Abstract
Heterogeneous nuclear ribonuclearproteins (hnRNPs) are nucleic acid-binding proteins and have critical roles in DNA repair, telomere regulation, and transcriptional gene regulation. Previously, we showed that hnRNP G has tumor-suppressive activity in human oral squamous cell carcinoma cells. Therefore, the identification of hnRNP G target genes is important for understanding the function of hnRNP G and its tumor suppressive activity. In this study, we identify a known tumor suppressor gene, thioredoxin-interacting protein (Txnip) gene as a novel target of hnRNP G. Expression of Txnip is upregulated by wild-type (wt) hnRNP G but not by a suppression-defective mutant hnRNP G (K22R) in human squamous cell carcinoma. Wt hnRNP G binds and transactivates the Txnip promoter in vivo, whereas the K22R mutant does not. Furthermore, overexpression of Txnip alone in cancer cells leads to the inhibition of anchorage-independent growth and in vivo tumorigenicity in immunocompromised mice, suggesting a reversion of the transformation phenotype. These studies indicate that hnRNP G promotes the expression of Txnip and mediates its tumor suppressive effect.
Keywords: hnRNP G, tumor suppression, transcriptional regulation, Txnip
Introduction
hnRNPs were initially described as chromatin-associated RNA binding proteins which play a major role in RNA processing [1]. However, recent studies have shown that the biological functions of hnRNPs are very diverse, including RNA turnover, telomere biogenesis, oncogenesis, and spermatogenesis [2]. hnRNP G (also known as RBMX) is a member of hnRNP family and was first identified as a nuclear protein with an apparent molecular weight of 43 kDa [3,4]. Its structural feature suggests that physical association of hnRNP G with RNA may be required for its role in RNA processing and metabolism [1,3,5]. hnRNP G is detected primarily in the nuclei of mammalian cells and localized on lampbrush chromosomes of amphibian oocytes, supporting its association with nascent RNA transcripts as part of the transcriptional complex [3]. Recent studies have demonstrated that hnRNP G alters pre-mRNA splicing patterns by antagonizing the effects of Tra2β, a splicing activator and functions as a transcription factor of sterol regulatory-element-binding protein-1c (SREBP-1c) [6,7]. Therefore, the level of hnRNP G expression and its biochemical activities may have profound and multifaceted effects on the global gene expression profile in cells and the maintenance of normal cellular homeostasis. Nevertheless hnRNP G remains one of the least characterized members of the hnRNP family.
We previously reported the tumor suppressive function of wt hnRNP G. Ectopic expression of wt hnRNP G in human cancer cells lacking endogenous hnRNP G expression resulted in severe retardation of proliferation, reduction of clonogenic efficiency, loss of anchorage-independent growth, and reduction of in vivo tumorigenicity in immunocompromised mice, indicating a reversal of the transformation phenotype [8]. Furthermore, hnRNP G is either absent or down-regulated in precancerous dysplastic as well as malignant oral lesions, suggesting the involvement of hnRNP G inactivation in early oral carcinogenesis [8].
Our microarray data showed significant upregulation of the known tumor suppressor Txnip gene by wt hnRNP G [8]. We thus investigated whether the expression of Txnip is regulated by hnRNP G and whether this upregulation is associated with the tumor suppressive function of hnRNP G. In this report, we demonstrate that Txnip is a novel hnRNP G target gene. Moreover, ectopic overexpression of Txnip alone in cancer cells inhibited tumor growth in vitro and in vivo. These results indicate that hnRNP G mediates tumor suppression in part by upregulating the expression of Txnip.
Materials and methods
Cells and the culture conditions
HEp-2, a human head and neck squamous cell carcinoma cell line, was purchased from the American Type Culture Collection, and grown in Minimum Essential Medium (MEM; Invitrogen) supplemented with 50 μg/ml streptomycin, 50 U/ml penicillin, and 10% serum.
Reverse transcription (RT)-PCR
Total RNA was isolated by TRIzol Reagent (Invitrogen), and synthesis of cDNA was performed in a 50 μl reaction containing 5 μg of RNA per the manufacturer’s instructions using SuperScriptII Reverse Transcriptase (Invitrogen). Amounts of hnRNP G and Txnip mRNA were determined by semi-quantitative RT-PCR. hnRNP G transcripts were amplified as described in our previous publication [8]. Txnip transcripts were amplified by using the forward primer, 5′-acgcttcttctggaagacca-3′ (nucleotides 223–242; relative to the first nucleotide of start codon) and the reverse primer, 5′-gccattggcaaggtaagtgt-3′ (nucleotides 656–675). Detailed PCR condition is found in supplementary materials and methods (S1).
