Abstract
The rostral ventromedial medulla (RVM) is involved in facilitation of spinal nociceptive processing and generation of hyperalgesia in inflammatory and neuropathic pain models. We hypothesized that the bilateral hyperalgesia that develops after repeated intramuscular injections of acidic saline is initiated and maintained by activation of descending facilitatory pathways from the RVM. Male Sprague-Dawley rats were implanted with intracerebral guide cannulae into the nucleus raphe magnus (NRM) or the nucleus gigantocellularis (Gi). Two injections of acidic saline into one gastrocnemius muscle 5 days apart leads to robust hyperalgesia after the second injection. Either ropivacaine (local anesthetic) or vehicle (control) was microinjected into the RVM prior to the first intramuscular acid injection, prior to the second injection, or 24h after the second injection. Mechanical withdrawal thresholds of the paw (von Frey filaments) and the muscle (tweezer) were measured before and 24h after induction of hyperalgesia. The withdrawal thresholds for both the paw (cutaneous secondary hyperalgesia) and muscle (primary hyperalgesia) were decreased 24h after the second intramuscular acid injection in the vehicle control groups. Administration of ropivacaine prior to the first intramuscular acid injection had no effect on development of either cutaneous or muscle hyperalgesia that develops after the second injection. However, neither cutaneous nor muscle hyperalgesia developed in the group treated with ropivacaine prior to the second intramuscular injection. Ropivacaine also significantly reversed the hyperalgesia in the group treated 24h after the second intramuscular acid injection. Thus, the RVM is critical for both the development and maintenance of hyperalgesia after muscle insult.
Keywords: pain, raphe magnus, gigantocellularis, facilitation
1. Introduction
Chronic widespread muscle (CWP) pain is a common problem with about 10–15% of the population affected at any given time (Gran 2003). People with CWP pain, like fibromyalgia, demonstrate a generalized decrease in mechanical thresholds (Quimby et al. 1988;Tunks et al. 1988;Granges and Littlejohn 1993;Graven-Nielsen et al. 2000;Staud et al. 2001a), temporal summation to thermal and deep mechanical stimulation (Sorensen et al. 1998;Staud et al. 2001b), and larger areas of referred pain after infusion of hypertonic saline into the muscle (Graven-Nielsen et al. 2000). Patients with CWP also have alterations in descending modulation that are interpreted as decreased endogenous pain inhibition (Kosek and Hansson 1997;Lautenbacher and Rollman 1997;Staud et al. 2002). Together these data suggest there is increased excitability in the central nervous system in people with CWP.
To model these central changes in CWP, we developed an animal model induced by repeated intramuscular acid injections that is characterized by robust muscle (primary), cutaneous (secondary), and visceral (secondary) hyperalgesia (Sluka et al. 2001;Miranda et al. 2004;Yokoyama et al. 2007). Spinal neurons show increased excitability characterized by a bilateral spread of the receptive field (Sluka et al. 2003) and bilateral increases in phosphorylation of the transcription factor CREB (Hoeger-Bement and Sluka 2003). In parallel there is an increase in the release of glutamate in the spinal cord after the second intramuscular injection of acid (Skyba et al. 2005a) and spinal blockade of NMDA receptors reverses the hyperalgesia that occurs after the second injection (Skyba et al. 2002). Thus, this model using repeated intramuscular acid injections mimics CWP with a strong central sensitization.
Secondary hyperalgesia is generally accepted to be maintained by changes in the central nervous system (Willis et al. 1996). Along with the spinal cord, it is now clear that descending influences from the rostral ventromedial medulla (RVM) are both inhibitory and facilitatory (Urban and Gebhart 1999;Porreca et al. 2002). Prior studies examining the role of the RVM show that both the nucleus raphe magnus (NRM) and the nucleus gigantocellularis (Gi) facilitate nociception and promote secondary hyperalgesia associated with tissue injury (Zhuo and Gebhart 1997;Urban et al. 1999;Terayama et al. 2002;Vera-Portocarrero et al. 2006a;Vera-Portocarrero et al. 2006b). Bilateral lesions of the Gi prevent secondary hyperalgesia of the paw induced by joint inflammation, mustard oil, nerve injury, or pancreatitis (Urban et al. 1999;Porreca et al. 2001;Vera-Portocarrero et al. 2006a). In contrast, primary hyperalgesia induced by intraplantar injection of carrageenan is unaffected by lesions of the Gi (Urban et al. 1999). In studies examining the NRM, electrical stimulation at low intensities (5–25µA) facilitates the tail flick latency and increases the response of the dorsal horn to noxious thermal stimuli (Zhuo and Gebhart 1990;Zhuo and Gebhart 1997). Visceral hyperalgesia produced by colon inflammation is reversed by microinjection of an NMDA receptor antagonist into NRM, suggesting increased excitation (Coutinho et al. 1998). Therefore, we hypothesized that the bilateral hyperalgesia that develops after repeated intramuscular injections of acidic saline is initiated and maintained by activation of descending facilitatory pathways from the NRM and Gi.
