Abstract
Resveratrol is a bioflavonoid which is known to inhibit cell proliferation and induce apoptosis in cancer cell lines at concentrations above 50uM. It also has colon cancer prevention activity in mouse models and possibly in humans. We have examined the effects of low concentrations of resveratrol on a specific signaling pathway, the Wnt pathway, which is activated in over 85% of sporadic colon cancers. Two colon cancer (HT29 and RKO) and one normal mucosa-derived (NCM460) cell lines were utilized. Cell proliferation was not affected by resveratrol at ≤40uM for HT29 and NCM460 and <20uM for RKO though Wnt signal throughput, as measured by a reporter construct, was reduced in RKO and NCM460 at concentrations as low as 10uM (p<0.001). This effect was most easily appreciated following Wnt pathway stimulation with Wnt3a conditioned medium and LEF1 or LEF1/β-catenin transfection. Resveratrol did not inhibit Wnt throughput in mutationally activated HT29. Low concentrations of resveratrol significantly decreased the amount and proportion of β-catenin in the nucleus in RKO (p=0.002) and reduced the expression of lgs and pygoI, regulators of β-catenin localization in all cells lines. Thus, at low concentrations, in the absence of effects on cell proliferation, resveratrol significantly inhibits Wnt signaling in colon-derived cells which do not have a basally activated Wnt pathway. This inhibitory effect may be due in part to regulation of intracellular β-catenin localization.
Keywords: Resveratrol, colon cancer, Wnt pathway, β-catenin, pygopus
BACKGROUND
Resveratrol
Resveratrol is a bioflavonoid found in the skin of dark grapes, red wine and peanuts. In vitro, this agent has been shown to have antioxidant activity and pro-apoptotic activity, and to inhibit proliferation of colon cancer cell lines [1]. Resveratrol is also reported to inhibit ribonucleotide reductase, ornithine decarboxylase [2] and IkappaB kinase [3] and is purported to act as a cancer prevention agent for a wide variety of human cancers including colon cancer [4]. The amount of resveratrol in red wine is 5 to13 micromoles/liter [5] and it is suggested that even one glass (100ml, ~1 micromole, 250ug) of Pinot Noir per day provides cancer prevention activity. However, a quantity as small as ¼ mg does not result in serum/plasma concentrations in the range where activity is seen in vitro. The suggestion that resveratrol has chemopreventive efficacy in humans, and the evidence that it has chemopreventive efficacy in animal models, at dosages which attain low plasma levels, is perplexing [6]. This could be explained, in part, if activity of resveratrol is present at lower uM concentrations than has previously been appreciated.
Colon cancer and Wnt signaling
Colon cancer is the third leading cause of cancer deaths among men and women in the United States [7]. The epidemiology of colon cancer suggests that dietary factors play a critical role in tumor initiation and promotion [8], with populations consuming a diet high in fiber, vegetables and fruits exhibiting a lower cancer incidence. Mutations in genes leading to activation of the Wnt signaling pathway are common in colon cancer, seen in over 85% of sporadic cases [9]. Signaling through the Wnt pathway begins with a WNT ligand which interacts with a cell surface frizzled (Fz) receptor to initiate the signal cascade [10]. Pathway activation prevents degradation of β-catenin which is translocated to the nucleus where it binds to members of the LEF/TCF family of HMG-box transcription factors [11], inducing transcription of growth regulatory genes such as myc [12], and cyclinD1 [13]. Other proteins including legless (lgs), pygopusI (pygoI) and pygopusII (pygoII) complex with LEF/TCFs and β-catenin and facilitate both the translocation of β-catenin into the nucleus and the induction of transcription by the active complex [14,15]. In colon carcinogenesis, mutations in either APC or β-catenin are early events which constitutively activate the Wnt pathway [16].
Resveratrol can modulate prostaglandin production and inhibit the expression of cyclooxygenase-2 [17,18] which is overexpressed in colon cancer. Cox2 inhibition may be one mechanism through which resveratrol is acting in relation to cancer chemoprevention [19]. However, the concentrations which display this activity in vitro are difficult to attain in vivo [20], and thus other mechanisms need to be explored. Since the Wnt pathway is critically important in the development of colon cancer, any inhibitory effects of resveratrol on this pathway are particularly relevant to explore. This study was undertaken to define whether low concentrations of resveratrol, concentrations below those which inhibit proliferation and induce apoptosis, have activity against colon-derived cells in vitro and, specifically, whether these low concentrations affected Wnt signaling. We report here that subapoptotic concentrations of resveratrol can inhibit Wnt signal throughput in colon-derived cells and that this effect may be due, in part, to regulation of β-catenin localization. This activity may contribute to resveratrol's cancer prevention activity.
METHODS AND MATERIALS
Cell lines
Three cell lines were utilized for these studies. NCM460 is a cell line derived from normal colonic mucosal cells which were obtained from Incell Corporation (San Antonio, Texas) [21]. This cell line was maintained in M3 Base cell culture medium complete (Incell, catalog number M300A-500) with 10% fetal bovine serum (FBS). There are no known apc or β-catenin mutations present in this cell line. RKO, a human colon cancer cell line, was kindly provided by Dr. Parker (UCLA). This cell line, originally obtained from a patient with hereditary non-polyposis colon cancer, displays microsatellite instability (MSI, or RER(+)) and, similar to NCM460, has no known Wnt pathway activation. It was maintained in culture in RPMI1640 media at 5% CO2 with 10% FBS. HT29, a colon cancer cell line with mutationally activated Wnt signaling due to a truncation mutation in apc, was obtained from the American Type Culture Collection (ATCC, Manassas, Virginia). It was maintained in DMEM media at 5% CO2 with 10% FBS. These three cell lines were selected for study in order to provide both normal-derived and cancer-derived cells, and both Wnt-activated and Wnt-non-activated cells for analysis.
