Abstract
Background
There is limited knowledge on the extent and dynamics of the mucosal response to commensal and probiotic species in the human intestinal lumen. This study aimed to identify the acute, time-dependent responses of intestinal mucosa to commensal Lactobacillus plantarum WCFS1 in vivo in two placebo-controlled human intervention studies in healthy volunteers. Transcriptional changes in duodenal mucosa upon continuous intraduodenal infusion of L. plantarum WCFS1 for one- and six h, respectively, were studied using oro- and nasogastric intubations with dedicated orogastric catheters and tissue sampling by standard flexible gastroduodenoscopy.
Results
One- and six-h exposure of small intestinal mucosa to L. plantarum WCFS1 induced differential expression of 669 and 424 gene reporters, respectively. While short-term exposure to L. plantarum WCFS1 inhibited fatty acid metabolism and cell cycle progression, cells switched to a more proliferative phase after prolonged exposure with an overall expression profile characterized by upregulation of genes involved in lipid metabolism, cellular growth and development. Cell death and immune responses were triggered, but cell death-executing genes or inflammatory signals were not expressed. Proteome analysis showed differential expression of several proteins. Only the microsomal protein 'microsomal triglyceride transfer protein' was regulated on both the transcriptional and the protein level in all subjects.
Conclusion
Overall, this study showed that intestinal exposure to L. plantarum WCFS1 induced consistent, time-dependent transcriptional responses in healthy intestinal mucosa. This extensive exploration of the human response to L. plantarum WCFS1 could eventually provide molecular support for specific or probiotic activity of this strain or species, and exemplifies the strength of the applied technology to identify the potential bio-activity of microbes in the human intestine.
Background
Many lactic acid bacteria (LAB) have a long history of use in the preservation of food ingredients [1]. In addition to their preservative effect, which is largely based on their effectiveness to convert the available carbon sources in the raw materials to lactic and acetic acid, LAB-fermentation generates many other relevant product characteristics like texture, flavor and stability. Various LAB species, given as food supplements, exert beneficial bioactivity in the intestine [2,3].
The study of interactions between the human host and microbes that confer a health promoting effect is mostly limited to an evaluation of the effects of the microbes on gastrointestinal or systemic symptom scores. Several studies described beneficial effects of dietary supplementation with various LAB, including specific strains of the species Lactobacillus plantarum on symptoms in patients suffering from irritable bowel syndrome (IBS) [4], in inflammatory bowel disease (IBD) patients [5], and on the intestinal barrier function in stressed conditions [6]. Detailed, descriptive studies of the human mucosal response to LAB are scarce. DiCaro et al. investigated the effects of L. rhamnosus GG on transcriptional responses of small intestinal mucosa in oesophagitis patients [7], delivering a myriad of information about the impact of a probiotic supplementation regimen on the human mucosal response to probiotics in a limited number (n = 3) of subjects suffering from an underlying medical condition.
The present study aimed to identify the response of healthy intestinal mucosa upon exposure to L. plantarum WCFS1, a single colony isolate from NCIMB8826 (National Collection of Industrial and Marine Bacteria, Aberdeen, UK). This strain was originally isolated from human saliva and is used as a model microbe in the study of host-microbe interactions, due to its established taxonomy, its ability to survive gastrointestinal (GI) transit [8] and its putative role as a probiotic species [9]. The availability of the complete genome sequence of L. plantarum WCFS1 [10] has strongly facilitated the study of its response to the conditions encountered in the mammalian GI tract.
In the present study, we applied a recently developed in vivo intestinal perfusion technique [11] to assess the transcriptional and proteomic response of intestinal mucosa to short (1 h) and extended (6 h) exposures to L. plantarum WCFS1 in healthy subjects. These studies focused on the proximal small intestine, because in healthy people, only small amounts of microbes reside in this intestinal region, whereas it encounters and perceives large amounts of food-derived microbes. The fact that only small amounts of microbes reside in that specific niche enables to determine effects of the L. plantarum WCFS1 exposures without interference of other mucosa-microbe interactions, as would be the case in the distal small intestine or the colon, which harbour a large microbial community. This manuscript describes, for the first time in healthy subjects, the responses of the intestinal mucosa to intraluminal microbes. Biological interpretation of the gene expression analysis revealed that especially genes associated with lipid metabolism, cellular proliferation, cell death and survival and immune responses were modulated. The impact of the microbes on transcription of genes involved in these processes is presented and discussed in detail.
Results
The mucosal responses analyzed and presented here represent the acute response of epithelial cells interacting with L. plantarum cells and their secreted products and that of cells from non-interacting, neighboring epithelial cells in the lamina propria, which are not directly exposed to luminal contents, and other cell types such as immune cells.
Study 1
We observed significant changes in 669 gene reporters; 225 genes upregulated and 444 gene reporters downregulated (see Additional file 1). The fold changes of these differentially expressed genes ranged from 0.56 to 1.33. A fold change of 0.56 means that 56% of the expression of the gene in the placebo intervention remains after the L. plantarum WCFS1 intervention, whereas a fold change of 1.33 means that the expression of that gene increased by 33% due to the L. plantarum WCFS1 exposure.
The biological implications of the altered transcriptional profiles were first identified using the pathway analysis software suite GenMapp. Table 1 shows the 24 pathways, based on the Gene Ontology database, that had a Z-score > 3, and 3 gene reporters or more were differentially expressed. Among these processes, protein biosynthesis and -metabolism, ribosome-associated processes, processes involved in protease activity and fatty acid oxidation seem to be the most important modulated biological processes
Table 1.
GenMAPP pathways affected by a 1-h exposure of duodenal mucosa to L. plantarum WCFS1
| GO Name | Changed | Measured | Total |
| caveola | 3 | 4 | 4 |
| Golgi stack | 3 | 10 | 12 |
| protein biosynthesis | 20 | 173 | 252 |
| peptidyl-prolyl cis-trans isomerase activity | 6 | 26 | 32 |
| protein metabolism | 3 | 13 | 16 |
| structural constituent of ribosome | 18 | 153 | 232 |
| isomerase activity | 11 | 77 | 96 |
| cytosolic large ribosomal subunit (sensu Eukarya) | 7 | 38 | 39 |
| cytoplasm | 35 | 512 | 636 |
| ribosome | 13 | 117 | 167 |
| heterogeneous nuclear ribonucleoprotein complex | 4 | 16 | 17 |
| translational initiation | 3 | 18 | 26 |
| endoplasmic reticulum | 22 | 234 | 271 |
| ER to Golgi transport | 4 | 18 | 20 |
| GTPase activity | 3 | 21 | 38 |
| regulation of translational initiation | 4 | 25 | 32 |
| fatty acid beta-oxidation | 3 | 12 | 13 |
| ubiquitin conjugating enzyme activity | 7 | 46 | 75 |
| chymotrypsin activity | 10 | 79 | 119 |
| catalytic activity | 6 | 96 | 210 |
| regulation of translation | 3 | 24 | 27 |
| metabolism | 19 | 243 | 408 |
| trypsin activity | 10 | 79 | 134 |
| cytosolic small ribosomal subunit (sensu Eukarya) | 5 | 29 | 30 |
Biological processes with relatively high numbers of gene reporters changed, based on the Gene Ontology (GO) database. The table shows the process title (GO name), the number of gene reporters changed changed (changed), measured with the microarrays (measured) and the total number of genes present in the pathway MAPP (total) (for detailed description of pathway selection, see Methods).
Study 2
The 6-h L. plantarum challenge significantly modulated the expression of 424 gene reporters; 383 were upregulated and 41 were downregulated (see Additional file 2). The fold changes of these differentially expressed genes ranged from 0.70 to 1.78. Genmapp pathway analysis showed that, based on Gene Ontology databases with the criteria set at Z-score > 5, and 3 gene reporters or more differentially expressed, 22 pathways were mediated by the intervention (Table 2). Processes which are associated with the MHC class I and II and other immune response and antigen processing related processes were modulated by the intervention, as well as several processes which are involved in energy metabolism (mainly fatty acid beta-oxidation, tricarboxylic acid cycle and electron transport chain).
