Abstract
We sought to develop and optimize a hybridoma-based technology for generating human hybridomas that secrete virus-specific monoclonal antibodies for clinical diagnosis and therapy. We developed a novel electrofusion protocol for efficiently fusing Epstein-Barr virus (EBV)-transformed human B cells with myeloma partners. We tested seven myeloma cell lines and achieved highest efficiency when the HMMA 2.5 line was used. We optimized the electrofusion process by improving cell treatments before and after electrofusion as well as varying cell ratios, fusion medium and other experimental parameters. Our fusion efficiency increased remarkably to 0.43%, a significant improvement over the efficiency of previous PEG-based or other electrofusion methods. Using the optimized protocol, we obtained human hybridomas that secrete fully human monoclonal antibodies against two major human respiratory pathogens: respiratory syncytial virus (RSV) and an influenza H3N2 vaccine virus strain. In conclusion, we have developed an efficient and routine approach for the generation of human hybridomas secreting functional human virus-specific monoclonal antibodies.
Keywords: Hybridomas, Antibodies, Monoclonal, Immunity, Humoral
1. Introduction
Human monoclonal antibodies (mAbs) possess many advantages over animal-derived antibodies for clinical applications, such as prevention or treatment of microbial infection, immunotherapy of toxins and diagnosis by antibody-targeted radioisotope imaging. The creation of human hybridoma cell lines that produced immunoglobulins by fusing human B cells with mouse myeloma cells was first reported in 1973 (Schwaber and Cohen, 1973). A year later, human-human hybridomas were described (Bloom and Nakamura, 1974). The first success in generating human mAbs with predefined specificity was reported in 1980 (Olsson and Kaplan, 1980). Olsson and Kaplan successfully fused human spleen cells from patients with Hodgkin's disease with human myeloma cells. Despite a significant number of human mAbs that have been described, current methods for isolation of fully human mAbs are inefficient, yielding unpredictable results. The development of a reliable and routine method for generating human mAbs faces a number of obstacles, such as the low immunoglobulin (Ig) production capability of most fusions and the rapid loss of Ig production and chromosomal instability of most human hybridomas.
Collection of antigen-specific B cells is the first important step of human hybridoma generation. Antigen-specific cells are generally rare in the peripheral blood. The fusion efficiency of current methods for hybridoma generation is not sufficient to immortalize rare cells from the numbers of cells that can be obtained by routine phlebotomy. Stevens reported that the frequency of B cells producing anti-tetanus IgG antibody in the circulation was only 1 × 10-4 at a time point two to four weeks after the booster injection (Stevens et al., 1979). Such a low frequency, combined with the fact that B cells usually represent less than 10% of the peripheral blood mononuclear cells (PBMC), and the low fusion efficiency of current fusion methods (on the order of 10-5 to 10-6) suggest that the chance of obtaining an antigen-specific human hybridoma is only on the order of 10-9 to 10-10. Consequently, the generation of human hybridoma cells secreting desirable human mAbs has proven difficult. Although human B cells can be immortalized by EBV transformation (Casali et al., 1986; Kozbor and Roder, 1981), conventional EBV-transformed immortalization is restricted to the CD21+ subset of B cells, and the resultant mAbs are predominantly of the IgM isotype. Moreover, EBV-transformed B cells generally grow poorly, they usually secrete low amounts of antibodies, and they are also difficult to clone because they exhibit chromosomal instability (Casali et al., 1986; Crawford and Ando, 1986; Roder et al., 1986; Steinitz et al., 1978). Recent studies suggest the addition of CpG during transformation can facilitate more efficient transformation (Bernasconi et al., 2002; Hartmann and Krieg, 2000; Traggiai et al., 2004).
The limited number of suitable fusion partners has hindered development of human mAbs by hybridoma technology. The mouse myelomas originally used for hybridoma work were not suitable for deriving human mAbs from human B cells because heterospecific hybrids often quickly reject the relevant human chromosomes. Investigators recently have isolated or generated new myeloma lines that are of interest for human hybridoma work. One new murine fusion partner cell line was transformed to co-express genes that encode murine interleukin-6 (mIL-6) and human telomerase catalytic subunit (hTERT) (Dessain et al., 2004). Murine IL-6 directly stimulates immunoglobulin production and the proliferation of the hybridoma. Human TERT can lengthen telomeres through the synthesis of the telomeric hexamer repeat sequence, thereby providing cells with unlimited replication capability and promoting karyotypic stability. Human myeloma cell lines also have been used for fusion, such as U266 (Olsson and Kaplan, 1980, 1983), U266-derived SKO-007 (Brodin et al., 1983; Cote et al., 1983; Olsson, 1983) and a recently isolated fully human myeloma designated Karpas 707H (Karpas et al., 2001). Finally, another class of fusion partner, heterohybridoma, has been constructed by the fusion of murine myeloma cells with human cells, for example the K6H6/B5 (Carroll et al., 1986), KR12 (Kozbor et al., 1984), HMMA 2.5 (Posner et al., 1987), and MFP-2 (Kalantarov et al., 2002; Kirman et al., 2002) cell lines. Although each of these lines can yield viable fusions, a highly efficient fusion protocol has not been described for any.
In this study, we developed an optimized electrofusion technique and demonstrated HMMA 2.5 to be the optimal partner cell line in this method. By optimizing the conditions of EBV transformation, the parameters of electrofusion and the treatment of hybridoma cell line cultures, we overcame the inherent limitations of current technologies for the routine generation of human hybridomas. Moreover, employing this optimized protocol, we successfully generated human hybridomas that secrete fully human mAbs against two major human respiratory pathogens.
2. Materials and methods
2.1. Cell lines and culture media
We tested seven different cell lines as myeloma fusion partners. A summary of the source of the lines is provided in Table 1. Details about each line follow. In every case, RPMI 1640 medium (Invitrogen) was used, with 50 μg/mL gentamicin (Invitrogen); additional additives are specified for each cell line below.
Table 1. Partner cell lines and culture media.
| Cell Line | Medium additives * | Source | References |
|---|---|---|---|
| KR12 | 15% FBS
20 μg/ml 6-thioguanine |
ATCC cat. no. CRL-8656 | Kozbor 1984, 1985 |
| K6H6/B5 | 10% FBS
2 mM glutamine |
ATCC cat. no. CRL-1823 |
Brown 1980,
Carroll 1986 |
| Karpas 707H | 10% FBS
2 mM glutamine |
Dr. Abraham Karpas
Department of Hematology Cambridge University, UK |
Karpas 2001 |
| SHM-D33 | 20% FBS
2 mM glutamine |
ATCC cat. no. CRL-1668 | Teng 1983 |
| Sp2/mIL-6
(hTERT) |
10% FBS
2 mM glutamine 1 mM sodium pyruvate |
Dr. Scott K. Dessain
The Whitehead Institute for Biomedical Research Cambridge, MA |
Dessain 2004 |
| HMMA 2.5 | 10% FBS
2 mM glutamine 0.1 mM non-essential amino acids 0.1 mM sodium pyruvate |
Dr. Marshall R Posner
Dana-Farber Cancer Institute Boston, MA |
Posner 1987 |
| MFP-2 | 10% Cosmic Calf serum
4 mM glutamine 0.1 mM non-essential amino acids 1 mM sodium pyruvate 1x vitamins |
Dr. Ilya Trakht
College of Physicians and Surgeons of Columbia University New York, NY |
Kalantarov 2002,
Kirman 2002 |
Each of the medium formulations was based on RPMI 1640, and contained 50 μg/ml gentamicin.
KR12 (ATCC CRL-8658; (Kozbor et al., 1982; Kozbor et al., 1984) is a myeloma-like cell line generated by fusing the human lymphoblastoid cell line KR-4, which is deficient in hypoxanthine phosphoribosyl transferase and resistant to ouabain, with the human plasmacytoma line RPMI8226. The medium for culture of KR12 cells was supplemented with 15% heat-inactivated fetal bovine serum (FBS), 20 μg/ml 6-thioguanine, 0.1 mM ouabain, and 2 mM glutamine.