Cell proliferation, cell cycle and cell size
Cell proliferation was determined utilizing an MTT assay. This assay measures the reduction of a tetrazolium component into an insoluble formazan product by the mitochondria of viable cells. Following 48 hour incubation with or without resveratrol, cells were incubated with MTT reagent for 2 hours and then lysed with detergent. Solubilized crystals produce a color which is read using an ELISA plate reader at a wavelength of 570 nm. The amount of color produced is directly proportional to the number of viable cells. For cell cycle analysis, cells were plated in a 6-well format and treated for 48 hours. Cells were recovered by trypsinization, washed with PBS, fixed in 70% ethanol and sorted overnight at −20°C. Fixed cells were washed ×3 in ice cold PBS and incubated with 500ul of a propidium iodide solution (50ug/ml) containing 50ug RNAseA for 30 minutes in the dark at room temperature. Cell cycle distribution was analyzed by flow cytometry using a Becton Dickinson FACScan system. To characterize cell size following treatment of RKO cells with resveratrol, a trypsinized suspension of spherical cells were placed in a hemocytometer and photographed. The cross-sectional area and distribution of trypan blue-excluding cells was calculated using Image-Pro Plus.
Wnt throughput assays
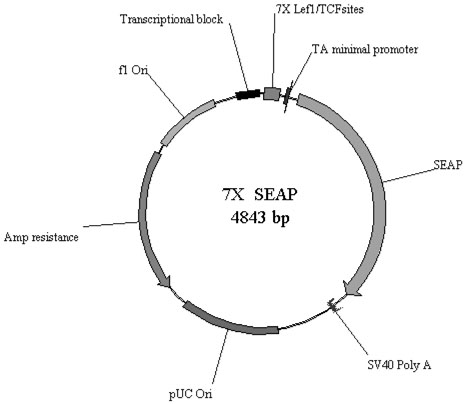
In order to evaluate changes in Wnt signaling at low dosages of resveratrol, the colon-derived cell lines were utilized with a Wnt pathway reporter construct designed in our laboratory containing seven LEF/TCF consensus binding sites preceding a secreted alkaline phosphatase (7X-SEAP) reporter (Figure 1). Activation of Wnt signaling leads to production of SEAP which is secreted into the culture media. SEAP is easily detected and quantified by sampling of the culture media, facilitating sampling without the need for disrupting transfected cells. Comparative studies of the 7X-SEAP reporter with the “super” TopFlash reporter documented equivalence in sensitivity. For the assays, cells were plated into 24 well plates and allowed to grow for 48 hours. Cells were typically between 70-90% confluent on the day of transfection. Separate cohorts of cells were treated with Wnt3a conditioned medium (CM; Willert, et al., http://www.stanford.edu/~rnusse/assays/W3aPurif.htm) or transfection with LEF1 or LEF1/β-catenin in order to increase Wnt signal throughput, thus facilitating detection of inhibitory activity by resveratrol. 1.2μg of reporter plasmid DNA and 0.12μg of LEF1 expression construct, β-catenin and/or inactive DNA (pBluescript) for a total mass of 1.44μg DNA per well was transfected using Lipofectamine2000 following manufacturers recommendations. Each condition was performed in triplicate. After 48 hours in culture with or without resveratrol, media was recovered and 20μl was assayed using Great EscAPe SEAP detection kit (BD Biosciences) in a 96 well format. Plates were read on a Dynatech Laboratories ML3000 microtiter plate luminometer. Experiments were performed at various concentrations of resveratrol ranging up to 20uM. All experiments were performed in conjunction with transfection of a control SEAP plasmid lacking LEF/TCF binding sites. Results were additionally normalized to cell counts which were performed to insure that cells were viable and not undergoing apoptosis. Results of the cell counts were consistent with the MTT data. For the very low (5uM) concentration resveratrol experiments RKO cells were co-cultured in 2D culture with stably-transfected Wnt3a producing L-cells for 48 hours, in 50:50 RPMI:DMEM media with 10% fetal bovine serum. Media was assayed for SEAP activity after 48 hours of exposure to 5uM resveratrol or equivalent v/v DMSO only control.
Figure 1.
Plasmid map of the 7×-SEAP reporter construct.
β-catenin localization and quantification
β-catenin localization was evaluated by confocal microscopy and fluorescence immunohistochemistry. Cells were grown in 4-well chamber slides (LabTek/Nunc, Rochester, NY) with 10uM and 20uM resveratrol and equivalent v/v DMSO (diluent for resveratrol) as controls. Cells were incubated for 48-72 hours, fixed, washed, and endogenous peroxidases inhibited by incubating with H2O2/methanol for 30 minutes. Cells were then stained with monoclonal anti-β-catenin antibodies (Transduction labs/BD Biosciences, San Jose, CA). Following counterstaining with an ABC immunohistochemistry kit (Santa Cruz Biotechnology, Santa Cruz, CA), peroxidase localization with a tyramide-fluorochrome system (Perkin Elmer, Boston, MA) was performed, and visualization was obtained by confocal fluorescence microscopy. Because the cell lines in the resting state, especially NCM460 and RKO, have relatively low levels of β-catenin, the experiments were repeated with the addition of Wnt3a conditioned medium (CM) to activate the Wnt pathway. Using fluorescence microscopy, 100 cells were visualized and the degree of β-catenin related fluorescence defined with image quantification software. Each cell nucleus was individually mapped as was the cell membrane of each cell. Cells in clusters without distinct cell borders were not evaluated. Nuclear staining across different resveratrol treatment groups was compared as was the calculated nuclear:cytoplasmic β-catenin ratio.
Expression of lgs, pygoI and pygoII
Following incubation with or without resveratrol as described above, cells were harvested for mRNA in order to define the expression of lgs, pygoI and pygoII by quantitative real-time PCR (qRT-PCR). Specific primer sets for each gene were employed and expression was compared to actin controls in all cases. Primer pairs were for lgs, pygoI and pygoII were obtained from Qiagen (Valencia, CA), catalog numbers QT00051604, QT00032081 and QT00097727, respectively. Temperatures utilized were 48°C for reverse transcription for 30 minutes and 95°C for DNA polymerase activation for 10 minutes. Each of fifty cycles were run as follows: 95°C denaturation for 15 seconds, 55°C annealing for 30 seconds, 60°C synthesis for 30 seconds. Actin was utilized as a housekeeping gene for data normalization.