2. Materials and Methods
All experiments in this study were approved by Animal Care and Use Committee at The University of Iowa in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council).
2.1. Induction of hyperalgesia
Male Sprague-Dawley rats (250–350 g, n=82) were deeply anesthetized with halothane or isofluorane (2–4 %) and injected twice 5 days apart (Day 0 and Day 5) with 100 µl of pH 4.0 saline into the gastrocnemius muscle of the left hind limb. This produces bilateral mechanical hyperalgesia of the paw that lasts for weeks (Sluka et al. 2001).
2.2. Placement of guide cannulae
Intracerebral guide cannulae were placed in the RVM 3 days prior to the first intramuscular injection of either vehicle or acidified saline (Day -3). The rats were anesthetized with isofluorane and positioned in a stereotaxic head holder. The skull was exposed and a small hole drilled for placement of guide cannulae. The cannulae were placed 3 mm above the nucleus raphe magnus (NRM) [intra-aural: −2.0 mm; mediolateral: 0.0 mm; dorsoventral: −6.5 mm from the surface] or bilaterally 3 mm above the nuclei gigantocellullari (Gi) [intra-aural: −2.0 mm; mediolateral: +/− 0.5 mm; dorsoventral: −6.2 mm from the surface]. The cannulae were secured to the skull with dental cement and rats were allowed to recover prior to testing.
To examine placement of the cannula, an equivalent volume of methylene blue dye was injected through the cannula at the end of the experiment. Rats were then euthanized, the brain removed and postifixed in 10% formalin at room temperature for at least 1 week. The day before cutting, brains were transferred to 30% sucrose. Then the brain was cross-sectioned at 35–40 µm on a cryostat and examined under a light microscope for placement of the cannula. Injection sites targeted to NRM also included the nucleus gigantocellulars pars alpha. Injection sites targeted to the Gi remained within the Gi. Previous studies in our laboratories show that 1 µl injections into the NRM can spread to include the nucleus reticularis gigantocellularis pars alpha (Kalra et al., 2001) and 0.5 µl injections into the Gi remain confined to the Gi (Urban et al., 1999
2.3. RVM Injections
Ropivacaine (0.5%) or vehicle (0.9% sterile saline) was administered through the guide cannulae at one of 3 time periods.
i. 15 min prior to the first intramuscular injection on Day 1; 1 µl for the NRM or 0.5 µl/side for the Gi was infused slowly over a period of 1 min through the guide cannula [NRM: n=6 vehicle, n=7 ropivacaine; Gi: n=6 vehicle, n=6 ropivicaine].
ii. 15 min prior to the second intramuscular injection on Day 5; 1 µl for the NRM or 0.5 µl/side for the Gi was infused slowly over a period of 1 min through the guide cannula [NRM: n=7 vehicle, n=8 ropivacaine; Gi: n=6 vehicle, n=6 ropivacaine].
iii. 24 h after the second intramuscular injection on Day 6, 1 µl for the NRM or 0.5 µl/side for the Gi was infused slowly over a period of 1 min through the guide cannula [NRM: n=7 vehicle, n=6 ropivacaine; Gi: n=6 vehicle; n=6 ropivacaine].
