of the 113 species listed, i.e. these synonymous codons have become committed to a given CUB orientation while others have not. There is a significant negative correlation between the proportion of codons committed to a given CUB orientation and the extent to which CUB varies (also apparent in Figure 5).
Internal relationship among codon bases in codon usage tables
Codons are defined by 3 nucleotides. Therefore, CUF Tables can be further analyzed as Nucleotide Usage Frequency (NUF) Tables.
The 113 CUF Tables in our material are based on 288 million codons and 690 K CDS. The number of codons in this collection is enough to provide reliable information about the general rules, if any, that determine nucleotide ratios and correlations in genomes.
There are some highly significant correlations among codon bases. The fractional frequency of each nucleotide base in every codon position correlates positively with its complementary codon (Table 2).
Table 2.
Positional nucleotide usage frequencies in 113 Species
| log(-C) | C1/# | C2/# | G3/# | C3/# | G1/# | G2/# | T2/# | T1/# | T3/# | A3/# | A2/# | A1/# |
| A1/# | -45.1 | -31.7 | -29.2 | -26.5 | -24.3 | -20.6 | 5.5 | 16.6 | 21.1 | 32.9 | 35.1 | 100.0 |
| A2/# | -23.1 | -21.4 | -19.5 | -15.4 | -20.9 | -27.0 | 1.1 | 13.9 | 14.5 | 18.5 | 100.0 | 35.1 |
| A3/# | -33.8 | -19.7 | -53.6 | -55.9 | -17.8 | -15.1- | 6.1 | 20.9 | 33.1 | 100.0 | 18.5 | 32.9 |
| T3/# | -25.0 | -12.2 | -50.9 | -56.0 | -16.7 | -18.7 | 6.4 | 24.9 | 100.0 | 33.1 | 14.5 | 21.1 |
| T1/# | -21.0 | -9.9 | -25.2 | -22.8 | -30.6 | -17.1 | 4.6 | 100.0 | 24.9 | 20.9 | 13.9 | 16.6 |
| T2/# | -10.3 | -13.0 | -6.6 | -6.3 | -1.3 | -7.2 | 100.0 | 4.6 | 6.4 | 6.1 | 1.1 | 5.5 |
| G2/# | 24.9 | 11.3 | 23.0 | 14.0 | 11.9 | 100.0 | -7.2 | -17.1 | -18.7 | -15.1 | -27.0 | -20.6 |
| G1/# | 12.4 | 12.4 | 18.6 | 17.5 | 100.0 | 11.9 | -1.3 | -30.6 | -16.7 | -17.8 | -20.9 | -24.3 |
| C3/# | 29.0 | 17.0 | 44.3 | 100.0 | 17.5 | 14.0 | -6.3 | -22.8 | -56.0 | -55.9 | -15.4 | -26.5 |
| G3/# | 32.9 | 15.3 | 100.0 | 44.3 | 18.6 | 23.0 | -6.6 | -25.2 | -50.9 | -53.6 | -19.5 | -29.2 |
| C2/# | 25.5 | 100.0 | 15.3 | 17.0 | 12.4 | 11.3 | -13.0 | -9.9 | -12.2 | -19.7 | -21.4 | -31.7 |
| C1/# | 100.0 | 25.5 | 32.9 | 29.0 | 12.4 | 24.9 | -10.3 | -21.0 | -25.0 | -33.8 | -23.1 | -45.1 |
C: Significance of correlation. – sign was added to negative correlations. log (-0) was regarded to be 100.
The sum of both complementary codon pairs (A+T and G+C) in every codon position is positively correlated to the sum of the same codon pair in the other two codon positions (Table 3). These correlations are valid for every species.
Table 3.
