Abstract
The mammalian target of rapamycin (mTOR) pathway plays a central role in regulating protein synthesis, ribosomal protein translation, and cap-dependent translation. Deregulations in mTOR signaling are frequently associated with tumorigenesis, angiogenesis, tumor growth and metastasis. This review highlights the role of the mTOR in anticancer drug resistance. We discuss the network of signaling pathways in which the mTOR kinase is involved, including the structure and activation of the mTOR complex and the pathways upstream and downstream of mTOR as well as other molecular interactions of mTOR. Major upstream signaling components in control of mTOR activity are PI3K/PTEN/AKT and Ras/Raf/MEK/ERK pathways. We discuss the central role of mTOR in mediating the translation of mRNAs of proteins related to cell cycle progression, those involved in cell survival such as c-myc, hypoxia inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF), cyclin A, cyclin dependent kinases (cdk1/2), cdk inhibitors (p21Cip1 and p27Kip1), retinoblastoma (Rb) protein, and RNA polymerases I and III. We then discuss the potential therapeutic opportunities for using mTOR inhibitors rapamycin, CCI-779, RAD001, and AP-23573 in cancer therapy as single agents or in combinations to reverse drug resistance.
Keywords: mTOR, drug resistance, p70S6K1, PI3K, AKT, MAP kinase, VEGF, CCI-779, RAD001 (everolimus), AP-23573, neurofibromatosis 1
1. Introduction
The mammalian target of rapamycin (mTOR, also known as rapamycin-associated protein [FRAP], rapamycin target [RAFT1], or sirolimus effector protein [SEP]) was identified and cloned shortly after the discovery of the two yeast genes, TOR1 and TOR2 (Brown et al., 1994; Chiu et al., 1994; Kunz et al., 1993; Sabatini et al., 1994). The structure of mTOR is highly conserved. Human mTOR has a 95% amino acid sequence identity to the mouse and rat TOR proteins. mTOR is a 289 kDa serine/threonine kinase and is a member of the large phosphatidylinositol 3-kinase (PI3K)-related kinase (PIKK) family and its catalytic kinase domain in the C-terminus is highly homologous to the lipid kinase domain of PI3K. The members of this family are involved in such basic cellular functions as cell proliferation, cell cycle progression, DNA damage checkpoints, and maintainance of telomere length. Dysfunction of PIKK-related kinases results in disorders such as cancer and immunodeficiency (Bjornsti and Houghton, 2004; Janus et al., 2005).
In cancer cells often receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR), fibroblast growth factor receptor (FGFR) or insulin-like growth factor 1 receptor (IGF-1R) are aberrantly actived and may trigger multiple cytoplasmic kinases including serine/threonine kinases (Faivre et al., 2006). These cellular signaling pathways promote cancer development independently or in parallel and/or through cross-talk and involve frequently, the PI3K/AKT kinase pathway and mitogen-activated protein kinase (MAPK) cascades (Tortora et al., 2007). Both these signaling cascades can regulate the function of mTOR (Faivre et al., 2006; Shaw and Cantley, 2006).
The ability of cancer cells to become resistant to anticancer agents remains a significant impediment to successful chemotherapy. Multiple mechanisms have been shown to contribute to cancer cell drug resistance, including reduction of drug accumulation, increase of thiol-containing biomolecules such as glutathione and metallothioneins, enhancement of the DNA damage repair, and multiple deficiencies in apoptosis induction (Estrela et al., 2006; Madhusudan and Middleton, 2005; Mollinedo and Gajate, 2006; Yu, 2006; Fojo, 2007; Broxterman and Georgopapadakou, 2007). Recently, also mTOR signaling has been implicated not only in tumor development, but also in drug resistance against chemotherapy and radiotherapy (Beuvink et al., 2005) and targeting of mTOR with rapamycin or its analogs has been shown to inhibit tumor growth and render tumors sensitive to chemotherapy in vivo (Dudkin et al., 2001; Frost et al., 2004; Stephan et al., 2004). In this review, we highlight recent findings on the involvement of the mTOR signaling pathway in cancer cell survival, proliferation and drug resistance.
2. mTOR signaling pathway
2.1. mTOR structure and activation
It has been shown that the TOR pathway is essential for cell growth and development in fruit flies, nematodes and mammals and dysfunction of the gene encoding TOR leads to lethality in all species. TOR regulates cellular functions, including translation, transcription, mRNA turnover, protein stability, actin cytoskeletal organization and autophagy (Inoki et al., 2005; Jacinto and Hall, 2003; Moretti et al., 2007).
Eukaryote TORs share 40%-60% identity in their primary sequence. mTOR comprises several conserved structural domains (Hay and Sonenberg, 2004; Janus et al., 2005). The N-terminus of mTOR consists of 20 tandemly repeated motifs including Huntingtin, elongation factor 3, the A subunit of protein phosphatase 2A (PP2A), and TOR (HEAT motifs). The C-terminus consists of mutated FRAP-ataxia-teleangiectasia (FAT), a transformation/transcription domain associated protein domain, an FKPB12-rapamycin binding (FRB) domain, a catalytic kinase domain, a probable autoinhibitory or repressor domain, and a FAT carboxy-terminal domain. The catalytic kinase domain in the C-terminus has sequence similarity with the catalytic domain of PI3K, but there is no experimental evidence that it displays lipid kinase activity.
There are two distinct TOR complexes in mammals, mTORC1 and mTORC2. mTORC1 is a heterotrimeric protein kinase that consists of the mTOR catalytic subunit and two associated proteins, raptor (regulatory associated protein of mTOR) and mLST8 (also known as GβL); whereas mTORC2 consists of four different subunits: mTOR, mLST8/GβL, rictor (rapamycin-insensitive companion of mTOR), and mSin1 (also known as mitogen-activated-protein kinase-associated protein 1) (Abraham, 2002). FKBP12-rapamycin interacts with and inhibits mTORC1, but does not affect mTORC2; thus the mTORC1 complex is rapamycin-sensitive, while mTORC2 is not. It has been clarified that mTORC1 is responsible for sensing nutrient signals, while mTORC2 is involved in actin remodeling. Increasing evidence indicates that mTORC1 pathway activation is a hallmark of cancer, but the molecular mechanisms by which mTORC1 is regulating cancer development remain largely to be elucidated.
It is well known that the inactivation of tuberous sclerosis 1 (TSC1), also known as harmartin, and tuberous sclerosis 2 (TSC2), also known as tuberin, which acts as a crucial negative regulator of mTORC1 (see Figure 1), can cause the tumor-prone syndrome tuberous sclerosis complex (TSC) and lymphangio leiomyomatosis (LAM) (Gao et al., 2002; Sabatini et al., 1994). The TSC1-TSC2 heterodimer is a GTPase activating protein for Rheb (Ras homologue enriched in brain) and plays an important role in mTORC1 activation. Inactivation of tumor suppressors such as PTEN (phosphatase and tensin homolog deleted on chromosome ten), serine-threonine kinase 11 (STK11, also known as LKB1), NF1 (neurofibromatosis 1) or p53 causes the inhibition of TSC1-TSC2, thus leading to the activation of mTOR pathway. For example, loss or mutation of PTEN activates AKT, which directly phosphorylates and inhibits TSC1-TSC2. The loss of STK11 suppresses AMPK (AMP-activated protein kinase), which normally mediates an activating phosphorylation of TSC1-TSC2 (Faivre et al., 2006). Neurofibromin, the product of NF1 gene, contains a small domain that shares significant homology with proteins belonging to the guanosine triphosphatase (GTPase) activating protein (GAP) family, involved in the negative regulation of small GTPase proteins like Ras. Loss of NF1 results in the accumulation of Ras in the GTP-bound state and causes AKT activation and TSC1-TSC2 inhibition via PI3K (Cichowski and Jacks, 2001; Houshmandi and Gutmann, 2007; Shaw and Cantley, 2006). Evidence in favour of p53 involvement in the regulation of mTOR activity is: 1) the inhibition of mTOR activity by p53 activation is mediated primarily by activation of AMPK (AMP-dependent protein kinase) in a p53-dependent fashion with the subsequent activation of the TSC1/TSC2 complex; 2) the activation of p53 increases the mRNA expression of both the PTEN and TSC2 genes (Feng et al., 2005). Thus, the inactivation of p53 leads to mTOR activation by TSC1-TSC2 inhibition. Some tumor-prone syndromes related with molecules in mTOR pathway including Cowden syndrome, tuberous sclerosis complex, lymphangioleiomyomatosis, neurofibromatosis 1, and Peutz-Jeghers syndrome are shown in detail in other reviews (Faivre et al., 2006).
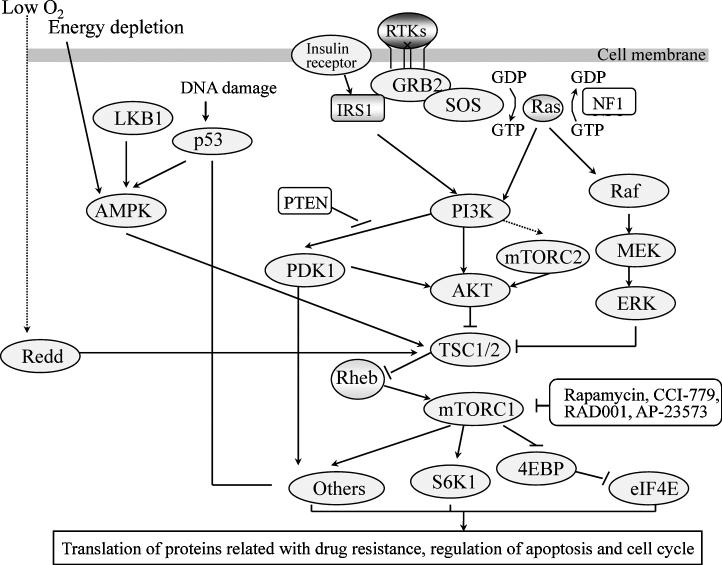
Figure 1. mTOR signaling pathways involved in the regulation of translation.