Table 2.
GenMAPP pathways affected by a 6-h exposure of duodenal mucosa to L. plantarum WCFS1
| GO Name | Changed | Measured | Total |
| energy pathways | 14 | 83 | 92 |
| Cytoplasm | 26 | 514 | 636 |
| oxidoreductase activity | 30 | 318 | 453 |
| MHC class II receptor activity | 5 | 11 | 31 |
| tricarboxylic acid cycle | 7 | 22 | 29 |
| fatty acid beta-oxidation | 5 | 12 | 13 |
| electron transport | 27 | 221 | 346 |
| antigen presentation\, exogenous antigen | 4 | 10 | 30 |
| antigen presentation\, endogenous antigen | 4 | 7 | 13 |
| antigen processing\, exogenous antigen via MHC class II | 4 | 10 | 30 |
| antigen processing\, endogenous antigen via MHC class I | 4 | 10 | 18 |
| endoplasmic reticulum | 26 | 235 | 271 |
| Mitochondrion | 29 | 365 | 464 |
| cytochrome-c oxidase activity | 5 | 16 | 27 |
| Microsome | 14 | 94 | 118 |
| unspecific monooxygenase activity | 6 | 23 | 23 |
| lipid metabolism | 9 | 104 | 129 |
| Glycolysis | 8 | 39 | 56 |
| Cytosol | 12 | 126 | 141 |
| mitochondrial membrane | 4 | 10 | 13 |
| inner membrane | 6 | 26 | 33 |
| hydrogen ion transporter activity | 3 | 26 | 33 |
Biological processes with relatively high numbers of gene reporters changed, based on the Gene Ontology (GO) database. The table shows the process title (GO name), the number of gene reporters changed changed (changed), measured with the microarrays (measured) and the total number of genes present in the pathway MAPP (total) (for detailed description of pathway selection, see Methods).
The proteome analyses of the tissue samples obtained in study 2 showed that two proteins differed consistently in all volunteers in response to the 6-h exposure to L. plantarum (data not shown). One of these spots was identified as microsomal triglyceride transfer protein (MTP), while the analysis failed to allow identification of the second spot. The microarray results showed that the expression of the gene encoding MTP (probeset 205675_at on the Affymetrix U113A microarray and indicated by UniGene reference Hs.195799) was increased by 19.3%, as a result of the exposure to L. plantarum. Paradoxically, the same gene appeared to be down-regulated by 36.8% in the 1 h L. plantarum intervention study, but this difference did not reach statistical significance. The tissue samples from study 1 were not subjected to proteome analyses.
Comparative analysis of study 1 and 2
Fifty-four genes were regulated in both the 1 h and 6 h perfusion microarray datasets. Of these genes, 4 were differentially expressed in the same direction in both studies (3 were upregulated and 1 downregulated), 2 were upregulated and downregulated, and 48 were downregulated and upregulated in study 1 and 2, respectively. Below, the findings of the two studies are compared in detail using the Ingenuity Pathways Analysis (IPA) software tool (see Availability and requirements section for URL). The statistical assessments of significance of representation of the metabolic and signaling pathways that are part of the IPA output are based on a right-tailed Fisher's Exact Test. This test calculates the probability that genes participate in a given pathway, relative to their occurrence in all other pathway annotations covered by Ingenuity.
Lipid biosynthesis and fatty acid metabolism
After 1 h of exposure to L. plantarum, three genes involved in lipid and fatty acid metabolism (ACSL5; CD36 antigen, and DEGS1) were downregulated (Figure 1). After 6 h exposure, the transcription regulators JUND, GTF2I and TSC22-D1, which are involved in protection from oxidative stress and lipid/fatty acid metabolism, GPX4, which is involved in protection against oxidative stress, the fatty acid binding protein FABP1, the thrombospondin receptor (or fatty acid translocase) CD36, six acyl-CoA genes and isocitrate dehydrogenase-1 (IDH1), which is one of the more "central" hub proteins were all upregulated. However, important central regulatory proteins such as the obesity gene leptin or PPAR-γ were not differentially expressed after 6 h exposure (Figure 2).
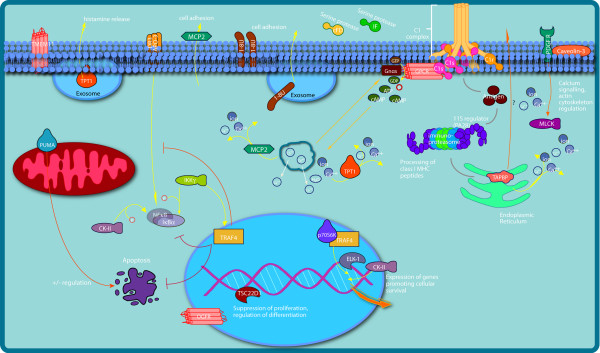
Figure 1.
Cellular responses after one hour exposure to L. plantarum. After continuous injection of L. plantarum WCFS1 for one hour into the proximal duodenum, proteins that participate in integrated signalling, metabolic and response pathways were modulated in intestinal mucosa. The left area shows genes encoding proteins involved in the regulation of cell death and survival, the central and right areas shows diverse signalling pathways and proteins that are involved in complement activation (immune response; may include genes expressed in immune cells). Note the involvement of nuclear proteins in modulation of cell death and survival.
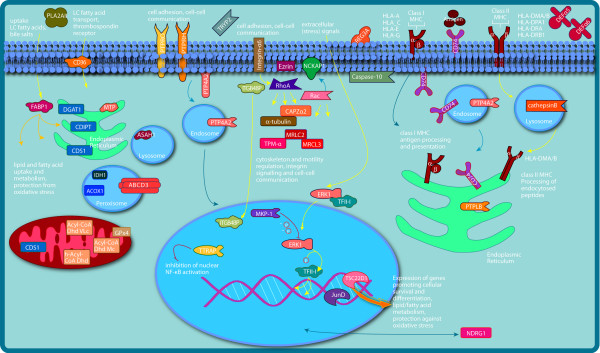
Figure 2.
Cellular responses after six hours exposure to L. plantarum. After continuous injection of L. plantarum WCFS1 for six hours into the proximal duodenum, proteins involved in fatty acid metabolism, cell motility and immune responses were modulated. Genes encoding proteins involved in fatty acid metabolism are in the upper left area of the image, those involved in cell motility (may include genes expressed in immune cells) are in the central area, and genes involved in innate and adaptive immune response are in the upper right area. Note the modulated ERK/MAPK/MKP-1 signalling pathway that extends into the nucleus.
Major transcription factors and proteins mediating cellular proliferation
After 1 h exposure to L. plantarum WCFS1, four major transcriptional regulators were all found to be downregulated: the oncogene product MYC (c-Myc), FOS (c-Fos), JUN (c-Jun) and the p65 subunit of the NF-κB transcription complex, RELA. From these responses, it appears that downregulation of genes modulating cellular metabolism and proliferation is the central mucosal response to the first perception of L. plantarum. None of these regulators were differentially expressed after the 6 h exposure.
Cell death and death-inducing factors
Several genes encoding proteins which are involved in regulation and modulation of cell death were regulated in both studies. The tumor necrosis factor receptors (TNFRs) TNFRS1A, which is a major receptor for TNF-α and a mediator of apoptosis and regulator of inflammation, and TNFRSF4 were downregulated after 1 h infusion of L. plantarum, whereas the TNF receptor TNFRSF25 (DR3) was upregulated after the 1-h exposure to L. plantarum WCFS1. Expression of the TNF-related proteins TNFAIP1 (B12), which is a membrane-located ion channel and a direct target of TNF-induced signaling, transforming growth factor alpha (TGF-α), and the TNFR inducer interleukin 1-β were downregulated as well after 1 h of exposure to L. plantarum WCFS1.