K6H6/B5 (ATCC CRL-1823; (Brown et al., 1980; William L Carroll, 1986) is a human-mouse heterohybridoma produced by fusing NS-1-Ag4 myeloma cells with malignant lymphoid cells isolated from a patient with nodular lymphoma. The culture medium was supplemented with 10% FBS and 2 mM glutamine.
Karpas 707H (Karpas et al., 2001) is a fully human myeloma cell line established from a patient with multiple myeloma. The culture medium was supplemented with 10% FBS and 2 mM glutamine.
SHM-D33 (ATCC CRL-1668; (Teng et al., 1983) was generated by fusing the human myeloma cell line FU-266 with the mouse myeloma cell line P3X63Ag8. The culture medium was supplemented with 20% FBS and 2 mM glutamine.
Sp2/mIL-6 (hTERT) (Dessain et al., 2004) was derived from the murine myeloma cell line Sp2/0 and modified to ectopically express murine interleukin-6 (mIL-6) and human telomerase (hTERT). The culture medium was supplemented with 10% FBS, 2 mM glutamine, and 1 mM sodium pyruvate.
HMMA 2.5 is a subclone of HMMA2.11TG/O that was selected by passaging the line in 6-thioguanosine, cloning by limiting dilution and selecting a clone with optimal fusion efficiency. The parental line HMMA2.11TG/O was generated by fusion of the mouse myeloma cell line P3X63Ag8.653 with bone marrow mononuclear cells from a patient with IgA myeloma (Posner et al., 1987). The culture medium was supplemented with 10% FBS, 2 mM glutamine, 0.1 mM non-essential amino acids, and 0.1 mM sodium pyruvate.
MFP-2 (Kalantarov et al., 2002; Kirman et al., 2002) is a trioma that was generated by fusing a murine myeloma cell line with a human myeloma cell line, yielding the intermediate heteromyeloma B6B11, followed by fusion with a human lymphocyte. The medium was supplemented with 10% Cosmic Calf serum (Hyclone), 4 mM glutamine, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, and vitamins.
2.2. PBMC preparation and EBV transformation
PBMC were obtained from donor blood by Ficoll density gradient centrifugation (Histopaque 1077-1, Sigma, St. Louis, MO). Human lymphocytes were stimulated for transformation using the TLR agonists CpG ODN 2006 or ODN 10103 (Coley Pharmaceutical Group, Canada) and supernatant from persistently infected and transformed B95-8 cells (ATCC CRL-1612) containing Epstein Barr virus. A total of 2 × 106 cells was cultured in 5% CO2 at 37 °C and monitored by microscopic inspection for transformation by the appearance of cell clusters.
2.3. Polyethylene glycol (PEG) mediated fusion
PEG-mediated fusion was performed using a conventional technique. Briefly, myeloma cells and EBV-transformed human B cells were washed with RPMI-1640 medium first, then washed in D-PBS without calcium or magnesium (Invitrogen). Pre-warmed PEG-1500 (Roche Diagnostics, Indianapolis, IN) was added to the cell pellet for re-suspension in a drop-wise manner, followed by drop-wise addition of 4 ml RPMI-1640. We added 10 ml RPMI-1640 and incubated the suspension at 37 °C in 5% CO2 for five minutes. The fused cells were washed once with RPMI-1640 medium, re-suspended in selection medium containing hypoxanthine/aminopterin/thymidine (HAT, Sigma), and seeded into 96-well plates at a density of approximately 6,000 transformed B cells/well.
2.4. Electrofusion
The electrofusion system used was a PA-4000/PA-101 apparatus with electrode FE-20/1000 fusion chambers (Cyto Pulse Sciences, Inc.). Fusion volume was 500 μl. The myeloma cells and EBV-transformed human B cells were washed with RPMI-1640 and Cytofusion medium (Cyto Pulse Sciences, Inc.). Instrument settings were as follows. Pre-fusion dielectrophoresis was performed for 15 seconds with an alternating current voltage of 70V at 0.8 MHz. Cells were electroporated with a single square-wave high-voltage direct current pulse lasting 0.04 milliseconds. The pulse frequencies and voltages we tested included a single pulse of 300V or multiple pulses of different decreasing voltages from 280V to 260V. Post-fusion dielectrophoresis was accomplished for 30 seconds using an alternating current voltage of 20V at 0.08 MHz. After fusion, cells were allowed to recover in the fusion electrode for 30 minutes at room temperature, harvested, and then washed once with RPMI-1640 prior to plating in multi-well plates for culture.
2.5. Hybridoma selection, calculation of yield, and cloning
After fusion, cells were seeded into 96-well microplates at approximately 6,000 B cells per well (for example 18,000 total cells when a 2:1 myeloma to B cell ratio was used in fusion) in complete RPMI-1640 medium containing 20% heat-inactivated FBS, 2.5 μg/ml amphotericin B, 2 mM L-glutamine, 1 mM sodium pyruvate, 50 μg/ml gentamicin, 60 μg/ml tylosin solution, 100 μM hypoxanthine, 0.4 μM aminopterin, 16 μM thymidine (HAT; Sigma) and 0.5 μM ouabain. After seven days of culture, cells were fed by removing 100 μl culture medium followed by addition of an equal volume of fresh medium containing 100 μM hypoxanthine/ 16 μM thymidine (HT; Sigma). The number of independent colonies in each well was counted 20 days after fusion. Fusion efficiency on a percentage basis was calculated as the total number of hybridoma colonies in each well, divided by the number of input B cells, and multiplied by 100. A total of 192 wells was assessed for each condition. After initial screening for immunoglobulin (Ig) production by an enzyme-linked immunosorbent assay (ELISA), the hybridoma cells from positive wells were expanded into 24-well plates and cultured in RPMI 1640 containing 20% heat-inactivated FBS, 2 mM glutamine, 1 mM sodium pyruvate and 50 μg/mL gentamicin. Supernatants of the expanded lines then were tested for specificity using an antigen-specific ELISA. The positive hybridoma cells were sub-cloned by serial limiting dilution in 96-well plates at 100, 10, and 0.3 cell-per-well density. The 0.3 cell-per-well limiting dilutions were performed twice to ensure that we generated clones.
2.6. Enzyme-linked immunosorbent assays (ELISAs)
Culture supernatants were screened by separate ELISAs for total human Ig, human IgG and antigen-specific antibodies. To detect human Ig or IgG, Immulon 2HB microtiter plates were pre-coated with unlabeled goat anti-human antibodies that detect both heavy-and light-chain (H+L) Ig molecules (Southern Biotechnology Associates, Birmingham, AL) and incubated overnight at 4 °C. Wells were blocked with D-PBS containing 5% nonfat milk, 2% goat serum and 0.2% Tween-20 at room temperature for two hours. Plates were washed with D-PBS containing 0.05% Tween-20. After washing, supernatant was added to each well and incubated for two hours at room temperature. For human Ig detection, alkaline phosphatase-conjugated goat anti-human Ig (H+L) (Southern Biotechnology Associates, Birmingham, AL) was used as secondary antibody. For detection of human IgG, alkaline phosphatase-conjugated goat anti-human IgG Fcγ fragment-specific antibodies (Jackson ImmunoResearch) were used as the secondary antibody. The enzymatic reaction was performed with phosphatase substrate tablets (Sigma) in 1 M Tris-HCl, 0.3 mM MgCl2 substrate buffer.
For the anti-RSV F protein ELISA, plates were pre-coated with 0.2 μg/ml purified recombinant RSV F protein incubated at 4 °C overnight, and then blocked with D-PBS containing 5% BSA, 2% goat serum, 0.2% Tween-20 at room temperature for 2 hours. For the anti-H3N2 influenza antigen ELISA, plates were pre-coated with 1 μg/ml H3N2 A/Sidney/5/97-like strain vaccine antigens, incubated at 4 °C overnight, and then blocked with D-PBS containing 5% nonfat milk, 2% goat serum, 0.2% Tween-20 at room temperature for 2 hours. The subsequent steps of the binding assay were the same as above.