When ligands such as growth factors or insulin bind their receptor tyrosine kinases (RTKs) the RTKs dimerize and activate their kinase activity, resulting in phosphorylation of tyrosine sites on adaptor proteins such as the insulin receptor substrate (IRS) family. The phosphorylated tyrosine residues serve as docking sites for adaptor proteins such as GRB2, which recruit Ras guanine-nucleotide-exchange factors (GEFs) such as SOS. SOS stimulates the exchange of GDP for GTP on Ras. GTP-Ras activates its downstream pathways including phosphatidylinositol 3-kinase (PI3K)/AKT and Raf/MEK/ERK pathways. Neurofibromin 1 (NF1) is one of the GTPase-activating proteins (GAPs), which binds to activated Ras and catalyse GTP hydrolysis, hence returns Ras to its inactive GDP-binding state. PTEN (phosphatase and tensin homolog deleted on chromosome 10) dephosphorylates PI(3,4,5)P3 back to PI(4,5)P2 to antagonize PI3K.
AKT is activated by PI3K and phosphorylated by 3-phosphoinositide-dependent protein kinase (PDK). AKT activation inhibits the tuberous sclerosis 1 (TSC1)-TSC2 heterodimer, which is a key negative regulator of mTOCR1 by suppressing a small GTP-binding protein, Ras homologue enriched in brain (Rheb). Growth factors, DNA damage, energy depletion, and hypoxia activate TSC1-TSC2 through different signaling pathways. The main function of the mTORC1 pathway is to regulate mRNA translation and ribosome biogenesis by phosphorylating and activating p70S6K1, which is an important regulator of cell size by controlling ribosomal protein translation. P70S6K1 inhibits IRS1 by directly phosphorylating it, thus forming a feedback loop. mTORC1 also phosphorylates eukaryotic translation initiation factor 4E binding protein (4EBP) and suppresses its function to inhibit the mRNA cap-binding protein eukaryotic initiation factor 4E (eIF4E). Growth factors regulate the activity of mTORC2 through PTKs. In addition to regulating the organization of actin, mTORC2 directly phosphorylates AKT on the hydrophobic site of its C-terminal, which is necessary for full AKT activation. Several other proteins, including cyclin dependent kinase 4 (CDK4)-cyclin D1 complexes, p27, pRb, and polymerases interact with mTOR. Thus, the mTOR pathway is involved in regulating the translation of proteins associated with drug resistance, controlling cell cycle progression and apoptosis and thereby contributes to cancer cell drug resistance.
2.2. Signaling upstream of mTOR
2.2.1. The PI3K/AKT signaling pathway
PI3K is composed of heterodimers of a p110 catalytic subunit and a p85 regulatory subunit (Cantley, 2002; Ward and Finan, 2003). RTKs interact with the p85 regulatory subunit of PI3K via the Src homology 2 (SH2) domain of p85, which dimerizes with p110 catalytic subunit of PI3K and localizes the p85/p110 heterodimer to the plasma membrane, while Ras interacts directly with the p110 catalytic subunit of PI3K in a GTP-dependent manner. RTKs can also indirectly activate PI3K via other proteins such as protein kinase C (PKC), SHP1, Rho, and Src (Hennessy et al., 2005). Upon activation, PI3K phosphorylates the D3 hydroxyl of PI(4)P and PI(4,5)P2 to produce PI(3,4)P2 and PI(3,4,5)P3, respectively. PI(3,4,5)P3 binds to a subset of proteins containing pleckstrin-homology (PH), FYVE, Phox (PX), C1 and C2 domains. PTEN phosphatase can dephosphorylate PI(3,4,5)P3 back to PI(4,5)P2. Thus, activation of PI3K and mutation of PTEN are frequent events in cancer (Jiang and Liu, 2008).
AKT/PKB is activated by the PI3K product PI(3,4,5)P3 through the binding of the AKT pleckstrin homology (PH) domain, which triggers AKT to localize to the plasma membrane where AKT is phosphorylated by phosphoinositide-dependent kinase 1 (PDK1) at Thr 308 (Datta et al., 1999; Engelman et al., 2006; Sarbassov et al., 2005). In addition, Akt can be phosphorylated at the hydrophobic C-terminal domain (Ser 473) by PDK2 for its full activation (Blume-Jensen and Hunter, 2001; Hennessy et al., 2005). Upon full activation, AKT translocates to the nucleus and directly phosphorylates and inhibits TSC1 and TSC2, which in turn activate mTOR and downstream targets (Faivre et al., 2006). Overexpression and mutation of AKT is observed in various cancers (Jiang and Liu, 2008) and is an anticancer drug target under active investigation (Broxterman and Georgopapadakou, 2007).
2.2.2. The Ras/Raf/MEK/ERK signaling pathway
The Ras/MAPK pathway targets TSC2 as discovered by the observation that expression of an activated allele of ras induces TSC2 phosphorylation (Roux et al., 2004). Ras proteins (H-, K- and N-Ras) function as a GDP/GTP-regulated switch and as an important oncoproteins. In normal quiescent cells, Ras is GDP-bound and inactive. Upon stimulation by growth factors, hormones or cytokines, the activated GTP-bound form of Ras binds to and activates Raf kinase (Repasky et al., 2004; Wolthuis and Bos, 1999). There are three different isoforms of Raf serine/threonine kinases in mammalian cells: A-Raf, B-Raf and C-Raf (RAF1). A-Raf and B-Raf have a tissue-restricted expression, while C-Raf is expressed ubiquitously in all tissues and cells.
Upon activation, Raf phosphorylates and activates MEK, which activates ERK/MAPK. ERK phosphorylates both cytosolic and nuclear substrates leading to regulation of gene expression, cytoskeletal rearrangements and metabolism (Schubbert et al., 2007; Roberts and Der, 2007). In addition to extracellular stimuli, loss of NF1 also causes accumulation of GTP-bound Ras and ultimately triggers activation of mTORC1 signaling (Johannessen et al., 2005). Also, the reduced expression of a conserved microRNA (let-7) that targets the 3′ untranslated region of H-ras, N-ras and K-ras mRNAs increased Ras activity (Johnson et al., 2005; Kolfschoten et al., 2005; Shaw and Cantley, 2006).
Ras and Raf are frequently mutated or aberrantly expressed in human cancer. For example, Ras mutations occur in pancreatic, papillary thyroid, colon, non-small cell lung, biliary tract, bladder, breast, cervix, endometrial, ovarian and liver cancer (Schubbert et al., 2007; Roberts and Der, 2007; Bamford et al., 2004). The most important isoform of Raf is B-raf, in which active form of mutations have been found in 27%∼70% of human malignant melanomas (Eberle et al., 2007), and 36%∼53% of thyroid, 5%∼22% of colon, and 30% of ovarian cancers (Garnett and Marais, 2004). It has been reported that in some cases B-Raf mutations may lead to heterodimerization with C-Raf and activation of ERK/MAPK signaling pathway (Garnett et al., 2005).
2.2.3. AMPK and other molecules
Nutrients represent an important regulator of mTOR activity (Tsang et al., 2007). Amino acid starvation results in a rapid dephosphorylation of S6K1 and 4E-BP1, whereas re-addition of amino acids restores S6K1 and 4E-BP1 phosphorylation in an mTORC1-dependent manner (Hay and Sonenberg, 2004). Nutrients may regulate TOR signaling through energy production in the form of ATP. AMP-activated protein kinase (AMPK) activity can be regulated by the cellular energy level through the balance in ATP:AMP ratio, which when low under nutrient deprivation activates AMPK. Activated AMPK on its turn can phosphorylate TSC2 at multiple serine and threonine sites leading to inactivation of mTOR (Inoki et al., 2003). In addition, since the Km of mTOR for ATP is in the mM range which is close to the intracellular ATP concentrations, mTOR activation may be influenced by the intracellular concentrations of ATP independent of the levels of amino acids, suggesting that mTOR serves as a homeostatic sensor of ATP (Dennis et al., 2001). Moreover, various environmental stressors also regulate mTOR signaling. For example, hypoxia and DNA damage down-regulate mTOR activity (Tsang et al., 2007). Upon hypoxia, hypoxia-inducible factor 1 (HIF-1) upregulates both REDD1 and REDD2 (Figure 1), which act upstream of TSC1 and TSC2 to inhibit mTORC1 signaling and protein synthesis. DNA damage may inhibit mTOR signaling via p53 expression and AMPK activation (Feng et al., 2005).
2.3. Signaling downstream of mTOR
mTOR is a central regulator of protein synthesis, cell proliferation, cell cycle progression and cell survival. The mTORC1 pathway regulates cell growth through its downstream effectors such as the regulators of translation 4EBP1 (eukaryotic translation initiation factor 4E binding protein 1) and p70S6K1 (ribosomal S6 kinase 1).
2.3.1. 4EBP1
4EBP1 represses the initiation of protein translation through its association with the mRNA cap-binding subunit of the eukaryotic translation initiation factor eIF4F (Sonenberg and Gingras, 1998). mTOR directly phosphorylates and inhibits 4EBP1 activity and may also indirectly inhibit protein serine/threonine phosphatase, which in turn dephosphorylates 4EBP1 during G1-to-S phase transition (Faivre et al., 2006). EBP1 is an inhibitor of eukaryotic translation initiation factor 4E (eIF4E), which is activated by various mitogenic stimuli. Overexpression of eIF4E is sufficient to induce cell transformation (Rousseau et al., 1996). The active form of mTOR phosphorylates 4EBP1 at several serine/threonine sites to promote the dissociation of eIF4E from 4EBP1. Free eIF4E can form the multisubunit eIF4F complex through binding to eIF4G (a large scaffolding protein), eIF4A (an ATP-dependent RNA helicase), and eIF4B, facilitating cap-dependent protein translation and inducing an increase in translation of mRNAs with regulatory elements in the 5′-untranslated terminal regions (5′-UTR) of its downstream target genes such as c-myc, ornithine decarboxylase and cyclin D1, which are required for G1-to-S phase transition (Faivre et al., 2006). In contrast, in quiescent cells or under growth-factor-deprived conditions, unphosphorylated 4EBP1 binds tightly to eIF4E, inhibiting initiation of protein translation. The inhibition of mTOR activation by nutrient deprivation or by rapamycin causes 4EBP1 dephosphorylation, which prevents protein translation. Inhibition of mTOR lead to downregulation of ribosome biogenesis and upregulation of catabolic processes such as autophagy, which is essential for adaptation and survival during starvation conditions (Jastrzebski et al., 2007; Mayer and Grummt, 2006).