Statistics
Means, standard deviations and standard errors of the mean (SEM) for data from all experimental groups were calculated and comparisons between groups were made with a two-sided unpaired t-test with the level of significance defined as α<0.05.
RESULTS
Proliferation and cell cycle analysis
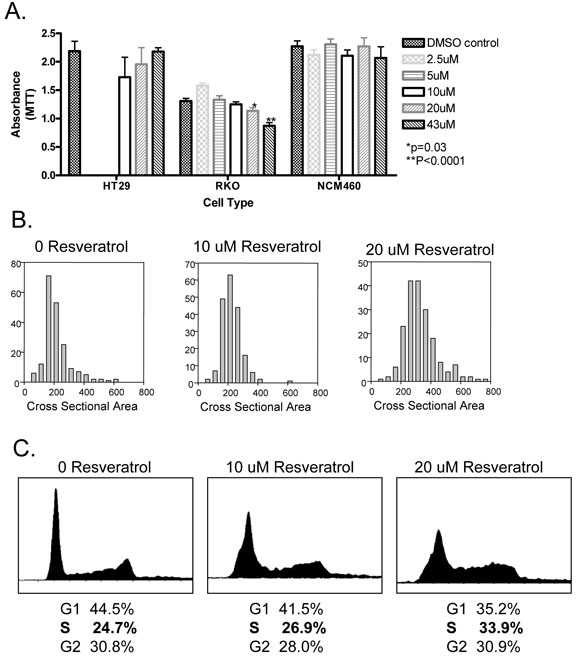
Resveratrol did not affect proliferation of HT29 and NCM460 at concentrations ranging from 2.5 uM to 40uM (Figure 2A). RKO was unaffected at 5uM and 10uM but exhibited a slight decrease in proliferation at 20uM (p=0.03) and a more significant decrease at 40uM (p<0.001). For RKO, cell size was noted to be slightly increased at 20uM (Figure 2B), possibly indicative of early induction of cell cycle arrest at this concentration for this particular cell line. Mean cell cross sectional area was 218.8 ± 92.5 μ2 with no resveratrol, 226.1 ± 66.5 μ2 at 10uM resveratrol and 331.7 ± 111.2 μ2 at 20uM resveratrol (p<0.0001). The effect of resveratrol on the cell cycle was examined by flow cytometry in RKO cells. Treatment with resveratrol increased the proportion of cells in S phase from 24.7% to 26.9% to 33.9% upon exposure to 10uM and 20uM respectively (Figure 2C), indicating cell cycle arrest at S phase.
Figure 2.
Panel A: Proliferation of HT29, RKO and NCM460 cells measured by MTT assay at varying concentrations of resveratrol. Proliferation was not determined for HT29 at 2.5uM and 5uM resveratrol. *p=0.03; **p<0.0001. Error bars represent SEM.
Panel B: RKO cell size as defined by cross sectional area in microns-squared. Y axis depicts # cells at each size cohort. 200 cells counted under each resveratrol condition.
Panel C: Cell cycle analysis of RKO cells treated with 10uM and 20uM resveratrol and compared to control (0uM resveratrol). The proportion of cells in S phase increases with increasing resveratrol concentration.
Evaluation of Wnt pathway activity
Wnt signal throughput was evaluated with the 7×-SEAP reporter construct for each of the cell lines (Table 1). Wnt signal throughput was induced in NCM460 cells following transfection of LEF1 or LEF1/β-catenin. Treatment with Wnt3a conditioned medium (CM) did not augment Wnt signal throughput further by itself, or in the setting of LEF1/β-catenin transfection, but was synergistic following LEF1 transcription inducing a small (1.38 fold) but significant (p=0.0048) increase. RKO cells responded to LEF1 and LEF1/β-catenin transfection with an increase in Wnt signal throughput and were extremely responsive to the addition of Wnt3a CM under all conditions, displaying up to a 30 fold increase. Despite intrinsic Wnt pathway activation, HT29 cells could be induced to further increase Wnt signal throughput following transfection with LEF1 and LEF1/β-catenin. HT29 cells were minimally responsive to Wnt3a CM either by itself or in combination with LEF1 or LEF1/β-catenin.
Table 1.
Effect of low concentrations of resveratrol on Wnt pathway throughput in colon-derived cell lines.