2.7. IgG production experiments
Fully cloned hybridoma lines were tested for their level of production of IgG. Cells were cultured in stationery flasks at a density of 105 cells per mL in complete medium in 5% CO2. Supernatants were collected at 24 hour time points for quantification in the human Ig capture ELISA assay, as above, with signals compared to that generated by a standard curve of purified human IgG. Production was calculated as the amount of antibody produced on a per-cell, per-hour basis.
3. Results
3.1. Determination of drug selection conditions for human hybridomas
To establish optimal drug treatment conditions for selection of human hybridomas, we determined the sensitivity and resistance to drug selection of both transformed B cells and myeloma fusion partner cell lines.
Ouabain resistance
Conventional primary B cells die in prolonged culture, but transformed B cells can survive prolonged culture and HAT selection. Human cells are sensitive to ouabain selection, therefore the selection of human hybridomas was carried out in the presence of ouabain to eliminate non-fused EBV-transformed B cells. We tested the sensitivity of EBV-transformed human B cells to differing concentrations of ouabain and found the minimum concentration for killing EBV-transformed human B cells to be 0.5 μM. More than 99% of EBV-transformed B cells were killed during seven days of culture in medium containing 0.5 μM ouabain (Fig. 1). We then tested the resistance of seven myeloma fusion partner cell lines to 0.5 μM ouabain. The results showed the Sp2/0 mIL-6 (hTERT), SDM-D33, HMMA 2.5 and KR12 cell lines grew normally after seven days of exposure to 0.5 μM ouabain. The KR12 line was found to secrete human light chain constitutively (data not shown) and was eliminated from further consideration as a partner cell candidate. Approximately 50% of MFP-2 cells and Karpas 707H cells survived after seven days of selection (Fig. 2). Although some literature suggested K6H6/B5 cells were resistant to ouabain, we found in preliminary experiments that the line was sensitive to 0.5 μM ouabain. We did not further evaluate K6H6/B5, since ouabain resistance is required for selection of hybridomas following fusion with EBV-transformed B cells.
Figure 1. Ouabain sensitivity of EBV-transformed human B cells.
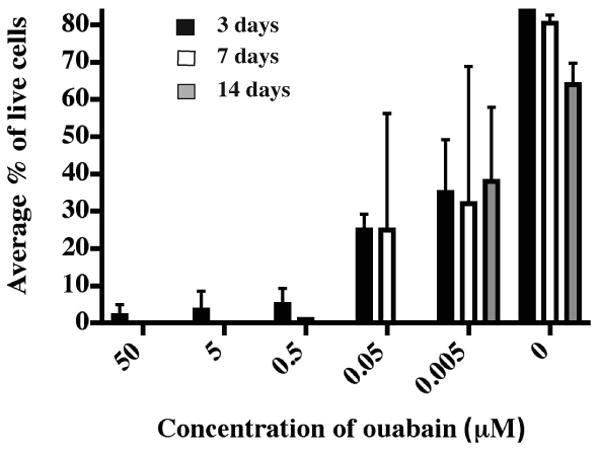
Two EBV-transformed B cell lines from separate donors were plated in 96-well plates with complete medium plus different concentrations of ouabain; 6,000 cells were plated in each well. A total of 100 cells were assessed for live/dead status using trypan blue dye exclusion at 3, 7 or 14 days after plating. Each condition had 4 replicates. Columns represent the average percentage of live cells; error bars represent SD.
Figure 2. Ouabain resistance of partner cell lines.
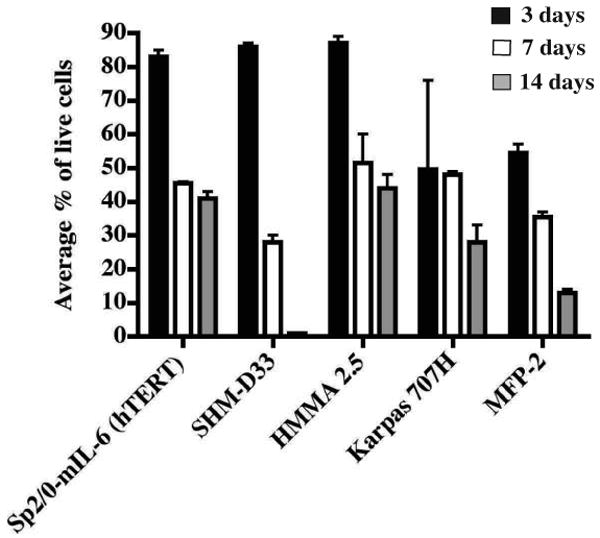
Five partner cell lines were tested for resistance to 0.5 μM ouabain. Each cell line was cultured in its own medium plus 0.5 μM ouabain in 96-well plates. Each well initially contained 12,000 cells. A total of 100 cells were assessed for live/dead status using trypan blue dye exclusion at 3, 7 or 14 days after plating. Each condition had 4 replicates. Columns represent the average percentage of live cells; error bars represent SD.
HAT resistance
We tested the resistance and sensitivity of myeloma partner cell lines to medium containing HAT and found that all cell lines were sensitive to the drug combination. EBV-transformed B cells were resistant to the HAT selection medium (data not shown).
Therefore Sp2/0mIL-6 (hTERT), SDH-D33, and HMMA 2.5 cells were found to have preferred features for use in this work, namely they are non-secreting, ouabain-resistant HAT-sensitive lines. These three lines were used as partner cell lines in subsequent fusion studies. The selection medium was finally formulated as HAT plus 0.5 μM ouabain.
3.2. CpG increased efficiency of EBV transformation of human B cells significantly
Synthetic oligodeoxynucleotides (ODNs) that contain immunostimulatory CpG motifs trigger an immunomodulatory cascade that involves B and T cells, natural killer cells and professional antigen-presenting cells. We added CpG ODNs to the EBV transformation medium for human B cells. We confirmed that the addition of CpG ODNs during EBV transformation performed in tissue culture flasks did significantly enhance the efficiency of transformation, and shortened transformation times by five to ten days. In order to enrich for the percentage of antigen-specific B cell numbers in pre-fusion B cell samples, we transformed smaller numbers of human B cells in multiple wells of 384-well plates using CpG and EBV (Table 2). The results indicated that addition of CpG remarkably increased efficiency of EBV transformation of human B cells.
Table 2. Efficiency of EBV transformation of human B cells in 384-well plates with or without CpG.
| PBMC per well | Number of wells with EBV-transformed cells of 48 wells tested (% wells), in the presence or absence of CpG | |||
|---|---|---|---|---|
| + CpG | - CpG | |||
| day 15* | day 22 | day 15 | day 22 | |
| 10,000 | 48 (100) | 48 (100) | 39 (81) | 39 (81) |
| 5,000 | 40 (83) | 40 (83) | 28 (58) | 32 (67) |
| 500 | 12 (25) | 12 (25) | 3 (6) | 3 (6) |
| 50 | 1 (2) | 1 (2) | 0 (0) | 0 (0) |
| 10 | 1 (2) | 1 (2) | 0 (0) | 0 (0) |
The day of culture following initiation of EBV transformation.
3.3. Selection of the optimal partner cell line for generating human hybridomas
We compared the relative fusion abilities of three partner cell lines, Sp2/0 mIL-6 (hTERT), SHM-D33 and HMMA 2.5 to form hybridoma cells with EBV-transformed human B lymphocytes. First, we compared the fusion efficiency of different myeloma partner cell lines with the same batch of EBV-transformed human B cells (Fig. 3). We then compared the fusion efficiency of a particular partner cell line with different batches of EBV-transformed human B cells (Table 3). The electrofusion protocol applied used a single DC pulse of 300V. A total of 3 × 106 transformed B cells was used in the fusion with a cell ratio of 2:1 (partner cell: transformed B cells). Hybridoma cells were selected in medium containing HAT plus 0.5 μM ouabain for seven days in 96-well plates. The number of hybridoma colonies for each condition was counted after 15-20 days of culture after fusion. The results suggested that HMMA 2.5 was the most suitable partner cell line for generation of human hybridoma cells when electrofusion was used. We therefore used this cell line for further optimization of the electrofusion protocol.