Under cellular immune-response stimulating conditions, translocation of the transcription factor NFκB to the nucleus promotes the expression of predominantly proinflammatory cytokines. The p65 (RelA) component of NF-κB was downregulated after 1 h exposure. Expression of one of the upstream regulators of NF-κB function, IKBKG (IKK-κ or NEMO), was upregulated. IKK phosphorylates IκB, thereby targeting IκB for proteolytic degradation and indirectly enabling nuclear translocation of NFκB. TNF receptor-associated factor 4 (TRAF4), which negatively regulates NF-κB activation upstream from IKK, was also upregulated after 1 h exposure.
In line with the overall down- or nonregulation of death-mediating pathways, one of the non-TNF proteins which belong to the group of direct inducers of cell death by extracellular signals, the Fas-activated serine/threonine kinase FASTK, was downregulated after 1 h exposure to L. plantarum.
The genes TPT1 (or histamine releasing factor), cytokine induced apoptosis inhibitor 1 (cIAP-1), mitochondrial PUMA (BBC3), cathepsin B (CTSB), ATP6V0A1, cathepsin L and LAMP2, all of which are associated with cell-death related responses, were differentially expressed after the 1 h exposure to L. plantarum WCFS1. We found that those genes involved in regulation of cell death during acute responses were not regulated or expressed after 6 h exposure and that no other death-regulating genes were expressed as well. Of the TNFR family, only TTRAP, the TRAF and TNF receptor-associated protein, was upregulated after 6 h exposure to L. plantarum.
Immune responses
After 1 h exposure to L. plantarum, interleukin-1 (IL-1), IL-1 receptors IL-1R and IL-1-like receptor IL1RL1 (ST2) were downregulated. The pro-inflammatory cytokine receptor IL8R1 was downregulated as well. No regulation of these genes was found after 6 h exposure.
Several genes activating innate immune responses were upregulated after 1 h exposure (Table 3). Among these was IF, the gene encoding the complement factor I, a serine protease which can activate the classical complement pathway of innate immune response. A second, CFD (adipsin) is also a serine protease and activator of the complement pathway. C1S, a third serine protease and activator of complement pathway is involved in activation of G-protein-coupled receptor pathway and contains a calcium-binding EGF domain. C1R is a protein similar to C1S and is also part of complement pathway. All the encoding genes were upregulated after 1-h exposure to L. plantarum WCFS1. In line with upregulation of C1S, downregulation of one of its inhibitors, SERPINE2, was observed.
Table 3.
Immune-related responses of human intestinal mucosal cells to L. plantarum WCFS1
| component | function | regulation after 1 hr exposure | regulation after 6 hrs exposure |
| IL-1 | interleukin-1 cytokine, induction immune responses |  |
- |
| IL-1R | receptor for interleukin-1 (IL-1) |  |
- |
| IF | complement factor I, serine protease, activator of complement pathway of innate immune response |  |
- |
| CFD (adipsin) | serine protease, activator of complement pathway of innate immune response |  |
- |
| C1R | serine protease, activator of complement pathway of innate immune response and GPCR 1 pathway |  |
- |
| C1S | serine protease, activator of complement pathway of innate immune response and GPCR 1 pathway |  |
- |
| C8G | part of antibacterial membrane attack complex (MAC) |  |
- |
| CD74 | TM receptor, antigen presentation, immune response and humoral defence | - |  |
| CD93 | cell-surface glycoprotein, part of complement component 1, activator of macrophage and cellular adhesion |  |
- |
| HLA-A * | regulation of NK cell cytotoxicity | - |  |
| HLA-C | endogenous antigen presentation | - |  |
| HLA-E | immune response regulation, protection from NK cells | - |  |
| HLA-G | regulatory/suppressive to T cells, cytokine tolerance | - |  |
| HLA-DMA | antigen presentation, detection of microbes, CLIP release | - |  |
| HLA-DMB | antigen presentation, detection of microbes, CLIP release | - |  |
| HLA-DPA1 | antigen presentation derived from extracellular proteins | - |  |
| HLA-DRA | antigen presentation derived from extracellular proteins |  |
 |
| HLA-DRB1 | antigen presentation derived from extracellular proteins | - |  |
1 G-protein coupled receptor
* HLA (human leukocyte antigen) are transmembrane receptors that are all part of MHC complex pathway, HLA-x are class I, HLA-Dxxx are class II paralogues.
Green triangles refer to downregulation, and red triangles to upregulation of genes related to immune responses.
The C8G gene which encodes the γ polypeptide of complement component 8 was downregulated in the 1 h exposure study. C8G is part of the antibacterial Membrane Attack Complex (MAC), a cytolytic protein complex involved in damaging bacterial cell membranes. Also CD93 (C1QR1), a cell-surface glycoprotein and part of complement component 1, was downregulated. In contrast to 1 h exposure, none of these genes were regulated after 6 h exposure.
Parts of the MHC class I pathway were found to be regulated after the 1 h exposure. The activating proteasome α and β subunits (PSME1/2) were among the few upregulated genes. The chaperone HSP70 which plays a role during processing of cytosolic antigens before uptake into the ER, was downregulated. Although no regulation of the TAP1 or TAP2 transporters was found, the TAP-binding protein (TAPBP) which mediates the interaction between de novo produced MHC class I molecules and the transporter associated with antigen processing (TAP), was upregulated. Only one of the MHC transmembrane (TM) receptors, HLA-DRA was regulated after 1 h exposure. The (downregulated) gene product is expressed in antigen-presenting cells (APCs) and is involved in immune response, exogenous antigen presentation and processing.
After 6 h exposure to L. plantarum WCFS1, the intestinal mucosa showed a different immune response-related expression profile. Several HLA-type TM receptors belonging to MHC class I and class II were upregulated (Table 3). The upregulated HLA-A TM receptor is involved in immune responses and modulates, upon interaction with natural killer (NK) cells, cytotoxicity. The HLA-E gene encodes an MHC-class protein with an immunoregulatory function; surface localization of the protein can protect expressing cells from NK cell-mediated lysis. The HLA-DMA TM receptor was among the class II receptors that were upregulated after 6 h exposure to L. plantarum WCFS1. This receptor is involved in immune response via antigen presentation and processing and in the detection of microbes. The TM receptor CD74 antigen was also upregulated after 6 h exposure. This receptor is involved in antigen presentation, immune response and humoral defense mechanism, but does also promote cell proliferation and negative regulation of apoptosis.
Q-PCR The microarray dataset was verified with real-time quantitative PCR (QPCR). The observed up- or downregulation of eight out of ten genes was confirmed. The Q-PCR analysis of two genes, GUCA2A and PCNA, did not confirm the microarray data. In addition, QPCR analysis of the gene representing MTP confirmed the microarray results.
Discussion
Human in vivo studies aiming to elucidate the interaction between microbes and the intestine are hampered by medical and ethical limitations. Previously, mainly animal models have been employed to investigate mucosal responses to microbes. In germ-free mice, colonization with commensal bacteria induced genes involved in nutrient absorption, mucosal barrier fortification, xenobiotic metabolism, angiogenesis and postnatal intestinal maturation [12], and innate immunity [13]. These studies clearly revealed that the exposure to commensal bacteria modulates the expression of many genes with diverse functions in general gut physiology. It is not clear how these findings relate to the human in vivo situation, in which a complex community of different microbes is present. Data on the impact of commensal microbes on the transcriptome of human gut mucosa in vivo is restricted to studies describing the effects of supplementation of L. GG [7] and of Bacillus clausii [14] on intestinal transcriptional responses in oesophagitis patients. In the present study, the impact of L. plantarum WCFS1 on gene transcription and pathway regulation was investigated in a human in vivo model in healthy volunteers that enabled to administer the microbes directly into the small intestine and sample intestinal tissue for transcriptional profiling. To the best of our knowledge this is the first example of a study that employs post-genomic technologies to unravel specific host-microbe interactions in healthy human subjects. In two subsequent intervention studies, effects of 1- and 6-h continuous injection of L. plantarum WCFS1 were investigated. An exposure time of 1-h was chosen as the 'short term' exposure time, whereas an exposure time of 6 h was considered as a prolonged exposure. Previous studies in our lab showed that after a 6-h period, problems may arise due to the long fasting period, during which subjects remain at rest and, hence, this was considered as the maximal time frame for the chosen experimental set-up.