2.4. Behavioral testing
Paw withdrawal and muscle withdrawal thresholds were tested for all groups of rats. In all groups, the paw withdrawal threshold was tested prior to the muscle withdrawal threshold. In some rats grip force was also tested (n = 24). For groups of rats receiving intra-RVM drug administration prior to the first or second intramuscular injection, testing was performed at three time periods: (1) before the first intramuscular injection of acidic saline or vehicle, (2) before the second intramuscular injection of acidic saline or vehicle and (3) 24 h after the second intramuscular injection of acidic saline or vehicle. The paw withdrawal thresholds and the muscle withdrawal thresholds were tested before microinjection of the RVM. For groups of rats receiving intra-RVM drug administration 24 h after the second intramuscular injection, the paw withdrawal threshold was determined before the first and second intramuscular injections, 24 h after the second intramuscular injection, and 30 min, 1 h, and 2 h following intra-RVM drug administration. The muscle withdrawal threshold in these rats was determined before the first and second intramuscular injections, 24 h after the second intramuscular injection, and 1 h and 2 h following intra-RVM drug administration.
2.4.1. Paw withdrawal threshold
Rats were tested for paw withdrawal threshold with von Frey filaments applied to the paw. The animals were placed in Lucite cubicles over a wire mesh and acclimated for 30 min before testing. A series of filaments with various bending forces (9.47, 12.74, 17.64, 35.28, 51.94, 76.44, 90.81, 122.5, 226.38, 486.08 mN) were applied to the plantar surface of the hindpaw until the rat withdrew from the stimulus. Each filament was applied twice. The lowest force at which a withdrawal response was obtained was taken as the paw withdrawal threshold. A decrease in withdrawal threshold was interpreted as secondary cutaneous hyperalgesia. There is significant statistical test-retest reliability with this method (Gopalkrishnan and Sluka 2000).
2.4.2. Muscle withdrawal threshold
Rats were tested for muscle withdrawal threshold with a pair of forceps applied to the gastrocnemius muscle as previously described (Skyba et al. 2005b). Rats were acclimated in a restraining device (a canvas garden glove with hole on the top) 3 times a day (1 h apart) for 2 days, each training session consisting of 5 min before intramuscular injection 1. The forceps were equipped with two strain gauges to measure force. To measure the muscle withdrawal threshold, animals were placed in the restrainer and the experimenter compressed the gastrocnemius muscle with the tip of the forceps while the hindlimb was extended.
Compression was continued until the animal withdrew the leg or vocalized. The maximum force applied at withdrawal was recorded as the muscle withdrawal threshold. Three trials five minutes apart at each time period were performed and averaged to obtain one reading per time period. This test does not have a maximum withdrawal threshold and is continued until the animal withdraws from the stimulus. A decrease in withdrawal threshold of the muscle was interpreted as primary muscle hyperalgesia.
2.4.3. Grip force measurement
Rats were tested for grip force of the hindpaw (n=24) with a two gauge grip strength unit (Kehl et al. 2000). The unit consists of two force gauges, 45° mesh grip, grasping bar and acrylic base. For testing the rat was held by the base of the tail. Gripping the rat by the tail, it was gently pulled towards the gauge until the grip was released. The maximum force exerted was recorded at the time the rat released its grip.
2.5. Statistical analysis
Mechanical withdrawal thresholds of paw were tested for differences between groups (vehicle and drug) with a non-parametric Kruskal-Wallis test. Differences across time were assessed with a signed rank test. Compression withdrawal thresholds for muscle and grip force were tested for differences across time and between groups with a repeated measures ANOVA. Post hoc testing was done with a Tukey’s test between groups and a paired t-test across time. Pre-injection groups were tested for an interaction between time of acid injection, intra-RVM injection group, and time of testing. The mechanical withdrawal threshold of the paw is presented as the median and 25th and 75th percentiles. The compression withdrawal thresholds of muscle and grip force are presented as mean +/− S.E.M. Data was considered significant if p< 0.05.