Cloning, construction of retroviral vectors, and infection of cells with the retroviruses
Full-length wt Txnip cDNA (1,264 bp) was obtained by PCR amplification of total cellular cDNA of normal human oral keratinocytes (NHOK) using the forward primer, 5′-gatcctcgagagttccatcatggtgatgtt-3′ containing the XhoI site (underlined) and the reverse primer 5′-ctagaagctttccaggaagagagacaaaaa-3′ containing the HindIII site (underlined). Detailed PCR condition is found in supplementary materials and methods (S2). The PCR fragments were then cloned into the pLNCX2 retrovirus expression plasmid (Clontech) digested with XhoI and HindIII. The resulting construct, pLNCX2-Txnip, was transfected into PA317 packaging cell line to harvest the viral vectors as described previously [8,9]. Cells were infected with the viral vetors and selected with G418 (100 μg/ml).
Western blot analysis
One hundred μg of total protein was electrophoretically separated by standard SDS-PAGE. The primary antibodies and dilutions used are as follows: hnRNP G (G-17, Santa Cruz Biotechnology; 1:200), Txnip (JY2, MBL, Woburn, MA; 1:1000) and actin (I-19, Santa Cruz Biotechnology; 1:200).
Txnip promoter constructs and luciferase assay
A 2,023 bp upstream region (−2,010 to +13; relative to transcription start site) of the Txnip gene was amplified by PCR using the sense primer, 5′-gatcctcgaggagaatcgctctaacccag-3 including a XhoI restriction site, and the antisense primer, 5-ctagaagcttaagaggggttagtttcaagc-3 including a HindIII restriction site, from genomic DNA of NHOK as described in supplementary materials and methods (S3). The PCR product was gel-purified, digested with XhoI and HindIII enzymes, and ligated into a promoter-less vector (pGL3-Basic vector; Promega) upstream of the luciferase reporter gene linearized by XhoI and HindIII enzymes. The resulting vector was named pGL3-Txp. Transfection and luciferase assay were carried out as described in supplementary materials and methods (S4). For normalization of transfection efficiency, the cells were also transfected with pRL-SV40 (Promega) containing the Renilla luciferase gene under SV40 promoter.
Deletion constructs of the Txnip promoter were generated by PCR cloning. The following primers were used to amplify various deletion mutants of the Txnip promoter from the pGL3-Txp construct: −902 (sense primer): 5′-gatcctcgag aggtaatgaggtaaatgggg-3′; −403 (sense primer): 5′-gatcctcgagtccttttacctcaaaacacc-3′; −245 (sense primer): 5′-gatcctcgagctctccttcctctccttcc-3′; −157 (sense primer): 5′-gatcctcgagacagcgatctcactgattg-3′; −1,537 (sense primer): 5′-gatcctcgagcatctcaatcttgggacatt-3′; −1067 (antisense primer): 5′-ctagaagcttaggatgcttcacttttca-3′; −625 (antisense primer): 5′-ctagaagcttctctgagggaaaggagaact-3′. All sense and antisense primers contained XhoI and HindIII (underlined) site, respectively, to facilitate cloning, and all clones were sequenced before analysis.
Chromatin immunoprecipitation (ChIP)
ChIP was performed using a ChIP assay kit (Upstate). The assay was carried out according to the manufacturer’s protocol. Immune complexes were obtained using 5 μg of hnRNP G antibody (G-17; Santa Cruz). Then, genomic DNA was isolated from the complexes and subjected to PCR using the following Txnip promoter primers: sense, 5′-tccttttacctcaaaacacc-3′; antisense, 5-aagaggggttagtttcaagc-3 as described in supplementary materials and methods (S5).
Assay of anchorage-independent growth in soft agar
To determine colony-forming efficiency in semi-solid medium, 1 × 104 cells were plated in 3 ml of culture medium containing 0.3% agarose over a base layer of serum-free MEM medium containing 0.5% agarose. Three weeks after incubation in a humidified incubator at 37° C at 5% CO2, colonies were stained with p-iodonitrotetrazolium violet (1 g/liter) and counted. The experiment was performed in triplicates with 60-mm dishes.