Figure 3. Comparison of fusion efficiency of three partner cell lines.
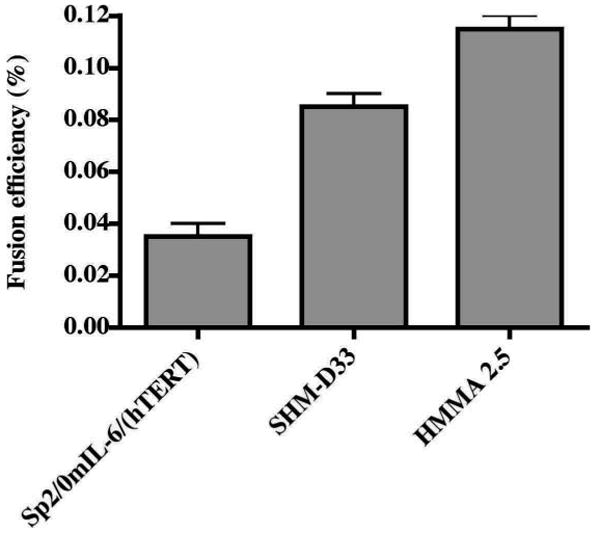
A suspension containing 6 × 106 cells of the Sp2/mIL-6/hTERT, SHM-D33 or HMMA 2.5 cell lines was fused individually with 3 × 106 cells of a single line of EBV-transformed human B cells. Fusions were distributed such that each well of 96-well plates received 6,250 B cells. Fusion products were selected by HAT plus 0.5 μM ouabain treatment for five days. The fusion efficiency (%) was calculated by counting the total number of colonies in each well, divided by the number of input B cells and multiplied by 100. A total of 192 wells was assessed for each condition. Each column represents the average percentage of fusion efficiency of two experiments; error bars represent the SD.
Table 3. Fusion efficiency for three fusion partner cell lines with EBV-transformed B cells.
| Partner cell line | Number of independent fusions tested | Fusion efficiency a (mean percentage of B cells fused ±SD) | Mean number of wells with 1 or more clones, of 192 wells tested (%) b |
|---|---|---|---|
| Sp2/0/mIL-6 (hTERT) | 2 | 0.04 ±0.005 | 140 (73) |
| SHM-D33 | 3 | 0.07 ±0.01 | 161 (84) |
| HMMA 2.5 | 6 | 0.13 ±0.01 | 188 (98) |
The cell ratio of transformed B cell and myeloma partner cells was 1:2. Fusions were dispersed into 96-well plates. The fusion efficiency on a percentage basis was calculated as the total number of hybridoma colonies in each well, divided by the number of input B cells, and multiplied by 100. A total of 192 wells was assessed for each condition.
Wells were scored positive if one or more hybridoma clones grew in the well. Each well was inoculated with an aliquot of the fusion suspension that contained approximately 6000 EBV-transformed B cells and 12,000 myeloma cells prior to fusion.
During these studies, we obtained an additional partner line, MFP-2, which was reported to be an ideal partner cell line for direct fusion with human B cells (Kalantarov et al., 2002; Kirman et al., 2002). The potential of this line as a fusion partner cell for EBV-transformed human B cells had not been reported. We therefore included MFP-2 as a partner cell line candidate and compared its fusion efficiency to that of HMMA 2.5 in both PEG-mediated fusion and electrofusion protocols. Following 0.5 μM ouabain selection, approximately 50% of MFP-2 cells survived after seven days. We therefore transfected a ouabain-resistance gene into the MFP-2 cell line, seeking to increase cell resistance under 0.5 μM ouabain selection. We compared the fusion efficiency of PBMCs with either the engineered MFP-2 line or HMMA 2.5 cells. MFP-2 cells exhibited higher fusion efficiency for PBMCs in both PEG-mediated fusion and electrofusion (Table 4). However, when fused with EBV-transformed human B cells, HMMA 2.5 was the more efficient line, with a two- to three-fold higher efficiency than the engineered MFP-2 cells using electrofusion (Table 5). Notably, the electrofusion method yielded an overall higher hybridoma yield than PEG-mediated fusion. Therefore, we selected HMMA 2.5 as the optimal partner cell for subsequent experiments.
Table 4. Fusion efficiency using PEG-mediated or electrofusion methods to fuse PBMCs with MFP-2 or HMMA 2.5 partner cell lines, in two independent fusions.
| Myeloma cell line | Fusion | PEG fusion | Electrofusion | ||
|---|---|---|---|---|---|
| Myeloma:PBMC ratio | Fusion efficiencya | Myeloma:PBMC ratio | Fusion efficiencya | ||
| MFP-2 | 1 | 1:3 | 0.03 | 1:3 | 0.006 |
| 2 | 1:3 | 0.01 | 2:1 | 0.070 | |
| HMMA 2.5 | 1 | nt | - | 1:3 | 0.014 |
| 2 | 1:3 | 0.0003 | 2:1 | 0.030 | |
Fusions were dispersed into 96-well plates. The fusion efficiency was calculated as specified in Table 3. It should be noted that only an estimated 5 to 10% of PBMCs are B cells, so the actual fusion efficiency of B cells was higher.
nt= not tested
Table 5. Fusion efficiency of EBV-transformed human B cell lymphoblastic cell lines (BCLC) with MFP-2 or HMMA 2.5 myeloma cell lines.
| Myeloma cell line | Fusion | PEG fusion | Electrofusion | ||
|---|---|---|---|---|---|
| Myeloma:BCLC ratio | Fusion efficiencya | Myeloma:BCLC ratio | Fusion efficiencya | ||
| MFP-2 | 1 | 1:3 | 0.07 | 2:1 | 0.12 |
| 2 | 1:3 | 0.08 | 2:1 | 0.15 | |
| HMMA 2.5 | 1 | 1:3 | 0.02 | 2:1 | 0.36 |
| 2 | 1:3 | 0.11 | 2:1 | 0.27 | |
EBV-transformed B cells were used in each fusion, then fusions were dispersed into 96-well plates. The fusion efficiency was calculated as specified in Table 3.
3.4. Selection of optimal electrofusion pulse parameters
We tested whether multiple direct current electrical pulses could enhance the overall fusion efficiency. HMMA 2.5 cells were fused with EBV-transformed human B cells to compare the effect of single or multiple pulses. The overall fusion efficiency decreased slightly when using multiple pulses, compared to a single pulse procedure (Fig. 4). The addition of an alternating current wave between multiple pulses, to hold B cells and myeloma cells in contact prior to repeat pulses, did not affect this reduction in fusion efficiency (Fig. 4). This negative effect likely was due to increased lysis of myeloma partner cells following multiple pulses. We used a single pulse for subsequent experiments.
Figure 4. Comparison of fusion efficiency using different pulse parameters for electrofusion.