2.3.2. P70S6K1
The serine/threonine kinase p70S6K1 (S6K1) is another important downstream target of mTOR. P70S6K1 can also be activated by TOR-insensitive signaling pathways such as PDK1, MAPK and stress-activated protein kinase (SAPK). Activated mTOR phosphorylates p70S6K1 and leads to the recruitment of the 40S ribosomal subunit into translating polysomes, which enhances the translation of mRNAs with a 5′-terminal oligopolypyrimidine (5′-TOP). The phosphorylation of p70S6K1 at Thr389 by mTOR is required for its activation since the substitution of this residue with alanine blocks its activity (Dennis et al., 1996). At least three phosphorylation sites have been identified in p70S6K1, and all of them can be blocked by mTOR inhibitors. The targets of p70S6K1 include ribosomal proteins, elongation factors, and insulin growth factor 2 (Faivre et al., 2006). In addition, p70S6K1 also phosphorylates the eIF4G and eIF4B units of the eIF4F complex. P70S6K1 also has a negative feedback loop to repress PI3K/AKT pathway by inhibiting insulin receptor substrate 1 (IRS1) and IRS2 expression (Haruta et al., 2000; Sabatini et al., 1994). P70S6K1 can phosphorylate the pro-apoptotic molecule BAD on Ser136 disrupting BAD binding to the mitochondrial death inhibitors Bcl-XL and Bcl2 (Faivre et al., 2006).
2.3.3. Other molecular interactions of mTOR
mTOR inhibitors can additionally block cell cycle progression and cell proliferation through disrupting cyclin dependent kinase 4 (CDK4)-cyclin D1 complexes. mTOR inhibition also upregulates CDK inhibitor p27 at both mRNA and protein levels, prolongs its half-life and facilitates the formation of complexes between p27 and CDK/cyclins (Hashemolhosseini et al., 1998; Kawamata et al., 1998). mTOR may also regulate protein synthesis at both transcriptional and translational levels through the function of polymerases (Pol) I and III in regulating RNA transcription (Mayer and Grummt, 2006; Mayer et al., 2004; James and Zomerdijk, 2004). Finally, mTOR activity also plays an important role in pRb synthesis and phosphorylation during cell cycle progression (White, 1997). Decrease of CDK4-cyclin D1 complexes by an mTOR inhibitor also inhibits pRb phosphorylation (Faivre et al., 2006; Baker et al., 2005; Ilyin et al., 2003) again highlighting the profound role of mTOR in regulation of the G1-to-S transition.
3. mTOR and drug resistance
3.1. mTOR signaling and cancer
mTOR mediates the translation of mRNAs related with cell cycle check-points, regulates the expression of survival factors such as c-myc, HIF-1α and vascular endothelial growth factor (VEGF) and is involved in the regulation of cyclin A, cyclin dependent kinases (cdk1/2), cdk inhibitors (p21Cip1 and p27Kip1), retinoblastoma (Rb) protein, RNA polymerases, protein phosphatases (PP2A, PP4 and PP6) and CLIP-170. mTOR also plays a key role in regulating cancer cell proliferation, apoptosis, cell migration and tumor angiogenesis (Huang et al., 2003a). Our previous studies have shown that p70S6K1 is an important molecule in regulating cell proliferation, tumor growth and angiogenesis through mediating HIF-1 and VEGF expression by its upstream pathways such as PI3K/AKT and ERK (Fang et al., 2005; Liu et al., 2006a; Meng et al., 2006; Skinner et al., 2004a; Zhong et al., 2004; Zhou et al., 2007; Gao et al., 2004a). In addition mTOR also regulates human double minute 2 protein phosphorylation and stability (Fang et al., 2006). Some carcinogens including arsenite can activate p70S6K1 through ROS production and the PI3K/AKT pathway (Skinner et al., 2004b; Gao et al., 2004b).
Thus the signaling pathways that activate mTOR are altered in many human cancers. For example, an increased copy number and somatic mutations of PIK3CA, the gene encoding the p110α catalytic subunit of PI3K, was observed in ovarian, cervical, gastric, ovarian, breast, colorectal, gastric, lung, hepatocellular, thyroid and endometrial cancers, glioblastomas and acute leukemia. The deletion and somatic mutations of the p85α regulatory subunit (PIK3R1) were found in primary human glioblastoma, colon and ovarian cancer (Jiang and Liu, 2007). The tumor suppressor PTEN is frequently mutated or lost in many kinds of cancers and the decreasing levels of PTEN expression are correlated with poor prognosis of cancers, such as ovarian, prostate and cervical cancer (Jiang and Liu, 2007). All these changes result in constitutive activation of AKT and consequently mTOR signaling.
Inactivating mutations of STK11/LKB1 are associated with the Peutz-Jeghers syndrome, which is characterized by gastrointestinal hamartomatous polyps and mucocutaneous pigmentation as well as an increased risk for gastrointestinal, breast and female genital tract cancers (Jenne et al., 1998; Hemminki et al., 1998; Bruwer et al., 1954). LKB1 also affects tumor angiogenesis, invasiveness and metastasis (Zhuang et al., 2006). Mutations of TSC2 lead to tuberous sclerosis syndrome, which is is an autosomal dominant disorder characterized by widespread hamartosis and TSC2 mutations may be a risk factor for developing tumors such as pancreatic islet-cell tumors and renal cell carcinoma (Sampson and Harris, 1994; Merritt et al., 2006; Yeung et al., 1994). Loss of TSC2 is also associated with tumor metastasis and development (Pacheco-Rodriguez et al., 2007).
P70S6K1 overexpression and activation has been reported in several tumor cell lines, it is amplified in some breast cancers (Couch et al., 1999; Holz and Blenis, 2005) and it is also associated with tumor angiogenesis and invasiveness (Pende et al., 2004; Skinner et al., 2004a). Recent study implicates that p70S6K1 can directly phosphorylate mTOR at threonine 2446/serine 2448, which has been shown previously to be part of a regulatory repressor domain (Holz and Blenis, 2005). 4EBP1 is another mTOR substrate that inhibits the function of eIF4E. It has been reported that in gastrointestinal cancer both eIF4E and 4EBP1 are frequently overexpressed, but the 4EBP1 levels are the most elevated in patients that have little or no metastatic disease (Martin et al., 2000). On the contrary, recent studies have found that p-4EBP1 is mainly expressed in poorly differentiated tumors and correlated with high-grade tumors and a poor prognosis in human breast tumors and ovarian cancer (Rojo et al., 2007; Castellvi et al., 2006). It has been demonstrated that eIF4E acts as an oncogene by mediating cell transformation (DeFatta and De Benedetti, 2003). The gene encoding eIF4E is amplified in head and neck carcinoma and breast cancers (Sorrells et al., 1998; Sorrells et al., 1999). Overexpression of eIF4E in experimental models dramatically alters cellular morphology, enhances proliferation and induces cellular transformation, tumorigenesis and metastasis and is associated with some solid tumors such as colon, breast and esophageal cancer (McClusky et al., 2005; Salehi and Mashayekhi, 2006; Rosenwald et al., 1999). The levels of eIF4E are also correlated to the progression of head and neck, bladder, gastric and breast cancer (McClusky et al., 2005; Nathan et al., 1999; Chen et al., 2004; Crew et al., 2000). eIF4F activation is an essential component of the malignant phenotype in breast carcinoma (Avdulov et al., 2004). In addition, other translation initiation factors that form the eIF4F complex might be implicated in tumorigenesis. For example, eIF4GI is amplified and overexpressed in squamous cell lung carcinomas (Brass et al., 1997; Mamane et al., 2006), and eIF4GI overexpression induces NIH 3T3 cells transformation (Fukuchi-Shimogori et al., 1997). eIF4A is overexpressed in human melanoma cells and in hepatocellular carcinomas (Mamane et al., 2006; Eberle et al., 1997; Shuda et al., 2000) and antisense RNA against translation factor eIF4A1 decreased the proliferation of human melanoma cell lines (Eberle et al., 2002).
3.2. Role of mTOR signaling pathway in drug resistance
The mTOR signaling pathway has been implicated in multiple anticancer drug resistance mechanisms. Many mutations in cancer such those in EGFR, Ras, PI3K and AKT confer survival signals and therefore the anti-apoptotic effects of mTOR and p70S6K1 signaling are a logical potential mechanisms of drug resistance. Some examples of mTOR pathway involvement in specific drug resistance mechanisms are listed below and summarized in Table 1:
-
■
The retinoid acid resistant NB4 promyelocytic cell lines exhibit defect in the regulation of 4EBP1 and 4EBP2 (Grolleau et al., 2000).
-
■
mTOR activation has been associated with vincristine resistance (Vanderweele and Rudin, 2005).
-
■
Recent evidence indicates that activation of PI3K pathway, either via loss of the tumor suppressor PTEN or through amplification of the PI3K encoding PIK3CA, can mediate trastuzumab resistance in breat cancer patients (Berns et al., 2007). The activated PI3K/AKT pathway promotes resistance to anti-estrogen drugs in breast cancer (Tokunaga et al., 2006; Clark et al., 2002; Campbell et al., 2001; Frogne et al., 2005). In addition, PI3K overexpression and PTEN reduction contribute to cisplatin resistance in ovarian cancer cells (Lee et al., 2005). Deregulated expression of PTEN also leads to breast cancer drug resistance. In prostate cancer cells, the PI3K/PTEN pathway also plays an important role in drug resistance (McCubrey et al., 2007).
-
■
PI3K can mediate the expression of MDR1, which is a transmembrane drug transporter (Tazzari et al., 2007; Lee, Jr. et al., 2004).
-
■
Constitutively active AKT is an important regulator of drug resistance against TRAIL (Chen et al., 2001; Bortul et al., 2003), cisplatin (Fraser et al., 2008; Liu et al., 2007; Lee et al., 2005; Gagnon et al., 2004), erlotinib (Tarceva®) (Yamasaki et al., 2007), taxol (Liu et al., 2006c), arsenic trioxide (Tabellini et al., 2005), all-trans-retinoic acid (Neri et al., 2003), etoposide and doxorubicin (Yu et al., 2008; Tanaka and Grossman, 2003). PI3K/AKT is also a crucial survival pathway in imatinib-resistant gastrointestinal stromal tumors (Bauer et al., 2007). The mTOR/p70S6K1 pathway is downstream of PTEN and AKT in inducing resistance to TRAIL in human glioblastoma (Panner et al., 2005). The Akt isoform, AKT1, is associated with taxol resistance in hepatoma, AKT2 is involved in cisplatin resistance in ovarian cancer cells, AKT2 and AKT3 are associated with cisplatin resistance in human uterine cancer cells (Gagnon et al., 2004; Yuan et al., 2003; Lin et al., 2003). Amplification and overexpression of AKT1 is involved in cisplatin resistance in human lung adenocarcinoma and rapamycin, the inhibitor of mTOR, reversed cisplatin resistance (Liu et al., 2007). A similar result was observed for cisplatin resistance in human breast cancer cells (Eckstein et al., 2008), indicating that AKT plays an important role in cisplatin resistance.