| Plus Wnt3 conditioned media | |||||||
|---|---|---|---|---|---|---|---|
| 1A |
1B |
Fold Change |
|||||
| control |
Lef1 |
Lef1/Beta- Catenin |
control |
Lef1 |
Lef1/Beta- Catenin |
||
| NCM |
2.9863 0.8413 |
8.7168 1.3529 p=0.0000 |
24.7085 13.1528 p=0.00237 |
1.0786 +/−0.0719 p=0.0963 |
1.3807 +/−0.2542 p=0.0048 |
1.0548 +/−0.1353 p=NS |
|
| NCM + 10uM RES |
80.22% +/−1.41 p=0.0019 |
80.21% +/−9.36% p=0.0345 |
75.21% +/−6.33 p=0.0044 |
0.7904 +/−0.0299 p=0.0055 |
0.8897 +/−0.0665 p=0.0017 |
0.7683 +/−0.0995 p=NS |
|
| NCM + 20 uM RES |
72.55% +/−9.53 p=0.0318 |
88.83% +/−9.67 p=NS |
71.52% +/−3.27 p=0.0171 |
0.7133 +/−0.0413 p=0.00054 |
0.8881 +/−0.0752 p=0.01318 |
0.7125 +/−0.0207 p=0.00139 |
|
| RKO |
0.0428+/− 0.0163 |
0.1646+/− 0.1142 p=0.0270 |
13.3303+/− 13.9838 p=0.0422 |
15.4351 +/4.0339 p=0.00000 |
30.7591 +/−9.5413 p=0.00002 |
3.2482 +/−1.1015 p=0.00056 |
|
| RKO + 10uM RES |
104.75% +/−5.95 p=NS |
81.46% +/−5.59 p=NS |
125.43% +/−8.76 p=0.0130 |
9.8299 +/−1.5027 p=NS |
27.7683 +/−0.8956 p=0.0036 |
1.9354 +/−0.1763 p=0.0210 |
|
| RKO + 20uM RES |
86.78 % +/− 1.01 p=0.NS |
53.76% +/− 8.06 p=0.003 |
91.77% +/− 14.7 p=0.NS |
10.5443 1.2293 p = 0.004 |
11.7152 0.8649 p=0.0117 |
2.3713 0.1014 p=0.0185 |
|
| HT29 |
0.0512 +/−0.0044 |
0.0969 +/−0.0127 p=0.0041 |
0.9086 +/−0.1719 p=0.0010 |
1.1315 +/−0.1064 p=0.NS |
1.0695 +/−0.0791 p=0.NS |
1.3715 +/−0.1075 p=.0417 |
|
| HT29 + 20uM RES |
118.35% 5.64 p=0.0364 |
107.22% 2.12 p=0.NS |
107.71% 17.99 p=0.NS |
1.3167 +/−0.1419 p=0.NS |
1.2329 +/−0.1240 p=0.NS |
1.5740 +/−0.2187 p=0.NS |
|
A: Wnt pathway throughput (SEAP activity). Absolute value +/− SD. For Resveratrol conditions % of non-Resveratrol activity +/− SD.
B: Fold Change due to Wnt3 conditioned media
Rows with cell line only listed: P value is for Wnt3a, Wnt3a/LEF1 or Wnt3a/LEF1/β-catenin vs. control.
Rows which list resveratrol concentration: P value is for comparison to similar condition without resveratrol.
NS=not significantly different (p>0.05)
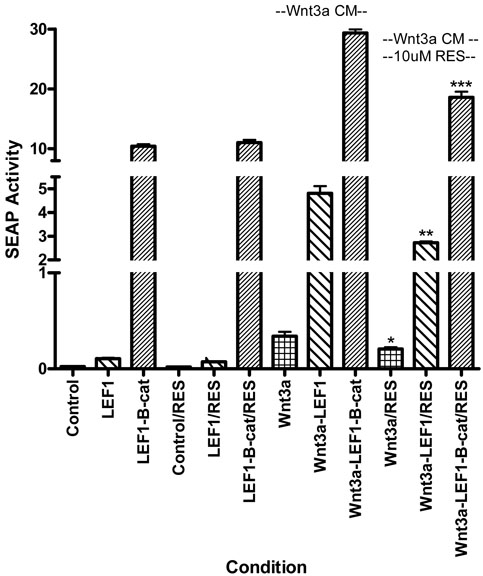
Low dose resveratrol, at 10uM and 20uM (concentrations at which no significant effect on cell proliferation was seen), significantly reduced Wnt signal throughput in NCM460 under every condition: without additional Wnt stimulation, following transfection of LEF1 and LEF1/β-catenin, and with the addition of Wnt3a CM. Wnt signal throughput was reduced by 20-30% (Table 1). 10uM and 20uM resveratrol did not affect Wnt throughput in unstimulated RKO cells but did significantly reduce it in nearly all conditions following augmentation of the Wnt signal through either transfection or treatment with Wnt3a CM. Resveratrol was ineffective in these cells only following LEF1/β-catenin transfection in the absence of Wnt3a CM (Table 1). The effect of 10uM resveratrol in reducing Wnt signal throughput in RKO cells in the presence of Wnt3a CM is depicted in Figure 3. Resveratrol trended to reduce Wnt throughput under control conditions (p=0.056) and significantly reduced Wnt throughput following LEF1 transfection (p=0.002) and following LEF1/β-catenin transfection (p<0.001). The lowest concentration of resveratrol tested in RKO cells was 5uM but no effect on Wnt throughput was seen at this concentration (Table 2). These latter experiments were conducted following exposure to Wnt3a producing cells in side-by-side co-culture rather than the addition of Wnt3a CM. HT29 cells were generally unresponsive to resveratrol and, while not significant, the trend at 20uM was for an increase, rather than a decrease in Wnt signal throughput (Table 1).
Figure 3.
Wnt throughput as measured by SEAP (secreted alkaline phosphatase) activity following transfection of the 7X-SEAP reporter in RKO cells under various conditions. The 3 left bars represent Wnt throughput (control, transfected LEF1 and transfected LEF1-β-catenin) in the absence of Wnt3a CM and resveratrol. The next 3 bars represent Wnt throughput (control, transfected LEF1 and transfected LEF1-β-catenin) in the absence of Wnt3a CM but in the presence of 10uM resveratrol. The next 3 bars represent Wnt throughput under the various conditions following the addition of Wnt3a CM. The 3 right bars represent Wnt throughput following Wnt3a CM in the presence of 10uM resveratrol. LEF1 transfection, LEF1/β-catenin transfection and the addition of Wnt3a CM each significantly increase Wnt throughput. Resveratrol decreases Wnt throughput under each transfection condition in the presence of Wnt3a (*p=0.056; **p=0.002; ***p<0.001). All data represents Wnt throughput as measured by SEAP activity rather than fold increase to facilitate comparisons between different subgroups. Error bars represent SEM.
Table 2.