Positional nucleotide usage frequencies in 113 Species
| log(-C) | C1+G1 | C3+G3 | C2+G2 | C2+T2 | C1+T1 | G2+T2 | C3+T3 | G1+T1 | G3+T3 | A3+C3 | A1+C1 | A3+G3 | A2+C2 | A1+G1 | A2+G2 | A2+T2 | A3+T3 | A1+T1 |
| A1+T1 | -100.0 | -38.6 | -38.3 | -8.9 | -7.4 | -4.5 | -3.6 | -1.9 | -0.5 | 0.5 | 1.9 | 3.6 | 4.5 | 7.4 | 8.9 | 38.3 | 38.6 | 100.0 |
| A3+T3 | -38.6 | -100.0 | -24.9 | -4.6 | -6.9 | -2.7 | -4.0 | -0.4 | -2.0 | 2.0 | 0.4 | 4.0 | 2.7 | 6.9 | 4.6 | 24.9 | 100.0 | 38.6 |
| A2+T2 | 38.3 | -24.9 | -100.0 | -7.1 | -12.8 | -2.9 | -2.3 | -1.1 | -0.2 | 0.2 | 1.1 | 2.3 | 2.9 | 12.8 | 7.1 | 100.0 | 24.9 | 38.3 |
| A2+G2 | -8.9 | -4.6 | -7.1 | -100.0 | -3.0 | -7.1 | -5.4 | -11.2 | -0.5 | 0.5 | 11.2 | 5.4 | 7.1 | 3.0 | 100.0 | 7.1 | 4.6 | 8.9 |
| A1+G1 | -7.4 | -6.9 | -12.8 | -3.0 | -100.0 | -0.2 | -3.7 | -0.2 | -0.7 | 0.7 | 0.2 | 3.7 | 0.2 | 100.0 | 3.0 | 12.8 | 6.9 | 7.4 |
| A2+C2 | -4.5 | -2.7 | -2.6 | -7.1 | -0.2 | -100.0 | -0.8 | -2.4 | -0.2 | 0.2 | 2.4 | 0.8 | 100.0 | 0.2 | 7.1 | 2.9 | 2.7 | 4.5 |
| A3+G3 | -3.6 | -4.0 | -2.3 | -5.4 | -3.7 | -0.8 | -100.0 | -1.3 | -1.0 | 1.0 | 1.3 | 100.0 | 0.8 | 3.7 | 5.4 | 2.3 | 4.0 | 3.6 |
| A1+C1 | -1.9 | -0.4 | -1.1 | -11.2 | -0.2 | -2.4 | -1.3 | -100.0 | -0.6 | 0.6 | 100.0 | 1.3 | 2.4 | 0.2 | 11 2 | 1.1 | 0.4 | 1.9 |
| A3+C3 | -0.5 | -2.0 | -0.2 | -0.5 | -0.7 | -0.2 | -1.0 | -0.6 | -100.0 | 100.0 | 0.6 | 1.0 | 0.2 | 0.7 | 0.5 | 0.2 | 2.0 | 0.5 |
| G3+T3 | 0.5 | 2.0 | 0.2 | 0.5 | 0.7 | 0.2 | 1.0 | 0.6 | 100.0 | -100.0 | -0.6 | -1.0 | -0.2 | -0.7 | -0.5 | -0.2- | -2.0- | -0.5 |
| G1+T1 | 1.9 | 0.4 | 1.1 | 11.2 | 0.2 | 2.4 | 1.3 | 100.0 | 0.6 | -0.6 | -100.0 | -1.3 | -2.4 | -0.2 | -11.2 | -1.1 | -0.4 | -1.9 |
| C3+T3 | 3.6 | 4.0 | 2.3 | 5.4 | 3.7 | 0.8 | 100.0 | 1.3 | 1.0 | -1.0 | -1.3 | -100.0 | -0.8 | -3.7 | -5.4 | 2.3 | 4.0 | -3.6 |
| G2+T2 | 4.5 | 2.7 | 2.9 | 7.1 | 0.2 | 100.0 | 0.8 | 2.4 | 0.2 | -0.2 | -2.4 | -0.8 | -100.0 | -0.2 | -7.1 | -2.9 | -2.7 | -4.5 |
| C1+T1 | 7.4 | 6.9 | 12.8 | 3.0 | 100.0 | 0.2 | 3.7 | 0.2 | 0.7 | -0.7 | -0.2 | -3.7 | -0.2 | -100.0 | -3.0 | -12.8 | -6.9 | -7.4 |
| C2+T2 | 8.9 | 4.6 | 7.1 | 100.0 | 3.0 | 7.1 | 5.4 | 11.2 | 0.5 | -0.5 | -11.2 | -5.4 | -7.1 | -3.0 | -100.0 | -7.1 | -4.6 | -8.9 |
| C2+G2 | 38.3 | 24.9 | 100.0 | 7.1 | 12.8 | 2.9 | 2.3 | 1.1 | 0.2 | -0.2 | -1.1 | -2.3 | -2.9 | -12.8 | -7.1 | -100.0 | -24.9 | -38.3 |
| C3+G3 | 38.6 | 100.0 | 24.9 | 4.6 | 6.9 | 2.7 | 4.0 | 0.4 | 2.0 | -2.0 | -0.4 | -4.0 | -2.7 | -6.9 | -4.6 | -24.9 | -100.0 | -38.6 |
| C1+G1 | 100.0 | 38.6 | 38.3 | 8.9 | 7.4 | 4.5 | 3.6 | 1.9 | 0.5 | -0.5 | -1.9 | -3.6 | -4.5 | -7.4 | -8.9 | -38.3 | -38.6 | -100.0 |
C: Significance of correlation. – sign was added to negative correlations. log (-0) was regarded to be 100
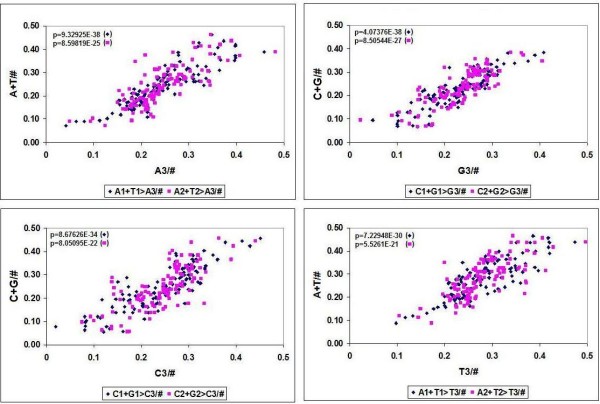
This strong positional correlation between codon bases suggests that it is possible to predict the frequency of usage of a nucleotide in the codon usage table from the frequencies of other nucleotides. Predictions regarding the third nucleotides in codons are especially interesting, because these are wobble bases for most amino acid codons.