-
■
The Raf/MAPK pathway is usually associated with cell proliferation and drug resistance in hematopoietic cells (McCubrey et al., 2007). It is reported that MEK and ERK can regulate MDR1 expression (Katayama et al., 2007). It has been reported that the activation of ERK and/or p38 MAPK is associated with resistance to cisplatin (Wang et al., 2007; Villedieu et al., 2006), endocrine therapy (Cui et al., 2006; Svensson et al., 2005), TRAIL (Lee et al., 2006), etoposide (Liu et al., 2006b), doxorubicin (Lin et al., 2005), gefitinib (Iressa®) (Han et al., 2005) or PD153035 (small molecule inhibitors of epidermal growth factor receptor) (Li et al., 2003), 5-fluorouracil (Zhao et al., 2006) and vincristine (Kisucka et al., 2001). The MAPKs also include c-Jun N-terminal kinase (JNK), which is shown to be involved in resistance to tamoxifen, cisplatin, doxorubicin, and mechlorethamine (Small et al., 2007; Wang et al., 2006b; Cui et al., 2006; Lin et al., 2005).
Table 1.
Molecular events in the mTOR pathway involved in cancer drug resistance
| Molecular events | Resistance to | Tumor/cell types | Refs |
|---|---|---|---|
| Overexpression of mTOR and p70S6K1 | TRAIL | Glioblastoma | (Panner et al., 2005) |
| Defect in the regulation of 4EBP1 and 4EBP2 | Retinoid acid | NB4 cell | (Grolleau et al., 2000) |
| MTOR activation | Vincristine | FL5.12 cells | (Vanderweele and Rudin, 2005) |
| PI3K regulates MDR1 expression | Vincristine | Leukemia | (Tazzari et al., 2007) |
| PI3K regulates MDR1 expression | Vincristine | Prostate cancer | (Lee, Jr. et al., 2004) |
| Activation of PI3K and AKT | Trastuzumab | Breast cancer | (Berns et al., 2007) |
| Activation of PI3K and AKT | Endocrine therapy | Breast cancer | (Tokunaga et al., 2006) |
| Constitutive AKT activation | Tamoxifen | Breast cancer | (Campbell et al., 2001; Clark et al., 2002; Frogne et al., 2005) |
| PIK3CA mRNA amplification and PTEN loss | Cisplatin | Ovarian cancer | (Lee et al., 2005) |
| AKT inhibits p53 phosphorylation | Cisplatin | Ovarian cancer | (Fraser et al., 2008) |
| Constitutive AKT activation | TRAIL | Prostate Cancer | (Chen et al., 2001) |
| Constitutive AKT activation | TRAIL | Leukemia | (Bortul et al., 2003) |
| Up-regulation of phosphorylated AKT | Erlotinib | Epidermoid cancer cell A-431 | (Yamasaki et al., 2007) |
| Activation of PI3K and AKT | Taxol | Hematopoietic cells | (Liu et al., 2006c) |
| Activation of PI3K and AKT | Arsenic trioxide | Leukemia | (Tabellini et al., 2005) |
| Activation of PI3K and AKT | All-trans-retinoic acid | Leukemia | (Neri et al., 2003) |
| Activation of PI3K and AKT | Imatinib | Gastrointestinal stromal tumor | (Bauer et al., 2007) |
| AKT phosphorylation and PTEN loss | Etoposide and doxorubicin | Gastric cancer | (Yu et al., 2008) |
| AKT phosphorylation and PTEN loss | Doxorubicin | Bladder cancer | (Tanaka and Grossman, 2003) |
| AKT1 amplification and overexpression | Cisplatin | Lung cancer | (Liu et al., 2007) |
| AKT1 activation | Taxol | Hepatoma cells | (Lin et al., 2003) |
| Constitutively active AKT2 expression | Cisplatin | Ovarian cancer | (Yuan et al., 2003) |
| AKT2 and AKT3 overexpression | Cisplatin | Uterine cancer | (Gagnon et al., 2004) |
| EGFR, AKT1 and ERK1 activation | Cisplatin | Breast cancer | (Eckstein et al., 2008) |
| Raf activation | Doxorubicin and paclitaxel | Breast cancer | (McCubrey et al., 2006) |
| MEK/ERK regulates MDR1 expression | Doxorubicin and paclitaxel | Colorectal cancer | (Katayama et al., 2007) |
| ERK-MKP-1 activation | Cisplatin | Ovarian cancer | (Wang et al., 2007) |
| ERK1/2 and p38 activation | Cisplatin | Ovarian cancer | (Villedieu et al., 2006) |
| ERK activation | Tamoxifen | Breast cancer | (Cui et al., 2006; Svensson et al., 2005) |
| ERK activation | TRAIL | Breast cancer | (Lee et al., 2006) |
| Activation of AKT and ERK | Etoposide | Gastric cancer | (Liu et al., 2006b) |
| Activation of AKT and ERK | ZD1839 or PD153035 | Gliomas | (Li et al., 2003) |
| ERK activation, JNK and p38MAPK inhibition | Doxorubicin | Prostate Cancer | (Lin et al., 2005) |
| ERK activation | Gefitinib | NSCLC | (Han et al., 2005) |
| ERK activation | 5-fluorouracil | Pancreatic cancer | (Zhao et al., 2006) |
| ERK activation | Vincristine | Leukemia | (Kisucka et al., 2001) |
| MKP-1/JNK activation | Cisplatin | MEF cells | (Wang et al., 2006b) |
| JNK activation | Tamoxifen | Breast cancer | (Cui et al., 2006) |
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; MDR1, multidrug resistance-associated protein 1; NSCLC, non-small cell lung cancer; MEFs, mouse embryonic fibroblasts.
4. Role of mTOR inhibitors in cancer treatment and modulation of drug resistance
4.1. mTOR inhibitors and tumor selectivity
Rapamycin (sirolimus) is a macrocyclic lactone product from the soil bacteria Streptomyces hygroscopicus. It was isolated and identified as an antifungal agent, especially active against Candida albicans. In the 1990s, it was approved by the FDA as an immunosuppressive agent. Later it was found to have antitumor and immunosupressive properties. Rapamycin and its analogs CCI-779 (temsirolimus), RAD001 (everolimus), and AP-23573 inhibit mTOR activation by binding to FK506-binding protein-12. These drugs are currently under the clinical evaluation for cancer treatment. Preclinical studies indicated that these compounds have cytostatic activity as a single agent in animal models and have synergistic effects in combination with conventional cytotoxic agents, with tamoxifen or with radiation. In clinical studies, these compounds have shown activity in many types of solid cancers (Granville et al., 2006). A very recent study demonstrated antitumor activity of rapamycin in patients with recurrent PTEN-deficient glioblastoma (Cloughesy et al., 2008).
In Phase II clinical studies, CCI-779 has been shown to have effects in patients with renal cell carcinoma and glioblastoma, who were previously treated with standard therapy (Galanis et al., 2005; Chan et al., 2005). The combination treatment of CCI-779 and the aromatase inhibitor letrozole is currently used for treating metastatic breast cancer in a Phase III study. RAD001 treatment enhanced chemotherapy effect in relapsed non-small cell lung cancer and refractory gastrointestinal stromal tumor (Granville et al., 2006). AP-23573 is a phosphorus-containing derivative of rapamycin, developed in both intravenous and oral formulations. Similar to CCI-779 and RAD001, AP-23573 has antiproliferative activity in a variety of PTEN-deficient cancer cell lines, including glioblastoma, prostate, breast, pancreas, lung and colon cancer cells (Rowinsky, 2004).
The activation status of upstream or downstream signaling pathways of mTOR might profoundly affect the response to treatment with mTOR inhibitors. Since insulin-like growth factors (IGF-I /II) and insulin specifically inhibit rapamycin-induced cell apoptosis (Thimmaiah et al., 2003), the combination of IGF receptor inhibitors with mTOR inhibitors may selectively repress growth of tumors with high IGF or insulin expression. In PTEN-deficient cancer cells, AKT activation enhances p70S6K1 activity and 4EBP phosphorylation, and increases c-Myc expression. These PTEN-deficient cells depend on the activation of mTOR for cell proliferation and are therefore more susceptible to the treatment with mTOR inhibitors (Neshat et al., 2001; Huang et al., 2003a; Shi et al., 2002). Similarly, tumors developing in patients with tuberous sclerosis may be sensitive to treatment with mTOR inhibitors. Ectopic expression of p53 or p21Cip1 protects cells from rapamycin-induced apoptosis through activation of apoptosis signal-regulating kinase 1 (ASK1) (Huang et al., 2003b), implicating that mTOR inhibitors would have a stronger therapeutic effect in the absence of p53 or p21 activity/expression.
4.2. mTOR inhibitors as modulators of drug resistance
As mentioned above, mTOR inhibitors, rapamycin or its analogs (Rapalogs) exert antitumor effect as a single agent and may have synergistic effects in combination with other chemotherapeutic drugs. These combinations are discussed below and are summarized in Table 2.