Effect of a very low concentration of resveratrol on Wnt throughput in RKO cells.
| Plus Wnt3 conditioned media | |||||||
|---|---|---|---|---|---|---|---|
| 1A |
1B |
Fold Change |
|||||
| control |
Lef1 |
Lef1/Beta- Catenin |
control |
Lef1 |
Lef1/Beta- Catenin |
||
| RKO |
0.0288+/− 0.0125 |
0.1826+/− 0.0205 p=0.0004 |
11.4874+/− 1.8236 p=0.0004 |
30.8831+/− 6.2975 p=0.0012 |
64.7230+/− 6.8884 p=0.0001 |
2.8199+/− 0.5251 p=0.0045 |
|
| RKO + 5uM RES |
ND |
ND |
ND |
27.1597+/− 5.7014 p=0.49 |
61.5837+/− 3.0671 p=0.51 |
2.9715+/− 0.1805 p=0.47 |
|
A: Wnt pathway throughput (SEAP activity). Absolute value +/− SD.
B: Fold Change due to Wnt3a co-culture
Top row: P value is for Wnt3a, Wnt3a/LEF1 or Wnt3a/LEF1/β-catenin vs. control.
Bottom row: P value is for comparison to similar condition without resveratrol.
Intracellular β-catenin localization
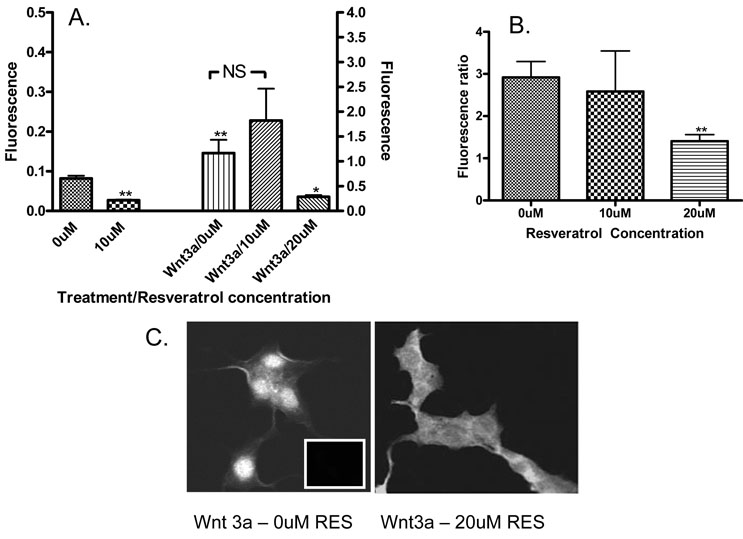
In order to determine whether resveratrol was modulating Wnt signaling by influencing β-catenin translocation to the nucleus, nuclear and cytoplasmic β-catenin was quantified for each cell line following 48 hours of treatment with different concentrations of resveratrol with and without Wnt3a conditioned medium. As anticipated based on the lack of effect on Wnt signal throughput, no change was seen in HT29. Similarly, no significant effect on β-catenin localization was seen in NCM460. In RKO cells, however, a significant reduction in the amount of nuclear β-catenin was seen at 10uM (p<0.001). Exposure to Wnt3a CM increased nuclear β-catenin (p<0.001) but this increase was abrogated by 20uM resveratrol (p<0.01 vs. Wnt3a CM with 0uM resveratrol, Figure 4A). To insure that these results represented changes in the distribution of β-catenin, rather than the total cellular content, the nuclear:cytoplasmic ratio was examined following Wnt3a stimulation and in the presence of 20uM resveratrol. A significant reduction in the nuclear:cytoplasmic ratio was seen in RKO cells (Figure 4B and 4C, p<0.001).
Figure 4.
Panel A: Quantity of nuclear β-catenin as measured by nuclear fluorescence intensity in RKO cells at 0uM and 10uM resveratrol and following the addition of Wnt3a CM at 0uM, 10uM and 20uM resveratrol. 10uM resveratrol reduces nuclear β-catenin (p<0.01) in the absence of Wnt3a stimulation. 20uM resveratrol reduces nuclear β-catenin following Wnt3a stimulation (p<0.05). Wnt3a alone increases the amount of nuclear β-catenin compared to non-stimulated baseline (p<0.01). Error bars represent SEM.
Panel B: Nuclear:cytoplasmic ratio of β-catenin in RKO cells following stimulation with Wnt3a CM as a ratio of fluorescence intensity. 20uM resveratrol significantly reduces the nuclear:cytoplasmic ratio (p<0.01). Error bars represent SEM.
Panel C: Visual representation of β-catenin fluorescence intensity and localization in RKO cells following stimulation with Wnt3a and exposure to 0uM (control) and 20uM resveratrol. Insert (left panel) depicts fluorescence intensity of secondary IgG antibody only control.
Expression of intracellular modulators of β-catenin localization
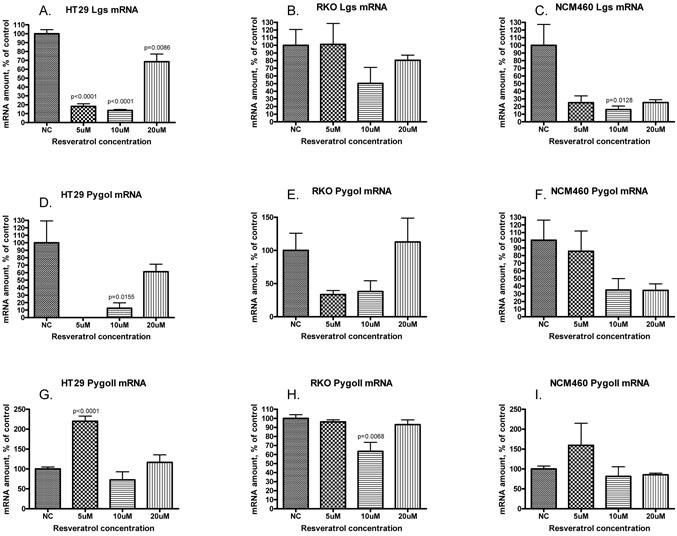
Quantitative real time PCR was utilized to define the effect of resveratrol at concentrations ranging from 5uM to 20uM on the expression of lgs, pygoI and pygoII in NCM460, RKO and HT29 (Figure 5). Lgs expression was significantly reduced in HT29 at all concentrations and at 10uM in NCM460. Other concentrations showed a non significant trend toward reduction, especially in NCM460. PygoI was reduced in all cell lines except at 20uM in RKO and 5uM in NCM460, though the only statistically significant decrease was seen at 10uM in HT29 (p=0.0155). PygoII overall was minimally changed, with a slight reduction at 10uM in RKO (p=0.0068) and an increase in mRNA levels in HT29 at 5uM (p<0.001).