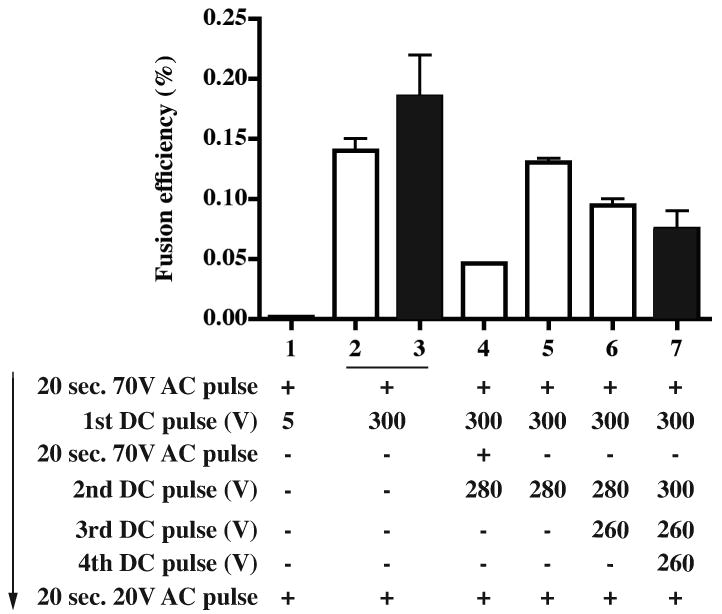
A total of 6 × 106 HMMA 2.5 cells were fused with 3 × 106 EBV-transformed B cells. Fusion products were selected in HAT plus 0.5 μM ouabain for five days in 96-well plates. AC stands for alternating current, DC for direct current. The electrical elements of the protocol are shown in order; + indicates pulse was used in the group, - indicates pulse was omitted. The number of resulting hybridoma colonies was counted 2 weeks after fusion. The fusion efficiency was determined as in Fig. 3. Experiment 1, in white; experiment 2 in black. The columns represent the average fusion efficiency (%) from two fusions; error bars represent the SD.
3.5. Co-culture with feeder cells did not increase the growth of human hybridoma cells after electrofusion
We investigated whether co-culture of hybridoma cells immediately after fusion would enhance the recovery of viable hybridoma cell lines. We have shown previously that co-culture of primary human B cells with murine EL4B5 thymoma cells or fibroblast cells expressing human CD154 (CD40 ligand) enhances proliferation and differentiation of antibody secreting B cells in culture (Weitkamp et al., 2003). Culture conditions and gamma irradiation procedures for the EL4B5 and CD154-expressing cells were described previously. Electrofusion was performed using HMMA 2.5 partner cells and EBV-transformed human B cells, and fusions were plated into three types of 96-well culture plates: 1) without feeder cells, 2) with 50,000 cells per well irradiated EL4B5 cells, or 3) with 10,000 cells per well irradiated CD154-expressing fibroblasts. We determined the effect of feeder cells on the growth of human hybridoma cells by determining the number of colonies formed from fusions when equal portions of the fusion reaction were cultured under the three conditions. The results showed that feeder cells did not increase the number of hybridomas generated by treatment with single or multiple electrofusion pulses (Fig. 5).
Figure 5. The effect of feeder cells on the growth of human hybridoma cells.
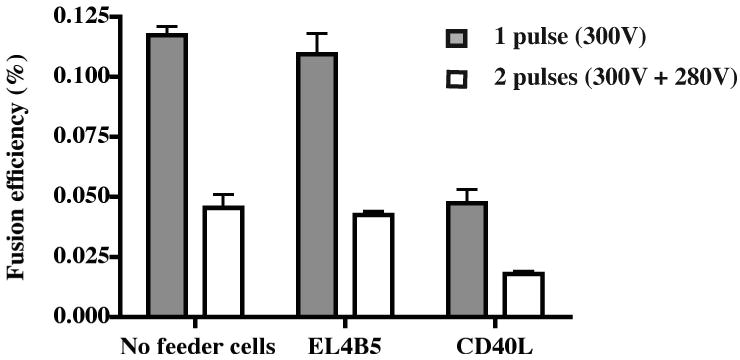
A total of 3 × 106 EBV-transformed human B cells were fused with HMMA 2.5 partner cells by electrofusion. The fused cells were plated into three types of 96-well plates: A) without feeder cells, B) with 50,000 EL4B5 cells per well, or C) with 10,000 human CD40L-expressing cells per well. EL4B5 and CD40L cells were irradiated at 1.86 Gy/min for 25 minutes. The fusion efficiency was determined as in Fig. 3. The columns represent the average fusion efficiency (%) from two experiments; error bars represent the SD.
3.6. Optimization of cell treatment conditions before and after fusion
The conditions of cell handling before and after fusion could affect efficiency of hybridoma formation. We sought to optimize additional parameters that could affect hybridoma yield significantly, such as wash intensity, wash buffer, incubation time and other handling conditions. Conventional treatment used PBS washes. In the optimized treatment, transformed B cells and partner cells were washed separately with fresh medium, followed by three washes with fusion medium to completely remove proteins and other medium additives that can alter the conductive properties of the fusion medium. After fusion, we added a recovery incubation period of 30 minutes for cells in the fusion chamber at 37 °C in 5% CO2 before collecting and plating in multi-well plates. With the modified pre- and post-fusion cell treatment, the overall fusion efficiency increased about three-fold from 0.15 to 0.43%. We again compared the fusion efficiency of this electrofusion protocol to that of PEG-mediated fusion using the same batch of EBV-transformed human B cells and HMMA 2.5 cells. Hybridoma production using the optimized electrofusion protocol coupled with optimized cell treatment was more than three-fold increased over conventional PEG fusion (Fig. 6). Although the fusion efficiency we observed with the PEG technique was significantly better than efficiencies previously reported (Steenbakkers et al., 1993), the electrofusion protocol still performed in a superior manner.
Figure 6. Comparison of fusion efficiency between experiments using conventional or optimized cell wash treatments.
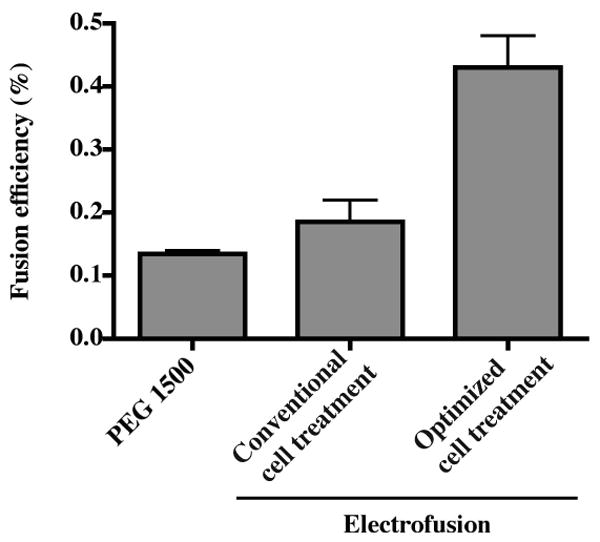
HMMA 2.5 cells were fused with EBV-transformed human B cells using previously determined optimal electrofusion parameters to test the effect of optimized cell treatment conditions before and after electrofusion, as detailed description in the results section. Electrofusion also was compared with PEG fusion. The cell ratio in the PEG fusion was 1:3 (partner cells : transformed B cells) and 2:1 in electrofusion. The input number of EBV-transformed B cells was 3 × 106 in all experiments. Hybridoma selection and colony counting were performed in the same way as previous experiments. The columns represent the average fusion efficiency (%) from two experiments; error bars represent the SD.
3.7. Comparison of fusion efficiency using different cell ratios
The mechanism of cell fusion caused by PEG and electrofusion differ significantly. Delivery of high voltage direct current pulses delivers a high level of energy to cells in the field, resulting in pore formation in the plasma cell membrane. The efficiency of pore formation depends on the energy delivered to cells, which corresponds in part to cell size. Electroporation is more efficient for large myeloma cells than for smaller B cells at the same electrical parameters. Since it was possible that the optimal cell ratio of myeloma to B cells differs between the two methods, we investigated the efficiency of fusion using varying cell ratios. The results showed that optimal cell ratio for electrofusion and PEG-mediated fusion did differ.
Our previous work had shown that a 1:3 myeloma to human B cell ratio is the optimal cell ratio for our PEG fusion method (data not shown). Electrofusion of MFP-2 or HMMA 2.5 myeloma cells with human PBMCs at varying ratios revealed that a partner cell to PBMC ratio of 2:1 was 2- to 10-fold more efficient for hybridoma formation compared with the 1:3 ratio that was optimal for PEG fusion. We then performed a series of additional experiments with differing cell ratios between myeloma cells and transformed human B cells. The results showed again that 2:1 was the optimal cell ratio in electrofusion. At the ratio of 1:1, an increased number of EBV-transformed B cells in the fusion did not increase the fusion efficiency remarkably (Table 6). These experiments demonstrated again that cell ratio is a critical factor affecting fusion efficiency, and that a myeloma cell to transformed human B cell ratio of 2:1 was the most efficient for our electrofusion protocol.