I used the correlation between the sum of complementary codon pairs in the 1st and 2nd codon positions to predict the wobble bases using the frequencies for 113 different species (Table 4, Figure 7). This is of course a prediction of the frequencies of the four wobble bases in all 64 possible codons and has no predictive value for individual wobble bases belonging to individual amino acids. All these correlation were of course carefully compared to corresponding random controls. Care was taken to ensure that the randomized control samples had the same size and distribution as the test samples. The sum of randomized fractions was kept equal to 1, as in the test samples. There were no correlations between the corresponding nucleotides in the control samples.
Table 4.
Wobble base prediction
| A3/# | = | A1+T1/# | × | 1.1004 | + | -0.2898 | p = | 9.3E-38 |
| A3/# | = | A2+T2/# | × | 1.39228 | + | -0.6003 | p = | 8.6E-25 |
| C3/# | = | C1+G1/# | × | 1.14274 | + | -0.3466 | p = | 8.7E-34 |
| C3/# | = | C2+G2/# | × | 1.42309 | + | -0.3182 | p = | 8.1E-22 |
| G3/# | = | C1+G1/# | × | 0.95213 | + | -0.2566 | p = | 4.1E-38 |
| G3/# | = | C2+G2/# | × | 1.23154 | + | -0.251 | p = | 8.5E-27 |
| T3/# | = | C1+G1/# | × | 0.99447 | + | -0.2019 | p = | 7.2E-30 |
| T3/# | = | C2+G2/# | × | 1.26235 | + | -0.4851 | p = | 5.5E-21 |
Figure 7.
Correlation between Codon Bases in Codons of 113 Species. The frequency of the four possible nucleotide bases (A, T, G, G) in the 3 possible codons positions (1st. 2nd, 3rd) were counted in 113 codon usage tables and plotted against each other. A1+T1 > A3 means the correlation between the sum of the 1st A plus 1st T frequencies and the 3rd A frequency (n = 113).
This simple but highly significant and species-independent positional relationship between NUFs provides further strong support for the view that the genetic code is the result of development and not at all a "frozen accident".
Correlation between individual codons
The detection of a strong internal pangenomic relationship among codons in the CUF Tables and the positional correlation among the base residues of these codons led to an even deeper correlation analysis. The correlations between every single codon frequency and every other codon frequency (64 × 64/2 = 2,048) were calculated using linear regression analysis [Additional file 5].
Further detailed analysis of the internal positional correlations between codons and codon bases revealed significant correlations between different codons, which are generally valid for every species in our collection.
I noticed that there is a pattern of positive/negative correlations in these tables corresponding to the codon letters and their positions in the codon. The general rules of this pattern are summarized in Figure 8.
Figure 8.
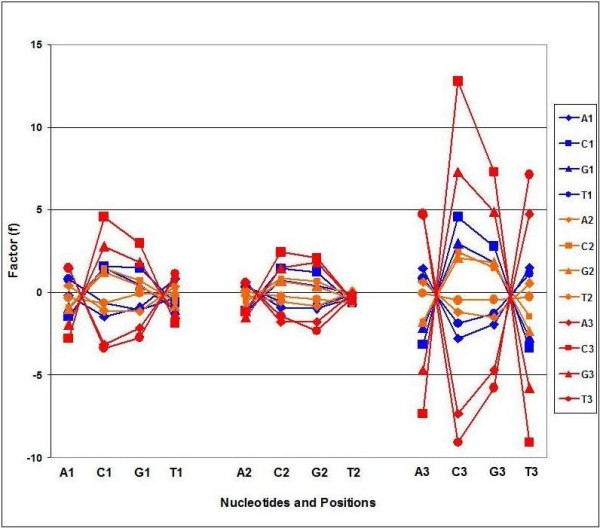
CUF – Pan-Genomic Codon Correlations. Codon frequencies were collected from 113 Codon Usage Frequency Tables and the correlation coefficients (C, 64 × 64) were calculated. f = -log C. A – sign was added to indicate negative correlations. The figure shows the f values between 4 × 4 codon letter combinations in 3 × 3 codon positions. Each symbol represent the mean of f values (n = 113). f < -2 and f > 2 correspond to statistically significant correlations.