Figure 5.
Expression of lgs (panels A, B, C), pygoI (panels D, E, F) and pygoII (panels G, H, I) measured by quantitative real-time PCR and represented as amount of mRNA, normalized to actin, and graphed as percent of control (y-axis). Cell lines tested included HT29 (panels A, D, G), RKO (panels B, E, H) and NCM460 (panels C, F, I). Resveratrol concentrations included 5uM, 10uM and 20uM (x-axis). Error bars represent SEM. Levels of significance noted when p<0.05.
DISCUSSION
We describe here that resveratrol inhibits Wnt signal throughput in RKO cells and NCM460 cells, both colon-derived cell lines lacking intrinsic activation of the Wnt pathway. These effects were noted at concentrations of resveratrol below those that inhibited cell proliferation. Additionally, pathway inhibition was seen under various conditions which increase Wnt throughput, such as treatment with Wnt3a conditioned medium or transfection with LEF1 and/or β-catenin. However, no effect on Wnt throughput was seen in HT29 which harbors a Wnt-activating mutation in apc.
In some cells (RKO), our data suggests that the effect of low concentrations of resveratrol on Wnt signaling may be at least partially due to inhibition of β-catenin nuclear translocation. A different plant flavonoid, apigenin has been shown to affect Wnt signaling by reducing nuclear β-catenin and the expression of c-myc and cyclinD1 in the prostates of TRAMP mice [22]. However, other mechanisms must be operative, as Wnt throughput inhibition was seen in NCM460 in the absence of demonstrable effects on β-catenin quantity or intracellular localization. Low concentrations of resveratrol also affect the expression of lgs, pygoI and, to a lesser extent, pygoII, the proteins for which are involved in β-catenin nuclear transport and localization. It is possible that resveratrol's activity in eliciting an S-phase arrest [23], in conjunction with reductions in lgs and pygoI, may affect the cellular distribution of β-catenin in a way that reduces Wnt signal throughput. The S-phase arrest mediated by resveratrol, confirmed here in RKO cells, occurs at lower concentrations than its cytotoxic and apoptotic effects [24-28]. Recently, it has been suggested that β-catenin transcriptional activity is regulated not only through the levels of protein degradation, but also via regulation of its nuclear localization [29]. During S-phase pause or arrest, APC protein is increased [30]. This occurs even in cells harboring truncating mutations since the N-terminal 20 amino acid segment of APC, which is essential for cell proliferation [31], binds to β-catenin serving as a cytoplasmic “sink” and preventing its translocation to the nucleus [30]. Thus, an increase in cytoplasmic APC would retain β-catenin in this compartment, reducing its transcription activating functions. Lgs and pygoI, alternatively, serve as nuclear “sinks” for β-catenin so a reduction in their abundance, as we demonstrate here following exposure to resveratrol, would additionally contribute to a reduction in β-catenin dependent Wnt throughput [15,32]. However, the fact that resveratrol had a measurable effect on β-catenin localization by immunohistochemistry only in RKO cells, while inhibiting Wnt signal throughput in both RKO and NCM460, and that the effects on lgs, pygoI and pygoII do not directly correlate with Wnt throughput inhibition, suggest that resveratrol's inhibition of Wnt signaling likely involves multiple mechanisms that may be partially cell type dependent.
The effect on Wnt signaling has implications for cancer treatment or cancer prevention, though resveratrol has various other effects in humans including beneficial activity related to cardiovascular disease [33], protective effects following brain damage and ischemia [34] and anti-aging properties [reviewed in 35]. In relation to the cancer chemopreventive effects, the concentrations attained in vivo are significantly lower than the concentrations required for inhibition of cell growth in vitro, raising the question as to how resveratrol exhibits this activity in animal models and perhaps in humans [36]. A recent pharmacokinetic study of single dose resveratrol confirmed that peak plasma concentrations of the parent compound following a single large 5g ingestion reached only 539ng/ml (2.4uM) [37]. Our finding that low concentrations of resveratrol can inhibit Wnt signaling may provide a partial explanation to this conundrum. Cooperativity with other biologically active flavanoids and polyphenols in wine, grapes and other nutritional sources and increased concentrations of resveratrol locally in the colon may also contribute to resveratrol's in vivo activity.
The data for prevention of colon cancer by resveratrol in animal models are somewhat contradictory, both in its efficacy as well as information regarding the effective dose range, though activity at low dosages, and therefore in circumstances where low serum concentrations are attained, has been reported. Tessotore [38] demonstrated activity of very low dose resveratrol of 0.2 mg/kg/day in reducing aberrant crypt foci (ACF) in the colon in an azoymethane-induced tumor model. In another carcinogen-based model, utilizing 1,2-dimethylhydrazine, resveratrol at 8 mg/kg/day reduced both ACF and colonic tumors [39,40]. In genetic models utilizing the APCmin/+ mouse, which harbors a single allele mutation in apc, and therefore has intrinsically activated Wnt signaling, Schneider [41] demonstrated profound activity at dosages as low as 0.3 mg/mouse/day in reducing intestinal tumors. In this study, expression of the Wnt target gene, cyclinD1, as well as other markers of cell cycling, were reduced. We have confirmed the effects of resveratrol on cell cycling in colon-derived cells in vitro in our study. Other reports suggest that resveratrol dosages as high as 90 mg/kg [42] are ineffective or that only extremely high dosages up to 500 mg/kg show activity [43]. Sale [44] utilized dosages of 60 mg/kg and 240 mg/kg, and found the former ineffective but the latter effective in inducing a more modest reduction in intestinal tumorigenesis. Overall, these studies indicate that resveratrol may have activity in both carcinogen-induced tumor models as well as in the Wnt activated APCmin/+ mouse, but that the effective dose is unclear, with activity reported utilizing dosages ranging from <1 mg/kg to 500 mg/kg per day. The effect of resveratrol on Wnt signaling in animal models has not been explored.