Table 6. Fusion efficiency of HMMA 2.5 myeloma partner cell with a single EBV-transformed human B cell lymphoblastic cell line (BCLC), using different cell ratios.
| Fusion | Number of input BCLC (×106) | Cell ratio (myeloma:BCLC) | Fusion efficiencya |
|---|---|---|---|
| 1 | 3 | 2:1 | 0.43 |
| 2 | 6 | 1:2 | 0.23 |
| 3 | 6 | 1:1 | 0.17 |
| 4 | 10 | 1:1 | 0.17 |
| 5 | 30 | 1:1 | 0.04 |
A single line of EBV-transformed B cells was used in all fusions, with differing numbers of input cells. Then fusions were dispersed into 96-well plates. The fusion efficiency was calculated as specified in Table 3.
3.8. Generation of human antigen-specific IgG mAbs against respiratory syncytial virus F protein and influenza H3N2
The principal goal of human hybridoma technology is to generate cell lines secreting antibodies specific for targets of medical interest. We sought to demonstrate the utility and efficiency of the optimized electrofusion protocol for generation of antibodies specific to the surface glycoproteins of two pathogenic human respiratory viruses. The first target we addressed was the respiratory syncytial virus (RSV) fusion (F) protein, a validated target for prophylaxis of high-risk human infants. PBMCs were isolated from a healthy adult donor. B cells were transformed using EBV and CpG, then fused with HMMA 2.5 myeloma cells using the final optimized electrofusion protocol. The resulting fusion was distributed into multi-well plates, and the supernatants were harvested when colonies formed in most wells (day 25) and tested for binding to RSV F protein using an F protein ELISA (Fig. 7), which identified a hybridoma line that secreted an anti-RSV F protein human mAb. We cloned the line by limiting dilution to yield the clone designated 6E4. Purified antibody secreted by the clone bound strongly to RSV F protein (Fig. 7).
Figure 7. Generation of hybridoma cells secreting human antibodies that bind respiratory syncytial virus (RSV) F protein.
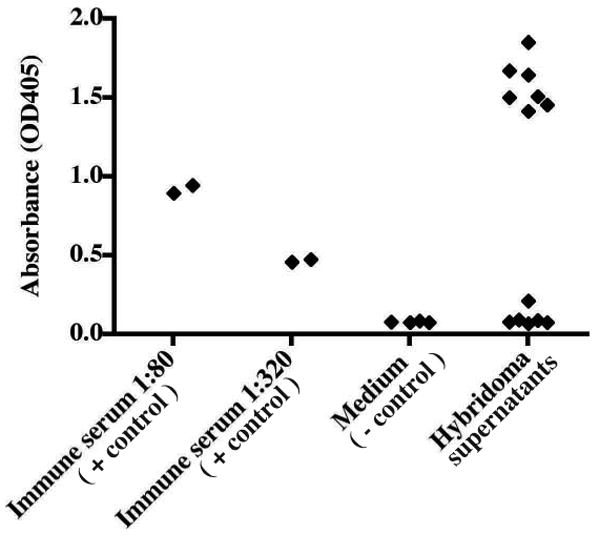
A total of 3 × 106 EBV-transformed human B cells and 6 × 106 HMMA 2.5 partner cells were fused using the electrofusion method. The supernatants from hybridoma lines appearing 2 weeks after cell fusion and selection in HAT + ouabain were screened with an RSV F-specific ELISA. A human RSV-immune serum was used as positive control; medium and blocking buffer was used to determine background signal.
As a second target, we chose surface glycoproteins of the 1997 H3N2 vaccine for influenza virus. EBV-transformed B cells from a previously immunized healthy adult donor and HMMA 2.5 myeloma cells were fused using the optimized fusion protocol. Several human hybridoma cell lines that secreted anti-H3N2 mAbs were identified and we cloned two, designated 15G5 and 1F12 (Fig 8). The result of an ELISA binding assay showed that the binding activity of these human mAbs to the H3N2 antigen was high and specific (Fig 9). Sequence analysis of the antibody variable region genes revealed that the two clones were identical.
Figure 8. Generation of hybridoma cells secreting human antibodies that bind the influenza A/H3N2/1997 virus vaccine strain.
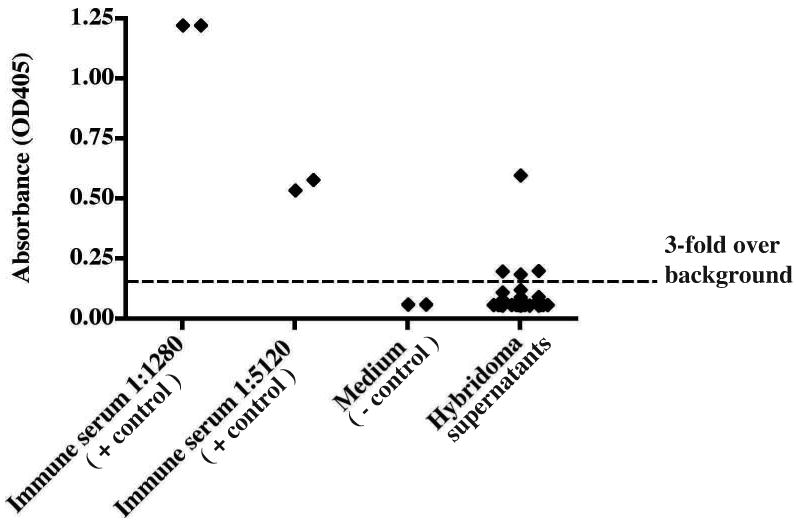
A total of 3 × 106 EBV-transformed human B cells and 6 × 106 HMMA 2.5 partner cells were fused using the electrofusion method. The supernatants from hybridoma lines appearing 2 weeks after cell fusion and selection in HAT + ouabain were screened with an H3N2-specific ELISA. Hybridoma wells were scored as positive when the optical density exceeded a threshold that was increased three-fold over background, indicated by dotted line.
Figure 9. Detection of antigen-specific binding for anti-H3N2 mAbs.
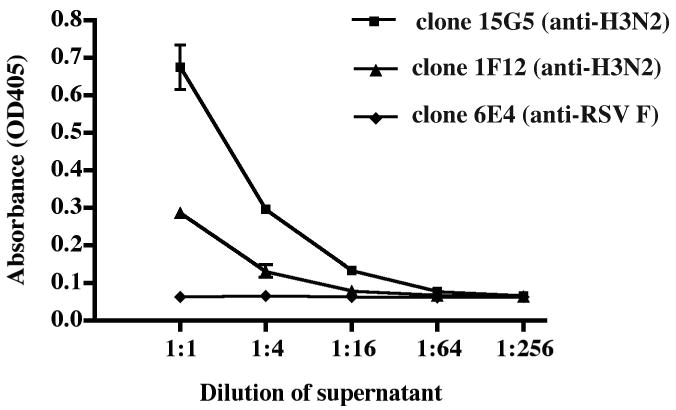
The 15G5 and 1F12 clones were determined to be H3N2-specific during preliminary screening of cell lines. After sub-cloning, they were incubated in 24 well-plates for 1 week, then the H3N2-binding ability of the clones was tested by ELISA. The negative control antibody was the anti-RSV F human mAb shown in Fig. 7.
3.8. Antibody production data
The goal of hybridoma formation generally is the production of IgG proteins. We tested 13 different human hybridoma cloned cell lines made by the optimized method for their level of production of IgG in culture. The amount of IgG produced was measured by capture ELISA, comparing binding signal to that of a standard curve produced by testing a dilution series of highly purified human IgG of known concentration. We normalized the production level to values on a per-cell and per-hour basis. The production level varied between cell lines, with the best level being 1.9 picograms IgG per hour per cell, and the average production level being 0.05 picograms IgG per hour per cell.