Table 2.
mTOR inhibitors in sensitization to chemotherapy
| Condition | Rapalogs | Drug sensitized | Refs |
|---|---|---|---|
| Basic research | |||
| Breast cancer with AKT activation | RAD001 | Letrozole and Fulvestrant | (Beeram et al., 2007) |
| Breast cancer with aberrant AKT activity | Rapamycin, CCI-799 | Tamoxifen | (DeGraffenried et al., 2004) |
| Bronchial and peripheral murine lung carcinomas | Rapamycin | HKI-272 (a TKI) | (Li et al., 2007) |
| Squamous cell carcinoma of the head and neck | CCI-799 | Erlotinib | (Jimeno et al., 2007) |
| PTEN-deficient and PTEN-intact glioblastoma cells | Rapamycin | Erlotinib | (Wang et al., 2006a) |
| Bcr/Abl-positive leukemia cells | Rapamycin, RAD001 | Imatinib | (Dengler et al., 2005) |
| Bcr/Abl-transformed myeloid and lymphoid cells | Rapamycin | Imatinib | (Mohi et al., 2004) |
| Small cell lung cancer | Rapamycin | Imatinib | (Tsurutani et al., 2005) |
| Small cell lung cancer | CCI-799 | Cisplatin | (Wu et al., 2005) |
| NSCLC | Rapamycin | Cisplatin | (Liu et al., 2007) |
| HER2/neu-overexpressing breast cancer cells | Rapamycin | Paclitaxel and carboplatin | (Mondesire et al., 2004) |
| B-cell neoplasm multiple myeloma (MM) | Rapamycin | Dexamethasone | (Stromberg et al., 2004) |
| PTEN-negative/Akt active prostate cancer | CCI-799 | Doxorubicin | (Grunwald et al., 2002) |
| Preclinical study | |||
| PTEN loss ErbB2-overexpressing breast cancer | RAD001 | Trastuzumab | (Lu et al., 2007) |
| Phase II study | |||
| Breast cancer | CCI-779 or RAD001 | Letrozole | (Chollet et al., 2006) |
TKI, EGFR tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer
Basic studies indicated that co-treatment with RAD001 and the aromatase inhibitor Letrozole or the selective estrogen receptor (ER) down-regulator Fulvestrant reversed AKT-mediated resistance and restored responsiveness to antiestrogen treatment (Beeram et al., 2007). Inhibition of mTOR activity also restored tamoxifen response in breast cancer cells with aberrant AKT Activity (DeGraffenried et al., 2004), suggesting a role of mTOR inhibitors in reversing hormone therapy for breast cancer.
The T790M mutation in EGFR has been identified in non-small cell lung cancer patients who are resistant to the EGFR inhibitor erlotinib. While, treatment with an irreversible EGFR tyrosine kinase inhibitor, HKI-272, inhibited only peripheral tumors but not bronchial tumors in a mouse lung cancer model model carrying the EGFR mutation, the combination of HKI-272 and rapamycin led to significant regression of both types of lung tumors in mice, indicating that this combination therapy may potentially benefit lung cancer patients with the EGFR mutations (Li et al., 2007). Similar results were obtained by the combined treatment with CCI-799 and erlotinib in a head and neck squamous cell carcinoma (Jimeno et al., 2007).
Rapamycin enhances the sensitivity of PTEN-deficient tumor cells to erlotinib in PTEN-deficient glioblastoma cells (Wang et al., 2006a). In addition, the combination of imatinib with rapamycin or RAD001 acts synergistically in Bcr-Abl-positive cells with moderate resistance to imatinib (Dengler et al., 2005). Rapamycin synergized with the TKI imatinib against BCR/ABL-transformed myeloid and lymphoid cells (Mohi et al., 2004). Combination treatment of imatinib with the PI3K inhibitor, LY294002 or rapamycin has synergistic effect in inducing apoptosis when compared to the treatment with imatinib, LY294002 or rapamycin alone in lung cancer cells (Tsurutani et al., 2005).
CCI-779 treatment restored the cisplatin sensitivity in small cell lung cancer cell lines selected for cisplatin resistance as well as cell lines derived from patients who failed to response to cisplatin treatment (Wu et al., 2005). In addition, we have shown that amplification and overexpression of AKT1 is associated with cisplatin resistance in lung tumor, while combination of cisplatin with LY294002 or rapamycin inhibits cisplatin-resistant tumor growth (Liu et al., 2007).
Rapamycin enhanced paclitaxel- and carboplatin-induced apoptosis in breast cancer cells, especially in HER2/neu-overexpressing cells, suggesting that the inhibition of mTOR may provide a potential promising approach for treatment in this tumor type (Mondesire et al., 2004).
Furthermore, inhibitors of mTOR sensitized multiple myeloma cells to apoptosis induced by dexamethasone (Stromberg et al., 2004) and reversed doxorubicin resistance conferred by PTEN mutation/Akt activation in prostate cancer cells (Grunwald et al., 2002).
Trastuzumab (Herceptin) is a humanized monoclonal antibody that targets ErbB2. When given with adjuvant chemotherapy, trastuzumab significantly improves disease-free survival following surgical removal of ErbB2-positive breast tumors. PTEN is required for the antitumor activity of trastuzumab and PTEN loss predicted poor clinical response to trastuzumab-based chemotherapy in patients. It is a clinically applicable strategy to combine trastuzumab with inhibitors of the mTOR pathway. A recent preclinical study indicates that the combinations of trastuzumab or the Akt inhibitor triciribine with RAD001 are promising regimens for treating trastuzumab resistant tumors caused by PTEN loss (Lu et al., 2007). In a phase II study in breast cancer, the combination of the aromatase inhibitor letrozole and CCI-779 or RAD001 has demonstrated better progression-free survival than the letrozole treatment alone (Chollet et al., 2006).
5. Conclusion and future directions
mTOR plays a central role in the cellular response to growth factors and receptor activation through PI3K/AKT and Raf/MEK signaling pathways and in the response to nutrients and stress through the AMPK/LKB1 signaling pathway. mTOR is involved in protein synthesis, cell proliferation, survival and multiple drug resistance mechansisms in cancer cells and upstream signaling molecules of mTOR including EGFR, IGF-1R, PI3K, PTEN, and AKT are frequently mutated in human cancer. Treatment with mTOR inhibitors as single agent can inhibit cancer cell proliferation and induce apoptosis and cell death. The Combination of mTOR inhibitors with other therapeutic agents has often synergistic effects in tumor growth inhibition in experimental models and in some clinical trials. These preliminary clinical data indeed indicate that mTOR inhibitors can modulate certain types of resistance in chemotherapy treatment refractory cancer. The inhibition of mTOR signaling therefore provides a strong lead to improve cancer treatment. Currently there are three promising rapamycin analogues in clinical trials for treating human cancer. The immediate future challenge is to determine whether or how these mTOR inhibitors can be applied in a highly tumor-specific way with little adverse effects and how to select the most sensitive patients among different genetic backgrounds. Also the molecular mechanisms of mTOR in conferring drug resistance still largely remain to be elucidated as well as the downstream targets of mTOR and p70S6K1 in regulating drug resistance remain to be identified.
Acknowledgements
This work was supported by CA109460 and CA123675 grants from National Cancer Institute, NIH; by American Cancer Society Research Scholar Grant 04-076-01-TBE.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham RT. Identification of TOR signaling complexes: more TORC for the cell growth engine. Cell. 2002;111:9–12. doi: 10.1016/s0092-8674(02)01009-7. [DOI] [PubMed] [Google Scholar]
- Avdulov S, Li S, Michalek V, Burrichter D, Peterson M, Perlman DM, et al. Activation of translation complex eIF4F is essential for the genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell. 2004;5:553–563. doi: 10.1016/j.ccr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Baker GL, Landis MW, Hinds PW. Multiple functions of D-type cyclins can antagonize pRb-mediated suppression of proliferation. Cell Cycle. 2005;4:330–338. [PubMed] [Google Scholar]
- Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br. J. Cancer. 2004;91:355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Duensing A, Demetri GD, Fletcher JA. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene. 2007;26:7560–7568. doi: 10.1038/sj.onc.1210558. [DOI] [PubMed] [Google Scholar]
- Beeram M, Tan QT, Tekmal RR, Russell D, Middleton A, De Graffenried LA. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann. Oncol. 2007;18:1323–1328. doi: 10.1093/annonc/mdm170. [DOI] [PubMed] [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O’Reilly T, Natt F, et al. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747–759. doi: 10.1016/j.cell.2004.12.040. [DOI] [PubMed] [Google Scholar]
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat. Rev. Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- Bortul R, Tazzari PL, Cappellini A, Tabellini G, Billi AM, Bareggi R, et al. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-kappaB activation and cFLIP(L) up-regulation. Leukemia. 2003;17:379–389. doi: 10.1038/sj.leu.2402793. [DOI] [PubMed] [Google Scholar]
- Brass N, Heckel D, Sahin U, Pfreundschuh M, Sybrecht GW, Meese E. Translation initiation factor eIF-4gamma is encoded by an amplified gene and induces an immune response in squamous cell lung carcinoma. Hum. Mol. Genet. 1997;6:33–39. doi: 10.1093/hmg/6.1.33. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Broxterman HJ, Georgopapadakou NH. Anticancer therapeutics: a surge of new developments increasingly target tumor and stroma. Drug Resist. Updates. 2007;10:182–193. doi: 10.1016/j.drup.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Bruwer A, Bargen JA, Kierland RR. Surface pigmentation and generalized intestinal polyposis; (Peutz-Jeghers syndrome) Proc. Staff. Meet. Mayo Clin. 1954;29:168–171. [PubMed] [Google Scholar]
- Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J. Biol. Chem. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Castellvi J, Garcia A, Rojo F, Ruiz-Marcellan C, Gil A, Baselga J, Cajal S. Phosphorylated 4E binding protein 1: a hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer. 2006;107:1801–1811. doi: 10.1002/cncr.22195. [DOI] [PubMed] [Google Scholar]
- Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2005;23:5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- Chen CN, Hsieh FJ, Cheng YM, Lee PH, Chang KJ. Expression of eukaryotic initiation factor 4E in gastric adenocarcinoma and its association with clinical outcome. J. Surg. Oncol. 2004;86:22–27. doi: 10.1002/jso.20037. [DOI] [PubMed] [Google Scholar]
- Chen X, Thakkar H, Tyan F, Gim S, Robinson H, Lee C, et al. Constitutively active Akt is an important regulator of TRAIL sensitivity in prostate cancer. Oncogene. 2001;20:6073–6083. doi: 10.1038/sj.onc.1204736. [DOI] [PubMed] [Google Scholar]
- Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc. Natl. Acad. Sci. (USA) 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet P, Abrial C, Tacca O, Mouret-Reynier MA, Leheurteur M, Durando X, Cure H. Mammalian target of rapamycin inhibitors in combination with letrozole in breast cancer. Clin. Breast Cancer. 2006;7:336–338. doi: 10.3816/CBC.2006.n.047. [DOI] [PubMed] [Google Scholar]
- Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell. 2001;104:593–604. doi: 10.1016/s0092-8674(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Clark AS, West K, Streicher S, Dennis PA. Constitutive and inducible Akt activity promotes resistance to chemotherapy, trastuzumab, or tamoxifen in breast cancer cells. Mol. Cancer Ther. 2002;1:707–717. [PubMed] [Google Scholar]
- Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS. Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch FJ, Wang XY, Wu GJ, Qian J, Jenkins RB, James CD. Localization of PS6K to chromosomal region 17q23 and determination of its amplification in breast cancer. Cancer Res. 1999;59:1408–1411. [PubMed] [Google Scholar]
- Crew JP, Fuggle S, Bicknell R, Cranston DW, De Benedetti A, Harris AL. Eukaryotic initiation factor-4E in superficial and muscle invasive bladder cancer and its correlation with vascular endothelial growth factor expression and tumour progression. Br. J. Cancer. 2000;82:161–166. doi: 10.1054/bjoc.1999.0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Parra I, Zhang M, Hilsenbeck SG, Tsimelzon A, Furukawa T, et al. Elevated expression of mitogen-activated protein kinase phosphatase 3 in breast tumors: a mechanism of tamoxifen resistance. Cancer Res. 2006;66:5950–5959. doi: 10.1158/0008-5472.CAN-05-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- DeFatta RJ, De Benedetti A. Translational upregulation of yes accompanies eIF4E-mediated oncogenic transformation. Int. J. Oncol. 2003;23:1709–1713. [PubMed] [Google Scholar]
- DeGraffenried LA, Friedrichs WE, Russell DH, Donzis EJ, Middleton AK, Silva JM, Roth RA, Hidalgo M. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin. Cancer Res. 2004;10:8059–8067. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- Dengler J, von Bubnoff N, Decker T, Peschel C, Duyster J. Combination of imatinib with rapamycin or RAD001 acts synergistically only in Bcr-Abl-positive cells with moderate resistance to imatinib. Leukemia. 2005;19:1835–1838. doi: 10.1038/sj.leu.2403848. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294:1102–1105. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Pullen N, Kozma SC, Thomas G. The principal rapamycin-sensitive p70(s6k) phosphorylation sites, T-229 and T-389, are differentially regulated by rapamycin-insensitive kinase kinases. Mol. Cell Biol. 1996;16:6242–6251. doi: 10.1128/mcb.16.11.6242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudkin L, Dilling MB, Cheshire PJ, Harwood FC, Hollingshead M, Arbuck SG, et al. Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin. Cancer Res. 2001;7:1758–1764. [PubMed] [Google Scholar]
- Eberle J, Fecker LF, Bittner JU, Orfanos CE, Geilen CC. Decreased proliferation of human melanoma cell lines caused by antisense RNA against translation factor eIF-4A1. Br. J. Cancer. 2002;86:1957–1962. doi: 10.1038/sj.bjc.6600351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle J, Krasagakis K, Orfanos CE. Translation initiation factor eIF-4A1 mRNA is consistently overexpressed in human melanoma cells in vitro. Int. J. Cancer. 1997;71:396–401. doi: 10.1002/(sici)1097-0215(19970502)71:3<396::aid-ijc16>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Eberle J, Kurbanov BM, Hossini AM, Trefzer U, Fecker LF. Overcoming apoptosis deficiency of melanoma-Hope for new therapeutic approaches. Drug Resist. Updates. 2007;10:218–234. doi: 10.1016/j.drup.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Eckstein N, Servan K, Girard L, Cai D, von Jonquieres G, Jaehde U, et al. Epidermal growth factor receptor pathway analysis identifies amphiregulin as a key factor for cisplatin resistance of human breast cancer cells. J. Biol. Chem. 2008;283:739–750. doi: 10.1074/jbc.M706287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev. Clin. Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat. Rev. Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- Fang J, Meng Q, Vogt PK, Zhang R, Jiang BH. A downstream kinase of the mammalian target of rapamycin, p70S6K1, regulates human double minute 2 protein phosphorylation and stability. J. Cell Physiol. 2006;209:261–265. doi: 10.1002/jcp.20749. [DOI] [PubMed] [Google Scholar]
- Fang J, Xia C, Cao Z, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19:342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc. Natl. Acad. Sci. (USA) 2005;102:8204–8209. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M, Bai T, Tsang BK. Akt promotes cisplatin resistance in human ovarian cancer cells through inhibition of p53 phosphorylation and nuclear function. Int. J. Cancer. 2008;122:534–546. doi: 10.1002/ijc.23086. [DOI] [PubMed] [Google Scholar]
- Frogne T, Jepsen JS, Larsen SS, Fog CK, Brockdorff BL, Lykkesfeldt AE. Antiestrogen-resistant human breast cancer cells require activated protein kinase B/Akt for growth. Endocr. Relat. Cancer. 2005;12:599–614. doi: 10.1677/erc.1.00946. [DOI] [PubMed] [Google Scholar]
- Frost P, Moatamed F, Hoang B, Shi Y, Gera J, Yan H, et al. In vivo antitumor effects of the mTOR inhibitor CCI-779 against human multiple myeloma cells in a xenograft model. Blood. 2004;104:4181–4187. doi: 10.1182/blood-2004-03-1153. [DOI] [PubMed] [Google Scholar]
- Fojo T. Multiple paths to a drug resistance phenotype: mutations, translocations, deletions and amplification of coding genes or promoter regions, epigenetic changes and microRNAs. Drug Resist. Updates. 2007;10:59–67. doi: 10.1016/j.drup.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Fukuchi-Shimogori T, Ishii I, Kashiwagi K, Mashiba H, Ekimoto H, Igarashi K. Malignant transformation by overproduction of translation initiation factor eIF4G. Cancer Res. 1997;57:5041–5044. [PubMed] [Google Scholar]
- Gagnon V, Mathieu I, Sexton E, Leblanc K, Asselin E. AKT involvement in cisplatin chemoresistance of human uterine cancer cells. Gynecol. Oncol. 2004;94:785–795. doi: 10.1016/j.ygyno.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J. Clin. Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am. J. Physiol .Cell. Physiol. 2004a;287:C281–C291. doi: 10.1152/ajpcell.00422.2003. [DOI] [PubMed] [Google Scholar]
- Gao N, Shen L, Zhang Z, Leonard SS, He H, Zhang XG, Shi X, Jiang BH. Arsenite induces HIF-1alpha and VEGF through PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol. Cell Biochem. 2004b;255:33–45. doi: 10.1023/b:mcbi.0000007259.65742.16. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Arrazola P, Hino O, Kobayashi T, Yeung RS, Ru B, Pan D. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat. Cell Biol. 2002;4:699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6:313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- Garnett MJ, Rana S, Paterson H, Barford D, Marais R. Wild-type and mutant B-RAF activate C-RAF through distinct mechanisms involving heterodimerization. Mol. Cell. 2005;20:963–969. doi: 10.1016/j.molcel.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Granville CA, Memmott RM, Gills JJ, Dennis PA. Handicapping the race to develop inhibitors of the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin pathway. Clin. Cancer Res. 2006;12:679–689. doi: 10.1158/1078-0432.CCR-05-1654. [DOI] [PubMed] [Google Scholar]
- Grolleau A, Wietzerbin J, Beretta L. Defect in the regulation of 4E-BP1 and 2, two repressors of translation initiation, in the retinoid acid resistant cell lines, NB4-R1 and NB4-R2. Leukemia. 2000;14:1909–1914. doi: 10.1038/sj.leu.2401904. [DOI] [PubMed] [Google Scholar]
- Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62:6141–6145. [PubMed] [Google Scholar]
- Han SW, Hwang PG, Chung DH, Kim DW, Im SA, Kim YT, et al. Epidermal growth factor receptor (EGFR) downstream molecules as response predictive markers for gefitinib (Iressa, ZD1839) in chemotherapy-resistant non-small cell lung cancer. Int. J. Cancer. 2005;113:109–115. doi: 10.1002/ijc.20550. [DOI] [PubMed] [Google Scholar]
- Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol. Endocrinol. 2000;14:783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivieres S, Mercep L, Ferrari S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J. Biol. Chem. 1998;273:14424–14429. doi: 10.1074/jbc.273.23.14424. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;391:184–187. doi: 10.1038/34432. [DOI] [PubMed] [Google Scholar]
- Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J. Biol. Chem. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- Houshmandi SS, Gutmann DH. All in the family: using inherited cancer syndromes to understand de-regulated cell signaling in brain tumors. J. Cell Biochem. 2007;102:811–819. doi: 10.1002/jcb.21506. [DOI] [PubMed] [Google Scholar]
- Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol. Ther. 2003a;2:222–232. doi: 10.4161/cbt.2.3.360. [DOI] [PubMed] [Google Scholar]
- Huang S, Shu L, Dilling MB, Easton J, Harwood FC, Ichijo H, Houghton PJ. Sustained activation of the JNK cascade and rapamycin-induced apoptosis are suppressed by p53/p21(Cip1) Mol. Cell. 2003b;11:1491–1501. doi: 10.1016/s1097-2765(03)00180-1. [DOI] [PubMed] [Google Scholar]
- Ilyin GP, Glaise D, Gilot D, Baffet G, Guguen-Guillouzo C. Regulation and role of p21 and p27 cyclin-dependent kinase inhibitors during hepatocyte differentiation and growth. Am. J. Physiol Gastrointest. Liver Physiol. 2003;285:G115–G127. doi: 10.1152/ajpgi.00309.2002. [DOI] [PubMed] [Google Scholar]
- Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat. Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat. Rev. Mol. Cell Biol. 2003;4:117–126. doi: 10.1038/nrm1018. [DOI] [PubMed] [Google Scholar]
- James MJ, Zomerdijk JC. Phosphatidylinositol 3-kinase and mTOR signaling pathways regulate RNA polymerase I transcription in response to IGF-1 and nutrients. J. Biol. Chem. 2004;279:8911–8918. doi: 10.1074/jbc.M307735200. [DOI] [PubMed] [Google Scholar]
- Janus A, Robak T, Smolewski P. The mammalian target of the rapamycin (mTOR) kinase pathway: its role in tumourigenesis and targeted antitumour therapy. Cell Mol. Biol. Lett. 2005;10:479–498. [PubMed] [Google Scholar]
- Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, et al. Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat. Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. PI3K/PTEN signaling in tumorigenesis and angiogenesis. Biochim. Biophys. Acta. 2008;1784:150–158. doi: 10.1016/j.bbapap.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Jimeno A, Kulesza P, Wheelhouse J, Chan A, Zhang X, Kincaid E, et al. Dual EGFR and mTOR targeting in squamous cell carcinoma models, and development of early markers of efficacy. Br. J. Cancer. 2007;96:952–959. doi: 10.1038/sj.bjc.6603656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen CM, Reczek EE, James MF, Brems H, Legius E, Cichowski K. The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc. Natl. Acad. Sci. (USA) 2005;102:8573–8578. doi: 10.1073/pnas.0503224102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, et al. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Katayama K, Yoshioka S, Tsukahara S, Mitsuhashi J, Sugimoto Y. Inhibition of the mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol. Cancer Ther. 2007;6:2092–2102. doi: 10.1158/1535-7163.MCT-07-0148. [DOI] [PubMed] [Google Scholar]
- Kawamata S, Sakaida H, Hori T, Maeda M, Uchiyama T. The upregulation of p27Kip1 by rapamycin results in G1 arrest in exponentially growing T-cell lines. Blood. 1998;91:561–569. [PubMed] [Google Scholar]
- Kisucka J, Barancik M, Bohacova V, Breier A. Reversal effect of specific inhibitors of extracellular-signal regulated protein kinase pathway on P-glycoprotein mediated vincristine resistance of L1210 cells. Gen. Physiol Biophys. 2001;20:439–444. [PubMed] [Google Scholar]
- Kolfschoten IG, van Leeuwen B, Berns K, Mullenders J, Beijersbergen RL, Bernards R, et al. A genetic screen identifies PITX1 as a suppressor of RAS activity and tumorigenicity. Cell. 2005;121:849–858. doi: 10.1016/j.cell.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Kunz J, Henriquez R, Schneider U, Deuter-Reinhard M, Movva NR, Hall MN. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993;73:585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Lee JT, Jr., Steelman LS, McCubrey JA. Phosphatidylinositol 3′-kinase activation leads to multidrug resistance protein-1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res. 2004;64:8397–8404. doi: 10.1158/0008-5472.CAN-04-1612. [DOI] [PubMed] [Google Scholar]
- Lee S, Choi EJ, Jin C, Kim DH. Activation of PI3K/Akt pathway by PTEN reduction and PIK3CA mRNA amplification contributes to cisplatin resistance in an ovarian cancer cell line. Gynecol. Oncol. 2005;97:26–34. doi: 10.1016/j.ygyno.2004.11.051. [DOI] [PubMed] [Google Scholar]
- Lee TJ, Lee JT, Park JW, Kwon TK. Acquired TRAIL resistance in human breast cancer cells are caused by the sustained cFLIP(L) and XIAP protein levels and ERK activation. Biochem. Biophys. Res. Commun. 2006;351:1024–1030. doi: 10.1016/j.bbrc.2006.10.163. [DOI] [PubMed] [Google Scholar]
- Li B, Chang CM, Yuan M, McKenna WG, Shu HK. Resistance to small molecule inhibitors of epidermal growth factor receptor in malignant gliomas. Cancer Res. 2003;63:7443–7450. [PubMed] [Google Scholar]
- Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Lin HL, Lui WY, Liu TY, Chi CW. Reversal of Taxol resistance in hepatoma by cyclosporin A: involvement of the PI-3 kinase-AKT 1 pathway. Br. J. Cancer. 2003;88:973–980. doi: 10.1038/sj.bjc.6600788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Chang SY, Hsieh DS, Lee CF, Yu DS. Modulation of mitogen-activated protein kinase cascades by differentiation-1 protein: acquired drug resistance of hormone independent prostate cancer cells. J. Urol. 2005;174:2022–2026. doi: 10.1097/01.ju.0000176476.14572.39. [DOI] [PubMed] [Google Scholar]
- Liu LZ, Hu XW, Xia C, He J, Zhou Q, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate epidermal growth factor-induced vascular endothelial growth factor and hypoxia-inducible factor-1alpha expression through activation of AKT and P70S6K1 in human ovarian cancer cells. Free Radic. Biol. Med. 2006a;41:1521–1533. doi: 10.1016/j.freeradbiomed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Liu LZ, Zhou XD, Qian G, Shi X, Fang J, Jiang BH. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007;67:6325–6332. doi: 10.1158/0008-5472.CAN-06-4261. [DOI] [PubMed] [Google Scholar]
- Liu SQ, Yu JP, Yu HG, et al. Activation of Akt and ERK signalling pathways induced by etoposide confer chemoresistance in gastric cancer cells. Dig. Liver Dis. 2006b;38:310–318. doi: 10.1016/j.dld.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen L, Ko TC, Fields AP, Thompson EA. Evi1 is a survival factor which conveys resistance to both TGFbeta- and taxol-mediated cell death via PI3K/AKT. Oncogene. 2006c;25:3565–3575. doi: 10.1038/sj.onc.1209403. [DOI] [PubMed] [Google Scholar]
- Lu CH, Wyszomierski SL, Tseng LM, Sun MH, Lan KH, Neal CL, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin. Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- Madhusudan S, Middleton MR. The emerging role of DNA repair proteins as predictive, prognostic and therapeutic targets in cancer. Cancer Treat. Rev. 2005;31:603–617. doi: 10.1016/j.ctrv.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- Martin ME, Perez MI, Redondo C, Alvarez MI, Salinas M, Fando JL. 4E binding protein 1 expression is inversely correlated to the progression of gastrointestinal cancers. Int. J. Biochem. Cell Biol. 2000;32:633–642. doi: 10.1016/s1357-2725(00)00007-8. [DOI] [PubMed] [Google Scholar]
- Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene. 2006;25:6384–6391. doi: 10.1038/sj.onc.1209883. [DOI] [PubMed] [Google Scholar]
- Mayer C, Zhao J, Yuan X, Grummt I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev. 2004;18:423–434. doi: 10.1101/gad.285504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClusky DR, Chu Q, Yu H, Debenedetti A, Johnson LW, Meschonat C, et al. A prospective trial on initiation factor 4E (eIF4E) overexpression and cancer recurrence in node-positive breast cancer. Ann. Surg. 2005;242:584–590. doi: 10.1097/01.sla.0000184224.55949.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Xia C, Fang J, Rojanasakul Y, Jiang BH. Role of PI3K and AKT specific isoforms in ovarian cancer cell migration, invasion and proliferation through the p70S6K1 pathway. Cell Signal. 2006;18:2262–2271. doi: 10.1016/j.cellsig.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Merritt JL, Davis DM, Pittelkow MR, Babovic-Vuksanovic D. Extensive acrochordons and pancreatic islet-cell tumors in tuberous sclerosis associated with TSC2 mutations. Am. J. Med. Genet. A. 2006;140:1669–1672. doi: 10.1002/ajmg.a.31351. [DOI] [PubMed] [Google Scholar]
- Mohi MG, Boulton C, Gu TL, Sternberg DW, Neuberg D, Griffin JD, et al. Combination of rapamycin and protein tyrosine kinase (PTK) inhibitors for the treatment of leukemias caused by oncogenic PTKs. Proc. Natl. Acad. Sci. (USA) 2004;101:3130–3135. doi: 10.1073/pnas.0400063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollinedo F, Gajate C. Fas/CD95 death receptor and lipid rafts: new targets for apoptosis-directed cancer therapy. Drug Resist. Updates. 2006;9:51–73. doi: 10.1016/j.drup.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, et al. Targeting mammalian target of rapamycin synergistically enhances chemotherapy-induced cytotoxicity in breast cancer cells. Clin. Cancer Res. 2004;10:7031–7042. doi: 10.1158/1078-0432.CCR-04-0361. [DOI] [PubMed] [Google Scholar]
- Moretti L, Yang ES, Kim KW, Lu B. Autophagy signaling in cancer and its potential as novel target to improve anticancer therapy. Drug Resist. Updates. 2007;10:135–143. doi: 10.1016/j.drup.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Nathan CO, Franklin S, Abreo FW, Nassar R, De Benedetti A, Glass J. Analysis of surgical margins with the molecular marker eIF4E: a prognostic factor in patients with head and neck cancer. J. Clin. Oncol. 1999;17:2909–2914. doi: 10.1200/JCO.1999.17.9.2909. [DOI] [PubMed] [Google Scholar]
- Neri LM, Borgatti P, Tazzari PL, Bortul R, Cappellini A, Tabellini G, et al. The phosphoinositide 3-kinase/AKT1 pathway involvement in drug and all-trans-retinoic acid resistance of leukemia cells. Mol. Cancer Res. 2003;1:234–246. [PubMed] [Google Scholar]
- Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc. Natl. Acad. Sci. (USA) 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Rodriguez G, Steagall WK, Crooks DM, Stevens LA, Hashimoto H, Li S, et al. TSC2 loss in lymphangioleiomyomatosis cells correlated with expression of CD44v6, a molecular determinant of metastasis. Cancer Res. 2007;67:10573–10581. doi: 10.1158/0008-5472.CAN-07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol. Cell Biol. 2005;25:8809–8823. doi: 10.1128/MCB.25.20.8809-8823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, et al. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repasky GA, Chenette EJ, Der CJ. Renewing the conspiracy theory debate: does Raf function alone to mediate Ras oncogenesis? Trends Cell Biol. 2004;14:639–647. doi: 10.1016/j.tcb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Rojo F, Najera L, Lirola J, Jimenez J, Guzman M, Sabadell MD, et al. 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin. Cancer Res. 2007;13:81–89. doi: 10.1158/1078-0432.CCR-06-1560. [DOI] [PubMed] [Google Scholar]