3. Results
Two injections of pH 4.0 saline (Day 0 and Day 5) into one gastrocnemius muscle of rats resulted in a significant bilateral decrease in paw withdrawal threshold (Fig. 1A, vehicle) and muscle withdrawal threshold 24 h after the second injection (Fig. 1B, vehicle), replicating previous findings (Sluka et al., 2001; Yokoyama et al., 2007). There was no effect on either hindpaw or muscle withdrawal thresholds in rats that received intra-RVM vehicle or ropivacaine before the first intramuscular acid injection of pH 4.0 saline (Fig. 1A, B) or in rats that received intra-RVM vehicle before the second intramuscular acid injection of pH 4.0 saline (Fig. 1D, E) or intra-RVM vehicle 24 h after second intramuscular acid injection of pH 4.0 saline (Fig. 1 G, H). There was no change in grip force 24 h after the second injection of acidic saline (forepaw: before the first intramuscular acid injection: 416 ± 13; 24 h after the second intramuscular acid injection: 425 ± 13; hindpaw: before the first intramuscular acid injection: 426 ± 17; 24 h after the second intramuscular acid injection 411 ± 15). Thus, two injections of pH 4.0 saline result in a decrease in withdrawal thresholds of the paw and muscle 24 h after the second injection and this decrease is interpreted as hyperalgesia.
Figure 1.
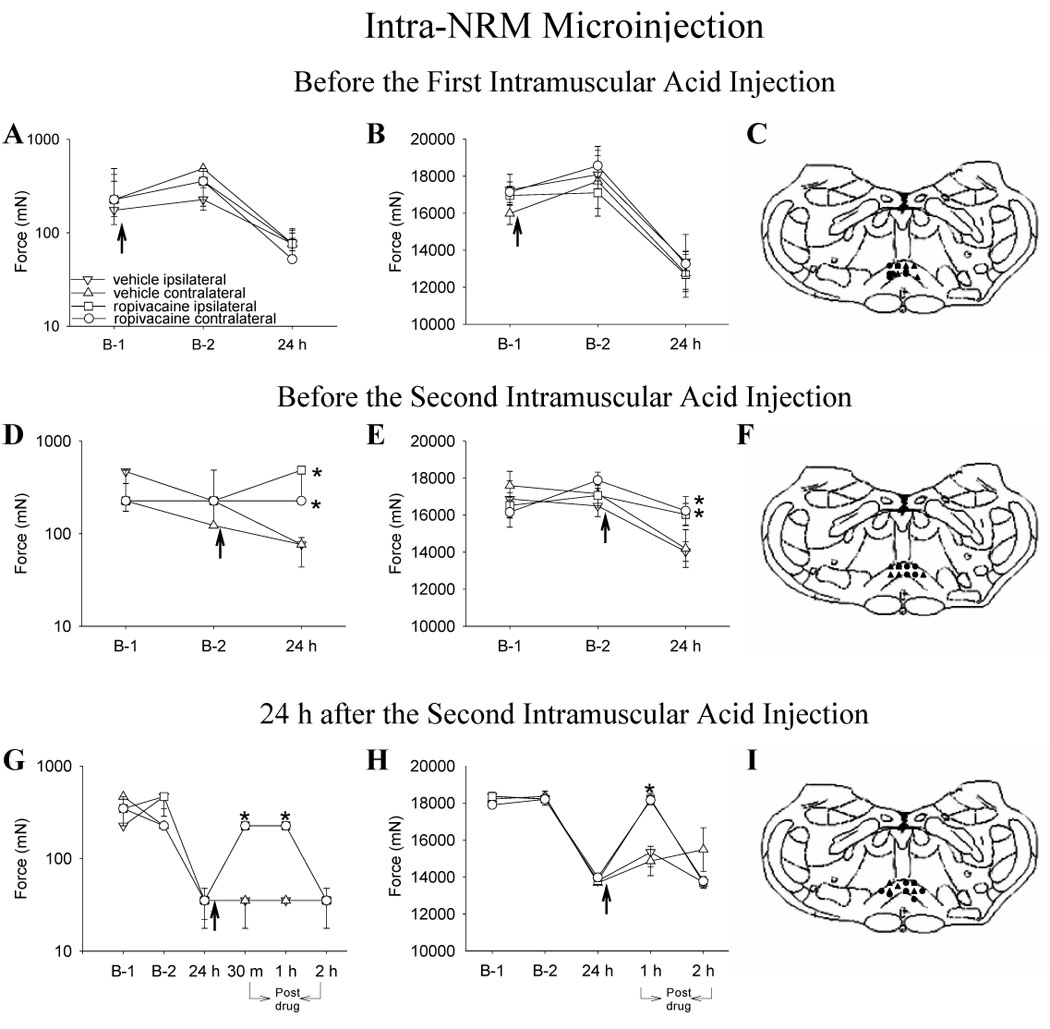
Line graphs representing mechanical withdrawal thresholds of the paw (A, D, G) and of the gastrocnemius muscle (B, E, H) from animals injected into the NRM with ropivacaine (circle) or vehicle (triangle) before injection 1 (A,B,C), before injection 2 (D,E,F) and 24 h after injection 2 (G,H,I) of acidic saline. The ipsilateral side is represented by closed symbols and the contralateral side by open symbols. The arrow represents the time when either ropivacaine or vehicle was injected into the NRM; i.e. immediately after baseline behavior testing and 15 minutes before intramuscular injection of acidic saline. A,B. Ropivacaine microinjected before first injection of acidic saline had no effect on the decreased withdrawal threshold of the paw and muscle 24 h after second injection of acidic saline. C. Sites of injection for the group receiving