There is a simple rule regarding codon correlations in the pangenome: there are positive correlations between complementary nucleotides and negative correlations between non-complementary nucleotides. This pattern of correlations is statistically significant in most combinations of nucleotide positions in codons. The correlations are statistically most significant between nucleotides in the 3rd codon positions.
Prediction of individual wobble bases
I used these correlations to predict individual wobble bases (all 64) from the 1st and 2nd letters of the codons (all 64). The possible correlations between a codon and the 16 possible permutations of the 4 1st and 2nd codon letters (64 × 4 × 4 = 1024) are listed in [Additional file 6].
Accuracy of codon predictions
I used the strongest correlations [Supplementary File 6] to predict codon frequencies, and the mean of several predictions was used as the averaged predicted value (p). Four different approaches were used to evaluate the predictions quantitatively.
The correlation between real (r) and predicted (p) values belonging to the same codons was significant (p < 0.05) in 54 cases but not the other 10 (Figure 9a).
Figure 9.
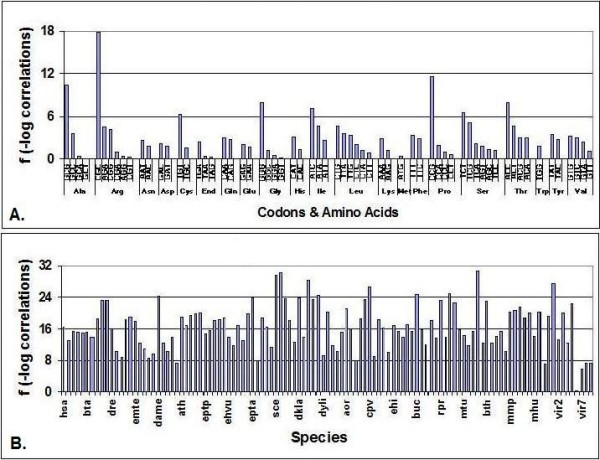
Accuracy of Codon Predictions – Amino Acid and Species Related Predictions. Codon frequencies (64) were predicted (p) in 113 species and compared to the real (r) values. The correlations between r and p were sorted for codons (A) and species (lB). The correlations were expressed as f values (-log correlation coefficient). An f > 1.5 can be regarded as statistically significant correlation.
The correlation between real (r) and predicted (p) values belonging to the same species was significant (p < 0.05) in all 113 cases and The p value was below 10E-07 in all but 2 species (Figure 9b).
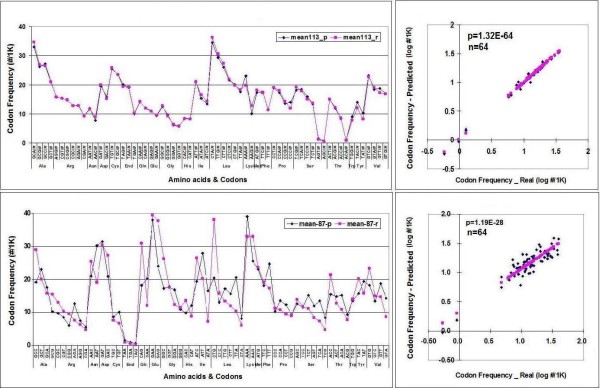
The average accuracy of individual CUF predictions in 113 species and 87 individual proteins was estimated by comparing the average real and predicted frequencies. The significance of the correlation between real and predicted CUF was 1.3E-64 when data from 113 species were averaged and compared (n = 64) and 1.9E-28 when data derived from 87 individual proteins (n = 64) were used (Figure 10).
Figure 10.
Accuracy of Codon Predictions in species and proteins. Codon frequencies were predicted in 113 species (A, B) and in 87 individual proteins(C, D). The average real (r) and predicted (p) codon frequencies were plotted (A, C) and correlations were analyzed (B, D).
Discussion
There are basically two approaches to measuring CUB. First, relative synonymous codon usage (RSCU) values can be calculated [5]. RSCU is the observed number of codon occurrences divided by the number expected if synonymous codons were used uniformly. Second, the relative merits of different codons can be assessed from the viewpoint of translational efficiency. This second approach led to the development of the Codon Adaptation Index (CAI, [6]). The CAI model assigns a parameter, termed 'relative adaptiveness', to each of the 61 codons (stop codons excluded). The relative adaptiveness of a codon is defined as its frequency relative to the most often-used synonymous codons and is computed from a set of highly expressed genes. The CAI is widely used even though the subjectivity involved in selecting the reference codons is well recognized [26,27].
My way of calculating CUB is very close to the original suggestion [5] and regards uniform codon usage as the "null hypothesis"; any deviation from this is the bias. This approach made it possible to avoid subjectivity and species limitations in choosing the reference set of codons, and I can build the concept of CUB on the massive foundation of statistical laws and the large collection of sequence data collected in Codon Usage Frequency Tables.