Determination of tumorigenicity in vivo
HEp-2 cells were infected with retroviral vectors, LNCX2 or LNCX2-Txnip in the presence of 8 μg/ml polybrene (Invitrogen), and infected cells were selected with G418. Then, 1.0 × 107 of selected cells were subcutaneously injected into the flank of immunocompromised mice (strain nu/nu, Charles River Laboratories). The animal study was performed as described previously [8].
Results
Txnip is upregulated by wild-type hnRNP G, but not by the tumor suppression-defective mutant hnRNP G (K22R)
Our previous study showed that the HEp-2 cells lack endogenous hnRNP G expression and are a proper cell line to test effect of exogenous hnRNP G on various biochemical processes [8,9]. Indeed, we demonstrated that wt hnRNP G elicited tumor suppressive activity, and its overexpression led to marked induction of Txnip mRNA in HEp-2 cells [8]. To further investigate whether the upregulation of Txnip is associated with the tumor suppressive activity of hnRNP G, we performed semi-quantitative RT-PCR and Western blot using HEp-2 cells overexpressing wt or tumor suppression-defective mutant hnRNP G (K22R) [8]. Both mRNA and protein levels of Txnip were significantly increased in cells overexpressing wt hnRNP G, but not in the control or the K22R overexpressing cells (Fig. 1A), suggesting that Txnip may be a downstream target of hnRNP G.
Figure 1. Effect of hnRNP G on the expression of Txnip.
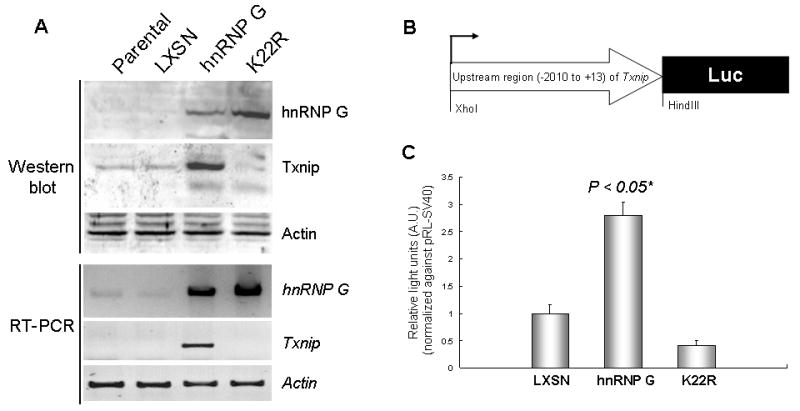
(A) Total protein and RNA were isolated from the parental HEp-2 cells and the HEp-2 cells expressing empty virus (LXSN), wt hnRNP G, or K22R hnRNP G mutant. Western blotting was performed using 100μg of protein for hnRNP G (43 kDa), Txnip (50 kDa), and actin (43 kDa). Amounts of hnRNP G and Txnip mRNA were determined by semi-quantitative RT-PCR with 25 amplification cycles. hnRNP G (1,165 bp), Txnip (453 bp) and actin (220 bp) were amplified from the same cDNA samples. Actin was used for the control in both Western and RT-PCR analysis. The assays were repeated twice and showed similar result. (B) Construction of pGL3-Txp reporter plasmid containing the 2,023 bp (−2010 to +13) Txnip upstream fragment. (C) The HEp-2 cells expressing LXSN, wt hnRNP G, or K22R hnRNP G mutant were co-transfected with pGL3-Txp and pRL-SV40. Luciferase activities derived from pRL-SV40 were used to normalize for transfection efficiency. The graph shows the mean of triplicated relative firefly luciferase light units normalized against those from pRL-SV40. The error bars indicate ±SD from three independent experiments. *Significantly different (P<0.05, Student’s T-test) from the other group, i.e., LXSN and K22R.
Activation of the Txnip promoter by wild-type hnRNP G
To investigate the effect of hnRNP G on the promoter function of Txnip, we constructed a luciferase reporter vector whose luciferase is driven by a 2,023-bp human Txnip promoter region (−2,010 to +13; Fig. 1B). As shown in Figure 1C, the activity of the Txnip promoter from the wt hnRNP G-expressing cells (HEp2/hnRNP G) was significantly greater than that from the control (HEp2/LXSN) and the K22R-expressing cells (HEp2/K22R). These results indicate that Txnip is transcriptionally upregulated by wt hnRNP G, but not by the K22R mutant.