The two studies presented here showed regulation of several hundreds of genes in small intestinal mucosa induced by intraluminal ingestion of L. plantarum. Exposure of proximal small intestinal mucosa for 1 h to L. plantarum provoked a transcriptional response, which at least in part appeared to be modified in the prolonged (6 h) exposure. The temporal changes in gene transcription during the first hours of intraluminal injection of L. plantarum as described in this manuscript provides an in-depth overview of the cellular host response to intraluminal non-pathogenic microbes. The observed counter-regulatory responses are important for the maintenance of normal gut homeostasis. This was confirmed by the observations of the proteomics assay in the prolonged exposure study, in which only two proteins were consistently found to be differentially present after the L. plantarum exposure compared to placebo. In the acute exposure study, no attempts were undertaken to investigate differences in protein profiles, because the exposure period of 1 h was expected to be too short to provoke substantial differences at the protein level.
Lipid and fatty acid uptake and metabolism participate in a diversity of cellular functions including membrane biogenesis, general metabolism and (bacterial) signaling. With increasing mucosal exposure time to L. plantarum, the transcriptional profile of lipid and fatty acid uptake and metabolism was also affected, reflecting an increase of fatty acid and lipid metabolism.
After 1 h perfusion, several genes involved in lipid and fatty acid metabolism or uptake and transport (ACSL5; CD36 antigen and DEGS1) were downregulated, suggesting that the mucosal cells did not actively take up or metabolize additional fatty acids and lipids during the interaction with L. plantarum in comparison to placebo-treated mucosa. In contrast, after the prolonged exposure of 6 h, three transcription regulators involved in lipid/fatty acid metabolism and protection from oxidative stress as a result of this metabolism (JUND, GTF21 and TSC22-D1) were upregulated in comparison to the placebo treatment (Figure 2). One important gene involved in protection against oxidative stress, glutathione peroxidase (GPX4), was also upregulated after 6-h exposure to L. plantarum.
The CD36 glycoprotein, which plays a role in cellular adhesion and in the regulation of fatty acid transport and uptake, and participates in cellular control of oxidative stress by binding to oxidized low-density lipoprotein [15], was downregulated after 1-h exposure, but was upregulated after 6 h exposure to L. plantarum. The fatty acid binding protein FABP1 was upregulated in the 6-h exposure study, as was the microsomal and ER-localized triglyceride transfer protein, MTP, which is discussed in more detail below. Hence, it is clear that the prolonged exposure to L. plantarum induced upregulation of genes involved in fatty acid uptake. Several genes participating in mitochondrial and peroxisomal fatty acid metabolism (e.g. acyl-CoA genes) or membrane sphingolipid metabolism (ASAH1) were upregulated as well (Figure 2).
Microsomal triglyceride transfer protein (MTP) was modulated on the transcriptional and on the protein level during the interaction with L. plantarum in the 6-h exposure study. MTP is involved in intracellular lipid transport and secretion, and is primarily expressed in liver and intestinal tissues [16]. Recently, MTP was also found to be involved in modulating the intestinal immune response to antigens by regulating type I CD1 molecules [17,18], which are major histocompatibility complex (MHC) class I-homologues. It was also shown that MTP affects antigen presentation and is able to regulate CD1a, CD1b and CD1c production, hence supporting the idea that MTP is important in the host response to microbial pathogens. This is in agreement with the other MHC-related responses observed in this study, as described below. Overall, mediation of MTP in response to extra- and intracellular signals may affect the mucosal response to pathogens, and may also affect lipid metabolism and plasma lipoprotein levels. MTP inhibitors may be used to promote weight loss [19], but they may have detrimental side effects, such as fat accumulation in the liver and small intestine. The observation that MTP was non-significantly downregulated in the 1-h exposure study, whereas it was upregulated in the extended, 6-h exposure study, presents a challenging situation to speculate on the eventual physiological consequences. The role of MTP in lipid metabolism, a process that was affected by L. plantarum, and its role in the immune response involving dendritic cells [18] could suggest that upon L. plantarum exposure, a mucosal response was triggered that initiated microbe sensing by dendritic cells, which was at least in part mediated by MTP, and stimulated lipid metabolism-related processes in intestinal mucosa.
The down-regulation of major transcription factors stimulating cell proliferation observed in the acute exposure study suggests that cell proliferation is suppressed after the first perception of L. plantarum cells. The initial down-regulation of the cellular cycle may be the result of a shift in cellular activity towards microbial perception, cell defense and immune responses. Indeed, both during acute (1 h exposure) and later responses, modulation of immune response pathways and cellular death, survival and proliferation was observed.
Transforming growth factor-β (TGF-β) is an important regulator of cell proliferation [20]. Although no regulation of TGF-β was observed in the datasets, the TGF-β-early-response genes TSC22 displayed exposure-time dependent differential regulation in time, with downregulation after 1 h (TSC-22D1, -3) and upregulation after 6 h (TSC-22-D1). TSC-22 is an important downstream component of TGF-β and PPARγ signaling during intestinal epithelial cell differentiation. Both TGF-β and PPARγ signaling pathways are important for inhibiting excessive epithelial cell proliferation in the intestine [21]. The downregulation of proliferation in the acute phase, after the 1-hr exposure to L. plantarum, appeared to be further enhanced by an upregulation of the proliferation-inhibiting gene product IFITM3. Generally, proliferation rates are primarily determined by two main and interacting pathways, i.e., the NFκB and the mitogen-activated protein (MAP) kinase pathways. After 6 h exposure, the MAP kinase ERK1 (MAPK3) was upregulated. In the cellular context, ERK1 may have acted as an inhibitor of cellular over-proliferation by downregulating important oncogene regulators such as c-Fos and by antagonistic effects on ERK2 function, a related MAPK, that mediates proliferative signals [22].
NFκB is one of the key regulators of both cell proliferation and apoptosis [23]. Remarkably, after 1 h treatment with L. plantarum, only down-regulation of the p65 subunit of NFκB was found, while no component of NFκB was differentially expressed after 6 h exposure. The exact role of NFκB in cell proliferation is not clear, but it was shown that NFκB is required for cell proliferation by mediating the expression of c-Myc [24], which stimulates the transcription of genes with an important role in cell cycle control, like cyclins, CDKs and E2F [25].
NFκB plays an important role in the regulation of apoptosis. It suppresses apoptosis by inducing the expression of several anti-apoptotic proteins and by modulating the expression or activity of pro-apoptotic proteins [25]. Paradoxically, NFκB also exerts pro-apoptotic activity [26]. NFκB itself exists as an inactive cytoplasmic complex, bound to inhibitory proteins of the IκB family. Upon stimulation, TRAFs recruit NFκB -inducible kinases (NIK) that phosphorylate the IKK complex that subsequently triggers ubiquitination of the IκB, which makes it possible for the NFκB complex to translocate from the cytoplasm to the nucleus, where it activates transcription of various genes [27]. One caspase recruitment domain protein, CARD10, induces NFκB activity through interactions with the IKK complex [28]. This CARD10, was upregulated after 6 h exposure, suggesting NFκB activity induction. However, the simultaneous upregulation of TTRAP, a gene that encodes a protein that inhibits NFκB by binding to TNF receptors [27] may have antagonized the presumptive CARD10 induction of NFκB complex.
