Abstract
During mating of Saccharomyces cerevisiae, two nuclei fuse to produce a single diploid nucleus. Two genes, KAR7 and KAR8, were previously identified by mutations that cause defects in nuclear membrane fusion. KAR7 is allelic to SEC71, a gene involved in protein translocation into the endoplasmic reticulum. Two other translocation mutants, sec63-1 and sec72Δ, also exhibited moderate karyogamy defects. Membranes from kar7/sec71Δ and sec72Δ, but not sec63-1, exhibited reduced membrane fusion in vitro, but only at elevated temperatures. Genetic interactions between kar7 and kar5 mutations were suggestive of protein–protein interactions. Moreover, in sec71 mutants, Kar5p was absent from the SPB and was not detected by Western blot or immunoprecipitation of pulse-labeled protein. KAR8 is allelic to JEMI, encoding an endoplasmic reticulum resident DnaJ protein required for nuclear fusion. Overexpression of KAR8/JEM1 (but not SEC63) strongly suppressed the mating defect of kar2-1, suggesting that Kar2p interacts with Kar8/Jem1p for nuclear fusion. Electron microscopy analysis of kar8 mutant zygotes revealed a nuclear fusion defect different from kar2, kar5, and kar7/sec71 mutants. Analysis of double mutants suggested that Kar5p acts before Kar8/Jem1p. We propose the existence of a nuclear envelope fusion chaperone complex in which Kar2p, Kar5p, and Kar8/Jem1p are key components and Sec71p and Sec72p play auxiliary roles.
INTRODUCTION
Nuclear fusion (karyogamy) is the last step in the mating pathway that culminates in the formation of a diploid cell. In preparation for mating in Saccharomyces cerevisiae, cells respond to the mating pheromone secreted by a and α cells, exit the mitotic cell cycle, and differentiate into mating-proficient cells. The mating cells grow directionally toward the selected mating partner, producing a cell with a characteristic mating projection often called a shmoo. Once contact between the partner cells is established, the mating pair undergoes cell fusion followed by nuclear fusion to form a diploid zygote (for review, see Sprague and Thorner, 1994; Herskowitz, 1995; Rose, 1996; Marsh and Rose, 1997).
The pathway of karyogamy in yeast proceeds by at least two major steps (Kurihara et al., 1994). First, cytoplasmic microtubules emanating from the spindle pole body (SPB) are required to bring the nuclei into close proximity (Byers and Goetsch, 1975; Byers, 1981), a process called congression. The SPB is embedded in the nuclear envelope, which otherwise remains intact throughout all phases of the cell cycle (Byers, 1981). Upon pheromone induction, the cytoplasmic microtubules emanating from the SPB position the nucleus close to the mating projection (Byers and Goetsch, 1975; Miller and Rose, 1998). Immediately after cell fusion, the cytoplasmic microtubules interconnect, the two nuclei move together, and the two SPBs become closely apposed (Byers and Goetsch, 1975; Meluh and Rose, 1990; Beh et al., 1997).
The second step of karyogamy entails the fusion of the nuclear membranes. Two membranes, the inner and outer nuclear envelopes, surround each nucleus. Therefore, the establishment of nuclear lumenal continuity requires that the two outer and two inner membranes become fused in register. Membrane fusion is also coupled to the fusion of the two SPBs, resulting in the formation of a single larger microtubule-organizing center (Byers and Goetsch, 1975). Based on ultrastructural analysis, Byers and Goetsch (1975) proposed that nuclear membrane fusion initiates along one edge of the two SPBs to generate a single diploid nucleus. Whether the SPB and the two sets of membranes fuse in one concerted event or in several stepwise events is not yet known (Rose, 1996).
Several mutations that block nuclear fusion have been isolated and characterized (Conde and Fink, 1976; Polaina and Conde, 1982; Kurihara et al., 1994). The mutants fall into two distinct classes corresponding to the two major events in the karyogamy pathway. Class I mutants are defective for nuclear congression, and class II mutants are defective for nuclear membrane fusion (Kurihara et al., 1994). All of the class I mutants are unable to bring the two nuclei into close proximity, and all contain mutations in genes that are involved with microtubule function (Kurihara et al., 1994).
In the class II mutants, kar2, kar5, kar7, and kar8, the two nuclei congress normally, but the nuclear membranes do not fuse (Kurihara et al., 1994). Mutations in these genes are also defective in an in vitro endoplasmic reticulum (ER)–nuclear envelope membrane fusion assay (Kurihara et al., 1994; Latterich and Schekman, 1994). Kar2p is the yeast homologue of the mammalian BIP/GRP78, a member of the Hsp70 chaperone family (Rose et al., 1989). Kar2p resides in the lumen of the ER–nuclear envelope and is essential for the translocation and folding of secretory precursors into the ER (Rose et al., 1989; Vogel et al., 1990; Sanders et al., 1992). Two observations suggest that Kar2p has a direct role in nuclear membrane fusion, which is independent of its role in translocation (Vogel et al., 1990; Vogel, 1993; Latterich and Schekman, 1994). First, temperature-sensitive KAR2 mutants show temperature-sensitive ER–nuclear envelope membrane fusion in vitro. Second, there is a striking lack of correlation between nuclear fusion and translocation defects for various kar2 alleles.
Kar5p is a novel integral ER–nuclear envelope membrane protein. Kar5p is predicted to have a carboxyl-terminal transmembrane domain, and protease protection analysis demonstrated that most of the protein is present in the lumen of the ER–nuclear envelope (Beh et al., 1997). Consistent with its role in nuclear fusion, Kar5p is induced by pheromone and localizes near the SPB (Beh et al., 1997). KAR5 was also identified in a screen for pheromone-induced genes (Erdman et al., 1998). A homologue of Kar5p, called Tht1p, has also been identified in Schizosaccharomyces pombe and shown to play a role in nuclear fusion (Tange et al., 1998).
In addition, Ng and Walter (1996) found that certain mutations in SEC63, SEC71, and SEC72 also result in zygotes with nuclear membrane fusion defects. These three genes all encode ER–nuclear envelope proteins with roles in protein translocation. Sec63p is an essential integral membrane protein with a large cytoplasmic domain and a smaller lumenal domain. The lumenal domain of Sec63p is composed of a DnaJ homology domain that interacts with Kar2p (Sadler et al., 1989; Brodsky and Schekman, 1993; Feldheim et al., 1993; Scidmore et al., 1993). SEC71 encodes a 206-residue, 31.5-kDa integral membrane glycoprotein in the Sec63p complex (Green et al., 1992; Brodsky and Schekman, 1993; Feldheim et al., 1993; Kurihara and Silver, 1993). Unlike SEC63 and most other components of the translocation machinery, SEC71 is not an essential gene; deletion mutations result in a temperature-sensitive growth defect and the accumulation of a subset of precursor proteins at the nonpermissive temperature (Feldheim et al., 1993; Kurihara and Silver, 1993). Sec72p is a 23-kDa peripheral membrane protein that is also a component of the Sec63p complex (Green et al., 1992; Brodsky and Schekman, 1993; Fang and Green, 1994; Feldheim and Schekman, 1994). SEC72 is also not essential for life but is required for the translocation of a subset of protein precursors (Feldheim and Schekman, 1994).
Recently, another gene with homology to DnaJ, called JEM1 (DnaJ-like protein of the ER membrane), was identified by the Yeast Genome Project. JEM1 encodes a 645–amino acid peripheral membrane protein associated with the lumenal region of the ER (Nishikawa and Endo, 1997; Nishikawa and Endo, 1998). The carboxyl-terminal domain of JEM1 contains a J domain with 47% identity to the Escherichia coli DnaJ protein. Disruption of JEM1 results in a bilateral karyogamy defect reminiscent of other class II karyogamy mutants (Nishikawa and Endo, 1997).
Here we show that KAR7 and KAR8 are allelic to SEC71 and JEM1, respectively. In agreement with Ng and Walter (1996), we find that other components of the translocation machinery, including SEC63 and SEC72, but not SEC61, are required for efficient nuclear fusion in vivo. However, membranes that are devoid of Sec71p and Sec72p showed only a temperature-sensitive reduction in membrane fusion competence in vitro. To investigate KAR7/SEC71’s role in karyogamy, we analyzed the basis of a previously noted genetic interaction between KAR7/SEC71 and KAR5, a gene specifically required for nuclear fusion. We conclude that KAR7/SEC71 is required for the synthesis and/or stability of Kar5p. To investigate KAR8/JEM1’s role we used dosage suppression experiments and electron microscopy (EM) analysis. We show that KAR8/JEM1 has a unique karyogamy function that cannot be substituted for by SEC63 or SCJ1. EM analysis showed that the nuclear fusion bridges seen in kar8/jem1Δ mutant zygotes are different from those observed in kar2, kar5, and kar7 mutants. Analysis of kar5Δ kar8/jem1Δ double mutants suggests that Kar8p functions downstream of Kar5p. We propose the existence of a nuclear fusion complex in which Kar5p, Kar8/Jem1p, and Kar2p are key components and Sec63p, Sec71p, and Sec72p play auxiliary roles.
MATERIALS AND METHODS
Microbial Techniques, General Methods, and Strains
Yeast media and genetic techniques were as previously described (Rose et al., 1990). Yeast and E. coli plasmid DNA minipreps were performed as described elsewhere (Rose et al., 1990). Yeast strains were transformed by the lithium acetate method (Ito et al., 1983). Limited plate matings were performed as described previously (Brizzio et al., 1996). Filter matings for the microscopic analysis of zygotes were performed as described elsewhere (Brizzio et al., 1996). Briefly, ∼5 × 106 cells in exponential phase from each parent were mixed onto a 45-μm nitrocellulose filter. The mating mixtures were then incubated for 2–3 h at 30°C. The cells were subsequently fixed in methanol:acetic acid (3:1) on ice for 1 h and washed two times with PBS. 4′,6′-Diamidino-2-phenylindole (DAPI) was added at 1 μg/ml for 5 min, and the cells were washed with PBS. Zygotes were then analyzed by differential interference contrast (DIC) and fluorescence microscopy (Axiophot; Carl Zeiss, Thornwood, NY). For sec63-201 × sec63-201 matings, zygotes were fixed in 3.7% formaldehyde diluted in PBS for 10 min and then washed two times with PBS before staining with DAPI.
Quantitative matings were performed as described previously (Rose et al., 1990). In brief, ∼3 × 106 cells in midexponential phase from each parent were mixed onto a 45-μm nitrocellulose filter. The mating mixtures were then incubated for 4 h at 23, 30, and at 35°C. Several dilutions were plated on YEPD, on appropriate plates for diploid selection and on YPG containing cycloheximide at 3 μg/ml. Rho° cycloheximide-resistant strains (ρ° cyh2) were generated as described previously (Rose et al., 1990).
The strains used in this study are listed in Table 1. Unless stated otherwise, all the strains are isogenic to S288C.
Table 1.