The origin and biological significance of CUB is not well understood, therefore I tried to find the rules (if any) of its evolutionary development and gain new insights about its possible function. I sort my findings into two main categories: I found
a.) some (few) signs of the evolutionary origin and development of CUB;
b.) unexpectedly large number of highly significant intern correlations between different codon residues (bases) at different codon positions (first, central, wobble) as well as between individual codons.
Inter-species variation in CUB is about 10%, but it is obvious that prokaryotes have significantly larger CUBs than eukaryotes. Bacteria may show the greatest bias because these primitive organisms are rich in highly-expressed genes and often use only one dominant codon. CUB decreases progressively with evolution and humans have the lowest bias (only about 20%). Evolutionary increase in codon number and genome complexity seems to reduce the CUB. It is noticeable that the average CUB (29.3 ± 1.1% (S.E.M.) n = 113) means that synonymous codon usage frequencies are 29.3% distant from the "all codons are equally good" hypothesis, and 70.7% distant from the "one codon is the best 'codon" alternative.
A more detailed qualitative analyzes of CUB is possible using a pan-genomic CUF Table. The original purpose of this virtual table was to create a reference for comparison of CUBs, but it turned out to reveal other codon-related connections too. The pan-genomic CUF Table is based on only 113 species, so it might be the first but not the last of its kind. It makes it possible to detect major, universal trends in codon usage behind small individual (or even species-wide) variations.
CUB is often correlated to the intensity of translation and has even been used to predict highly-expressed genes [6]. It is also known to be related to tRNA copy number, and co-evolution of tRNA gene composition and codon usage bias in genomes has been suggested [28]. I found a very strong correlation between the number of synonymous codons and the frequency of the amino acids they encoded, as well as the CUB. More synonymous codons encode more amino acids of the same kind and cause greater bias. This (rather logical) connection is not described in the literature, probably because the definition of CUB is very different from mine.
I tried to define a kind of "phylogenetic tree" of CUBs using the pan-genomic CUF table as reference. The significance of correlations between species-specific CUF and pan-genomic CUF gave a qualitative, theoretical measure of distances between codon usages. However this correlation-based approach did not successfully detect any recognizable, species-related evolutionary pattern.
Estimation of codon commitments through evolution showed that some codons are clearly over-utilized while other are avoided in most species. This finding is compatible with the concept of dominant and suppressed codons, but without stating that this difference is the result of evolution [29].
The non-randomness of synonymous codon usage is widely accepted today, and it has been suggested that independent forces (such as tRNA pool size [30]) have a role in the reading frame and there are contextual constraints on synonymous codon choice [31-33].
Other lines of evidence suggest that the Genetic Code itself (the 64 codons in toto as a system) has an inherited, internal structure [34,35]. Statistical studies on the nucleotide compositions of codons and of different codon positions support this concept [36-41].
I searched for the origin end development of codon bias and I found an extensive network of internal correlations between codons of a species and the nucleotides that define them. The correlations described in this article are:
- Correlation between the frequency of any single codon residue (base) at any codon position (first, central, wobble) and the frequency of any other single codon residue (base) at any other codon position (also first, central, wobble);
- Correlation between the sum of frequencies of any two codon residues (bases) at any two codon positions ((first, central, wobble) and the sum of any two other codon residues (bases) at any two other codon positions (also first, central, wobble);
- Correlation between A+T, G+C frequencies at the 1st, 2nd codon positions and A+T, G+C frequencies at the 3rd codon position;
- Correlations between any two codons.
There seems to be a simple rule behind all these statistically significant correlations: the correlation between any two nucleotides at any two codon positions is positive if the two nucleotides are complementary to each other and negative if they are not (illustrated in Figure 8).
The large number of statistically highly significant correlations made it possible to predict the frequencies of synonymous codons (in 113 species and 87 individual proteins) from the general overall frequencies of codons. The reliability of predictions was tested.
Conclusion
The cumulative Codon Usage Frequency of any codon is strongly dependent on the cumulative Codon Usage Frequency of other codons belonging to the same species. The rules of this codon dependency are the same for all species and reflect WC base pair complementarity. This internal connectivity of codons indicates that all synonymous codons are integrated parts of the Genetic Code with equal importance in maintaining its functional integrity. The so-called codon bias is a bias caused by the protein-centric view of the genome.
Competing interests
The author declares that they have no competing interests.
Authors' contributions
JB is the only author.