To further identify a critical hnRNP G regulatory region in the Txnip promoter, we generated various deletion constructs as described in Figure 2A. The deletion constructs were transiently transfected into HEp2/hnRNP G cells and luciferase activity for each construct was measured (Fig. 2B). Deletions beyond −245 did not affect transcriptional activity, suggesting the hnRNP G acts at a site within this 258 bp region (from +13 to −245). To further confirm the importance of this region for hnRNP G regulation, the Txnip promoter construct −245 to +13 was cotransfected with pLXSN vector expressing wt hnRNP G or mutant K22R into HEp-2 cells (Fig. 2C). The cells cotransfected with wt hnRNP G showed significantly increased luciferase activity compared to controls (the promoter construct alone and cotransfection with the empty vector) and to those cotransfected with pLXSN expressing mutant K22R (Fig. 2C). These findings indicate that the region responsible for hnRNP G-mediated transactivation of Txnip lies between −245 to +13 bp.
Figure 2. Identification of a minimal Txnip promoter region responsible for hnRNP G.
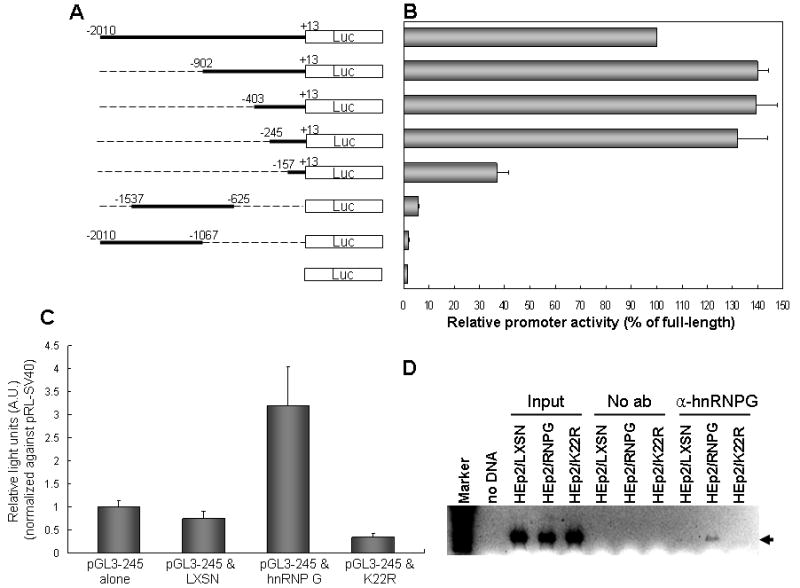
(A) Schematic representation of the pGL3 vectors containing the full-length (−2010 to +13) or deleted hnRNP G promoter regions. (B) The reporter vectors were transfected into the HEp-2 cells expressing wt hnRNP G, and luciferase activities from each vector were measured. Relative promoter activity is expressed as percentage against luciferase value from the full-length Txnip promoter. Each experiment was performed with a triplicate dish. The error bars indicate ±SD from three independent experiments. (C) The HEp-2 cells were co-transfected with the pGL3-245 vector containing 258 bp Txnip upstream fragment (−245 to +13) and LXSN, LXSN-hnRNP G, or K22R hnRNP G mutant. pRL-SV40 was also transfected into the same cells to normalize for transfection efficiency. The graph shows the mean of triplicated relative firefly luciferase light units normalized against those from pRL-SV40. The error bars indicate ±SD from three independent experiments. (D) Physical association of hnRNP G with the Txnip promoter in vivo was determined by ChIP assay. Cellular hnRNP G-DNA complexes were immunoprecipitated using hnRNP G antibody, and the presence of Txnip promoter in the complex was determined by PCR of Txnip promoter region (−245 to +13). Aarrow indicates amplified Txnip promoter DNA fragment. The assay was performed twice, and similar result was obtained from the experiments.
To further confirm the direct involvement of hnRNP G in the transcriptional activation of Txnip, ChIP assay was performed (Fig. 2D). The critical (−403 to +13) region was PCR-amplified from hnRNP G-immunoprecipitated complexes recovered from control (HEp2/LX), HEp2/hnRNPG, or HEp2/K22R cells. The PCR fragment representing the critical region was observed only from immunoprecipitated complexes recovered from the HEp2/hnRNPG cells, indicating that hnRNP G physically associates with the Txnip promoter in vivo and that Txnip is a direct transcriptional target of hnRNP G.