Yeast strains used in this study
| Strain | Genotype |
|---|---|
| MS5 | MATa ura3-52 leu2-3 leu2-112 |
| MS10 | MATa ura3-52 leu2-3, leu2-112 ade2-101 |
| MS16 | MATα ade2-101 trp1-Δ1 |
| MS22 | MATa ade2-101 trp1-Δ1 lys2-801 |
| MS23 | MATα ade2-101 trp1-Δ1 lys2-801 |
| MS31 | MATα trp1-Δ1 |
| MS52 | MATα ura3-52 leu2-3 leu2-112 trp1-Δ1 |
| MS1111 | MATa ura3-52 leu2-3 leu2-112 ade2-101 kar2-1 |
| MS1554 | MATa ura3-52 leu2-3, leu2-112 ade2-101 his3-Δ200 |
| MS2705 | MATΔ∷LEU2 ura3-52 leu2-3, leu2-112 trp1-Δ1 kar8-1333 [YCpMATα] |
| MS2706 | MATΔ∷LEU2 ura3-52 leu2-3, leu2-112 trp1-Δ1 kar8-1333 |
| MS3259 | MATa ura3-52 leu2-3, leu2-112 ade2-101 his3-Δ200 kar7-1039 |
| MS3260 | MATa ura3-52 leu2-3 leu2-112 his3-Δ200 ade2-101 kar8-1333 |
| MS3534 | MATa ura3-52 leu2-3, leu2-112 ade2-101 his3-Δ200 lys2-801 kar5-1162 |
| MS3536 | MATα ura3-52 lys2-801 his3-Δ200 ade2-101 leu2-3 leu2-112 kar8-1333 |
| MS3537 | MATα ura3-52 ade2-101 his3-Δ200 lys2-801 kar5-486 |
| MS3538 | MATα ura3-52 ade2-101 his3-Δ200 lys2-801 kar5-1162 |
| MS3539 | MATα ura3-52 ade2-101 his3-Δ200 lys2-801 kar7-1039 |
| MS3577 | MATα ura3-52 leu2-3, leu2-112 trp1-Δ1 kar5-Δ2∷LEU2 |
| MS3823 | MATa ura3-52 ade2-101 kar7-1039 |
| MS3826 | MATα ura3-52 leu2-3, leu2-112 ade2-101 kar7-1039 |
| MS3856 | MATα ura3-52 leu2-3, leu2-112 trp1-Δ1 ρo cyh2 |
| MS3908 | MATa ura3-52 leu2-3, leu2-112 ade2-101 his3-Δ200 SEC71∷YIp-LEU2 |
| MS3910 | MATa ura3-52 leu2-3, leu2-112 ade2-101 his3-Δ200 sec71-Δ1∷URA3 |
| MS3911 | MATα ura3-52 ade2-101 his3-Δ200 lys2-801 sec71-Δ1∷URA3 |
| MS3927 | MATα ura3-52 leu2-3, leu2-112 trp1-Δ1 sec71-Δ1∷URA3ρo cyh2 |
| MS3928 | MATa ura3-52 leu2-3 leu2-112 kar8-1333 |
| MS3986 | MATa ura3-52 leu2-3, leu2-112 ade2-101 his3-Δ200 lys2-801 kar5-Δ2∷LEU2 (pRS426) |
| MS3987 | MATa ura3-52 leu2-3, leu2-112 his3-Δ200 kar5-Δ2∷LEU2 (pMR3142) |
| MS3989 | MATa ura3-52 leu2-3, leu2-112 ade2-101 lys2-801 kar5-1039 (pMR3142) |
| MS3991 | MATa ura3-52 leu2-3, leu2-112 ade2-101 lys2-801 kar7-1039 (pMR3142) |
| MS4020 | MATa ura3-52 ade2-101 leu2-3, leu2-112 kar2-1 (pMR3142) |
| MS4021 | MATa ura3-52 leu2-3 kar1-1 (pMR3142) |
| MS4060 | MATα ura3-52 ade2-101 his3-Δ200 lys2-801 kar7-1039 ρo cyh2 |
| MS4076 | MATa ura3-52 leu2-3 leu2-112 kar8-Δ1∷LEU2 |
| MS4326 | MATα ura3-52 leu2-3 leu2-112 his3-Δ200 ade2-101 trp1-Δ1 |
| MS4338 | MATα ura3-52 leu2-3 leu2-112 trp1-Δ1 his3-Δ200 kar8-Δ1∷LEU2 |
| MS4342 | MATa ura3-52 leu2-3 leu2-112 his3-Δ200 kar8-Δ1∷LEU2 |
| MS4359 | MATα ura3-52 leu2-3 leu2-112 ade2-101 his3-Δ200 kar5-Δ1∷URA3 kar8-Δ1∷LEU2 |
| MS4360 | MATa ura3-52 leu2-3 leu2-112 ade2-101 his3-Δ200 trp1-Δ1 kar5-Δ1∷URA3 kar8-Δ1∷LEU2 |
| MY2248a | MATα ura3-52 leu2-3 leu2-112 ade2-101 trp1-Δ1 sec63-1 |
| MY2339 | MATα ura3-52 ade2-101 his4-539 sec61-2 |
| MY2341 | MATa ura3-52 leu2-3, leu2-112 ade2-101 sec61-2 |
| MY2653a | MATα ura3-52 leu2-3 leu2-112 trp1-Δ1 sec63-4 |
| MY2808 | MATa trp1Δ sec63-1 |
| MY3564 | MATα ura3-52 sec63-1 ρo cyh2 |
| MY3594 | MATa ura3-52 ade2-101 his4-539 trp1-Δ1 sec62-1 |
| MY3676 | MATα ura3-52 ade2-101 his4-539 sec61-2 ρo cyh2 |
| MY3678 | MATα ura3-52 leu2-3, leu2-112 his4-539 sec62-1 ρo cyh2 |
| MY3917 | MATα ura3-52 ade2-101 his3-Δ200 lys2-801 trp1-Δ1 sec72-Δ∷HIS3 |
| MY3918 | MATa ura3-52 leu2Δ1 ade2-101 his3-Δ200 sec72-Δ∷HIS3 |
| MY3931 | MATα ura3-52 ade2-101 his3-Δ200 lys2-801 trp1-Δ1 sec72-Δ∷HIS3 ρo cyh2 |
| MY4169a | MATα ura3-52 leu2-Δ1 ade2-101 lys2-801 trp1-Δ1 his3-Δ200 sec63-Δ∷HIS3 (SEC63-URA 2μ) |
| MLY1600a | MATα ura3-52 leu2-3, leu2-112 pep4-Δ∷URA3 |
| MLY1601a | MATa ura3-52 leu2-3, leu2-112 pep4-Δ∷URA3 gls1-1 |
| MLY1651a | MATα ura3-52 leu2-3, leu2-112 pep4-Δ∷URA3 sec63-1 |
| MLY1652a | MATα ura3-52 leu2-3, leu2-112 pep4-Δ∷URA3 gls1-1 sec63-1 |
| MLY1889a | MATa ura3-52 leu2-3, leu2-112 pep4-Δ∷URA3 gls1-1 sec71-Δ |
| MLY1890a | MATα ura3-52 leu2-3, leu2-112 pep4-Δ∷URA3 sec71-Δ |
| MLY1891a | MATa ura3-52 leu2-3, leu2-112 pep4-Δ∷URA3 gls1-1 sec72-Δ |
| MLY1892a | MATα ura3-52 leu2-3, leu2-112 pep4Δ∷URA3 sec72-Δ |
| DNY65b | MATa sec63-201 ura3-Δ99 leu2-Δ99 his3-Δ200 leu2-Δ1 trp1-Δ99 ade2-101 (pDN106) |
| DNY66b | MATα sec63-201 ura3-Δ99 leu2-Δ99 his3-Δ200 leu2-Δ1 trp1-Δ99 ade2-101 (pDN106) |
Obtained from R. Schekman laboratory.
Obtained from Davis Ng (P. Walter Laboratory).
Strain Construction and Plasmids
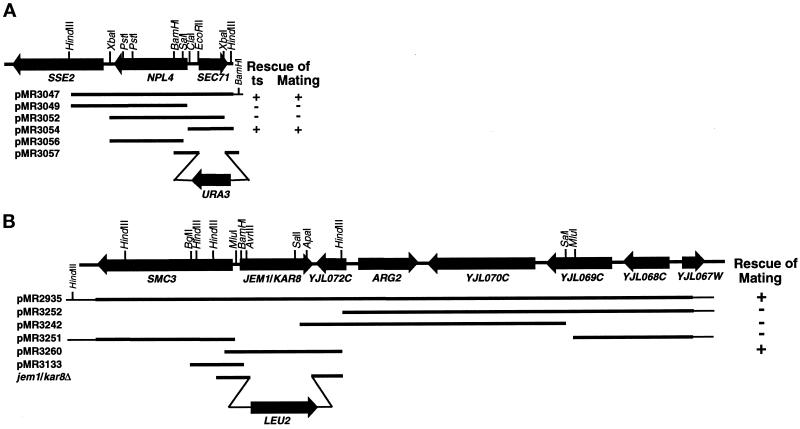
Plasmid pMR3056, used for linkage analysis between the SEC71 locus and kar7-1039, was constructed by cloning a 1.5-kb XbaI–SalI restriction fragment from the SEC71 locus (see Figure 2) into pRS405 YIp-LEU2 vector (Sikorski and Hieter, 1989). Plasmid pMR3056 was linearized with PstI before transformation of MS1554 to generate strain MS3908.
Figure 2.
(A) Restriction map of KAR7/SEC71 and surrounding region on chromosome II. Shown are several subclones generated to further define KAR7. Bars represent the DNA fragments present in the different plasmids. The ability (+) or inability (−) of the different plasmids to suppress the mating defect is indicated to the right. Shown at the bottom is the structure of plasmids pMR3056 and pMR3057 used in the linkage analysis and the generation of sec71Δ by one-step gene replacement respectively. (B) Restriction map of KAR8/JEM1 and surrounding region on chromosome X. One of the original plasmids (pMR2935) able to rescue the kar8-1333 mating defect is depicted. Several subclones were generated and tested for rescuing activity. Bars represent the DNA fragments present in the different plasmids. The ability (+) or inability (−) of the different plasmids to suppress the mating defect is indicated to the right. Shown at the bottom is the structure of plasmid pMR3133 used in the linkage analysis of JEM1 and the structure of the jem1/kar8Δ allele generated by one-step gene replacement.
Generation of a sec71Δ allele (sec71-Δ1::URA3) was done by one-step gene replacement (Scherer and Davis, 1979). Plasmid pMR3057 was made by cloning 513-bp EcoRI–BamHI and 286-bp BamHI–XbaI restriction fragments from pMR3047 (see Figure 2) into pRS406 YIp-URA3 vector (Sikorski and Hieter, 1989) cut with XbaI–EcoRI. Plasmid pMR3057 was linearized with BamHI before transformation of MS1554 and MS1683 to generate MS3910 and MS3911, respectively. This construct results in a 577-bp deletion that removes the SEC71 promoter regions and 173 amino acids of SEC71 coding region, leaving just 34 carboxyl-terminal amino acids. Generation of the sec71-Δ1::URA3 allele was confirmed by Southern blotting as previously described (Hoffman and Winston, 1987; Rose et al., 1990).
For linkage analysis between KAR8 and JEM1, plasmid pMR3133 was constructed to direct genomic integration at the KAR8 locus. Plasmid pMR3133 was made by cloning a genomic 1.2-kb BglII–BamHI restriction fragment from pMR2935 into pRS405, which had been cut with BamHI (see Figure 2). Plasmid pMR3133 was linearized with MluI before transformation of MS52.
Generation of the kar8/jem1Δ::LEU2 allele was done by one-step gene replacement. A disruption plasmid was generated by cloning 806-bp HindIII–ApaI and 647-bp AvrII–HindIII restriction fragments into pRS405 YIp-LEU2 vector (Sikorski and Hieter, 1989), and subsequently linearized with HindIII before transformation of MS5, to generate strain MS4076. This allele removes 642 amino acids internal to KAR8/JEM1, leaving 24 amino-terminal and 26 carboxyl-terminal amino acids. Generation of the kar8/jem1Δ allele was confirmed by Southern blotting as previously described (Sambrook et al., 1989; Rose et al., 1990). Strains MS4338 and MS4342 were generated by sporulation of a cross between MS4076 and MS4326.