4. Discussion
These studies defined an optimized protocol for generating human mAb-secreting hybridoma cell lines and demonstrated that the protocol is valuable and practical for the routine generation of human mAb-secreting hybridomas. We incorporated high-efficiency EBV-transformation using CpG treatment, determined HMMA 2.5 as the most suitable partner cell line of seven myeloma cell lines, improved the parameters of electrofusion and achieved remarkably enhanced efficiency of fusion and generation of viable hybridomas.
Using EBV-transformed human B cells in fusions can increase hybrid formation as much as 25-fold compared to that of using untransformed PBMC (Kozbor et al., 1982; Kozbor and Roder, 1984). However, a minority of B cells derived from peripheral blood lymphocytes can be infected by EBV, which used as a receptor the CD21 molecule present on a subset of B cells, and only about 3% of the infected cells can be immortalized (Sugden and Mark, 1977). Recently, it has been found that the addition of CpG oligonucleotide in the EBV transformation medium increased the efficiency of B cell immortalization from about 1-2% to 30-100% (Bernasconi et al., 2002; Hartmann and Krieg, 2000; Traggiai et al., 2004). CpG ODNs are internalized rapidly by immune cells and interact with Toll-like receptor 9, activating cells by initiating an immunostimulatory cascade (Klinman, 2004). Our results confirmed that the addition of CpG oligonucleotides during EBV transformation significantly increases the transformation efficiency. This procedure also reduces the time required for transformation.
The nature of the fusion partner is a critical determinant of efficiency for generating human hybridomas. We used a number of partners with interesting features to determine the optimal cell line for use in electrofusion. Most of the lines exhibited limitations. In our fusion experiments using Karpas 707H, we found that this cell line exhibited low fusion efficiency and weak resistance to ouabain. The KR12 cell line was highly resistant to ouabain, but the line secretes human Ig chains that interfere with mAb production. The MFP-2 line was most efficient at fusing non-transformed human B cells, however we showed that the highest efficiencies of fusion were achieved using EBV-transformed cells and the HMMA 2.5 line.
HMMA 2.5 is a human-mouse cell line that was generated by fusing mouse myeloma cell line P3x6Ag8.653 with bone marrow mononuclear cells of a patient with IgA myeloma (Posner et al., 1987). Using this partner cell line, an average fusion efficiency of one in 17,000 fused cells was achieved when fused with human PBMC, pokeweed mitogen-stimulated PBMC, and EBV-transformed polyclonal B cells. Employing HMMA 2.5, a number of hybridomas secreting human anti-tetanus monoclonal IgM and IgG antibodies were cloned. Earlier studies supported the prediction that fusion between heterohybridoma cell lines and human lymphocytes would yield stable hybridomas (Raison et al., 1982).
The electric field-induced cell fusion technique was reported in 1982 (Zimmermann and Vienken, 1982). Electrofusion has a number of theoretical advantages over PEG-mediated fusion. The efficiency of electrofusion is considerably higher than that of PEG fusion. PEG fusion has an average fusion efficiency of 5 × 10-6, whereas previous methods of electrofusion achieved efficiencies up to 4 × 10-4 (Foung and Perkins, 1989; Glassy, 1988; Ohnishi et al., 1987; Schmitt et al., 1989; Steenbakkers et al., 1993; Vienken and Zimmermann, 1985; Zimmermann and Vienken, 1982). Theoretically, electrofusion can be applied to induce hybrid formation between any two cells. However, the success of electrofusion depends on the nature of the fusion partner and the type and state of activation of B cells. Each electrofusion parameter needs to be optimized with the particular cells being used. There is a high degree of heterogeneity between different fusion partner cells, or even between subpopulations within a particular cell population. The growth conditions such as the concentration of CO2, growth medium composition, and the state of B cell activation can affect hybridoma formation efficiency (Zimmermann et al., 1990). Historically, electrofusion-mediated efficiencies of myelomas with human B cells have been quite low, for example, the fusion efficiency of the HMMA 2.5 precursor HMMA2.11TG/0 of 1/17,000 was previously described (Posner et al., 1987). In this study, we optimized the conditions for hybridoma production in an iterative fashion, increasing the fusion efficiency to 0.43%. This efficiency is quite high, and proved effective in generating human mAbs to human respiratory tract pathogens, even from subjects not recently infected or immunized. The optimized protocol offers an effective approach for generation of desirable full-length human mAbs.
Acknowledgments
We are grateful to Dr. Abraham Karpas, Cambridge University, UK for the Karpas 707H cell line; to Dr. Scott K. Dessain, The Whitehead Institute for Biomedical Research, Cambridge, MA for the SP2/0 mIL-6 (hTERT) cell line; to Dr. Marshall R Posner, Dana-Farber Cancer Institute, for HMMA2.5; to Dr. Ilya Trakht, Columbia University, for the MFP-2 cell line. We wish to appreciate Dr. He-ping Yan (Vanderbilt University) for helpful discussions. We also appreciate Rebecca Conner (Vanderbilt), and Alan King and Richard Walter (Cyto Pulse Sciences, Columbia, MD) for technical assistance and advice. This work was supported by NIH R01 AI-48677, NIH U19 AI-057229, and NIH U54 AI057157 (the NIAID Region IV Southeast Regional Center of Excellence in Emerging Infections and Biodefense). Clinical support was provided by NIH NCRR M01 RR000095 (the Vanderbilt General Clinical Research Center).
Footnotes
Abbreviations used in the paper: monoclonal antibody (mAb), Epstein-Barr virus (EBV), oligodeoxynucleotides (ODNs), peripheral blood mononuclear cells (PBMC), murine interleukin-6 (mIL-6), human telomerase catalytic subunit (hTERT), polyethylene glycol (PEG), respiratory syncytial virus (RSV)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- Bloom AD, Nakamura FT. Establishment of a tetraploid, immunoglobulin-producing cell line from the hybridization of two human lymphocyte lines. Proc Natl Acad Sci U S A. 1974;71:2689. doi: 10.1073/pnas.71.7.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin T, Olsson L, Sjogren HO. Cloning of human hybridoma, myeloma and lymphoma cell lines using enriched human monocytes as feeder layer. J Immunol Methods. 1983;60:1. doi: 10.1016/0022-1759(83)90329-0. [DOI] [PubMed] [Google Scholar]
- Brown S, Dilley J, Levy R. Immunoglobulin secretion by mouse X human hybridomas: an approach for the production of anti-idiotype reagents useful in monitoring patients with B cell lymphoma. J Immunol. 1980;125:1037. [PubMed] [Google Scholar]
- Carroll WL, Lowder JN, Streifer R, Warnke R, Levy S, Levy R. Idiotype variant cell populations in patients with B cell lymphoma. J Exp Med. 1986;164:1566. doi: 10.1084/jem.164.5.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P, Inghirami G, Nakamura M, Davies TF, Notkins AL. Human monoclonals from antigen-specific selection of B lymphocytes and transformation by EBV. Science. 1986;234:476. doi: 10.1126/science.3020687. [DOI] [PubMed] [Google Scholar]
- Cote RJ, Morrissey DM, Houghton AN, Beattie EJ, Jr, Oettgen HF, Old LJ. Generation of human monoclonal antibodies reactive with cellular antigens. Proc Natl Acad Sci U S A. 1983;80:2026. doi: 10.1073/pnas.80.7.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DH, Ando I. EB virus induction is associated with B-cell maturation. Immunology. 1986;59:405. [PMC free article] [PubMed] [Google Scholar]