Cell death induction during interactions with microbes occurs primarily through TLR signaling and expression of death-inducing cytokines, such as tumor necrosis factor (TNF) or Fas ligand (FasL) with concomitant increased expression of the appropriate death-signaling receptors. One receptor of the TNF family, TNFRSF25, was upregulated during acute response (1 h exposure). TNFRSF25, also called death receptor 3 (DR3), induces NFκB activation and thereby mediates the activation of apoptosis [29].
Although several genes associated with cell death were found to be differentially expressed, none of these are known to stimulate or execute cell death. Importantly, with progressing duration of exposure to the microbes, cells did not appear to maintain the cellular "gestation" phase with downregulated metabolism that characterized the acute 1-h response to L. plantarum but rather, to switch to a more proliferative phase with an overall expression profile characterized by upregulation of genes involved in cellular growth, proliferation and development. Notwithstanding this shift to cellular proliferation, monitoring of bacterial presence was still evident, as can be deduced from the upregulated expression of antimicrobial α-defensins and the major histocompatibility complex (MHC) antigen processing and presentation pathway.
Important MHC and other innate immune system-related receptors and effectors were differentially expressed after 1 and 6 h exposure, respectively. After the 1 h exposure to L. plantarum, genes encoding major complement component 1 proteins were upregulated, representing a group of proteins that mediate the "classical pathway" of the complement immune response. In contrast, the C8G gene, which encodes the γ polypeptide of complement component 8 was downregulated. This gene is part of the antibacterial Membrane Attack Complex (MAC), a cytolytic protein complex involved in damaging bacterial cell membranes. The observation that this antibacterial protein was down-regulated might imply that the immune system in the intestinal mucosa can perceive L. plantarum within 1 h and recognize these as commensal and non-pathogenic bacteria.
Prolonged infusion of L. plantarum induced MHC antigen processing and presentation pathways indicating that mucosal epithelia actively performed a bacterial monitoring and identification process. CD74, one of the major receptors, which can be bound by bacteria, was one of the upregulated genes. This receptor is involved in antigen presentation and immune responses [30], but does also promote cell proliferation and negative regulation of apoptosis.
Several MHC-I and MHC-II transmembrane receptors were upregulated after the 6 h exposure. After prolonged exposure to lactic acid bacteria, CD74 and HLA-DMA and -B were upregulated. These receptors participate in the MHCII pathway of antigen endocytosis, processing and presentation [31,32]. The increased expression of HLA-E indicates that epithelial cells were actively protected from potentially damaging immune system responses. Increasing the amount of HLA-E present at the cell surface renders cells less sensitive to natural killer-cell-mediated lysis. Stabilization of HLA-E expression is among others mediated by HLA-A2 [33] and the expression of this protein is also upregulated after 6 h.
Summarizing, complement 1 was induced during acute responses and MHC antigen processing and presentation pathways were being expressed after 6 h exposure, which suggests the activation of bacterial monitoring and identification process. Based on the microarray data, no inflammatory immune responses were executed. Rather, the expression profile after 6 h of exposure to L. plantarum is in agreement with cellular proliferation. However, it should be noted that after 6 h perfusion, the Paneth-cell-specific defensin α5 was upregulated, which indicates that the luminal microbes were perceived and their numbers controlled by immune responses from the crypt regions of the intestinal mucosa.
The Q-PCR analyses were in agreement with the microarray data. The gene expression results of eight out of ten genes were confirmed, which is in line with the consensus that the Affymetrix microarray platform provides a reliable platform to measure gene expression [34]. The observed differences between these two technologies are in line with the observations of others [34], and are mostly caused by differences in probe sequence and thus target location.
The present manuscript provides an in-depth overview of the initial transcriptional response of healthy intestinal mucosa upon its interaction with live bacterial cells of L. plantarum, describing time-dependent changes in transcriptional responses after 1 and 6 h of bacterial exposure to L. plantarum. The observed changes were modest, as genes were affected by maximally 78%. This is in concordance with the impact of an intervention study by our group in humans, in which also only modest impacts on fold changes, up to doubling of the gene transcripts, was observed [11]. The biological observations of the present study were in line with those observed in previous studies on the effects of a 30-d supplementation with the probiotic microorganisms L. rhamnosus GG and of Bacillus clausii on transcriptional responses of small intestinal mucosa in humans [7,14]. Immune modulation, cell growth, and cell-cell signaling were mediated at the transcriptome level by all three microbes (B. clausii, L. rhamnosus GG and L. plantarum WCFS1). In addition, our study revealed that L. plantarum WCFS1 mediates fatty acid uptake- and metabolism, contributing not only to lipid metabolism but also to immune response modulation as discussed above. It should be noted that, although the authors of the 30-d supplementation studies state that effects of probiotics on healthy duodenum were investigated, the use of medication (proton pump inhibitors) as well as the small sample size (n = 3 in each group) hamper the generalization of their results and, hence, comparison with the results of the present study may not be justified. We would like to emphasize that the effects on gene transcription, observed in the present study, may not be specific for L. plantarum WCFS1. As indicated above, some biological effects were also induced by other microbes. Future studies should reveal whether L. plantarum WCFS1 induces specific and unique responses in the gastrointestinal tract.
In conclusion, the present study showed that L. plantarum WCFS1 induced time-dependent transcriptional changes in intestinal mucosa in healthy subjects. Among a variety of biological processes, especially lipid metabolism, cellular proliferation, cell death and survival and immune responses were modulated. The gene expression profiles suggest that cell death and pro-inflammatory responses were triggered, but not executed. This extensive exploration of the human response to L. plantarum WCFS1 may eventually provide molecular support for specific or probiotic activity of this strain and/or species, and exemplifies the strength of the applied technology for the identification of the potential bio-activity of microbes in the human intestine.
Methods
This study encompassed two human intervention studies, which are described below under 'study 1' and 'study 2', respectively. Both studies were approved by the University Hospital Maastricht Ethical Committee, and conducted in full accordance with the principles of the 'Declaration of Helsinki' (52nd WMA General Assembly, Edinburgh, Scotland, Oct 2000). All subjects gave their written informed consent prior to their inclusion into the study.
Preparation of L. plantarum WCFS1
L. plantarum WCFS1 was grown on MRS medium under anaerobic conditions, following standard laboratory procedures. Fifteen minutes prior to each experiment with L. plantarum WCFS1, 1011 freshly prepared L. plantarum WCFS1 were resuspended in 600 mL physiological saline solution, containing 10 g/L glucose, at 37°C.
Study 1
Subjects
Eight healthy non-smoking volunteers (24 ± 4 y) without a history of GI symptoms and free of any medication, were investigated on two separate occasions in a randomized placebo-controlled crossover study.
Protocol
After an overnight fast, mucosal tissue samples were obtained from the horizontal part of the duodenum, approximately 15 cm distal to the pylorus, by standard flexible gastroduodenoscopy and a perfusion catheter was placed orogastrically in the proximal small intestine, as described previously [11]. Briefly, a catheter that enabled to inject a fluid 5 cm distal to the pylorus was inserted during a gastroduodenoscopy procedure. Catheter positioning was performed under short interval fluoroscopic control. No sedatives were given to the volunteers. Subsequently, during the first 180 min of the experiment, a physiological saline solution containing 10 g/L glucose was infused continuously at 10 ml/min using a peristaltic pump. After this period, which allowed establishment of steady state conditions in intestinal fluid fluxes, physiological saline containing 10 g/L glucose with or without, in total, 1 × 1011 L. plantarum WCFS1 was infused for one hour. Fluids were maintained at 37°C using a shaking water bath. Subjects remained in the supine position until the end of the experiment, and food or beverage consumption was not allowed during the experiment. After the perfusion experiment, the perfusion catheter was removed by gently pulling out the catheter. A second gastroduodenoscopy was performed exactly 15 minutes after the perfusion experiment to obtain tissue samples from the same intestinal region as earlier that day. The entire protocol was repeated on another day, 13 to 16 days after the first experiment, to randomly investigate the effects of placebo or L. plantarum WCFS1. In all tissue samples, gene expression levels were measured using genome-wide microarrays (Affymetrix U133A; see below). No samples were taken for proteome analysis purposes, because changes at the protein level were not expected to occur within the 1-h exposure time to L. plantarum WCFS1.