This is the first report describing a Wnt inhibitory activity of resveratrol. While the precise mechanisms require further study, resveratrol does affect β-catenin nuclear localization in some cells and the expression of genes encoding proteins involved in this process. The effects on Wnt signaling are seen at concentrations of resveratrol below those that induce apoptosis and inhibit cellular proliferation. Wnt inhibition appears to be restricted to cell lines without a mutationally activated Wnt pathway. While most colon cancers have a mutationally activated Wnt pathway, Wnt signaling is also of paramount importance in regulating proliferation of normal colonic mucosa and in the regulation of the colonic stem cell compartment [45, 46]. Because of this, the effects of resveratrol in non-mutationally activated cell lines may be most relevant in relation to colon cancer prevention rather than treatment. Further studies in animal models and humans on the influence of resveratrol on the Wnt pathway are warranted.
Acknowledgements
Supported by CA82450 (RFH).
REFERENCES
- 1.Szenda B, Tyihak E, Kiraly-veghely Z. Dose-dependent effects of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Experimental and molecular medicine. 2000;32:88–92. doi: 10.1038/emm.2000.16. [DOI] [PubMed] [Google Scholar]
- 2.Khanduja KL, Bharadwaj A, Kaushik G. Resveratrol inhibits N-nitrosodiethylamine-induced ornathine decarboxylase and cyclooxygenase in mice. J Nutr Sci Vitaminol. 2004;50:61–65. [PubMed] [Google Scholar]
- 3.Holmes-McNary M, Baldwin AS., Jr. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the I-k-B kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- 4.Bauer JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Natures Reviews Drug Discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 5.“Benton Lane Winery”. at http://www.avalonwine.com/Benton-Lane-Winery.php.
- 6.Gescher AJ, Steward WP. Relationship between mechanisms, bioavailability and preclinical chemopreventive efficacy of resveratrol: A conundrum. Cancer Epi, Biomarkers & Prevention. 2003;12:953–957. [PubMed] [Google Scholar]
- 7.“Cancer facts and figures”. American Cancer Society: 2007. at http://www.cancer.org. [Google Scholar]
- 8.Mason JB. Nutritional chemoprevention of colon cancer. Sem Gastroint Dis. 2002;13:143–153. [PubMed] [Google Scholar]
- 9.Robbins DH, Itzkowitz SH. The molecular basis of colon cancer. Med Clin NA. 2002;86:1467–95. doi: 10.1016/s0025-7125(02)00084-6. [DOI] [PubMed] [Google Scholar]
- 10.Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes & Development. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 11.Van de Watering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Yuma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. [DOI] [PubMed] [Google Scholar]
- 12.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, daCosta LT, Morin PJ, Volgelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 13.Testu O, McCormick F. Beta-catenin regulates expression of cyclinD1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- 14.Thompson BJ. A complex of Armadillo, Legless, and Pygopus coactivates dTCF to activate wingless target genes. Curr Biol. 2004;14:458–466. doi: 10.1016/j.cub.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 15.Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell. 2002;109:47–60. doi: 10.1016/s0092-8674(02)00679-7. [DOI] [PubMed] [Google Scholar]
- 16.Polakis P. The many ways of Wnt in cancer. Curr Opin Genet Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Patel B, Patel S, Hoffman R. Inhibition of cyclo-oxygenase-2 expression in mouse macrophages by 4-(3-methyl-but-1-enyl)-3,5,3',4'-tetrahydroxystilbene, a resveratrol derivative from peanuts. Phythother Res. 2005;19:552–555. doi: 10.1002/ptr.1698. [DOI] [PubMed] [Google Scholar]
- 18.Kundu JK, Shin YK, Kim SH, Surh YJ. Resveratrol inhibits phorbol ester-induced expression of COX-2 and activation of NF-kappaB in mouse skin by blocking IkappaB kinase activity. Carcinogenesis. 2006;27:1465–1474. doi: 10.1093/carcin/bgi349. [DOI] [PubMed] [Google Scholar]
- 19.Shankar S, Singh G, Srivastava RK. Chemoprevention by resveratrol: molecular mechanisms and therapeutic potential. Front Biosci. 2007;12:4839–4854. doi: 10.2741/2432. [DOI] [PubMed] [Google Scholar]
- 20.Bookcock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–52. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 21.Mayer MP, Stauffer JS, Manzano LA, Tanzer LR, Merriman RL. NCM460, A Normal Human Colon Mucosal Epithelial Cell Line. In Vitro Cell Dev Biol: Animal. 1996;32:315–317. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- 22.Shukla S, MacLennan GT, Flask CA, Fu P, Mishra A, Resnick MI, Gupta S. Blockade of beta-catenin signaling by plant flavonoid apigenin suppresses prostate carcinogenesis in TRAMP mice. Cancer Res. 2007;67:6925–35. doi: 10.1158/0008-5472.CAN-07-0717. [DOI] [PubMed] [Google Scholar]
- 23.Wolter F, Akoglu B, Clausnitzer A, Stein J. Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J Nutrition. 2001;131:2197–2203. doi: 10.1093/jn/131.8.2197. [DOI] [PubMed] [Google Scholar]