- Dessain SK, Adekar SP, Stevens JB, Carpenter KA, Skorski ML, Barnoski BL, Goldsby RA, Weinberg RA. High efficiency creation of human monoclonal antibody-producing hybridomas. J Immunol Methods. 2004;291:109. doi: 10.1016/j.jim.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Foung SK, Perkins S. Electric field-induced cell fusion and human monoclonal antibodies. J Immunol Methods. 1989;116:117. doi: 10.1016/0022-1759(89)90319-0. [DOI] [PubMed] [Google Scholar]
- Foung SK, Perkins S, Raubitschek A, Larrick J, Lizak G, Fishwild D, Engleman EG, Grumet FC. Rescue of human monoclonal antibody production from an EBV-transformed B cell line by fusion to a human-mouse hybridoma. J Immunol Methods. 1984;70:83. doi: 10.1016/0022-1759(84)90392-2. [DOI] [PubMed] [Google Scholar]
- Glassy M. Creating hybridomas by electrofusion. Nature. 1988;333:579. doi: 10.1038/333579a0. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- Hsu FJ, Kwak L, Campbell M, Liles T, Czerwinski D, Hart S, Syrengelas A, Miller R, Levy R. Clinical trials of idiotype-specific vaccine in B-cell lymphomas. Ann N Y Acad Sci. 1993;690:385. doi: 10.1111/j.1749-6632.1993.tb44039.x. [DOI] [PubMed] [Google Scholar]
- Kalantarov GF, Rudchenko SA, Lobel L, Trakht I. Development of a fusion partner cell line for efficient production of human monoclonal antibodies from peripheral blood lymphocytes. Hum Antibodies. 2002;11:85. [PubMed] [Google Scholar]
- Karpas A, Dremucheva A, Czepulkowski BH. A human myeloma cell line suitable for the generation of human monoclonal antibodies. Proc Natl Acad Sci U S A. 2001;98:1799. doi: 10.1073/pnas.98.4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirman I, Kalantarov GF, Lobel LI, Hibshoosh H, Estabrook A, Canfield R, Trakht I. Isolation of native human monoclonal autoantibodies to breast cancer. Hybrid Hybridomics. 2002;21:405. doi: 10.1089/153685902321043936. [DOI] [PubMed] [Google Scholar]
- Klinman DM. Use of CpG oligodeoxynucleotides as immunoprotective agents. Expert Opin Biol Ther. 2004;4:937. doi: 10.1517/14712598.4.6.937. [DOI] [PubMed] [Google Scholar]
- Kozbor D, Lagarde AE, Roder JC. Human hybridomas constructed with antigen-specific Epstein-Barr virus-transformed cell lines. Proc Natl Acad Sci U S A. 1982;79:6651. doi: 10.1073/pnas.79.21.6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozbor D, Roder JC. Requirements for the establishment of high-titered human monoclonal antibodies against tetanus toxoid using the Epstein-Barr virus technique. J Immunol. 1981;127:1275. [PubMed] [Google Scholar]
- Kozbor D, Roder JC. In vitro stimulated lymphocytes as a source of human hybridomas. Eur J Immunol. 1984;14:23. doi: 10.1002/eji.1830140105. [DOI] [PubMed] [Google Scholar]
- Kozbor D, Tripputi P, Roder JC, Croce CM. A human hybrid myeloma for production of human monoclonal antibodies. J Immunol. 1984;133:3001. [PubMed] [Google Scholar]
- Ohnishi K, Chiba J, Goto Y, Tokunaga T. Improvement in the basic technology of electrofusion for generation of antibody-producing hybridomas. J Immunol Methods. 1987;100:181. doi: 10.1016/0022-1759(87)90188-8. [DOI] [PubMed] [Google Scholar]
- Olsson L. Monoclonal antibodies in clinical immunobiology. Derivation, potential, and limitations. Allergy. 1983;38:145. doi: 10.1111/j.1398-9995.1983.tb01599.x. [DOI] [PubMed] [Google Scholar]
- Olsson L, Kaplan HS. Human-human hybridomas producing monoclonal antibodies of predefined antigenic specificity. Proc Natl Acad Sci U S A. 1980;77:5429. doi: 10.1073/pnas.77.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson L, Kaplan HS. Human--human monoclonal antibody-producing hybridomas: technical aspects. Methods Enzymol. 1983;92:3. doi: 10.1016/0076-6879(83)92004-9. [DOI] [PubMed] [Google Scholar]
- Ostberg L, Pursch E. Human X (mouse X human) hybridomas stably producing human antibodies. Hybridoma. 1983;2:361. doi: 10.1089/hyb.1983.2.361. [DOI] [PubMed] [Google Scholar]
- Posner MR, Elboim H, Santos D. The construction and use of a human-mouse myeloma analogue suitable for the routine production of hybridomas secreting human monoclonal antibodies. Hybridoma. 1987;6:611. doi: 10.1089/hyb.1987.6.611. [DOI] [PubMed] [Google Scholar]
- Raison RL, Walker KZ, Halnan CR, Briscoe D, Basten A. Loss of secretion in mouse-human hybrids need not be due to the loss of a structural gene. J Exp Med. 1982;156:1380. doi: 10.1084/jem.156.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder JC, Cole SP, Kozbor D. The EBV-hybridoma technique. Methods Enzymol. 1986;121:140. doi: 10.1016/0076-6879(86)21014-9. [DOI] [PubMed] [Google Scholar]
- Schmitt JJ, Zimmermann U, Neil GA. Efficient generation of stable antibody forming hybridoma cells by electrofusion. Hybridoma. 1989;8:107. doi: 10.1089/hyb.1989.8.107. [DOI] [PubMed] [Google Scholar]
- Schwaber J, Cohen EP. Human x mouse somatic cell hybrid clone secreting immunoglobulins of both parental types. Nature. 1973;244:444. doi: 10.1038/244444a0. [DOI] [PubMed] [Google Scholar]
- Steenbakkers PG, van Wezenbeek PM, Olijve W. Immortalization of antigen selected B cells. J Immunol Methods. 1993;163:33. doi: 10.1016/0022-1759(93)90236-z. [DOI] [PubMed] [Google Scholar]
- Steinitz M, Koskimies S, Klein G, Makela O. Establishment of specific antibody producing human lines by antigen preselection and EBV-transformation. Curr Top Microbiol Immunol. 1978;81:156. doi: 10.1007/978-3-642-67448-8_25. [DOI] [PubMed] [Google Scholar]
- Stevens RH, Macy E, Morrow C, Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979;122:2498. [PubMed] [Google Scholar]
- Sugden B, Mark W. Clonal transformation of adult human leukocytes by Epstein-Barr virus. J Virol. 1977;23:503. doi: 10.1128/jvi.23.3.503-508.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng NN, Lam KS, Calvo Riera F, Kaplan HS. Construction and testing of mouse--human heteromyelomas for human monoclonal antibody production. Proc Natl Acad Sci U S A. 1983;80:7308. doi: 10.1073/pnas.80.23.7308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traggiai E, Becker S, Subbarao K, Kolesnikova L, Uematsu Y, Gismondo MR, Murphy BR, Rappuoli R, Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat Med. 2004;10:871. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vienken J, Zimmermann U. An improved electrofusion technique for production of mouse hybridoma cells. FEBS Lett. 1985;182:278. doi: 10.1016/0014-5793(85)80315-x. [DOI] [PubMed] [Google Scholar]
- Weitkamp JH, Kallewaard N, Kusuhara K, Feigelstock D, Feng N, Greenberg HB, Crowe JE., Jr Generation of recombinant human monoclonal antibodies to rotavirus from single antigen-specific B cells selected with fluorescent virus-like particles. J Immunol Methods. 2003;275:223. doi: 10.1016/s0022-1759(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Carroll William L, T K, Dilley Jeanette, Levy Ronald. Mouse x human heterohybridomas as fusion partners with human B cell tumors. J Immunol Methods. 1986;89:61. doi: 10.1016/0022-1759(86)90032-3. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Gessner P, Schnettler R, Perkins S, Foung SK. Efficient hybridization of mouse-human cell lines by means of hypo-osmolar electrofusion. J Immunol Methods. 1990;134:43. doi: 10.1016/0022-1759(90)90110-h. [DOI] [PubMed] [Google Scholar]
- Zimmermann U, Vienken J. Electric field-induced cell-to-cell fusion. J Membr Biol. 1982;67:165. doi: 10.1007/BF01868659. [DOI] [PubMed] [Google Scholar]