To construct a KAR8/JEM1 2μ plasmid (pMR3270), a 3.3-kb HindIII fragment containing KAR8/JEM1 from pMR2935 was subcloned into pRS426 YEp-URA3 (Sikorski and Hieter, 1989). Plasmids pDF14 (LEU2 2μ SEC63) and pDF15 (URA3 2μ SEC63) were kindly provided by the R. Schekman laboratory (University of California, Berkeley, CA) and have been previously described (Feldheim et al., 1993). Plasmid pCen63, a CEN URA3 plasmid containing SEC63, was obtained from the P. Silver laboratory (Harvard University, Boston, MA). The KAR8/JEM1 2μ plasmid pMR3352 and the KAR8/JEM1-CEN plasmid pMR3369 were made by subcloning a 3.3-kb HindIII fragment containing KAR8/JEM1from pMR2935 into pRS425 YEp-LEU2 and pRS415 YCp-LEU2, respectively (Sikorski and Hieter, 1989). The SCJ1 2μ plasmid pPS720 (Silberstein et al., 1998) was kindly provided by Reid Gilmore (University of Massachusetts Medical School, Worcester, MA).
Cloning of KAR7
In addition to its mating defect, kar7-1039 showed a temperature-sensitive defect for growth at 37°C. Linkage analysis was used to show that the mating and the temperature-sensitive phenotypes were tightly linked. A kar7-1039 strain (MS3539) was crossed to a wild-type strain (MS10), and in 30 tetrads analyzed, there was cosegregation of the temperature-sensitive phenotype (Ts−) with the mating defect (2 Kar+:2 Kar−Ts−), indicating a genetic distance of <1.6 centimorgans (cM). The temperature-sensitive phenotype of kar7-1039 was then used to clone KAR7 by complementation. A yeast centromere-based (YCp50) genomic library (Rose et al., 1987) was transformed into a kar7-1039 strain (MS3259). Twenty thousand Ura+ transformants were selected at 30°C and screened for growth at 37°C. Fourteen positives were isolated. They all shared DNA fragments in common and complemented both the temperature-sensitive growth defect and the mating defect when reintroduced in the kar7-1039 strain (MS3259). A 3.9-Kb HindIII–HindIII restriction fragment present in all 14 clones was subcloned into pRS416 (Sikorski and Hieter, 1989). This plasmid (pMR3047) was then tested for its ability to complement the temperature-sensitive growth defect and the mating defect of kar7-1039 (MS3259). As indicated in Figure 2A, this fragment still retained both complementing activities. The sequence of the ends of the insert in pMR3047 was determined using Sequenase (United States Biochemical, Cleveland, OH) and the T3 and T7 primers (from pBluescript; Stratagene, La Jolla, CA), following the manufacturer’s instructions. Examination of the DNA sequence and consultation of the GenBank database showed that the cloned DNA contained the SEC71 and NPL4 genes and part of an uncharacterized gene, SSE2. To precisely define KAR7, several subclones were generated and tested for complementation of both defects (Figure 2A). Cloning of 2.9-kb HindIII–ClaI, 2.6-kb XbaI–XbaI, and 1-kb ClaI–HindIII restriction fragments into the pRS416 YCp-URA3 vector generated plasmids pMR3049, pMR3052, and pMR3054, respectively. Plasmid pMR3054 containing just SEC71 retained both complementing activities, suggesting that KAR7 was identical to the previously characterized gene SEC71. To verify that KAR7 is identical to SEC71, linkage analysis was performed. A KAR7 leu2 strain (MS1554) was transformed with the integration plasmid pMR3056 (see Figure 2A). A stable Leu+ transformant (MS3908) was crossed to a kar7-1039 leu2 strain (MS3826), and tetrad analysis was performed. The temperature-sensitive (Ts−) phenotype of kar7-1039 was used to follow KAR7 in this cross. In all 24 tetrads analyzed, there was cosegregation of Leu− with the Ts− phenotype (2 Leu+:2 Ts−Leu−), indicating a genetic distance between SEC71 and KAR7 of <2.1 cM.
Cloning of KAR8
KAR8 was cloned by complementation of the mating defect of kar8-1333. A MATa kar8-1333 strain (MS3260) was transformed with a yeast centromere-based YCp50 genomic library (Rose et al., 1987). Twenty-four thousand Ura+ transformants were replica printed onto lawns of the MATα kar8-1333 strain (MS2705) and allowed to mate for 3 h at 30°C on rich media. The colonies were then replica printed to synthetic minimal medium plates to select for diploids. Nine positives were isolated. Complementing plasmids were isolated, amplified in E. coli, and retransformed into MS3260. Seven of these clones completely rescued kar8-1333 and shared common genomic fragments, as determined by restriction digest analysis. Two other unlinked clones showed only partial suppression, and they were not studied further. To map KAR8 on the yeast physical map, a 1.8-kb BglII genomic fragment from one of the positive clones was hybridized to a lambda prime yeast genomic grid (Riles et al., 1993). By this method KAR8 was physically mapped to chromosome X near ARG3. To further define KAR8, several subclones were generated and tested for the ability to rescue the kar8-1333 mating defect (see Figure 2B). Plasmids pMR3252 and pMR3251 were derived from pMR2935 by deleting 5.6-kb HindIII and 8.5-kb MluI restriction fragments, respectively. Plasmids pMR3242 and pMR3260 were made by subcloning a 6.6-kb SalI–SalI and a 3.3-kb HindIII–HindIII into pRS416. Figure 2B shows that pMR3260, containing a single open reading frame recently identified as JEM1 (Nishikawa and Endo, 1997), retained complementing activity. To further test whether KAR8 is identical to JEM1, linkage analysis was performed. A KAR8 leu2 strain (MS52) was transformed with the integration plasmid pMR3133. A stable Leu+ transformant was crossed to a kar8-1333 leu2 strain (MS3260), and tetrad analysis was performed. In 16 tetrads analyzed, the Leu− phenotype and the mating defect cosegregated (2 Leu+:2 Mating−Leu−), indicating a distance between the JEM1 and KAR8 of <3.1 cM.
In Vitro ER Membrane Fusion Assay
Reagents used for measuring in vitro ER membrane fusion have been described before (Latterich and Schekman, 1994). Membranes isolated from wild-type, sec63-1, sec71Δ, and sec72Δ strains were tested for fusion competence as described before (Kurihara et al., 1994; Latterich and Schekman, 1994). Microsomal membranes were isolated from the following strains grown at 24°C: wild-type gls1 (MLY1601), wild-type GLS1 (MLY1600), sec71Δ gls1 (MLY1889), sec71Δ GLS1 (MLY1890), sec72Δ gls1 (MLY1891), sec72Δ GLS1 (MLY1892), sec63-1 GLS1 (MLY1651), and sec63-1 gls1 (MLY1652). These membranes were then tested for fusion competence by incubating donor and acceptor membranes (75 μg total protein each) at 24 and 37°C in the presence of an ATP regeneration system in a final volume of 50 μl for 1 h.
Immunological Techniques
Kar5p staining was performed by indirect immunofluorescence using polyclonal affinity-purified anti-Kar5p antibodies as previously described on strains MS3987, MS3986, MS4201, MS4020, MS3991, and MS3989 (Beh et al., 1997). For Western analysis 10 ml of early exponential cultures (5 × 106-1.5 × 107 cells/ml) of MS3987 and MS3991 in synthetic complete media lacking uracil were treated with α-factor at 6 μM for 120 min. Total protein extracts were prepared as described elsewhere (Ohashi et al., 1982). Proteins were electrophoretically separated using a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. Western blotting using affinity-purified anti-Kar5p antibodies was performed as previously described (Beh et al., 1997). For 35S pulse analysis, strains MS3986, MS3987, and MS3991 were grown to early exponential phase in synthetic complete media lacking uracil and then treated as described by Gammie et al. (1999), the only modification being that the strains were grown at 23°C and pulse labeled for 5 min. For immunoprecipitation of pulse-labeled extracts, the anti-Kar5p antibody was used at a concentration of 1:10.
Rescue of SEC63 Temperature-sensitive and Null Alleles
sec63-1 (MY2248) and sec63-4 (MY2653) mutant strains were transformed with pCen63, pMR3270, and pRS426 YEp-URA3 (Sikorski and Hieter, 1989). Three transformants were grown on synthetic media lacking uracil at 23°C for 2 d and then tested for growth at 23, 30, and 37°C on plates. To determine whether a sec63Δ strain could be rescued by overexpression of JEM1, strain MY4169 containing a functional copy of SEC63 on a URA3 plasmid (obtained from the R. Schekman laboratory) was transformed with either SEC63 2μ LEU2 (MR3253), JEM1-CEN LEU2 (MR3369), JEM1 2μ LEU2 (MR3352), or vector control LEU2-CEN plasmid (pRS415). The transformed strains were then patched on YPD and grown overnight and then replica plated to 5-fluoro-orotic acid, synthetic media lacking uracil, or leucine and incubated at 23, 30, and 37°C.
EM Analysis
For EM, mating mixtures were prepared as previously described (Kurihara et al., 1994). Permanganate fixation used to enhance membranous structures was also performed as previously described (Kurihara et al., 1994; Gammie et al., 1998). Serial sections of 70 or 90 nm were stained with lead citrate and examined in a Jeol (Tokyo, Japan) 100C transmission electron microscope at 80 kV.
RESULTS
KAR7 Is Allelic to SEC71
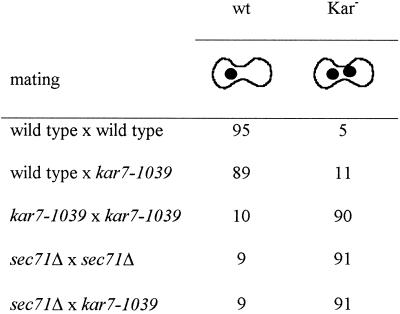
The kar7-1039 mutation was identified as a bilateral class II karyogamy mutant (Kurihara et al., 1994). Diploid formation was reduced eightfold in a kar7-1039 bilateral mating compared with wild type (Kurihara et al., 1994). Figure 1B displays the typical class II karyogamy phenotype of kar7-1039, with zygotes containing two closely apposed unfused nuclei. In contrast, wild-type zygotes have a single fused nucleus (Figure 1A). Table 2 presents a quantitative analysis of the karyogamy phenotype. As expected, a wild-type × wild-type mating resulted mostly in zygotes with a single diploid nucleus. In contrast, the kar7-1039 × kar7-1039 mating, but not the kar7-1039 × wild-type mating, resulted in mostly Kar− zygotes (90%).
Figure 1.
(A and B) Phenotype of class II Kar− zygotes. Shown are examples of wild-type (A) and class II Kar− (B) zygotes, respectively. Zygotes from filter matings between wild-type strains (MS1554 × MS23) or between kar7-1039 strains (MS3259 × MS3539) were analyzed by microscopy. Each image shows the nucleus by DAPI fluorescence and the zygote morphology by DIC. (C) Temperature-sensitive defect of kar7-1039. Streaks of wild-type (MS1554), kar7-1039 (MS3259), and sec71Δ (MS3910) strains were incubated at 30°C (left panel) or 37°C (right panel).
Table 2.
Nuclear fusion defect of kar7-1039 and sec71Δ by microscopic analysis of zygotes
Mating mixtures were stained with DAPI, and the phenotypes of the zygotes were observed by microscopy. Numbers represent percentages of wild-type (wt) or karyogamy defective class II (Kar−) zygotes. At least 150 zygotes were analyzed. The matings were as follows: wild type × wild type (MS1554 × MS23); wild type × kar7-1039 (MS22 × MS3539); kar7-1039 × kar7-1039 (MS3823 × MS3539); sec71Δ × sec71Δ (MS3910 × MS3911); and sec71Δ × kar7-1039 (MS3910 × MS3539).