Overexpression of Txnip suppresses tumor cell growth in vitro and in vivo
To investigate a direct tumor suppressive effect of Txnip, we constructed retroviral vectors expressing Txnip (LNCX2-Txnip) and determined the effect of Txnip on the HEp-2 cells lacking both endogenous hnRNP G and Txnip. The cells were infected with LNCX2-Txnip or insertless vector (LNCX2), and selected with G418. G418-resistant cells were pooled and screened for the Txnip overexpression by Western blot analysis. As shown in Figure 3, the level of Txnip in the HEp-2 cells infected with retrovirus expressing Txnip was significantly increased compared to parental and empty virus-infected control counterparts (no infection and LNCX2).
Figure 3. Ectopic expression of exogenous Txnip in HEp-2 cells.
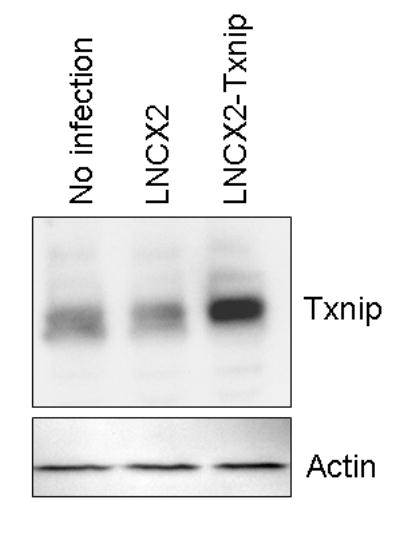
HEp-2 cells were infected with control (LNCX2) or Txnip expression (LNCX2-Txnip) vector and selected with 100 μg/ml G418. Total protein was isolated, and 100μg of protein was used for Western analysis for Txnip and actin.
To assess the effect of Txnip on the malignant properties of HEp-2 cells, we compared the ability of the parental HEp-2 and of those infected with LNCX2 or LNCX2-Txnip to form colonies in soft agar (Fig. 4A and 4B). HEp-2 cells infected with LNCX2-Txnip showed a significant reduction in the colony-forming efficiency in soft agar compared with the controls, i.e., parental cells and those infected with LNCX2 (Fig. 4A). Also, HEp-2 cells overexpressing Txnip produced smaller cell colonies than the cells expressing empty vector (Fig. 4B). Thus, overexpression of Txnip in the cancer cells reduces the ability of cells to produce colonies in soft agar medium.
Figure 4. Effect of Txnip on the tumor growth of HEp-2 cells in vitro and in vivo.
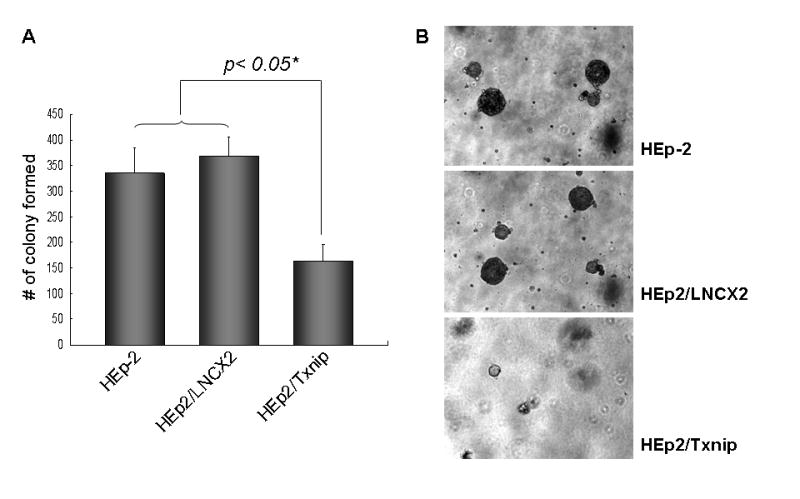
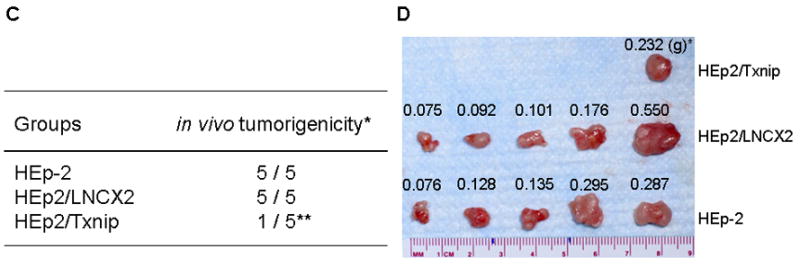
(A) The effect of Txnip on anchorage-independent growth was determined with parental and HEp-2 cells infected with LNCX2 or LNCX2-Txnip. After the infection, infected cell were selected with G418. Then, only selected cells (104) were plated in semi-solid agar and cultured for three weeks. Colonies were stained and counted. The assay was performed in triplicate with 60-mm dishes. *Significantly different (P<0.05, Student’s T-test) from the other group, i.e., parental and LNCX2. (B) Representative colony formation (original magnification, 40×). (C) Parental and G418 selected HEp-2 cells expressing LNCX2 or Txnip were injected subcutaneously into five nude mice. *Number of mice bearing tumors out of total number of mice injected. **Statistically different from the other groups, i.e., parental HEp-2 and LNCX2 (P<0.05, χ2 test of independence). (D) Photographs show the surgically removed tumors from all animals. * Weight of tumor (gram)
We also determined the effects of Txnip on the tumorigenic potential of HEp-2 cells in vivo (Fig. 4C and 4D). HEp-2 cells infected with LNCX2 or LNCX-Txnip were selected with 100 μg/ml G418. The drug-resistant cells were injected subcutaneously into nude mice. The parental HEp-2 cells and those expressing the empty vector (LNCX2) showed 100% efficiency of tumor formation 16 days post-injection. The cells expressing exogenous Txnip demonstrated significantly reduced efficiency (20%; 1/5) for the same period of observation (Fig. 4C and 4D). This tumor suppression was similar to that observed with hnRNP G [8]. Taken together, these results indicate that Txnip plays a growth inhibitory role in the cancer cells and tumors in vitro and in vivo.
Discussion