vehicle (triangle) or ropivaicaine (circle) before the first intramuscular acid injection. D, E. Ropivacaine microinjected before the second injection of acidic saline prevented the decrease in withdrawal thresholds of the paw and muscle. F. Sites of injection for the group receiving vehicle (triangle) or ropivacaine (circle) prior to the second intramuscular acid injection. G,H. Ropivacaine microinjected 24 h after second injection of acidic saline reversed the withdrawal threshold of the paw and muscle for 1 h. I. Sites of injection for the group receiving vehicle (triangle) or ropivacaine (circle) 24 h after the second intramuscular acid injection. The mechanical withdrawal thresholds of the paw are represented as the median with 25th and 75th percentiles and the compression withdrawal thresholds of the muscle are represented as the mean ± SEM. *, significantly greater than vehicle controls
3.1. Effects of ropivacaine administration before the first intramuscular injection
Injection of ropivacaine into the NRM or Gi prior to the first intramuscular injection of pH 4.0 saline on Day 0 had no effect on either hindpaw or muscle hyperalgesia that develops 24 h after the second intramuscular injection of pH 4.0 saline when compared to vehicle controls (Fig. 1A, B, Fig. 2A, B). Thus, despite ropivacaine injection into either the NRM or Gi prior to the first intramuscular injection of pH 4.0 saline, both paw and muscle withdrawal thresholds decreased to the same extent after the second intramuscular injection of pH 4.0 saline as those receiving vehicle injection into the NRM or Gi.
Figure 2.
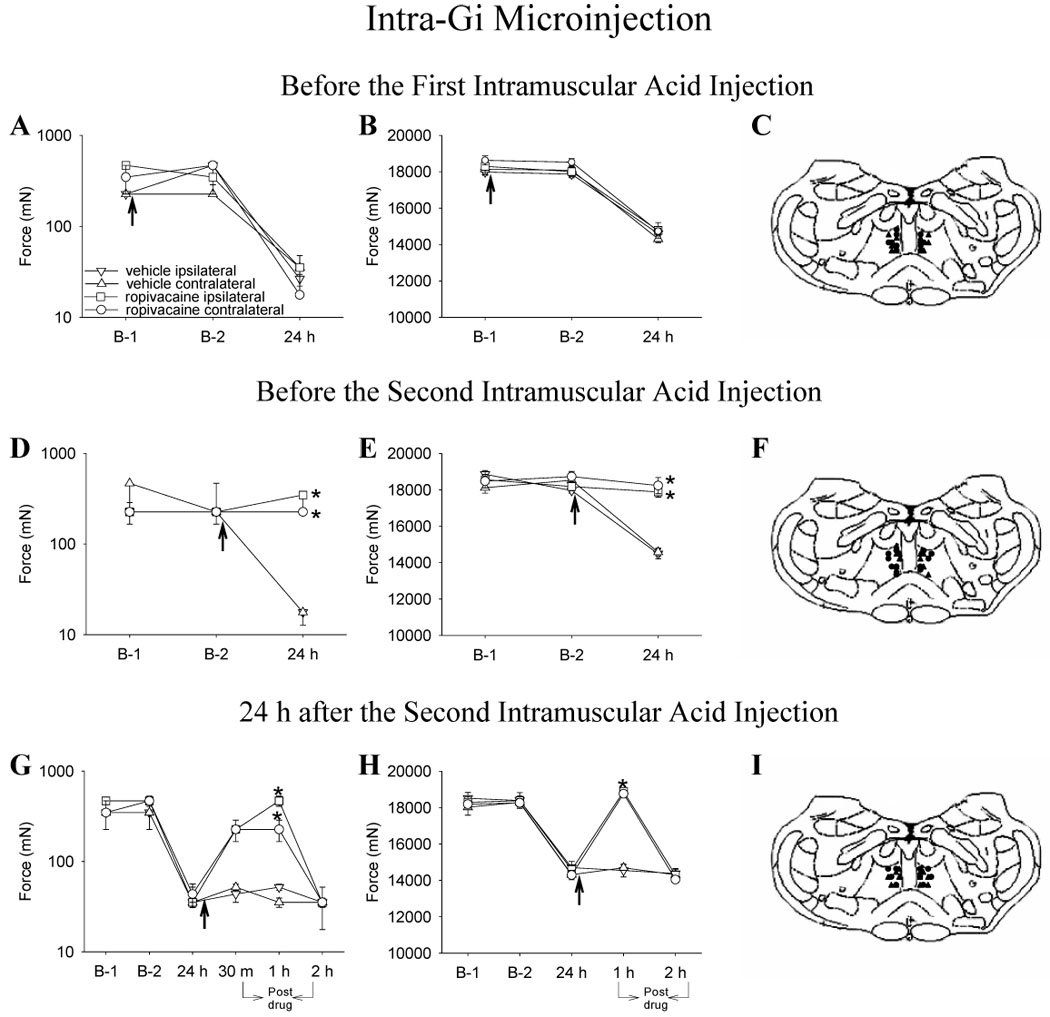
Line graphs representing mechanical thresholds of the paw (A, D, G) and of the gastrocnemius muscle (B, E, H) from animals injected into the Gi with ropivacaine (circle) or vehicle (triangle) before injection 1 (A,B,C), before injection 2 (D,E,F) and 24 h after injection 2 (G,H,I) of acidic saline. The ipsilateral side is represented by closed symbols and the contralateral side by open symbols. The arrow represents the time when either ropivacaine or vehicle was injected into the NRM; i.e. immediately after baseline behavior testing