In addition to its mating defect, the kar7-1039 mutant exhibited a tightly linked temperature-sensitive growth defect at 37°C (Figure 1C and MATERIALS and METHODS). The growth defect of kar7-1039 was then used to clone KAR7 by complementation (see MATERIALS and METHODS). Fourteen candidate plasmids were isolated. A subclone (pMR3047) containing a 3.9-kb HindIII–HindIII restriction fragment present in all 14 clones was able to complement both the temperature-sensitive growth defect and the mating defect of kar7-1039 (Figure 2A). Sequencing analysis revealed that the subcloned DNA corresponded to a region of the genome that contains SEC71, NPL4, and part of SSE2. Further subcloning showed that the previously characterized gene SEC71 alone on plasmid pMR3054 complemented both defects, suggesting that KAR7 is allelic to SEC71 (Figure 2A). Linkage analysis demonstrated that the KAR7 locus is tightly linked to SEC71, further supporting the identity of KAR7 as SEC71 (see MATERIALS and METHODS). We therefore generated MATa and MATα sec71Δ strains and found that they exhibited a karyogamy defect identical to that of kar7-1039 (Table 2). In addition, as previously reported, sec71Δ was viable, resulting only in a temperature-sensitive growth defect (Figure 1C; Feldheim et al., 1993; Kurihara and Silver, 1993).
Mutations in a Subset of ER Translocation Proteins Result in a Karyogamy Defect
Given that KAR7 is allelic to SEC71, which encodes a component of the protein translocation machinery that includes Kar2p, we independently tested whether mutations in other components of the translocation machinery resulted in karyogamy defects.
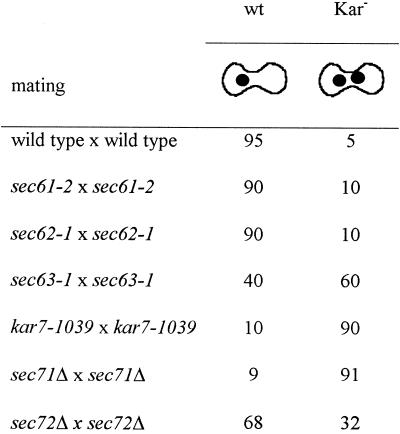
The results of quantitative matings with mutants defective for protein translocation are shown in Table 3. In each case, (sec61-2, sec62-1, sec63-1, kar7-1039, sec71Δ, and sec72Δ), the mutant strains were mated against strains harboring the same mutations. All the mutants tested showed a reduction in the mating efficiency indicated by the reduction in the percentage of diploids formed (Table 3). To determine whether the reduction in mating efficiency was due to a defect in nuclear fusion, cytoductant analysis and microscopic analysis of the zygotes were performed. Cytoductants are haploid cells with the nucleus of one parent but the cytoplasm from both. In wild-type matings, >95% of the zygotes have a diploid nucleus, and the number of cytoductants produced is very low. In contrast, the presence of two unfused nuclei in Kar− zygotes greatly increases the frequency of cytoductant buds. As shown in Table 3, the sec63-1, kar7-1039, and sec71Δ matings were the only matings that showed a cytoductant:diploid ratio significantly higher than the wild-type × wild-type mating. The nuclear fusion defect was considerably more severe for the sec71 alleles than for sec63-1. In addition, it is striking that the frequency of cytoductants was correlated with temperature in inverse ways for the two mutants. For sec71Δ and especially for kar7-1039, the frequency of cytoductants increased with temperature, in parallel with the decrease in diploid formation. In contrast, for sec63-1 the frequency of cytoductants decreased with increasing temperature, as the defect in translocation becomes more severe. One interpretation of this observation is that Sec63p’s role in nuclear fusion is not related to its function in translocation.
Table 3.
Quantitative mating experiments of various protein translocation-defective mutants
| Diploids (%) | C:D ratio | |
|---|---|---|
| Wild type | ||
| 23°C | 49 | 0.0009 |
| 30°C | 49 | 0.0008 |
| 35°C | 21 | 0.0003 |
| sec61-2 | ||
| 23°C | 27 | 0.0018 |
| 30°C | 7 | 0.0018 |
| 35°C | 2 | 0.0017 |
| sec62-1 | ||
| 23°C | 38 | 0.0038 |
| 30°C | 6 | 0.076 |
| 35°C | 6 | 0.0083 |
| sec63-1 | ||
| 23°C | 5 | 0.22 |
| 30°C | 3 | 0.028 |
| 35°C | 0.2 | 0.011 |
| kar7-1039 | ||
| 23°C | 9 | 0.02 |
| 30°C | 3 | 0.52 |
| 35°C | 0.1 | 1.2 |
| sec71Δ | ||
| 23°C | 5 | 0.19 |
| 30°C | 2 | 0.25 |
| 35°C | 1 | 0.53 |
| sec72Δ | ||
| 23°C | 24 | 0.0013 |
| 30°C | 15 | 0.0041 |
| 35°C | 11 | 0.001 |
Quantitative mating experiments performed at 23, 30, and 35°C. Percent diploid formation represents the number of diploids measured on appropriate selection plates divided by the number of viable cells measured on YPD plates × 100. C:D ratio reports the number of cytoductants measured on YPG cycloheximide plates divided by the number of diploids formed. The matings were as follows: wild type (MS1554 × MS3856); sec61-2 (MY2341 × MY3676); sec62-1 (MY3594 × MY3678); sec63-1 (MY2808 × MY3564); kar7-1039 (MS3259 × MS4060); sec71Δ (MS3910 × MS3927); and sec72Δ (MY3918 × MY3931). In general, matings against a wild-type strain gave a greater mating efficiency than mating involving the mutants themselves and a C:D ratio comparable to the wild-type × wild-type mating. However, sec63-1, kar7-1039, and sec71Δ strains resulted in a C:D ratio of 10−2–10−3 even when these mutants were mated against wild type.
To examine nuclear fusion directly, we stained zygotes with DAPI and examined the nuclei microscopically (Table 4). By this assay only the sec71 mutants showed a very strong defect (90% Kar− zygotes). The sec63-1 mating showed an intermediate defect (60% Kar− zygotes). The sec72Δ mating showed ∼32% mutant zygotes, indicating a mild defect in nuclear fusion, although the cytoductant:diploid ratio was not significantly higher than the wild-type mating (Tables 3 and 4). The difference between the two assays is not clear but could be explained if the zygotes that exhibited the nuclear fusion defect were inviable. Alternatively, the nuclear fusion defect might be temporary, and the nuclei eventually fuse. Finally, the sec61-2 and sec62-1 matings showed only 10–15% Kar− zygotes. These data demonstrate that mutations in SEC71 and, to a lesser extent, SEC63 and SEC72 lead to defects in nuclear fusion.
Table 4.
Nuclear fusion defect of protein translocation mutants by microscopic analysis of zygotes
Mating mixtures were stained with DAPI, and the phenotypes of the zygotes were observed by microscopy. Numbers represent percentages of wild-type (wt) or karyogamy-defective class II (Kar−) zygotes. At least 100 zygotes were analyzed. The sec61-2, sec62-1, and sec63-1 strains were pregrown at 23°C. Filter matings were performed for 2.5 h at 30°C except for sec63-1, which was performed for 4 h at 23°C. The matings were as follows: wild type × wild type (MS1554 × MS23); sec61-2 × sec61-2 (MY2341 × MY2339); sec62-1 × sec62-1 (MY3594 × MY3678); sec63-1 × sec63-1 (MY2808 × MY3564); kar7-1039 × kar7-1039 (MS3823 × MS3539); sec71Δ × sec71Δ (MS3910 × MS3911); and sec72Δ × sec72Δ (MY3918 × MY3917).
Membranes Defective in Sec71p or in Sec72p, but Not Sec63p, Show a Membrane Fusion Defect In Vitro
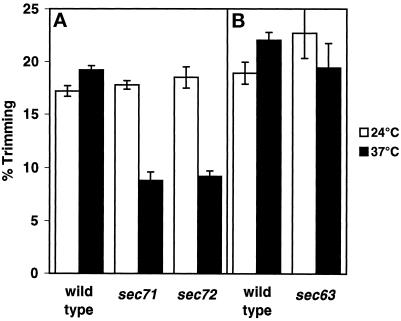
Previous studies demonstrated that the class II karyogamy genes are required for membrane fusion in vitro (Kurihara et al., 1994; Latterich and Schekman, 1994). We therefore decided to analyze the fusion competence of membranes isolated from strains deleted for SEC71 and SEC72 or containing the sec63-1 mutation. In the ER membrane fusion assay, microsomal membrane fractions are derived from strains that either lack or contain glucosidase I (Gls1p), the enzyme that is responsible for initiating deglucosylation of newly synthesized glycoproteins (Latterich and Schekman, 1994). Yeast prepro-α-factor translocated into the lumen of the glucosidase I–deficient microsomal membranes (gls1; donor membrane) becomes processed to the deglucosylated form only when donor membranes fused with glucosidase-containing (GLS1) ER membranes. Quantifying the amount of trimmed and untrimmed pro-α-factor serves as a direct measure of membrane fusion (Latterich and Schekman, 1994). Microsomal membranes from wild-type, sec71Δ, sec72Δ, and sec63-1 strains were tested for fusion competence by incubating donor and acceptor membranes at 24 and 37°C in the presence of an ATP regeneration system for 1 h.
Both the wild-type and the sec63-1 membranes exhibited similar levels of fusion at 24 and 37°C (Figure 3B). In contrast, both sec71Δ and sec72Δ membranes exhibited a similar temperature-sensitive fusion defect, approximately half of that of wild-type at 37°C (Figure 3A). The partial fusion defect was not caused by membrane rupture, because the glycosylated pro-α-factor translocated into the sec71Δ or sec72Δ mutant membranes remained protease protected after the incubation at 37°C. The fusion defect of the sec71Δ strain was comparable to that of the kar7-1039 strain tested before (Kurihara et al., 1994). Therefore, we concluded that both Sec71p and Sec72p, but not Sec63p, are necessary for efficient membrane fusion at elevated temperature. However, the two gene products are not required for in vitro membrane fusion at the lower temperature of 24°C. These results suggest that Sec71p and Sec72p play a role in stabilizing the fusion machinery rather than being directly required for the fusion reaction. In addition, because the assay is done under conditions in which there is neither protein synthesis nor translocation, we concluded that the role of Sec71p and Sec72p in membrane fusion is independent of their role in translocation.
Figure 3.
sec71Δ and sec72Δ, but not sec63-1, membranes show a defect in the in vitro ER–nuclear membrane fusion assay at 37°C. (A) Donor and acceptor membranes (75 μg protein each) prepared from wild-type strains (MLY1601 and MLY1600), strains deleted for the SEC71 gene (MLY1889 and MLY1890), or strains deleted for the SEC72 gene (MLY1891 and MLY1892) were combined in the presence of an ATP regeneration system in a total volume of 50 μl and held on ice. Reactions were incubated for 60 min at 24 or 37°C. (B) In a separate experiment, donor and acceptor membranes prepared from wild-type strains MLY1600 and MLY1601 and sec63-1 strains MLY1651 and MLY1652) were combined and incubated as above. In all cases, the experiments were repeated three times, and the mean values and SDs are shown. All strains were grown at 24°C before membrane isolation. The amount of glucose trimming, indicative of the successful fusion of membranes, was assessed as described previously (Latterich and Schekman, 1994).
Kar5p, a Protein Specifically Required for Nuclear Fusion, Is Absent in kar7-1039
KAR5 encodes a pheromone-inducible ER–membrane protein that is important for nuclear membrane fusion (Beh et al., 1997). Interestingly, one allele of KAR5, kar5-1162, showed unlinked noncomplementation with kar7-1039 (Kurihara et al., 1994). That is, a mating diploid of the form kar5-1162/KAR5 SEC71/kar7-1039 has a mating defect, even though both mutations are recessive. Unlinked noncomplementation is indicative of two proteins that functionally interact. In a few well-documented examples, the proteins were shown to physically interact (e.g., α- and β-tubulin; Stearns and Botstein, 1988).