Supplementary Material
CUB Tables – Summary of 113 Species
Calculation of Codon Usage Bias (CUB) – Explanation and Example. The 64 codons were sorted in to 21 subgroups (fractions) corresponding to the 20 coded amino acids and the stop signal. The sum of synonymous codon frequencies were always regarded as 100% i.e. the sum of all codon frequencies is 2100% (color coded columns). The fractional frequency (CUFij %) of a synonymous codon is the contribution of that codon to this 100%. The theoretical, natural frequencies of the synonymous codons is regarded as equal to each other (for example the natural fractional frequency of each synonymous codon of Arg is 100%/6 = 16.7%). The difference between this theoretical (calculated) frequency and the real (counted) fractional frequency of a codon is the CUBij %. However it is necessary to use the |CUBij %| value instead to be able to calculate and compare the total CUB values of entire proteins (i.e. the sum of 64 CUB values). A theoretical extreme case of codon usage is when only one of all synonymous codons is used (CUB% 1 max column). The maximal possible CUB of all codons will in this case be 2416.7%, which is regarded as the CUFmax. In the real case of Homo sapiens the sum of fractional frequencies is 456, which is 18.9% of the theoretical CUBmax.
Correlation Analyses of Codon Usage Bias (CUB) in 113 Species. CUBs of 113 species (each containing 64 values) were compared to the virtual CUB values in the Pan-Genomic Codon Usage Table by linear regression analyses. The – log C values were used as a measure of similarity and are indicated by horizontal bars at the right edge of the table. C: significance of correlation. The subgroups, corresponding to larger phylogenetic categories, are color coded and mean values for the groups are also indicated. The numbers of species in the subgroups are given in the "Mean" rows.
CUB commitment and variation. The 64 codons in 113 species were sorted according to the size and +/- orientation of their CUB. Some manual adjustments were made to segregate the data into four approximately symmetrical subgroups (corresponding to the color codes).
CUF- Pan-Genomic Codon Correlations. Codon frequencies were collected from 113 Codon Usage Frequency Tables and the significances of correlations (C, 64 × 64) were calculated. (n = 113). The table displays the -log C values. A – sign was added to the -log C value to indicate negative correlations. Significant positive correlations (values > 2) are indicated by bold numbers and gray background, while significant negative correlations (values < -2) are indicated by italic numbers and pink background. The collected data are sorted into 4 × 4 × 3 × 3 = 144 different subgroups corresponding to the 4 × 4 codon letter combinations and the 3 × 3 codon positions (red letters).
Prediction of Wobble Bases. List of correlations between the frequency of a codon and the frequency of other codons, which contain the 4 × 4 permutations of codons at the 1st and 2nd codon positions. 64 times 16 equations were calculated from these correlations. Only the strongest correlations, those used in codon predictions, are listed and color coded. Positive correlations are indicated by bold letter in blue background and negative correlations are given by italic letters in pink background. F is as defined in fig. 9.
Acknowledgments
Acknowledgements
The continuous support and editorial help of Dr P S Agutter is very much acknowledged.
References
- Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM. The sounds of silence: synonymous mutations affect function. Pharmacogenomics. 2007;8:527–532. doi: 10.2217/14622416.8.6.527. [DOI] [PubMed] [Google Scholar]
- Codon Usage Database – NCBI-GenBank Flat File Release 156.0 http://www.kazusa.or.jp/codon/ [October 15 2006].
- Gouy M, Gautier C. Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res. 1982;10:7055–7074. doi: 10.1093/nar/10.22.7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Li WH. An evolutionary perspective on synonymous codon usage in unicellular organisms. J Mol Evol. 1986;24:28–38. doi: 10.1007/BF02099948. [DOI] [PubMed] [Google Scholar]
- Sharp PM, Tuohy TM, Mosurski KR. Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res. 1986;14:5125–5143. doi: 10.1093/nar/14.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Li WH. The codon Adaptation Index – a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987;15:1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains W. Codon distribution in vertebrate genes may be used to predict gene length. J Mol Biol. 1987;197:379–388. doi: 10.1016/0022-2836(87)90551-1. [DOI] [PubMed] [Google Scholar]
- Eyre-Walker A. Synonymous codon bias is related to gene length in Escherichia coli: selection for translational accuracy? Mol Biol Evol. 1996;13:864–872. doi: 10.1093/oxfordjournals.molbev.a025646. [DOI] [PubMed] [Google Scholar]