Study 2
Subjects
Seven healthy non-smoking volunteers (28 ± 6 y), without a history of gastrointestinal complaints and free of any medication, participated in a randomized placebo-controlled crossover study.
Protocol
After an overnight fast, an intraduodenal feeding catheter (naso-intestinal tube, Flocare® Bengmark, Nutricia Healthcare S.A., Chatel-St. Denis, Switzerland) was placed nasogastrically following the manufacturers instructions. No sedatives were given to the volunteers. After positioning of the catheter in the small intestine (tube tip positioned 5–10 cm distal to the pylorus), a physiological saline solution containing 10 g/L glucose and a total of 1012 L. plantarum WCFS1 or, randomly on another test day, only physiological saline solution containing 10 g/L glucose, was injected continuously at 6.7 ml/min for 6 h. The concentration of bacteria present in the fluid was the same in both studies, which was important in order to allow proper comparisons between the different studies. The infusion rate was less than that in study 1, to avoid injecting too large amounts of fluid and bacteria, which could have induced gastrointestinal symptoms. Fluids were maintained at 37°C using a shaking water bath. Subjects remained in the supine position until the end of the experiment, and food or beverage consumption was not allowed during the experiment. After this 6-h period, tissue samples were obtained from the horizontal part of the duodenum, approximately 15 cm distal to the pylorus, by standard flexible gastroduodenoscopy, at approximately 15 cm distal to the pylorus. In all tissue samples, gene expression levels were measured using genome-wide microarrays (Affymetrix U133A; see below). In duplicate tissue samples, differential proteome analyses were performed using the CyDIGE method (2D gel-electrophoresis with minimal fluorescent labeling) with Maldi-TOF MS (see below).
Microarray analyses
RNA isolation Total RNA was isolated using a TRIzol lysis assay. Next, total RNA of each separate tissue sample was hybridized onto GeneChip microarrays (HG U133A; Affymetrix Inc, Santa Clara, Ca, USA) according to the manufacturer's instructions. Hence, for study 1, 32 RNA samples were hybridized (tissue samples from 8 subjects, obtained before and after the intervention with L. plantarum WCFS1 and placebo, respectively) and for study 2, 14 RNA samples (obtained from 7 subjects, taken after to exposure to L. plantarum WCFS1) were hybridized onto the chips. Briefly, 1 mL TRIzol (Invitrogen Life Technologies b.v., Breda, The Netherlands) and 10 μL β-mercaptoethanol (VWR International b.v., Amsterdam, The Netherlands) were added to each frozen tissue sample and shaken with a minibeadbeater for 30 seconds. 200 μL Chloroform (Sigma Aldrich Chemie b.v., Zwijndrecht, The Netherlands) was added and the samples were incubated for 3 minutes, followed by phase separation using centrifugation at 21000 g for 15 minutes. The upper aqueous phase was taken and 500 μL 70% Ethanol was added. Subsequently, the extracted RNA was purified using the RNeasy Mini Kit (Qiagen Benelux b.v., Venlo, The Netherlands) with extra DNA digestion by on-column RNase-Free DNase treatment (Qiagen Benelux b.v., Venlo, The Netherlands). RNA purity was determined using nanodrop equipment (ND-1000 spectrophotometer, Isogen Life Science B.V., IJsselstein, The Netherlands). Only RNA samples with a 260/280 ratio between 1.9 and 2.1 were considered for further analysis. RNA integrity was determined using Bioanalyzer technology (Bioanalyzer 2100, Agilent, Santa Clara, USA). RNA samples with RNA integrity numbers (RIN) above 8.0 were considered for further analysis.
Microarrays
Total RNA was hybridized onto GeneChip microarrays (Affymetrix Inc, Santa Clara, Ca, USA) according to the manufacturers' instructions. For this analysis, five micrograms of total RNA from each sample, and the one-cycle labeling system were applied as recommended by the manufacturer (Affymetrix Inc, Santa Clara, Ca, USA). Microarrays were scanned using a GeneChip Instrument System (Affymetrix Inc, Santa Clara, Ca, USA). The microarray data calculations to obtain detailed information on differentially expressed genes are described in the supplementary material (see Additional file 3).
Pathway analysis
The genes analyzed and fold changes were loaded into GenMapp [35] and MAPPFinder [36] and into the GOurmet and Ingenuity Pathway Analysis IPA: (see Availability and requirements section for URL)software packages to evaluate the transcripts in relation to known biological processes, molecular function and cellular component based on Gene Ontology (GO) terms and local maps. Only gene transcripts with either their average intensities for the placebo and treated groups above 500 or average intensities for one of these groups above 1000 and a 10 percent up or down regulation, respectively, were used to obtain a ranked list of pathways with differentially expressed genes.
MappFinder software was used to select the MAPPs with relatively high numbers of differentially expressed genes, which were affected by Lactobacillus plantarum WCFS1 compared to placebo. The ranking of regulated pathways was indicated by the individual Z-scores. The Z-score increases if higher numbers of changing genes are found, taking into account the number of genes present in the MAPP that are represented on the array, and the total number of genes involved in the concerning MAPP. MAPPs were selected for further study if the group results (Lacobacillus plantarum WCFS1 compared to placebo) reached an arbitrary Z-score of at least 3 on that MAPP for study 1 and at least 5 for study 2, and at least 3 genes were differentially expressed in that pathway. Different Z-score cut-off points were used for the two studies because effects in study 2 were more pronounced and revealed regulation of more biological pathways, thus allowing the use of a more stringent statistical cut-off in the pathway analysis.
Pathway visualization
Further refinement and biological interpretation of reconstructed pathways was performed by comparison with published information and pathways available from NCBI Entrez Gene info (see Availability and requirements section for URL), the Protein, Signaling, Transcriptional Interactions & Inflammation Networks Gateway database pSTIING, (see Availability and requirements section for URL) and BioCarta charts (see Availability and requirements section for URL). Based on the combined information from IPA and pSTIING and published data, a graphical representation of the microarray results in terms of their encoded and interacting proteins and pathways was reconstructed using the image ("toolbox") palette provided by Biocarta.
Q-PCR First Strand cDNA was synthesized using the iScript cDNA Synthesis kit (Bio-Rad, Veenendaal, The Netherlands) according to the manufacturer's instructions. 250 and 500 ng total RNA was used as template for the cDNA reaction of Study 1 en Study 2 respectively. The difference in starting quantities was the consequence of the smaller amount of RNA available for study 1, which was due to a smaller size of the tissue samples obtained in this study. The cDNA was diluted with RNase free H2O to a concentration of 5 and 10 ng/μl respectively. IQ Sybr Green Supermix (Bio-Rad, The Netherlands) was used for the Q-PCR. Each Q-PCR reaction of the Q-PCR contained 12,5 μl iQ Sybr Green Supermix, 1 μl of 10 μM gene-specific forward and reverse primers, 2 μl cDNA template solution and 8,5 μl sterile water. The following primers were used. Housekeeping genes were 18SrRNA: gtaacccgttgaaccccatt, ccatccaatcggtagtagcg; GAPDH: tgcaccaccaactgcttagc, ggcatggactgtggtcatgag and Calnexin: ccactgctcctccttcatctcc, cggtatcgtctttcttggctttgg. Genes study 1; GUCA2A: gggttgggaaactcaggaactttg, tacaggcagcgtaggcacag; PCNA: gccactccactctcttcaacgg, tggtgacagaaaagacttcagtatatgc; CD36: ggaatctgtcctattgggaaagtcactgc, ctgggttttcaactggagaggcaaagg; FOS: ctgtgtctcttttctctttctccttagtc, tccagcaccaggttaattccaataatg; DUSP1: agcagaggcgaagcatcatc, acggtggtggtggaggtg. Genes study 2; PLA2G2A: gcagaagtcaactgtgtgagtgtg, gggagggagggtatgagagagg; DEFA6: ggctcaacaagggctttcac, gtatgggacacacgacagtttc; CDS1: tggattcattgctgcctatgtgttatc, ctttagaaagggtggaagtgagtaagtc; REG3A: gctgtcccaaaggctccaagg, atcacatcactgctactccactcc; CD24: gtatttgggaagtgaagactggaagc, agtgttctaaatgtggctattctgatcc. Gene study 1 and 2; MTP: ggacctagcacagaggaatcag, ccaaatccaccagtttcttgaagc. Reactions were run on the My IQ Single Color Real Time PCR Detection System (Bio-Rad, Veenendaal, The Netherlands). The cycling conditions comprised 3 minutes at 95°C and 40 cycles at 95°C for 10 seconds and 60°C for 45 seconds followed by a melting program. The CT values were normalized using the IQ5 Optical System Software version 2.0 (Bio-Rad, Veenendaal, The Netherlands). Gene-transcripts were analyzed using a multivariate Gaussian linear regression, similar to the microarray analysis, with the difference of having the sample concentration, the test day, the perfusion procedure, repeats, and the best housekeeping gene among 18S ribosomal RNA, GAPDH and calnexin included. Detailes of the Q-PCR calculations are provided in Additional file 3.