- Rosenwald IB, Chen JJ, Wang S, Savas L, London IM, Pullman J. Upregulation of protein synthesis initiation factor eIF-4E is an early event during colon carcinogenesis. Oncogene. 1999;18:2507–2517. doi: 10.1038/sj.onc.1202563. [DOI] [PubMed] [Google Scholar]
- Rousseau D, Gingras AC, Pause A, Sonenberg N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene. 1996;13:2415–2420. [PubMed] [Google Scholar]
- Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. (USA) 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowinsky EK. Targeting the molecular target of rapamycin (mTOR) Curr. Opin. Oncol. 2004;16:564–575. doi: 10.1097/01.cco.0000143964.74936.d1. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Salehi Z, Mashayekhi F. Expression of the eukaryotic translation initiation factor 4E (eIF4E) and 4E-BP1 in esophageal cancer. Clin. Biochem. 2006;39:404–409. doi: 10.1016/j.clinbiochem.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Sampson JR, Harris PC. The molecular genetics of tuberous sclerosis. Hum. Mol. Genet. 1994;3:1477–1480. doi: 10.1093/hmg/3.suppl_1.1477. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schubbert S, Shannon K, Bollag G. Hyperactive Ras in developmental disorders and cancer. Nat. Rev. Cancer. 2007;7:295–308. doi: 10.1038/nrc2109. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shi Y, Gera J, Hu L, Hsu JH, Bookstein R, Li W, Lichtenstein A. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–5034. [PubMed] [Google Scholar]
- Shuda M, Kondoh N, Tanaka K, Ryo A, Wakatsuki T, Hada A, et al. Enhanced expression of translation factor mRNAs in hepatocellular carcinoma. Anticancer Res. 2000;20:2489–2494. [PubMed] [Google Scholar]
- Skinner HD, Zheng JZ, Fang J, Agani F, Jiang BH. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J. Biol. Chem. 2004a;279:45643–45651. doi: 10.1074/jbc.M404097200. [DOI] [PubMed] [Google Scholar]
- Skinner HD, Zhong XS, Gao N, Shi X, Jiang BH. Arsenite induces p70S6K1 activation and HIF-1alpha expression in prostate cancer cells. Mol. Cell Biochem. 2004b;255:19–23. doi: 10.1023/b:mcbi.0000007257.67733.3b. [DOI] [PubMed] [Google Scholar]
- Small GW, Shi YY, Higgins LS, Orlowski RZ. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res. 2007;67:4459–4466. doi: 10.1158/0008-5472.CAN-06-2644. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Gingras AC. The mRNA 5′ cap-binding protein eIF4E and control of cell growth. Curr. Opin. Cell Biol. 1998;10:268–275. doi: 10.1016/s0955-0674(98)80150-6. [DOI] [PubMed] [Google Scholar]
- Sorrells DL, Black DR, Meschonat C, Rhoads R, De Benedetti A, Gao M, et al. Detection of eIF4E gene amplification in breast cancer by competitive PCR. Ann. Surg. Oncol. 1998;5:232–237. doi: 10.1007/BF02303778. [DOI] [PubMed] [Google Scholar]
- Sorrells DL, Ghali GE, Meschonat C, DeFatta RJ, Black D, Liu L, et al. Competitive PCR to detect eIF4E gene amplification in head and neck cancer. Head Neck. 1999;21:60–65. doi: 10.1002/(sici)1097-0347(199901)21:1<60::aid-hed8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Stephan S, Datta K, Wang E, Li J, Brekken RA, Parangi S, et al. Effect of rapamycin alone and in combination with antiangiogenesis therapy in an orthotopic model of human pancreatic cancer. Clin. Cancer Res. 2004;10:6993–7000. doi: 10.1158/1078-0432.CCR-04-0808. [DOI] [PubMed] [Google Scholar]
- Stromberg T, Dimberg A, Hammarberg A, Carlson K, Osterborg A, Nilsson K, et al. Rapamycin sensitizes multiple myeloma cells to apoptosis induced by dexamethasone. Blood. 2004;103:3138–3147. doi: 10.1182/blood-2003-05-1543. [DOI] [PubMed] [Google Scholar]
- Svensson S, Jirstrom K, Ryden L, Roos G, Emdin S, Ostrowski MC, Landberg G. ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene. 2005;24:4370–4379. doi: 10.1038/sj.onc.1208626. [DOI] [PubMed] [Google Scholar]
- Tabellini G, Cappellini A, Tazzari PL, Fala F, Billi AM, Manzoli L, et al. Phosphoinositide 3-kinase/Akt involvement in arsenic trioxide resistance of human leukemia cells. J. Cell Physiol. 2005;202:623–634. doi: 10.1002/jcp.20153. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Grossman HB. In vivo gene therapy of human bladder cancer with PTEN suppresses tumor growth, downregulates phosphorylated Akt, and increases sensitivity to doxorubicin. Gene Ther. 2003;10:1636–1642. doi: 10.1038/sj.gt.3302056. [DOI] [PubMed] [Google Scholar]
- Tazzari PL, Cappellini A, Ricci F, Evangelisti C, Papa V, Grafone T, et al. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia. 2007;21:427–438. doi: 10.1038/sj.leu.2404523. [DOI] [PubMed] [Google Scholar]
- Thimmaiah KN, Easton J, Huang S, Veverka KA, Germain GS, Harwood FC, Houghton PJ. Insulin-like growth factor I-mediated protection from rapamycin-induced apoptosis is independent of Ras-Erk1-Erk2 and phosphatidylinositol 3′-kinase-Akt signaling pathways. Cancer Res. 2003;63:364–374. [PubMed] [Google Scholar]
- Tokunaga E, Kimura Y, Mashino K, Oki E, Kataoka A, Ohno S, et al. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer. 2006;13:137–144. doi: 10.2325/jbcs.13.137. [DOI] [PubMed] [Google Scholar]
- Tortora G, Bianco R, Daniele G, et al. Overcoming resistance to molecularly targeted anticancer therapies: rational drug combinations based on EGFR and MAPK inhibition for solid tumours and haematological malignancies. Drug Resist. Updates. 2007;10:81–100. doi: 10.1016/j.drup.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang CK, Qi H, Liu LF, Zheng XF. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov. Today. 2007;12:112–124. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Tsurutani J, West KA, Sayyah J, Gills JJ, Dennis PA. Inhibition of the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway but not the MEK/ERK pathway attenuates laminin-mediated small cell lung cancer cellular survival and resistance to imatinib mesylate or chemotherapy. Cancer Res. 2005;65:8423–8432. doi: 10.1158/0008-5472.CAN-05-0058. [DOI] [PubMed] [Google Scholar]
- Vanderweele DJ, Rudin CM. Mammalian target of rapamycin promotes vincristine resistance through multiple mechanisms independent of maintained glycolytic rate. Mol. Cancer Res. 2005;3:635–644. doi: 10.1158/1541-7786.MCR-05-0063. [DOI] [PubMed] [Google Scholar]
- Villedieu M, Deslandes E, Duval M, Heron JF, Gauduchon P, Poulain L. Acquisition of chemoresistance following discontinuous exposures to cisplatin is associated in ovarian carcinoma cells with progressive alteration of FAK, ERK and p38 activation in response to treatment. Gynecol. Oncol. 2006;101:507–519. doi: 10.1016/j.ygyno.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhou JY, Wu GS. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 2007;67:11933–11941. doi: 10.1158/0008-5472.CAN-07-5185. [DOI] [PubMed] [Google Scholar]
- Wang MY, Lu KV, Zhu S, Dia EQ, Vivanco I, Shackleford GM, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006a;66:7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- Wang Z, Xu J, Zhou JY, Liu Y, Wu GS. Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Res. 2006b;66:8870–8877. doi: 10.1158/0008-5472.CAN-06-1280. [DOI] [PubMed] [Google Scholar]
- Ward SG, Finan P. Isoform-specific phosphoinositide 3-kinase inhibitors as therapeutic agents. Curr. Opin. Pharmacol. 2003;3:426–434. doi: 10.1016/s1471-4892(03)00078-x. [DOI] [PubMed] [Google Scholar]
- White RJ. Regulation of RNA polymerases I and III by the retinoblastoma protein: a mechanism for growth control? Trends Biochem. Sci. 1997;22:77–80. doi: 10.1016/s0968-0004(96)10067-0. [DOI] [PubMed] [Google Scholar]
- Wolthuis RM, Bos JL. Ras caught in another affair: the exchange factors for Ral. Curr. Opin. Genet. Dev. 1999;9:112–117. doi: 10.1016/s0959-437x(99)80016-1. [DOI] [PubMed] [Google Scholar]
- Wu C, Wangpaichitr M, Feun L, Kuo MT, Robles C, Lampidis T, Savaraj N. Overcoming cisplatin resistance by mTOR inhibitor in lung cancer. Mol. Cancer. 2005;4:25. doi: 10.1186/1476-4598-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki F, Johansen MJ, Zhang D, et al. Acquired resistance to erlotinib in A-431 epidermoid cancer cells requires down-regulation of MMAC1/PTEN and up-regulation of phosphorylated Akt. Cancer Res. 2007;67:5779–5788. doi: 10.1158/0008-5472.CAN-06-3020. [DOI] [PubMed] [Google Scholar]
- Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc. Natl. Acad. Sci. (USA) 1994;91:11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q. Restoring p53-mediated apoptosis in cancer cells: new opportunities for cancer therapy. Drug Resist. Updates. 2006;9:19–25. doi: 10.1016/j.drup.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Yu HG, Ai YW, Yu LL, Zhou XD, Liu J, Li JH, et al. Phosphoinositide 3-kinase/Akt pathway plays an important role in chemoresistance of gastric cancer cells against etoposide and doxorubicin induced cell death. Int. J. Cancer. 2008;122:433–443. doi: 10.1002/ijc.23049. [DOI] [PubMed] [Google Scholar]
- Yuan ZQ, Feldman RI, Sussman GE, Coppola D, Nicosia SV, Cheng JQ. AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK1: implication of AKT2 in chemoresistance. J. Biol. Chem. 2003;278:23432–23440. doi: 10.1074/jbc.M302674200. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Shen S, Guo J, Chen H, Greenblatt DY, Kleeff J, et al. Mitogen-activated protein kinases and chemoresistance in pancreatic cancer cells. J. Surg. Res. 2006;136:325–335. doi: 10.1016/j.jss.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Zhong XS, Zheng JZ, Reed E, Jiang BH. SU5416 inhibited VEGF and HIF-1alpha expression through the PI3K/AKT/p70S6K1 signaling pathway. Biochem. Biophys. Res. Commun. 2004;324:471–480. doi: 10.1016/j.bbrc.2004.09.082. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Liu LZ, Fu B, Hu X, Shi X, Fang J, Jiang BH. Reactive oxygen species regulate insulin-induced VEGF and HIF-1alpha expression through the activation of p70S6K1 in human prostate cancer cells. Carcinogenesis. 2007;28:28–37. doi: 10.1093/carcin/bgl085. [DOI] [PubMed] [Google Scholar]
- Zhuang ZG, Di GH, Shen ZZ, Ding J, Shao ZM. Enhanced expression of LKB1 in breast cancer cells attenuates angiogenesis, invasion, and metastatic potential. Mol. Cancer Res. 2006;4:843–849. doi: 10.1158/1541-7786.MCR-06-0118. [DOI] [PubMed] [Google Scholar]