- 24.Joe AK, Liu H, Zuzui M, Vural ME, Ziao D, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis and changes in biomarker expression in human cancer cell lines. Clin Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- 25.Delamas D, Passilly-Degrace P, Jannin B, Cherkaoui M, Latruffe N. Resveratrol, a chemopreventive agent, disrupts the cell cycle control of human SW480 colorectal tumor cells. Int J Mol Med. 2002;10:193–199. [PubMed] [Google Scholar]
- 26.Park JW, Choi YJ, Jang MA, Lee YS, Jun DY, Suh SI, Baek WK, Suh MH, Jin IN, Kwon TK. Chemopreventive agent resveratrol, a natural product derived from grapes, reversibly inhibits progression through S and G2 phases of the cell cycle in U937 cells. Cancer Left. 2001;61:179–189. doi: 10.1016/s0304-3835(00)00658-3. [DOI] [PubMed] [Google Scholar]
- 27.Bernhard D, Tinhofer I, Tonko M, Hubl H, Ausserlechner MJ, Greil R, Kofler R, Csordas A. Resveratrol causes arrest in the S-phase prior to Fas-independent apoptosis in CEM-C7H2 acture leukemia cells. Cell Death Differ. 2000;7:834–842. doi: 10.1038/sj.cdd.4400719. [DOI] [PubMed] [Google Scholar]
- 28.Haider UG, Sorescu D, Griendling KK, Volmar AM, Dirsch VM. Resveratrol increases serine 15-phosphorylated by transcriptionally impaired p53 and induces a reversible DNA replication block in serum-activated vascular smooth muscle cells. Mol Pharmacol. 2003;63:925–932. doi: 10.1124/mol.63.4.925. [DOI] [PubMed] [Google Scholar]
- 29.Krieghoff E, Behrens J, Mayr B. Nucleo-cytoplasmic distribution of beta-catenin is regulated by retention. J Cell Sci. 2006;119:1453–1463. doi: 10.1242/jcs.02864. [DOI] [PubMed] [Google Scholar]
- 30.Schneikert J, Grohmann A, Behrens J. Truncated APC regulates the transcriptional activity of beta-catenin in a cell cycle dependent manner. Hum Mol Genet. 2007;16:199–209. doi: 10.1093/hmg/ddl464. [DOI] [PubMed] [Google Scholar]
- 31.Schneikert J, Behrens J. Truncated APC is required for cell proliferation and DNA replication. Int J Cancer. 2006;119:74–79. doi: 10.1002/ijc.21826. [DOI] [PubMed] [Google Scholar]
- 32.Townsley FM, Cliffe A, Bienz M. Pygopus and legless target armadillo/betacatenin to the nucleus to enable it transcriptional co-activator function. Nat Cell Biol. 2004;6:626–633. doi: 10.1038/ncb1141. [DOI] [PubMed] [Google Scholar]
- 33.Hung LM, Chen JK, Huang SS, Lee RS, Su MJ. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc Res. 2000;47:549–55. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 34.Virgili M, Contestabile A. Partial neuroprotection of in vivo excitotoxic brain damage by chronic administration of the red wine anti-oxidant agent, transresveratrol in rats. Neurosci Lett. 2000;281:123–6. doi: 10.1016/s0304-3940(00)00820-x. [DOI] [PubMed] [Google Scholar]
- 35.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: The in vivo evidence. Nature Reviews: Drug Discovery. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 36.Gescher AJ, Steward WP. Relationship between mechanisms, bioavailability and preclinical chemopreventive efficacy of resveratrol: A conundrum. Cancer Epi, Biomarkers & Prevention. 2003;12:953–957. [PubMed] [Google Scholar]
- 37.Boocook DJ, Faust GES, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff A, Gescher AJ, Steward WP, Brenner DE. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epi Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- 38.Tessitore L, Davit A, Sarotto I, Cadeni G. Resveratrol depresses the growth of colorectal aberrant crypt foci by affecting bax and p21CIP expression. Carcinogenesis. 2000;21:1619–1622. [PubMed] [Google Scholar]
- 39.Sengottuvelan M, Viswanathan P, Nalini N. Chemopreventive effect of trans-resveratrol – a phytoalexin against colonic aberrant crypt foci and cell proliferation in 1,2-dimethylhydrazine induced colon carcinogenesis. Carcinogenesis. 2006;27:1038–1046. doi: 10.1093/carcin/bgi286. [DOI] [PubMed] [Google Scholar]
- 40.Sengottuvelan M, Nalini N. Dietary supplementation of resveratrol suppresses colonic tumor incidence in 1,2 dimethylhydrazine-treated rats by modulating biotransforming enzymes and aberrant crypt foci development. Br J Nurtition. 2006;96:145–153. doi: 10.1079/bjn20061789. [DOI] [PubMed] [Google Scholar]
- 41.Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defenserelated gene expression in an animal model of human familial adenomatous polyposis. Nutrition and Cancer. 2001;39:102–107. doi: 10.1207/S15327914nc391_14. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler C, Rainwater L, Whelan J, McEntee MF. Dietary resveratrol does not affect intestinal tumorigenesis in ApcMin/+ mice. J Nutrition. 2004;134:5–10. doi: 10.1093/jn/134.1.5. [DOI] [PubMed] [Google Scholar]
- 43.Gignac EA, Bourquin LD. Influence of resveratrol and sulindac on intestinal tumor numbers in Min mice. FASEB J. 2001;15:A630. [Google Scholar]
- 44.Sale S, Tunstall RG, Ruparelia KC, Potter GA, Steward WP, Gescher AJ. Comparison of the effects of the chemopreventive agent resveratrol and its synthetic analog trans 3,4,5,4-tetramethoxystilbene (DMU-212) on adenoma development in the ApcMin/+ mouse and cyclooxygenase-2 in human derived colon cancer cells. Int J Cancer. 2005;115:194–201. doi: 10.1002/ijc.20884. [DOI] [PubMed] [Google Scholar]
- 45.de Lau W, Barker N, Clevers H. WNT signaling in the normal intestine and colorectal cancer. Front Biosci. 2007;12:471–91. doi: 10.2741/2076. [DOI] [PubMed] [Google Scholar]
- 46.Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005;306:357–63. doi: 10.1016/j.yexcr.2005.02.022. [DOI] [PubMed] [Google Scholar]