In this report, we demonstrate for the first time that hnRNP G elicits tumor suppressive activity in human cancer cells, in part, by upregulating a known tumor suppressor gene, Txnip. Our results demonstrate that Txnip is overexpressed in HEp-2 human squamous cell carcinoma cells expressing wild-type hnRNP G, but not in those overexpressing the K22R hnRNP G mutant which lacks tumor-suppressive activity [8]. Ectopic expression of Txnip alone in the same cells results in decreased ability to form replicating colonies in soft agar. Furthermore, Txnip overexpression significantly reduced the tumorigenic potential of the cancer cells in nude mice. Therefore, upregulation of Txnip by wild-type hnRNP G is closely associated with the tumor suppressive activity of hnRNP G.
As shown in the present study, hnRNP G transactivates the promoter of Txnip gene and associates with the promoter in vivo, indicating that hnRNP G is a novel transcription factor for Txnip. Furthermore, overexpression of wild-type hnRNP G increased the activity of the minimal Txnip promoter (from −403 to +13) while overexpression of the tumor suppression defective K22R mutant failed to enhance promoter activity. These results strongly suggest that upregulation of Txnip by hnRNP G is a plausible underlying mechanism of hnRNP G mediated tumor suppression. Although our results clearly indicate that hnRNP G transactivates the Txnip gene through the minimal Txnip promoter region, it is not clear at the present time whether hnRNP G can directly bind to this region. In fact, there is no report describing the presence of a DNA binding domain in hnRNP G protein. Apart from its function in RNA splicing, the other physiological roles of hnRNP G have yet to be fully understood and may include tumor suppression and DNA repair mechanisms [8,9]. Recent studies have shown that several hnRNP family proteins, such as D0B, E2BP, and K, are able to bind to the promoters of various cellular genes [10–12]. It is therefore interesting to note the DNA end binding ability of recombinant hnRNP G [9]. Moreover, a recent study showed that hnRNP G regulates the activity of the sterol regulatory element-binding protein-1c promoter [7]. Together, these reports suggest that, like other hnRNP proteins, hnRNP G may function as a transcription factor. However, we could not rule out the possibility that hnRNP G may regulate Txnip promoter activity through another DNA-binding protein. hnRNP G has been shown to associate with hnRNP C and tra-2β [5,13,14].
Txnip was originally identified as a vitamin D3-up-regulated gene in the human HL-60 promyelocytic cell line [15]. Txnip expression is also induced by various stresses, including H2O2, irradiation, heat shock, serum starvation, and treatment with transforming growth factor-β [16,17]. Reduced Txnip expression is found in various tumor tissues [17,18], suggesting a close association between reduced Txnip expression and tumorigenesis. The HcB-19 mouse strain, which has a spontaneous mutation in the Txnip gene, demonstrated an increased incidence of hepatocellular carcinoma, suggesting that Txnip is a candidate tumor suppressor gene in vivo [19]. Fibroblasts of Txnip-deficient mice proliferate more rapidly and express less p27kip1 cyclin-dependent kinase inhibitor compared with wild-type fibroblasts suggesting a possible mechanism for tumor suppression by Txnip [20]. Consistent with this observation, we also observed that the level of p27kip1 protein was significantly enhanced by ectopic expression of wild-type hnRNP G (data not shown). Considering that a histone deacetylase inhibitor, clinically used for the treatment of cancer, induced the expression of Txnip and caused the suppression of cancer cell growth [18], the upregulation of Txnip gene expression may be an important mechanism, at least in part, by which hnRNP G elicits tumor suppressive activity.