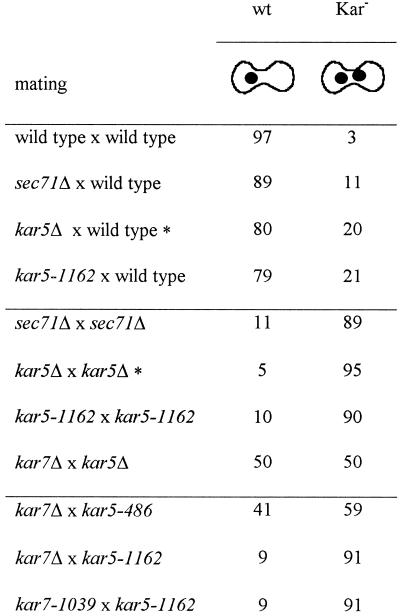
To further characterize KAR5 and SEC71 interaction, we performed matings between various kar5 and sec71 mating partners and analyzed the karyogamy phenotype by microscopy. As shown in Table 5, any kar5 × sec71 mutant mating is worse than wild-type × wild-type, kar5 × wild-type, or sec71 × wild-type matings. In particular, when kar5-1162 was mated to either kar7-1039 or sec71Δ, the result was a very strong karyogamy defect, similar to that of each mutant mated by itself (∼90% Kar− zygotes; Table 5). This behavior is in contrast to that of other kar mutations wherein crosses between the different bilateral mutants yielded wild-type zygotes (Kurihara et al., 1994). Therefore, we concluded that mutations in KAR5 and in SEC71 are “synthetic bilateral”; that is, mutation in one gene appears to cause a defect in the function of both proteins. One interpretation of these genetic data is that there is a functional interaction between Kar5p and Sec71p.
Table 5.
sec71 and kar5 mutants show synthetic bilateral nuclear fusion defects
Mating mixtures were stained with DAPI, and the phenotypes of the zygotes were observed by microscopy. Numbers represent percentages of wild-type (wt) or karyogamy-defective class II (Kar−) zygotes. At least 100 zygotes were analyzed. The matings were as follows: wild type × wild type (MS1554 × MS23); sec71Δ × wild type (MS3910 × MS16); kar5-1162 × wild type (MS3534 × MS23); sec71Δ × sec71Δ (MS3910 × MS3911); kar5-1162 × kar5-1162 (MS3534 × MS3538); kar7Δ × kar5Δ (MS3910 × MS3577); kar7Δ × kar5-486 (MS3910 × MS3537); kar7Δ × kar5-1162 (MS3910 × MS3538); and kar7-1039 × kar5-1162 (MS3823 × MS3538).
Data taken from Beh et al. (1997).
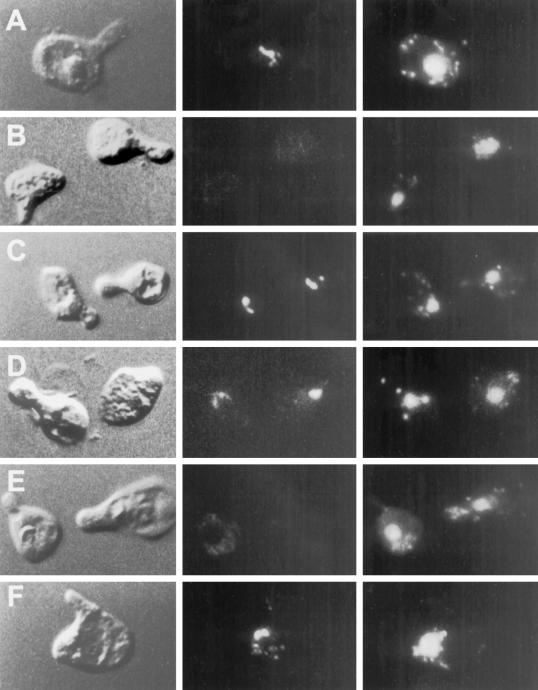
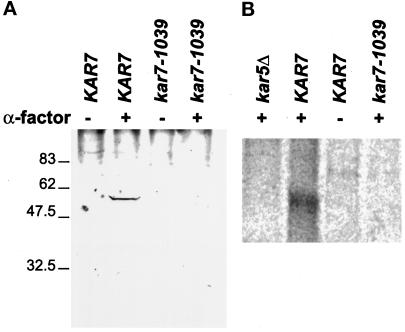
To examine the nature of the interaction between Kar5p and Sec71p, we analyzed the localization of Kar5p in kar7-1039 and other kar mutants (Figure 4). In wild-type cells that have been induced with mating pheromone, Kar5p localizes in the vicinity of the SPB (Figure 4A; Beh et al., 1997). Surprisingly, we found that Kar5p did not localize to the SPB in kar7-1039 (Figure 4E). Of 51 kar7-1039 shmoos examined, 49 did not exhibit the characteristic Kar5p staining. Kar5p was present and correctly localized in the kar1-1, kar2-1, and kar8-1333 mutants, indicating that the defect is specific for kar7-1039 (Figure 4, C, D, and F). The presence or absence of Kar5p in kar7-1039 was further investigated by Western analysis of total protein extract (Figure 5A). Kar5p was detected after pheromone induction of a KAR7 strain containing a KAR5 2μ plasmid (wild-type control; Figure 5A, lane 2). In contrast, no Kar5p was detected in isogenic pheromone-induced kar7-1039 cells containing the KAR5 2μ plasmid (Figure 5A, lane 4). This result suggested that either Kar5p is not synthesized under these conditions or that Kar5p is rapidly degraded. To determine whether KAR7 is required for the transcription of KAR5, we performed a Northern blot of a kar7-1039 strain containing the KAR5 2μ plasmid. We found that KAR5 mRNA was made in the kar7-1039 strain upon induction with α-factor, equivalent to the KAR7 control strain. Thus, KAR7 is not required for transcription of KAR5. To address whether Kar7p is required for the synthesis or stability of Kar5p, KAR7 and kar7-1039 strains were pulse labeled with 35S, and Kar5p was immunoprecipitated. In the pheromone-induced KAR7 strain, Kar5p was readily detected after 5 min of pulse labeling (Figure 5B, lane 2). In contrast, in the pheromone-induced kar7-1039 mutant, Kar5p could not be detected under equivalent conditions (Figure 5B, lane 4). Thus KAR7/SEC71 appears to be required for the normal synthesis of Kar5p. From these experiments, we cannot distinguish whether Kar5p is synthesized at a very decreased rate or very rapidly degraded after synthesis. These results also do not rule out the possibility, suggested from other experiments, that some functional Kar5p is made in the kar7 mutant. We conclude that KAR7/SEC71 is required for the synthesis and/or stability of Kar5p.
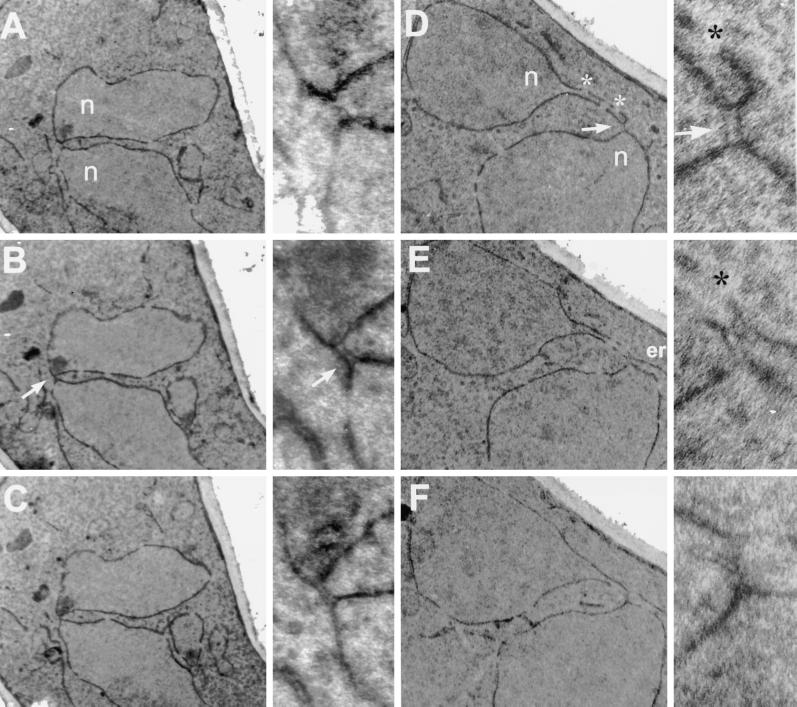
Figure 4.
Kar5p is mislocalized in kar7-1039 but not in other karyogamy mutants. In each series (A–F), the left panel shows the morphology of the cell (shmoo) by DIC. The middle panel shows the Kar5p immunofluorescence, and the right panel shows the nucleus stained with DAPI. (A) Kar5 shmoos (MS3987); (B) kar5Δ2 shmoos (MS3986); (C) kar1-1 shmoos (MS4021); (D) kar2-1 shmoos (MS4020); (E) kar7-1039 shmoos (MS3991); (F) kar8-1333 shmoos (MS3989). Cells were treated with α-factor for 2–2.5 hr before preparation for immunofluorescence. A and B are reproduced from Beh et al. (1997) J. Cell. Biol. 139, 1063–1076, by copyright permission of The Rockefeller University Press.
Figure 5.
Kar5p was not detected in the kar7-1039 mutant. (A) Western blot analysis. The strains kar5Δ2 and kar7-1039 transformed with 2μ KAR5 plasmid (MS3987 and MS3991) were treated (+) or left untreated (−) with α-factor for 2 hr. Total protein extracts were analyzed by Western blot using affinity-purified anti-Kar5p antibodies. (B) Pulse labeling. MS3986 and MS3987, kar5Δ2 strains transformed with either vector (lane 1) or 2μ KAR5 plasmid (lanes 2 and 3), and MS3991, a kar7-1039 strain transformed with 2μ KAR5 plasmid (lane 4), were 35S pulsed for 5 min at 23°C after treatment (+) or no treatment (−) with α-factor. Pulse-labeled extracts were immunoprecipitated with crude anti-Kar5p antibodies, run on a polyacrylamide gel, and visualized by PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
KAR8 Is Identical to JEM1
The kar8-1333 mutation was identified as a class II mutation in the same screen as kar7-1039 (Kurihara et al., 1994). The kar8-1333 mutation results in a strong bilateral mating defect in which diploid formation is reduced at least 270-fold compared with wild type (Kurihara et al., 1994). The kar8-1333 mutation also results in membrane fusion defects assayed by the in vitro ER membrane fusion assay (Kurihara et al., 1994).
KAR8 was cloned by complementation of the kar8-1333 mating defect (see MATERIALS and METHODS). Seven candidate plasmids that completely restored mating ability were isolated. These plasmids shared common restriction fragments from a genomic location that was physically mapped to chromosome X near ARG3. Subcloning and deletion analysis showed that a plasmid (pMR3260) containing a recently characterized gene, JEM1, was able to rescue the kar8-1333 mating defect (Figure 2B). Linkage analysis showed a tight linkage between the isolated complementing DNA containing JEM1 and the KAR8 locus, indicating that KAR8 is allelic to JEM1 (see MATERIALS and METHODS). To confirm this, we generated MATa and MATα kar8/jem1Δ strains and found that they exhibited a karyogamy defect indistinguishable from that of kar8-1333 (Table 6).
Table 6.
Nuclear fusion defect of kar8-1333 and jem1/kar8Δ by microscopic analysis of zygotes
Mating mixtures were stained with DAPI, and the phenotypes of the zygotes were observed by microscopy. Numbers represent percentages of wild-type (wt) or karyogamy-defective class II (Kar−) F zygotes. At least 200 zygotes were analyzed. The matings were as follows: wild type × wild type (MS1554 × MS52); wild type × kar8-1333 (MS1554 × MS3536); kar8-1333 × kar8-1333 (MS3260 × MS3536); jem1Δ × jem1Δ (MS4342 × MS4338); and jem1Δ × kar8-1333 (MS4342 × MS3536).