- Wan X, Xu D, Zhou J. A new informatics method for measuring synonymous codon usage bias. In: Dagli C, Buczak A, Ghosh J, Embrechts M, Ersoy E, editor. Intelligent engineering systems through artificial neural networks. Vol. 13. ASME Press, New York, NY; 2003. pp. 1101–1018. [Google Scholar]
- Ma J, Campbell A, Karlin S. Correlations betweenShine-Dalgarno sequences and gene features such as predicted expression levels and operon structures. J Bacteriol. 2002;184:5733–5745. doi: 10.1128/JB.184.20.5733-5745.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobry JR, Gautier C. Hydrophobicity, expressivity and aromaticity are the major trends of amino-acid usage in 999 Escherichia coli chromosome-encoded genes. Nucleic Acids Res. 1994;22:3174–3180. doi: 10.1093/nar/22.15.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Ding D, Tao X, Dafu D. The relationship between synonymous codon usage and protein structure. FEBS Lett. 1998;434:93–96. doi: 10.1016/S0014-5793(98)00955-7. [DOI] [PubMed] [Google Scholar]
- D'Onofrio G, Ghosh TC, Bernardi G. The base composition of the genes is correlated with the secondary structures of the encodedproteins. Gene. 2002;300:179–187. doi: 10.1016/S0378-1119(02)01045-4. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol. 1981;151:389–409. doi: 10.1016/0022-2836(81)90003-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982;158:573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- Bulmer M. Coevolution of codon usage and transfer RNAabundance. Nature. 1987;325:728–730. doi: 10.1038/325728a0. [DOI] [PubMed] [Google Scholar]
- Kanaya S, Yamada Y, Kudo Y, Ikemura T. Studies of codon usage and tRNA genes of 18 unicellular organisms and quantification of Bacillus subtilis tRNAs: gene expression level and species- specific diversity of codon usage based on multivariate analysis. Gene. 1999;238:143–155. doi: 10.1016/S0378-1119(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Sueoka N. Directional mutation pressure, selective constraints, and genetic equilibria. J Mol Evol. 1999;34:95–114. doi: 10.1007/BF00182387. [DOI] [PubMed] [Google Scholar]
- Sueoka N. Two aspects of DNA base composition: G+C content and translation-coupled deviation from intra-strand rule of A = T and G = C. J Mol Evol. 1999;49:49–62. doi: 10.1007/PL00006534. [DOI] [PubMed] [Google Scholar]
- Bernardi G. Compositional constraints and genome evolution. J Mol Evol. 1986;24:1–11. doi: 10.1007/BF02099946. [DOI] [PubMed] [Google Scholar]
- Karlin S, Mrazek J. What drives codon choices in human genes? J Mol Biol. 1996;262:459–472. doi: 10.1006/jmbi.1996.0528. [DOI] [PubMed] [Google Scholar]
- Antezana MA, Kreitman M. The nonrandom location of synonymous codons suggests that reading frame-independent forces have patterned codon preferences. J Mol Evol. 1999;49:36–43. doi: 10.1007/PL00006532. [DOI] [PubMed] [Google Scholar]
- Sueoka N, Kawanishi Y. DNA G+C content of the third codon position and codon usage biases of human genes. Gene. 2000;261:53–62. doi: 10.1016/S0378-1119(00)00480-7. [DOI] [PubMed] [Google Scholar]
- Sequence Manipulation Suite Codon Usage http://bioinformatics.org/sms2/codon_usage.html
- KEGG Kyoto Encyclopedia of Genes and Genomes., KEGG Release 40.0. 2006. http://www.genome.jp/kegg/
- Jansen R, Bussemaker HJ, Gerstein M. Revisiting the codon adaptation index from a whole-genome perspective: analyzing the relationship between gene expression and codon occurrence in yeast using a variety of models. Nucleic Acids Research. 2003;31:2242–2251. doi: 10.1093/nar/gkg306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin S, Campbell AM, Mrazek J. Comparative DNA analysis across diverse genomes. Annu Rev Genet. 1998;32:185–225. doi: 10.1146/annurev.genet.32.1.185. [DOI] [PubMed] [Google Scholar]
- Rocha EPC. Codon usage bias from tRNA's point of view: redundancy, specialization, and efficient decoding for translation optimization. Genome Res. 2004;14:2279–2286. doi: 10.1101/gr.2896904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten RJ. Forbidden synonymous substitutions in coding regions. Mol Biol Evol. 1993;10:205–220. doi: 10.1093/oxfordjournals.molbev.a039996. [DOI] [PubMed] [Google Scholar]
- Antezana MA, Kreitman M. The nonrandom location of synonymous codons suggests that reading frame-independent forces have patterned codon preferences. J Mol Evol. 1999;49:36–43. doi: 10.1007/PL00006532. [DOI] [PubMed] [Google Scholar]
- Lipman DJ, Wilbur WJ. Contextual constraints on synonymous codon choice. J Mol Biol. 1983;163:363–376. doi: 10.1016/0022-2836(83)90063-3. [DOI] [PubMed] [Google Scholar]
- Ticher A, Graur D. Nucleic acid composition, codon usage, and the rate of synonymous substitution in protein-coding genes. J Mol Evol. 1989;28:286–298. doi: 10.1007/BF02103424. [DOI] [PubMed] [Google Scholar]
- Fedorov A, Saxonov S, Gilbert W. Regularities of context-dependent codon bias in eukaryotic genes. Nucleic Acids Res. 2002;30:1192–1197. doi: 10.1093/nar/30.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro JC, Benyo B, Sansom C, Szlavecz A, Fordos G, Micsik T, Benyo Z. A common periodic table of codons and amino acids. Biochem Biophys Res Commun. 2003;306:408–15. doi: 10.1016/S0006-291X(03)00974-4. [DOI] [PubMed] [Google Scholar]