The effect of Txnip on apoptosis is unclear. Several apoptogenic agents, such as HDAC inhibitors and dexamethasone, readily increase Txnip expression [18,21]. However, the effect of Txnip overexpression on apoptosis is cell type-specific. For instance, the overexpression of Txnip is not sufficient to induce apoptosis in various human carcinoma cell lines [17,22], whereas the Txnip overexpression in a murine T-cell lymphoma cell line results in apoptosis [21]. We did not detect any relevant indication of apoptosis in the HEp-2 cells overexpressing Txnip (data not shown). Interestingly, however, we observed apoptosis in the HEp-2 cells overexpressing wild-type hnRNP G as determined by the presence of DNA fragmentation and activated/cleaved caspase-3 (data not shown). These findings suggest the presence of multiple tumor suppression pathways which are induced by hnRNP G.
Taken together, Txnip is the first hnRNP G target gene linked to tumor suppression, thereby further elucidating the molecular mechanism of tumor suppression induced by hnRNP G.
Supplementary Material
Footnotes
This work was supported by the grants DE15902 and DE14147 funded by the National Institute of Dental and Craniofacial Research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dreyfuss G, Matunis MJ, Piuol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- 2.Krecic AM, Swanson MS. hnRNP complexes: composition, structure, and function. Curr Opin Cell Biol. 1999;11:363–371. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- 3.Soulard M, Della Valle V, Siomi MC, Piñol-Roma S, Codogno P, Bauvy C, Bellini M, Lacroix JC, Monod G, Dreyfuss G. HnRNP G: sequence and characterization of a glycosylated RNA-binding protein. Nucleic Acids Res. 1993;21:4210–4217. doi: 10.1093/nar/21.18.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delbridge ML, Lingenfelter PA, Disteche CM, Graves JA. The candidate spermatogenesis gene RBMY has a homologue on the human X chromosome. Nat Genet. 1993;22:223–224. doi: 10.1038/10279. [DOI] [PubMed] [Google Scholar]
- 5.Soulard M, Barque JP, Della Valle V, Hernandez-Verdun D, Masson C, Danon F, Larsen CJ. A novel 43-kDa glycoprotein is detected in the nucleus of mammalian cells by autoantibodies from dogs with autoimmune disorders. Exp Cell Res. 1991;193:59–71. doi: 10.1016/0014-4827(91)90538-6. [DOI] [PubMed] [Google Scholar]
- 6.Nasim MT, Chernova TK, Chowdhury HM, Yue BG, Eperon IC. HnRNP G and Tra2beta: opposite effects on splicing matched by antagonism in RNA binding. Hum Mol Genet. 2003;12:1337–1348. doi: 10.1093/hmg/ddg136. [DOI] [PubMed] [Google Scholar]
- 7.Takemoto T, Nishio Y, Sekine O, Ikeuchi C, Nagai Y, Maeno Y, Maegawa H, Kimura H, Kashiwagi A. RBMX is a novel hepatic transcriptional regulator of SREBP-1c gene response to high-fructose diet. FEBS Lett. 2007;581:218–222. doi: 10.1016/j.febslet.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Shin KH, Kang MK, Kim RH, Christensen R, Park NH. Heterogeneous nuclear ribonucleoprotein G (hnRNP G) demonstrates tumor suppressive effect against oral squamous cell carcinoma cells. Clin Cancer Res. 2006;12:3222–3228. doi: 10.1158/1078-0432.CCR-05-2656. [DOI] [PubMed] [Google Scholar]
- 9.Shin KH, Kim RH, Kang MK, Kim R, Kim S, Lim PK, Yochim JM, Baluda MA, Park NH. p53 promotes the fidelity of DNA end-joining activity by, in part, enhancing the expression of heterogeneous nuclear ribonucleoprotein G. DNA Repair. 2007;6:830–840. doi: 10.1016/j.dnarep.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolnay M, Vereshchagina LA, Tsokos GC. Heterogeneous nuclear ribonucleoprotein D0B is a sequence-specificDNA-binding protein. Biochem J. 1999;338:417–425. [PMC free article] [PubMed] [Google Scholar]