KAR8/JEM1 Genetically Interacts with KAR2
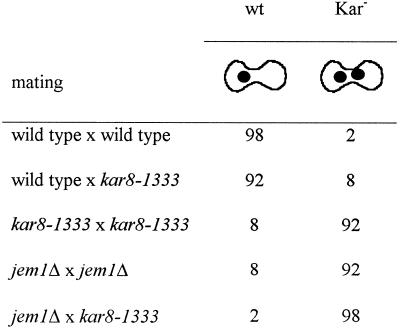
Because KAR8/JEM1 is a DnaJ homologue, and DnaJ proteins are predicted to interact with Hsp70/DnaK, we investigated whether Kar8/Jem1p performs its karyogamy function by interacting with Kar2p, the ER-lumenal BiP/Hsp70 of yeast. To this end, we examined whether overexpression of KAR8/JEM1 from a 2μ plasmid could relieve the mating defect of kar2-1, a point mutant in KAR2, which specifically results in a strong unilateral karyogamy defect (Table 7). A kar2-1 × wild-type mating resulted in mostly Kar− zygotes (74%). When a kar2-1 strain transformed with a KAR8/JEM1 2μ plasmid was mated to wild type, we observed a greater than twofold increase in the number of Kar+ zygotes (from 26 to 62%). Thus, overexpression of KAR8/JEM1 suppressed the unilateral mating defect of a kar2-1 mutant.
Table 7.
Dosage suppression of kar2, kar8, and sec63 mutants
Mating mixtures were stained with DAPI, and the phenotypes of the zygotes were observed by microscopy. Numbers represent percentages of wild-type (wt) or karyogamy-defective class II (Kar−) zygotes. At least 200 zygotes were analyzed. The matings were as follows: wild type × wild type (MS1554 × MS52) transformed with a vector control (pRS426); kar2-1 × wild type (MS1111 × MS31) in which MS1111 was transformed with vector (pRS426), JEM1-CEN (pMR3260), JEM1 2μ (pMR 3270), SEC63-CEN (pCen63), SEC63 2μ (pDF15), or SCJ1 2μ (pPS720); kar8-1333 × kar8-1333 (MS 3928 × MS3536) transformed with vector (pRS426), SEC63-CEN (pCen63), SEC63 2μ (pDF15), or SCJ1 2μ (pPS720); and sec63-201 × sec63-201 (DNY65 × DNY66) transformed with vector (pRS425), JEM1-CEN (pMR 3369), JEM1 2μ (pMR3352), or SEC63 2μ (pDF14). In the kar2-1 × wild-type cross, only the kar2-1 parent was transformed with the indicated plasmids. In the case of the kar8-1333 × kar8-1333 and sec63-201 × sec63-201 matings, both parents were transformed with the indicated plasmids. Data for the table were gathered from several independent experiments.
Given that Sec63p interacts with Kar2p during protein translocation, we asked whether overexpression of SEC63 could similarly relieve the karyogamy defect of kar2-1. As shown in Table 7, a kar2-1 strain containing a SEC63 2μ plasmid showed only a slight increase in the occurrence of Kar+ phenotype (33% Kar+ compared with 26% for vector control). We then asked whether overexpression of SEC63 could relieve the karyogamy defect of kar8-1333. As shown in Table 7, a kar8-1333 × kar8-1333 mating in which both mating partners carry a SEC63 2μ plasmid did not show a significant increase in the number of Kar+ zygotes (13% Kar+ compared with 10% for the vector control). We therefore concluded that SEC63 overexpression did not relieve the bilateral kar8-1333 karyogamy defect. To further investigate whether SEC63 and JEM1 have overlapping karyogamy functions, we also tested whether JEM1 overexpression could relieve the karyogamy defect of a sec63-201 × sec63-201 mating. The sec63-201 allele is a truncation mutant of SEC63 that results in a strong mating defect (Ng and Walter, 1996). As shown in Table 7, JEM1 overexpression had no effect on the karyogamy phenotype of sec63-201 × sec63-201 zygotes (48% Kar+ zygotes when JEM1 was overexpressed compared with 50% for the vector control). In contrast, 80% of zygotes were Kar+ when SEC63 was overexpressed. Taken together, these results suggest that SEC63 and KAR8/JEM1 perform distinct and different roles in karyogamy. Moreover, because KAR8/JEM1 overexpression suppresses the kar2-1 mating defect to a much greater degree than SEC63, we propose that Kar2p specifically interacts with Kar8/Jem1p for nuclear fusion.
Cells deleted for KAR8/JEM1 did not show a detectable growth defect (Nishikawa and Endo, 1997; our unpublished observations). However, deletion of JEM1 in combination with a deletion of SCJ1, encoding another lumenal ER DnaJ homologue, resulted in a temperature-sensitive phenotype, suggesting that KAR8/JEM1 has a vegetative function that is partially redundant with that of SCJ1 (Nishikawa and Endo, 1997; our unpublished observations). Recent evidence suggests that SCJ1 and to a lesser extent JEM1 are required for protein folding in the ER lumen under stress conditions (Silberstein et al., 1998). Given that SCJ1 and KAR8/JEM1 may have overlapping functions during vegetative growth, we next determined whether they might interact during mating. Because SCJ1 is not required for karyogamy (Nishikawa and Endo, 1997), and the kar8/jem1 defect is very strong, an increased severity of the karyogamy defect in the double mutant might not be readily apparent. Therefore, we determined whether increased dosage of SCJ1 suppresses the Kar− phenotype of kar2 and kar8 mutations. As shown in Table 7, overexpression of SCJ1 did not suppress the karyogamy defect of either kar2 or kar8 mutants. These results imply that SCJ1 cannot substitute for KAR8/JEM1 and that Kar8/Jem1p’s suppression of kar2 mutations is specific.
scj1Δ has been shown to be synthetically lethal with kar2-159, suggesting that SCJ1 may have a role in protein translocation (Schlenstedt et al., 1995) like SEC63. Given the apparent overlap of vegetative function between Kar8/Jem1p and Scj1p, we next wanted to determine whether KAR8/JEM1 also has vegetative functions in common with SEC63. To address this question we tested whether overexpression of KAR8/JEM1 could substitute for SEC63 or alleviate the temperature sensitivity of two sec63 alleles. The growth of sec63-1 and sec63-4 strains transformed with vector control, KAR8/JEM1 2μ, or a SEC63 CEN plasmid was assessed at 23, 30, and 37°C. Whereas sec63 mutants containing a SEC63 CEN plasmid grew at all temperatures, strains containing the KAR8/JEM1 2μ plasmid or vector control did not grow at 37°C, indicating that KAR8/JEM1 function could not substitute for SEC63. In addition, we found that high-copy KAR8/JEM1 could not restore viability to a sec63 null at any temperature (our unpublished results). These data suggest that SEC63 and JEM1 have different vegetative functions.
Ultrastructural Analysis of Class II Mutants Reveals Two Distinct Phenotypic Classes
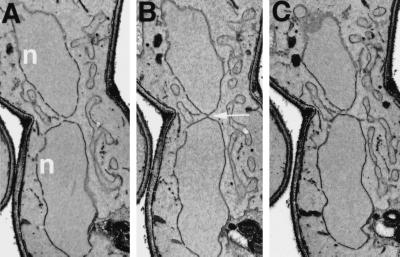
EM examination of class II mutants showed that although the nuclei did not fuse, they made direct contact through one or two membranous bridges that spanned the gap between the two nuclei (Kurihara et al., 1994). To extend the data reported by Kurihara et al. (1994), we carefully examined the phenotype of kar8 mutant zygotes by EM. In 35 kar8Δ zygotes in which the nuclei were closely apposed, 12 had a bridge in which a lumen could be easily seen. Figure 6 shows electron micrographs from serial sections of two kar8Δ zygotes. The bridges that connected the two nuclei contained a significant lumen that traversed as many as six serial sections, as much as 400 nm (Figure 6, A–C and D–F). The lumen of the bridges also appeared to be continuous with the lumen between the inner and outer nuclear envelopes. In contrast, the morphology of the bridges in kar2, kar5, and kar7/sec71 mutants was different from that in the kar8/jem1 mutants. In kar2, kar5, and kar7 mutant zygotes, the bridges had no apparent lumen and were entirely contained within a single section of 70 nm (Kurihara et al., 1994; Beh, 1996). Based on the different morphologies of fusion bridges, we reasoned that Kar2p, Kar5p, Kar7p, and Kar8p might be required at different steps during nuclear fusion, and more specifically, that Kar8p function is required later in the nuclear fusion pathway. To test this idea, we constructed a kar5Δ kar8Δ double mutant and examined the morphology of the membranous bridges in the mutant zygotes. If Kar5p acts before Kar8p, then the bridges in the kar5Δ kar8Δ double mutant zygotes should resemble those in the kar5Δ mutant. If Kar8p acts before Kar5p, then the double mutant should resemble the kar8Δ mutant. Of 100 kar5Δ kar8Δ mutant zygotes examined, 5 had fusion bridges, all of which resembled that of kar5Δ single mutants (Figure 7, A–C). These observations are consistent with the idea that Kar8p acts downstream of Kar5p.
Figure 6.
Electron micrographs of serial section of two kar8-1333 zygotes. The kar8-1333 mating partners used in this study were MS2705 and MS2706. (A–C) Micrographs of three consecutive serial sections (of seven) through a kar8-1333 mutant zygote shown at two different magnifications. (D–F) Micrographs of sections 1, 3, and 5 (of five) of another kar8-1333 mutant zygote also shown at two different magnifications. Each section is 70 nm thick. n, nuclei; *, nuclear pores; arrows, kar8-1333 bridges between the two nuclei.
Figure 7.
Electron micrographs of serial sections through a kar5Δ kar8Δ double mutant zygote. The kar5Δ kar8Δ mating partners used in this study were MS4359 and MS4360. (A–C) Micrographs of three consecutive 90-nm-thick serial sections through a kar5Δ kar8Δ mutant zygote. n, nuclei; arrows, morphology of the bridges observed in kar5Δ kar8Δ zygotes.
DISCUSSION
We cloned two genes, KAR7 and KAR8, involved in the fusion of the nuclear envelopes during karyogamy, and describe their genetic interactions with KAR2 and KAR5. We found KAR7 to be allelic to SEC71, a gene important for the posttranslational transport of a subset of protein precursors into the ER. In addition to KAR2 and SEC71, we and others have found that two other components of the translocation machinery, SEC63 and SEC72, are also required for efficient nuclear fusion in vivo (Ng and Walter, 1996). However, in vitro, membranes from the sec63-1 mutant showed no defect, and membranes lacking Sec71p and Sec72p showed reduced fusion competence only at elevated temperature. These results suggest that Sec63p is not directly required for nuclear membrane fusion and that Sec71p and Sec72p are required to stabilize a protein or complex required for nuclear membrane fusion. Genetic interactions between kar7/sec71 and kar5 mutations suggested that Kar7/Sec71p and Kar5p interact during nuclear fusion. Characterization of Kar5p in the kar7/sec71 mutants suggests that Kar7/Sec71p is required for the synthesis and/or stability of Kar5p. Cloning of KAR8 revealed that it is allelic to JEM1, which encodes an ER DnaJ-like protein shown recently to be required for nuclear fusion (Nishikawa and Endo, 1997). KAR8/JEM1 is therefore the second DnaJ homologue involved in nuclear fusion. Suppression experiments suggest that KAR8/JEM1 has a unique function in karyogamy acting in conjunction with KAR2, which cannot be substituted by SEC63 or SCJ1.