Proteomics analysis
Intestinal biopsies were prepared for protein analysis by sonication in a lysis buffer, and subjected to the 2-D Clean Up kit (Amersham Biosciences, Freiburg, Germany) to remove non-protein material. Protein concentrations were measured using the 2-D Quant Kit (Amersham Biosciences, Freiburg, Germany). The samples were further processed for two-dimensional fluorescence difference gel electrophoresis with the Ettan™ DIGE technique, following the manufacturers instructions [37]. Protein mixtures were separated by 2D electrophoresis, according to their iso-electric points, using the Ettan IPGphor 3 isoelectric Focusing System, and to their molecular weights using an EttanDalt12 electrophoresis system. Samples from the placebo intervention and from the L. plantarum intervention were loaded on one IPG-strip, together with a pooled internal standard. After the 2D-electrophoresis, the gels were scanned with a Typhoon confocal laser scanner (Typhoon 9410 Variable Mode Imager, Amersham Biosciences), and the scanned images were loaded into DeCyder software (Decyder 2D, Amersham Biosciences, Freiburg, Germany) to analyse the differences in protein profiles. Differentially expressed protein spots were picked with the Ettan Spot picker (Amersham Biosciences, Freiburg, Germany). Excised spots were subjected to mass-fingerprint identification using Maldi-TOF MS analysis as described elsewhere [38].
Availability and requirements
Microarray data are available in the ArrayExpress database http://www.ebi.ac.uk/arrayexpress under the experiment accession number E-MEXP-1328.
NCBI Entrez Gene info: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
pSTIING: http://pstiing.licr.org/
BioCarta charts: http://www.biocarta.com
Authors' contributions
FT conceived of the study, prepared and carried out the human experiments, and drafted the manuscript. PVB performed the bioinformatical- and pathway analyses, and contributed to the draft manuscript. PL was involved in the design of the study and performed the statistical analyses. AK carried out the RNA isolations and the Q-PCR analyses. WDV participated in the design of te study, and helped to draft the manuscript. MK conceived of the study, participated in its design and coordination and helped to draft the manuscript. RJB conceived of the study, participated in its design and coordination, performed the gastroduodenoscopy procedures, and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Genes study 1. Genes regulated by a 1-h exposure time to L. plantarum WCFS1. 669 Gene reporters were differentially expressed by the intervention; 225 genes were upregulated and 444 gene reporters were downregulated by L. plantarum WCFS1.
Genes study 2. Genes regulated by a 6-h exposure time to L. plantarum WCFS1. 424 Gene reporters were differentially expressed by the intervention; 383 genes were upregulated and 41 gene reporters were downregulated by L. plantarum WCFS1.
Microarray and QPCR data calculations. Detailed description of the calculations of the microarray data and the quantitative RT-PCR analysis.
Acknowledgments
Acknowledgements
The authors wish to thank dr. D. Jonkers (Maastricht University, Maastricht, The Netherlands) for preparing the L. plantarum WCFS1 supplements. This study was supported by a grant from the Top Institute Food and Nutrition (TIFN C007; FT, PVB, AK, WDV, MK, RJB). PL was funded by Maastricht University.
Contributor Information
Freddy J Troost, Email: f.troost@intmed.unimaas.nl.
Peter van Baarlen, Email: p.vanbaarlen@ncmls.ru.nl.
Patrick Lindsey, Email: p.lindsey@gen.unimaas.nl.
Andrea Kodde, Email: a.kodde@intmed.unimaas.nl.
Willem M de Vos, Email: devos@wur.nl.
Michiel Kleerebezem, Email: michiel.kleerebezem@nizo.nl.
Robert-Jan M Brummer, Email: robert.brummer@oru.se.
References
- Molin G. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am J Clin Nutr. 2001;73:380S–385S. doi: 10.1093/ajcn/73.2.380s. [DOI] [PubMed] [Google Scholar]
- Saxelin M, Tynkkynen S, Mattila-Sandholm T, de Vos WM. Probiotic and other functional microbes: from markets to mechanisms. Curr Opin Biotechnol. 2005;16:204–211. doi: 10.1016/j.copbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Marco ML, Pavan S, Kleerebezem M. Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol. 2006;17:204–210. doi: 10.1016/j.copbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Saggioro A. Probiotics in the treatment of irritable bowel syndrome. J Clin Gastroenterol. 2004;38:S104–6. doi: 10.1097/01.mcg.0000129271.98814.e2. [DOI] [PubMed] [Google Scholar]
- Geier MS, Butler RN, Howarth GS. Inflammatory bowel disease: current insights into pathogenesis and new therapeutic options; probiotics, prebiotics and synbiotics. Int J Food Microbiol. 2007;115:1–11. doi: 10.1016/j.ijfoodmicro.2006.10.006. [DOI] [PubMed] [Google Scholar]
- White JS, Hoper M, Parks RW, Clements WD, Diamond T, Bengmark S. The probiotic bacterium Lactobacillus plantarum species 299 reduces intestinal permeability in experimental biliary obstruction. Lett Appl Microbiol. 2006;42:19–23. doi: 10.1111/j.1472-765X.2005.01800.x. [DOI] [PubMed] [Google Scholar]
- Di Caro S, Tao H, Grillo A, Elia C, Gasbarrini G, Sepulveda AR, Gasbarrini A. Effects of Lactobacillus GG on genes expression pattern in small bowel mucosa. Dig Liver Dis. 2005;37:320–329. doi: 10.1016/j.dld.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Vesa T, Pochart P, Marteau P. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment Pharmacol Ther. 2000;14:823–828. doi: 10.1046/j.1365-2036.2000.00763.x. [DOI] [PubMed] [Google Scholar]
- Pavan S, Desreumaux P, Mercenier A. Use of mouse models to evaluate the persistence, safety, and immune modulation capacities of lactic acid bacteria. Clin Diagn Lab Immunol. 2003;10:696–701. doi: 10.1128/CDLI.10.4.696-701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP, Leer R, Tarchini R, Peters SA, Sandbrink HM, Fiers MW, Stiekema W, Lankhorst RM, Bron PA, Hoffer SM, Groot MN, Kerkhoven R, de Vries M, Ursing B, de Vos WM, Siezen RJ. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci U S A. 2003;100:1990–1995. doi: 10.1073/pnas.0337704100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troost FJ, Brummer RJ, Haenen GR, Bast A, van Haaften RI, Evelo CT, Saris WH. Gene expression in human small intestinal mucosa in vivo is mediated by iron-induced oxidative stress. Physiol Genomics. 2006;25:242–249. doi: 10.1152/physiolgenomics.00114.2005. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292:1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- Sonnenburg JL, Chen CT, Gordon JI. Genomic and Metabolic Studies of the Impact of Probiotics on a Model Gut Symbiont and Host. PLoS Biol. 2006;4:e413. doi: 10.1371/journal.pbio.0040413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Caro S, Tao H, Grillo A, Franceschi F, Elia C, Zocco MA, Gasbarrini G, Sepulveda AR, Gasbarrini A. Bacillus clausii effect on gene expression pattern in small bowel mucosa using DNA microarray analysis. Eur J Gastroenterol Hepatol. 2005;17:951–960. doi: 10.1097/00042737-200509000-00011. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Tao N, Chung KN, Heuser JE, Lublin DM. Endocytosis of oxidized low density lipoprotein through scavenger receptor CD36 utilizes a lipid raft pathway that does not require caveolin-1. J Biol Chem. 2003;278:45931–45936. doi: 10.1074/jbc.M307722200. [DOI] [PubMed] [Google Scholar]