- 11.Tay N, Chan SH, Ren EC. Identification and cloning of a novel heterogeneous nuclear ribonucleoprotein C-like protein that functions as a transcriptional activator of the hepatitis B virus enhancer II. J Virol. 1992;66:6841–6848. doi: 10.1128/jvi.66.12.6841-6848.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomonaga T, Levens D. Heterogeneous Nuclear Ribonucleoprotein K Is a DNA-binding transactivator. J Biol Chem. 1995;270:4875–4881. doi: 10.1074/jbc.270.9.4875. [DOI] [PubMed] [Google Scholar]
- 13.Choi YD, Grabowski PJ, Sharp PA, Dreyfuss G. Heterogeneous nuclear ribonucleoproteins: role in RNA splicing. Science. 1986;231:1534–1539. doi: 10.1126/science.3952495. [DOI] [PubMed] [Google Scholar]
- 14.Venables JP, Elliott DJ, Makarova OV, Makarov EM, Cooke HJ, Eperon IC. RBMY, a probable human spermatogenesis factor, and other hnRNP G proteins interact with Tra2beta and affect splicing. Hum Mol Genet. 2000;9:685–694. doi: 10.1093/hmg/9.5.685. [DOI] [PubMed] [Google Scholar]
- 15.Chen KS, DeLuca HF. Isolation and characterization of a novel cDNA from HL-60 cells treated with 1,25-dihydroxyvitamin D-3. Biochim Biophys Acta. 1994;1219:26–32. doi: 10.1016/0167-4781(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 16.Junn E, Han SH, Im JY, Yang Y, Cho EW, Um HD, Kim DK, Lee KW, Han PL, Rhee SG, Choi I. Vitamin D3 up-regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol. 2000;164:6287–6295. doi: 10.4049/jimmunol.164.12.6287. [DOI] [PubMed] [Google Scholar]
- 17.Han SH, Jeon JH, Ju HR, Jung U, Kim KY, Yoo HS, Lee YH, Song KS, Hwang HM, Na YS, Yang Y, Lee KN, Choi I. VDUP1 upregulated by TGF-beta1 and 1,25-dihydorxyvitamin D3 inhibits tumor cell growth by blocking cell-cycle progression. Oncogene. 2003;22:4035–4046. doi: 10.1038/sj.onc.1206610. [DOI] [PubMed] [Google Scholar]
- 18.Butler LM, Zhou X, Xu WS, Scher HI, Rifkin RA, Marks PA, Richon VM. The histone deacetylase inhibitor SAHA arrests cancer cell growth, up-regulates thioredoxin-binding protein-2, and down-regulates thioredoxin. Proc Natl Acad Sci USA. 2002;99:11700–11705. doi: 10.1073/pnas.182372299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheth SS, Castellani LW, Chari S, Wagg C, Thipphavong CK, Bodnar JS, Pontonoz T, Attie AD, Lopaschuk GD, Lusis AJ. Thioredoxin-interacting protein deficiency disrupts the fasting-feeding metabolic transition. J Lipid Res. 2005;46:123–134. doi: 10.1194/jlr.M400341-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Jeon JH, Lee KN, Hwang CY, Kwon KS, You KH, Choi I. Tumor suppressor VDUP1 increases p27(kip1) stability by inhibiting JAB1. Cancer Res. 2005;65:4485–4489. doi: 10.1158/0008-5472.CAN-04-2271. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Rong YP, Malone MH, Davis MC, Zhong F, Distelhorst CW. Thioredoxin-interacting protein (txnip) is a glucocorticoid-regulated primary response gene involved in mediating glucocorticoid-induced apoptosis. Oncogene. 2006;25:1903–1913. doi: 10.1038/sj.onc.1209218. [DOI] [PubMed] [Google Scholar]
- 22.Nishinaka Y, Nishiyama A, Masutani H, Oka S, Ahsan KM, Nakayama Y, Ishii Y, Nakamura H, Maeda M, Yodoi J. Loss of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 in human T-cell leukemia virus type I-dependent T-cell transformation: implications for adult T-cell leukemia leukemogenesis. Cancer Res. 2004;64:1287–1292. doi: 10.1158/0008-5472.can-03-0908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