Protein Translocation Components and Nuclear Fusion
A combination of genetic and biochemical approaches have been used to identify factors involved in protein translocation across the ER in yeast (see review of Lyman and Schekman, 1996). SEC61, SEC62, and SEC63 encode essential ER integral membrane proteins with multiple membrane-spanning domains (Deshaies and Schekman, 1987, 1989; Rothblatt et al., 1989; Sadler et al., 1989; Stirling et al., 1992). In contrast, SEC71 encodes an ER transmembrane protein required for growth at 37°C, and SEC72 encodes a peripheral ER membrane protein that is not essential at any temperature (Feldheim et al., 1993; Kurihara and Silver, 1993; Feldheim and Schekman, 1994). Although SEC71 and SEC72 are nonessential genes, they are important for the translocation of a subset of protein precursors (Green et al., 1992; Feldheim et al., 1993; Kurihara and Silver, 1993).
Together with Sss1p and Sbh1p, Sec61p forms the core translocation pore complex (Esnault et al., 1994; Panzner et al., 1995). Sec63p is found in complexes with Sec62p, Sec71p, and Sec72p (called the Sec62/Sec63p complex) and with Sec71p, Sec72p, and Kar2p (called the Sec63p–BiP complex; Deshaies et al., 1991; Brodsky and Schekman, 1993). In translocation, Sec62p, Sec71p, and Sec72p seem to act early, probably as a membrane-bound receptor that binds the precursor proteins before the precursor–Sec61p complex interaction (Sanders et al., 1992; Feldheim et al., 1993; Fang and Green, 1994). Sec63p interacts with Kar2p through its DnaJ domain, and together they act both early, to activate the pore to receive precursor, and late, to facilitate the release of the translocating precursor into the lumenal side of the ER (Lyman and Schekman, 1995, Lyman and Schekman 1997).
Kar2p was the first protein shown to be required for both ER protein translocation and nuclear fusion (Polaina and Conde, 1982; Rose et al., 1989; Vogel et al., 1990; Sanders et al., 1992). We found that KAR7 is allelic to SEC71 and that mutations affecting two other translocation components, sec63-1, and to a lesser extent sec72Δ, resulted in moderate defects in nuclear fusion. These results confirm the basic observations of Ng and Walter (1996) that a truncation mutation of SEC63 caused a severe karyogamy defect and that SEC71 and SEC72 are also required for nuclear fusion. However, we found that sec72Δ had a much less severe defect in karyogamy than mutations in KAR7/SEC71. Furthermore, we found that deletions of kar7/sec71 and sec72 resulted in only a moderate temperature-sensitive defect in vitro. These results suggest that nuclear membrane fusion does not specifically require either of these proteins, but instead they may serve to stabilize a fusion complex. Furthermore, the finding that membranes from the sec63-1 mutant were not defective in vitro suggests that this protein is also not directly required.
In contrast to the other genes required for translocation, we did not detect significant karyogamy defects in mutant strains containing sec61-2 or sec62-1. This was true even though the matings were performed under semipermissive conditions in which the temperature-sensitive mutations were clearly reducing the overall efficiency of mating. Therefore, it is very unlikely that the nuclear fusion defects arise from defects in translocation per se. We conclude that some, but not all, of the components of the translocation machinery are used in the nuclear membrane fusion pathway.
Sec71p’s Role in Nuclear Fusion Might Be Mediated through Kar5p
Why does nuclear fusion require components of the protein translocation machinery? Do the shared components directly participate in the fusion process, or are they required to assemble or stabilize the real fusogenic apparatus? To address some of these issues, we investigated the genetic interaction between mutations in KAR7/SEC71 and KAR5, a pheromone-inducible gene that seems to be specifically required for nuclear fusion (Beh et al., 1997). KAR7/SEC71 and KAR5 mutants exhibit a “synthetic bilateral” mating defect. That is, in matings between the two different mutants, one or the other mutant behaves as if it were defective for both proteins. Kar5p, which normally localizes to the SPB (the site for nuclear fusion), is absent in kar7-1039 but not in other kar mutant strains. Furthermore, both Western blot and pulse label analysis could not detect Kar5p in kar7-1039. Therefore we concluded that the karyogamy defect of kar7/sec71 mutants is most likely due to reduced levels of Kar5p, consistent with the genetic data.
The failure to detect Kar5p in the kar7-1039 mutant after pulse labeling at the permissive temperature suggests that Kar7/Sec71p is required for the synthesis of Kar5p. One obvious possibility is that Kar7p/Sec71p is required for the translocation of Kar5p. Our inability to detect a precursor is consistent with two models. First, Kar5p might be synthesized, but not translocated, resulting in its rapid degradation in the mutant. Alternatively, translation and translocation might be tightly coupled, such that Kar5p is not synthesized in the kar7/sec71 mutant. If so, the synthesis and translocation of Kar5p would be unusual in being strongly dependent on Sec71p and not on other components of the translocation machinery.
However, other data suggest a more complex role for Kar7/Sec71p in nuclear fusion. First, in vivo, the kar5Δ mutant exhibited a somewhat more severe nuclear fusion defect than kar7/sec71Δ. Second, in the nuclear envelope–ER membrane fusion assay, the kar5Δ mutant exhibited a much more severe defect than kar7/sec71Δ. In both cases the kar7/sec71Δ defect was temperature sensitive. Taken together these data suggest that residual Kar5p was present and functional in the kar7/sec71Δ mutant, but its activity was compromised at the higher temperature. This would be consistent with a separate assembly and stabilization function for Kar7/Sec71p.
A role for Kar7/Sec71p in assembly and stabilization during nuclear fusion would be similar to its role in protein translocation. One of the functions of the Sec62p/Sec63p/Sec71p/Sec72p complex is to stimulate the formation of the Sec61p complex required for protein translocation (Hanein et al., 1996). Likewise, a subset of these proteins including Sec71p and Sec72p might be required for the assembly of a protein complex, including Kar5p, which mediates nuclear membrane fusion. Alternatively, Kar7/Sec71p and Sec72p might function as auxiliary components of a complex that is directly involved in the fusion mechanism. The in vitro assay identified Kar2p, Kar5p, and Kar8p as being required for ER–nuclear envelope membrane fusion (Kurihara et al., 1994; Latterich and Schekman, 1994). Possibly these proteins form a chaperone complex, stabilized by Kar7/Sec71p and Sec72p, that help mediate membrane fusion. A chaperone complex might be required to facilitate conformational changes of the nuclear fusion complex during membrane fusion.
Kar2p and DnaJ Partners
The interaction between Kar2p and Sec63p through the DnaJ homology domain has been well documented genetically and biochemically (Feldheim et al., 1992; Brodsky and Schekman, 1993; Scidmore et al., 1993). The sec63-1 mutation maps to the DnaJ loop and disrupts the interaction with Kar2p (Nelson et al., 1993; Brodsky and Schekman, 1993). Accordingly, sec63-1 causes a strong protein translocation defect both in vivo and in vitro. Although sec63-1 caused a moderate defect in karyogamy, in vivo, it had no effect on nuclear envelope–ER fusion, in vitro. The specificity of the genetic interaction between sec63-1 and kar2 mutations has been extensively explored (Scidmore et al., 1993). Remarkably, sec63-1 was synthetically lethal with kar2 alleles that have a severe defect in translocation but not with alleles that have a more severe defect in nuclear fusion. Taken together with the biochemical data, these results suggests that Sec63p is the DnaJ partner for Kar2p’s role in protein translocation, but that Sec63p is not likely to be the major DnaJ partner for Kar2p’s role in nuclear fusion.
In contrast to Sec63p, there is strong evidence in favor of Kar8/Jem1p playing a major role in nuclear fusion. First, unlike sec63-1, kar8/jem1 mutants exhibit a strong defect in nuclear fusion both in vivo and in vitro (Kurihara et al., 1994; Nishikawa and Endo, 1997). Second, overexpression of Kar8/Jem1p, but not Sec63p, suppressed the karyogamy defect of kar2-1. Taken together, these results suggest that Kar8/Jem1p is the major DnaJ partner for Kar2p in nuclear fusion.
Nevertheless, the defects observed for both sec63-1 and sec63-201 (Ng and Walter, 1996) suggest that Sec63p does play a significant role in nuclear fusion. Given that Sec63p interacts with Kar2p for translocation, it is possible that the mutant proteins interact with Kar2p in a way that then interferes with Kar2p’s later interaction with Kar8p. This seems unlikely given that the sec63-1 mutation blocks the interaction between Sec63p and Kar2p (Brodsky and Schekman, 1993). Interestingly, sec63-201 and sec63-1 affect different domains of Sec63p, with sec63-201 truncating the carboxyl-terminal 27 residues of the cytoplasmic domain and sec63-1 mapping to the lumenal DnaJ domain. In vitro experiments suggest that these two domains of Sec63p have different functions during translocation (Lyman and Schekman, 1995). An intriguing possibility is that Sec63p’s role in nuclear fusion is mediated through its cytoplasmic domain and not through its DnaJ domain. If so, Sec63p’s role in nuclear fusion would likely be independent of Kar2p, consistent with our genetic data. In addition, Ng and Walter (1996) reported a mutation, sos1-1, which suppressed the translocation, but not the karyogamy, defect of sec63-201.
Regardless of the specific function of Sec63p, a second issue concerns when each DnaJ protein might act during nuclear fusion. Based on the genetic data, it seems unlikely that Kar8/Jem1p and Sec63p interact with Kar2p to perform similar functions at the same step in the nuclear fusion process. More likely Kar8/Jem1p and Sec63p have different roles, and each may be required at different steps in the karyogamy pathway. In this model, Kar2p may act at more than one step in nuclear fusion, possibly interacting first with one DnaJ and then the other. In this model, mutations in each DnaJ homologue might have more or less severe effects depending on the stringency of the requirement for that step in vivo and in vitro.
In this regard, it is striking that the membrane bridges observed by EM in kar2 and in kar5 mutants are quite different from the bridges observed for the kar8/jem1 mutant. The kar8/jem1 bridges had distinct lumens extending >400 nm of the nuclear surface. In contrast, kar2 and kar5 bridges had no detectable lumen and extended over less than one section, most likely no more than 10 nm. Given the morphology of the kar8/jem1 membranes, it is tempting to propose that Kar8/Jem1p acts later in the pathway, after the initial stages of membrane fusion. The presence of large bridges in kar8 mutants may indicate that Kar8/Jem1p is required to resolve such structures at a late step in nuclear membrane fusion. Kar2p, Kar5p, and Kar7/Sec71p may be required for the formation of the initial structures leading to kar8/jem1 bridges. If Kar2p acts at more than one step in the nuclear fusion pathway, as it does in translocation, then the EM analysis would only reveal its earliest point of action. Alternatively, the kar8/jem1 bridges may reflect aberrant intermediates that form as a result of the loss of Kar8/Jem1p function. In either case, the fact that the kar8/jem1 bridges do not form in a kar5 mutant suggests that the kar8/jem1 bridges arise from a later step in the fusion pathway.
Conclusion
Several ER–nuclear envelope proteins are required for the fusion of the nuclear envelope during conjugation of S. cerevisiae. Three proteins, Kar2p, Kar5p, and Kar8/Jem1p, are clearly required for nuclear fusion, both in vitro and in vivo. Three other proteins, Kar7/Sec71p, Sec72p, and Sec63p, appear to play secondary roles, with Kar7/Sec71p and Sec72p acting to stabilize a complex required for nuclear fusion. Of these, Kar2p, Kar7/Sec71p, Sec72p, and Sec63p have dual roles in protein translocation and nuclear fusion. In contrast, two proteins with major roles in protein translocation, Sec61p and Sec62p, were not required for nuclear fusion. These results suggest the existence of a novel chaperone complex, including Kar5p, Kar2p, and Kar8/Jem1p, and possibly Kar7/Sec71p and Sec72p required for nuclear fusion, which is distinct from the chaperone complexes mediating protein translocation and folding.