- Hagan DL, Kienzle B, Jamil H, Hariharan N. Transcriptional regulation of human and hamster microsomal triglyceride transfer protein genes. Cell type-specific expression and response to metabolic regulators. J Biol Chem. 1994;269:28737–28744. [PubMed] [Google Scholar]
- Brozovic S, Nagaishi T, Yoshida M, Betz S, Salas A, Chen D, Kaser A, Glickman J, Kuo T, Little A, Morrison J, Corazza N, Kim JY, Colgan SP, Young SG, Exley M, Blumberg RS. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–539. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]
- Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, Khurana A, Kronenberg M, Johnson C, Exley M, Hussain MM, Blumberg RS. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren JA, King VL, Campbell SL, Hickman MA. Biologic activity of dirlotapide, a novel microsomal triglyceride transfer protein inhibitor, for weight loss in obese dogs. J Vet Pharmacol Ther. 2007;30 Suppl 1:33–42. doi: 10.1111/j.1365-2885.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- Huang SS, Huang JS. TGF-beta control of cell proliferation. J Cell Biochem. 2005;96:447–462. doi: 10.1002/jcb.20558. [DOI] [PubMed] [Google Scholar]
- Gupta RA, Sarraf P, Brockman JA, Shappell SB, Raftery LA, Willson TM, DuBois RN. Peroxisome proliferator-activated receptor γ and transforming growth factor-β pathways inhibit intestinal epithelial cell growth by regulating levels of TSC-22. J Biol Chem. 2003;278:7431–7438. doi: 10.1074/jbc.M208076200. [DOI] [PubMed] [Google Scholar]
- Vantaggiato C, Formentini I, Bondanza A, Bonini C, Naldini L, Brambilla R. ERK1 and ERK2 mitogen-activated protein kinases affect Ras-dependent cell signaling differentially. J Biol. 2006;5:14. doi: 10.1186/jbiol38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- Piva R, Belardo G, Santoro MG. NF-kappaB: a stress-regulated switch for cell survival. Antioxid Redox Signal. 2006;8:478–486. doi: 10.1089/ars.2006.8.478. [DOI] [PubMed] [Google Scholar]
- Vermeulen K, Berneman ZN, Van Bockstaele DR. Cell cycle and apoptosis. Cell Prolif. 2003;36:165–175. doi: 10.1046/j.1365-2184.2003.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Kaltschmidt C, Hofmann TG, Hehner SP, Droge W, Schmitz ML. The pro- or anti-apoptotic function of NF-kappaB is determined by the nature of the apoptotic stimulus. Eur J Biochem. 2000;267:3828–3835. doi: 10.1046/j.1432-1327.2000.01421.x. [DOI] [PubMed] [Google Scholar]
- Pype S, Declercq W, Ibrahimi A, Michiels C, Van Rietschoten JG, Dewulf N, de Boer M, Vandenabeele P, Huylebroeck D, Remacle JE. TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated factors (TRAFs), and that inhibits nuclear factor-kappa B activation. J Biol Chem. 2000;275:18586–18593. doi: 10.1074/jbc.M000531200. [DOI] [PubMed] [Google Scholar]
- Wang L, Guo Y, Huang WJ, Ke X, Poyet JL, Manji GA, Merriam S, Glucksmann MA, DiStefano PS, Alnemri ES, Bertin J. Card10 is a novel caspase recruitment domain/membrane-associated guanylate kinase family member that interacts with BCL10 and activates NF-kappa B. J Biol Chem. 2001;276:21405–21409. doi: 10.1074/jbc.M102488200. [DOI] [PubMed] [Google Scholar]
- Wen L, Zhuang L, Luo X, Wei P. TL1A-induced NF-kappaB activation and c-IAP2 production prevent DR3-mediated apoptosis in TF-1 cells. J Biol Chem. 2003;278:39251–39258. doi: 10.1074/jbc.M305833200. [DOI] [PubMed] [Google Scholar]
- Jensen PE, Weber DA, Thayer WP, Westerman LE, Dao CT. Peptide exchange in MHC molecules. Immunol Rev. 1999;172:229–238. doi: 10.1111/j.1600-065X.1999.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Narayan K, Chou CL, Kim A, Hartman IZ, Dalai S, Khoruzhenko S, Sadegh-Nasseri S. HLA-DM targets the hydrogen bond between the histidine at position beta81 and peptide to dissociate HLA-DR-peptide complexes. Nat Immunol. 2007;8:92–100. doi: 10.1038/ni1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DA, Evavold BD, Jensen PE. Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science. 1996;274:618–620. doi: 10.1126/science.274.5287.618. [DOI] [PubMed] [Google Scholar]
- Nattermann J, Nischalke HD, Hofmeister V, Ahlenstiel G, Zimmermann H, Leifeld L, Weiss EH, Sauerbruch T, Spengler U. The HLA-A2 restricted T cell epitope HCV core 35-44 stabilizes HLA-E expression and inhibits cytolysis mediated by natural killer cells. Am J Pathol. 2005;166:443–453. doi: 10.1016/S0002-9440(10)62267-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales RD, Luo Y, Willey JC, Austermiller B, Barbacioru CC, Boysen C, Hunkapiller K, Jensen RV, Knight CR, Lee KY, Ma Y, Maqsodi B, Papallo A, Peters EH, Poulter K, Ruppel PL, Samaha RR, Shi L, Yang W, Zhang L, Goodsaid FM. Evaluation of DNA microarray results with quantitative gene expression platforms. Nat Biotechnol. 2006;24:1115–1122. doi: 10.1038/nbt1236. [DOI] [PubMed] [Google Scholar]
- Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet. 2002;31:19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- Doniger SW, Salomonis N, Dahlquist KD, Vranizan K, Lawlor SC, Conklin BR. MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol. 2003;4:R7. doi: 10.1186/gb-2003-4-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manual EDIGEU. Ettan DIGE User Manual 18-1173-17 Edition AB. 2002.
- Bouwman F, Renes J, Mariman E. A combination of protein profiling and isotopomer analysis using matrix-assisted laser desorption/ionization-time of flight mass spectrometry reveals an active metabolism of the extracellular matrix of 3T3-L1 adipocytes. Proteomics. 2004;4:3855–3863. doi: 10.1002/pmic.200400861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Genes study 1. Genes regulated by a 1-h exposure time to L. plantarum WCFS1. 669 Gene reporters were differentially expressed by the intervention; 225 genes were upregulated and 444 gene reporters were downregulated by L. plantarum WCFS1.
Genes study 2. Genes regulated by a 6-h exposure time to L. plantarum WCFS1. 424 Gene reporters were differentially expressed by the intervention; 383 genes were upregulated and 41 gene reporters were downregulated by L. plantarum WCFS1.
Microarray and QPCR data calculations. Detailed description of the calculations of the microarray data and the quantitative RT-PCR analysis.