- Biro JC. Protein folding information in nucleic acids which is not present in the genetic code. Ann N Y Acad Sci. 2006;1091:399–411. doi: 10.1196/annals.1378.083. [DOI] [PubMed] [Google Scholar]
- Frank GK, Makeev VJ. G and T nucleotide contents show specie-invariant negative correlation for all three codon positions. J Biomol Struct Dyn. 1997;14:629–639. doi: 10.1080/07391102.1997.10508163. [DOI] [PubMed] [Google Scholar]
- Wang J. The base contents of A, C, G or U for the three codon positions and the total coding sequences show positive correlation. J Biomol Struct Dyn. 1998;16:51–57. doi: 10.1080/07391102.1998.10508226. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Gupta SK, Sundararajan VS, Ghosh TC. Compositional correlation studies among the three different codon positions in 12 bacterial genomes. Biochem Biophys Res Commun. 1999;266:66–71. doi: 10.1006/bbrc.1999.1774. [DOI] [PubMed] [Google Scholar]
- D'Onofrio G, Bernardi G. A universal compositional correlation among codon positions. Gene. 1992;110:81–88. doi: 10.1016/0378-1119(92)90447-W. [DOI] [PubMed] [Google Scholar]
- Wada A. Compliance of genetic code with base-composition deflecting pressure. Adv Biophys. 1992;28:135–158. doi: 10.1016/0065-227X(92)90024-L. [DOI] [PubMed] [Google Scholar]
- Gutiérrez G, Oliver JL, Marín A. Dinucleotides and G+C content in human genes: opposite behavior of GpG, GpC, and TpC at II-III codon positions and in introns. J Mol Evol. 1993;37:131–136. doi: 10.1007/BF02407348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CUB Tables – Summary of 113 Species
Calculation of Codon Usage Bias (CUB) – Explanation and Example. The 64 codons were sorted in to 21 subgroups (fractions) corresponding to the 20 coded amino acids and the stop signal. The sum of synonymous codon frequencies were always regarded as 100% i.e. the sum of all codon frequencies is 2100% (color coded columns). The fractional frequency (CUFij %) of a synonymous codon is the contribution of that codon to this 100%. The theoretical, natural frequencies of the synonymous codons is regarded as equal to each other (for example the natural fractional frequency of each synonymous codon of Arg is 100%/6 = 16.7%). The difference between this theoretical (calculated) frequency and the real (counted) fractional frequency of a codon is the CUBij %. However it is necessary to use the |CUBij %| value instead to be able to calculate and compare the total CUB values of entire proteins (i.e. the sum of 64 CUB values). A theoretical extreme case of codon usage is when only one of all synonymous codons is used (CUB% 1 max column). The maximal possible CUB of all codons will in this case be 2416.7%, which is regarded as the CUFmax. In the real case of Homo sapiens the sum of fractional frequencies is 456, which is 18.9% of the theoretical CUBmax.
Correlation Analyses of Codon Usage Bias (CUB) in 113 Species. CUBs of 113 species (each containing 64 values) were compared to the virtual CUB values in the Pan-Genomic Codon Usage Table by linear regression analyses. The – log C values were used as a measure of similarity and are indicated by horizontal bars at the right edge of the table. C: significance of correlation. The subgroups, corresponding to larger phylogenetic categories, are color coded and mean values for the groups are also indicated. The numbers of species in the subgroups are given in the "Mean" rows.
CUB commitment and variation. The 64 codons in 113 species were sorted according to the size and +/- orientation of their CUB. Some manual adjustments were made to segregate the data into four approximately symmetrical subgroups (corresponding to the color codes).
CUF- Pan-Genomic Codon Correlations. Codon frequencies were collected from 113 Codon Usage Frequency Tables and the significances of correlations (C, 64 × 64) were calculated. (n = 113). The table displays the -log C values. A – sign was added to the -log C value to indicate negative correlations. Significant positive correlations (values > 2) are indicated by bold numbers and gray background, while significant negative correlations (values < -2) are indicated by italic numbers and pink background. The collected data are sorted into 4 × 4 × 3 × 3 = 144 different subgroups corresponding to the 4 × 4 codon letter combinations and the 3 × 3 codon positions (red letters).
Prediction of Wobble Bases. List of correlations between the frequency of a codon and the frequency of other codons, which contain the 4 × 4 permutations of codons at the 1st and 2nd codon positions. 64 times 16 equations were calculated from these correlations. Only the strongest correlations, those used in codon predictions, are listed and color coded. Positive correlations are indicated by bold letter in blue background and negative correlations are given by italic letters in pink background. F is as defined in fig. 9.