ACKNOWLEDGMENTS
We thank members of the laboratory for discussions. We thank Randy Schekman for strains, space, and support for the some of the experiments described here. We are very grateful to Davis Ng and Peter Walter for sec63-201 strains and to Pam Silver and Reid Gilmore for SCJ1 plasmids. We thank Joe Goodhouse for assistance with EM. This work was supported by National Institutes of Health grant GM-37739 to M.D.R. V.B., W.K., C.T.B., and D.H. were partially supported by National Institutes of Health institutional training grants in genetics and cell and molecular biology. S.S.L.A. was partially supported by European Molecular Biology Organization long-term fellowship ALTF97721.
REFERENCES
- Beh CT. Functions of the Yeast Endoplasmic Reticulum. Ph.D. Thesis. Princeton, NJ: Princeton University; 1996. [Google Scholar]
- Beh CT, Brizzio V, Rose MD. KAR5 encodes a novel pheromone-inducible protein required for homotypic nuclear fusion. J Cell Biol. 1997;139:1063–1076. doi: 10.1083/jcb.139.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzio V, Gammie AE, Nijbroek G, Michaelis S, Rose MD. Cell fusion during yeast mating requires high levels of a-factor mating pheromone. J Cell Biol. 1996;135:1727–1739. doi: 10.1083/jcb.135.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Schekman R. A Sec63p-BiP complex from yeast is required for protein translocation in a reconstituted proteoliposome. J Cell Biol. 1993;123:1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B. Multiple roles of the spindle pole bodies in the life cycle of Saccharomyces cerevisiae. In: von Wittstein D, Friis J, Kielland-Brandt M, Stenderup A, editors. Molecular Genetics of Yeast. Copenhagen: Munksgaard; 1981. pp. 119–131. [Google Scholar]
- Byers B, Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation in Saccharomyces cerevisiae. J Bacteriol. 1975;124:511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J, Fink GR. A mutant of Saccharomyces cerevisiae defective for nuclear fusion. Proc Natl Acad Sci USA. 1976;73:3651–3655. doi: 10.1073/pnas.73.10.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Sanders SL, Feldheim DA, Schekman R. Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature. 1991;349:806–808. doi: 10.1038/349806a0. [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Schekman R. A yeast mutant defective at an early stage in import of secretory precursors into the endoplasmic reticulum. J Cell Biol. 1987;105:633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Schekman R. SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J Cell Biol. 1989;109:2653–2664. doi: 10.1083/jcb.109.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman S, Lin L, Malczynski MD, Snyder M. Pheromone-regulated genes required for yeast mating differentiation. J Cell Biol. 1998;140:461–483. doi: 10.1083/jcb.140.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault Y, Feldheim D, Blondel MO, Schekman R, Kepes F. SSS1 encodes a stabilizing component of the Sec61 subcomplex of the yeast protein translocation apparatus. J Biol Chem. 1994;269:27478–27485. [PubMed] [Google Scholar]
- Fang H, Green N. Nonlethal sec71-1 and sec72-1 mutations eliminate proteins associated with the Sec63p-BiP complex from S. cerevisiae. Mol Biol Cell. 1994;5:933–942. doi: 10.1091/mbc.5.9.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D, Rothblatt J, Schekman R. Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol Cell Biol. 1992;12:3288–3296. doi: 10.1128/mcb.12.7.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D, Schekman R. Sec72p contributes to the selective recognition of signal peptides by the secretory polypeptide translocation complex. J Cell Biol. 1994;126:935–943. doi: 10.1083/jcb.126.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D, Yoshimura K, Admon A, Schekman R. Structural and functional characterization of Sec66p, a new subunit of the polypeptide translocation apparatus in the yeast endoplasmic reticulum. Mol Biol Cell. 1993;4:931–939. doi: 10.1091/mbc.4.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie A, Brizzio V, Rose M. Distinct morphological phenotypes of cell fusion mutants. Mol Cell Biol. 1998;9:1395–1410. doi: 10.1091/mbc.9.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie A, Stewart BG, Scott CF, Rose MD. The two forms of karyogamy transcription factor Kar4p are regulated by differential initiation of transcription, translation, and protein turnover. Mol Cell Biol. 1999;19:817–825. doi: 10.1128/mcb.19.1.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green N, Fang H, Walter P. Mutants in three novel complementation groups inhibit membrane protein insertion into and soluble protein translocation across the endoplasmic reticulum membrane of Saccharomyces cerevisiae. J Cell Biol. 1992;116:597–604. doi: 10.1083/jcb.116.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanein D, Matlack KE, Jungnickel B, Plath K, Kalies KU, Miller KR, Rapoport TA, Akey CW. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell. 1996;87:721–732. doi: 10.1016/s0092-8674(00)81391-4. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Hoffman CS, Winston F. A ten minute DNA preparation from yeast releases autonomous plasmids for transformation of E. coli. Gene. 1987;57:267–272. doi: 10.1016/0378-1119(87)90131-4. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara LJ, Beh CT, Latterich M, Schekman R, Rose MD. Nuclear congression and membrane fusion: two distinct events in the yeast karyogamy pathway. J Cell Biol. 1994;126:911–923. doi: 10.1083/jcb.126.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T, Silver P. Suppression of a sec63 mutations identifies a novel component of the yeast endoplasmic reticulum translocation apparatus. Mol Biol Cell. 1993;4:919–930. doi: 10.1091/mbc.4.9.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latterich M, Schekman R. The karyogamy gene KAR2 and novel proteins are required for ER-membrane fusion. Cell. 1994;78:87–98. doi: 10.1016/0092-8674(94)90575-4. [DOI] [PubMed] [Google Scholar]
- Lyman SK, Schekman R. Interaction between BiP and Sec63p is required for the completion of protein translocation into the ER of Saccharomyces cerevisiae. J Cell Biol. 1995;131:1163–1171. doi: 10.1083/jcb.131.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman SK, Schekman R. Polypeptide translocation machinery of the yeast endoplasmic reticulum. Experientia. 1996;52:1042–1049. doi: 10.1007/BF01952100. [DOI] [PubMed] [Google Scholar]
- Lyman SK, Schekman R. Binding of secretory precursor polypeptides to a translocon subcomplex is regulated by BiP. Cell. 1997;88:85–96. doi: 10.1016/s0092-8674(00)81861-9. [DOI] [PubMed] [Google Scholar]
- Marsh L, Rose MD. The pathway of cell and nuclear fusion during mating in S. cerevisiae. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces: Cell Cycle and Cell Biology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 827–888. [Google Scholar]
- Meluh PB, Rose MD. KAR3, a kinesin-related gene required for yeast nuclear fusion. Cell. 1990;60:1029–1041. doi: 10.1016/0092-8674(90)90351-e. [DOI] [PubMed] [Google Scholar]
- Miller RK, Rose MD. Kar9p is a novel cortical protein required for cytoplasmic microtubule orientation in yeast. J Cell Biol. 1998;140:377–390. doi: 10.1083/jcb.140.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MK, Kurihara T, Silver P. Extragenic suppressors of mutations in the cytoplasmic C-terminus of SEC63 define five genes in Saccharomyces cerevisiae. Genetics. 1993;134:159–173. doi: 10.1093/genetics/134.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DTW, Walter P. ER membrane protein complex required for nuclear fusion. J Cell Biol. 1996;132:499–510. doi: 10.1083/jcb.132.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S, Endo T. The yeast JEM1p is a DnaJ-like protein of the endoplasmic reticulum membrane required for nuclear fusion. J Biol Chem. 1997;272:12889–12892. doi: 10.1074/jbc.272.20.12889. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Endo T. Reinvestigation of the functions of the hydrophobic segment of Jem1p, a yeast endoplasmic reticulum membrane protein mediating nuclear fusion. Biochem Biophys Res Commun. 1998;244:785–799. doi: 10.1006/bbrc.1998.8342. [DOI] [PubMed] [Google Scholar]
- Ohashi A, Gibson J, Gregor I, Schatz G. Import of proteins into mitochondria. The precursor of cytochrome c1 is processed in two steps, one of them heme-dependent. J Biol Chem. 1982;257:13042–13047. [PubMed] [Google Scholar]
- Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995;81:561–570. doi: 10.1016/0092-8674(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Polaina J, Conde J. Genes involved in the control of nuclear fusion during the sexual cycle of Saccharomyces cerevisiae. Mol Gen Genet. 1982;186:253–258. doi: 10.1007/BF00331858. [DOI] [PubMed] [Google Scholar]
- Riles L, Dutchik JE, Baktha A, McCauley BK, Thayer EC, Leckie MP, Braden VV, Depke JE, Olson MV. Physical maps of the six smallest chromosomes of Saccharomyces cerevisiae at a resolution of 2.6 kb pairs. Genetics. 1993;134:81–150. doi: 10.1093/genetics/134.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MD. Nuclear fusion in the yeast, Saccharomyces cerevisiae. Annu Rev Cell Dev Biol. 1996;12:663–695. doi: 10.1146/annurev.cellbio.12.1.663. [DOI] [PubMed] [Google Scholar]
- Rose MD, Misra LM, Vogel JP. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell. 1989;57:1211–1221. doi: 10.1016/0092-8674(89)90058-5. [DOI] [PubMed] [Google Scholar]
- Rose MD, Novick P, Thomas JH, Botstein D, Fink GR. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods of Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Rothblatt JA, Deshaies RJ, Sanders SL, Daum G, Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989;109:2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler I, Chiang A, Kurihara T, Rothblatt J, Way J, Silver P. A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J Cell Biol. 1989;109:2665–2675. doi: 10.1083/jcb.109.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanders SL, Whitfield KM, Vogel JP, Rose MD, Schekman RW. Sec61p and BIP directly facilitate polypeptide translocation into the ER. Cell. 1992;69:353–365. doi: 10.1016/0092-8674(92)90415-9. [DOI] [PubMed] [Google Scholar]
- Scherer S, Davis RW. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci USA. 1979;76:4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlenstedt G, Harris S, Risse B, Lill R, Silver PA. A yeast DnaJ homologue, Scj1p, can function in the endoplasmic reticulum with BiP/Kar2p via a conserved domain that specifies interactions with Hsp70s. J Cell Biol. 1995;129:979–988. doi: 10.1083/jcb.129.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scidmore MA, Okamura HH, Rose MD. Genetic interactions between KAR2 and SEC63, encoding eukaryotic homologues of DnaK and DnaJ in the endoplasmic reticulum. Mol Biol Cell. 1993;4:1145–1159. doi: 10.1091/mbc.4.11.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberstein S, Schlenstedt G, Silver PA, Gilmore R. A role for the DnaJ homologue Scj1p in protein folding in the yeast endoplasmic reticulum. J Cell Biol. 1998;143:921–933. doi: 10.1083/jcb.143.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague GFJ, Thorner JW. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae. In: Jones EW, Pringle JR, Broach JR, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1994. pp. 657–744. [Google Scholar]
- Stearns T, Botstein D. Unlinked noncomplementation: isolation of new conditional-lethal mutations in each of the tubulin genes of Saccharomyces cerevisiae. Genetics. 1988;119:249–260. doi: 10.1093/genetics/119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling CJ, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell. 1992;3:129–142. doi: 10.1091/mbc.3.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tange Y, Horio T, Shimanuki M, Ding DQ, Hiraoka Y, Niwa O. A novel fission yeast gene, tht1(+), is required for the fusion of nuclear envelopes during karyogamy. J Cell Biol. 1998;140:247–258. doi: 10.1083/jcb.140.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J. KAR2, the Yeast Homologue of Mammalian BiP/GRP78. Ph.D. Thesis. Princeton, NJ: Princeton University; 1993. [Google Scholar]
- Vogel JP, Misra LM, Rose MD. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol. 1990;110:1885–1895. doi: 10.1083/jcb.110.6.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]