and 15 minutes before intramuscular injection of acidic saline. A,B. Ropivacaine microinjection before first injection of acidic saline had no effect on the decreased withdrawal threshold of the paw and muscle 24 h after second injection of acidic saline. C. Sites of injection for the group receiving vehicle (triangle) or ropivaicaine (circle) before the first intramuscular acid injection. D, E. Ropivacaine microinjected before the second injection of acidic saline prevented the decrease in withdrawal thresholds of the paw and muscle. F. Sites of injection for the group receiving vehicle (triangle) or ropivaicaine (circle) before the second intramuscular acid injection. D, E. Ropivacaine microinjected 24 h after second injection of acidic saline reversed the decreased withdrawal threshold of the paw and muscle for 1 h. F. Sites of injection for the group receiving vehicle (triangle) or ropivaicaine (circle) 24 h after the second intramuscular acid injection. D, E. The mechanical withdrawal thresholds of the paw are represented as the median with 25th and 75th percentiles and the compression withdrawal thresholds of the muscle are represented as the mean ± SEM. *, significantly greater than vehicle controls
3.2. Effects of ropivacaine administration before the second intramuscular injection
The withdrawal threshold of the paw and muscle were significantly greater 24 h after the second intramuscular injection of acidic saline in the group injected with ropivacaine into the Gi or Gi when compared to the group injected with vehicle into the NRM or Gi. That is, the hyperalgesia that normally develops was blocked. Paw withdrawal thresholds after intra-NRM ropivacaine were significantly greater 24 h after the second intramuscular acid injection (ipsilateral paw: χ2= 16.990, p= 0.001; contralateral paw: χ2= 15.649, p= 0.001) compared with the group that received intra-NRM vehicle prior to the second intramuscular acid injection or those receiving intra-NRM ropivacaine prior to the first intramuscular injection of acidic saline (p= 0.0001) (Fig. 1D). For changes in the muscle withdrawal threshold after intra-NRM injections, an overall effect for changes in muscle withdrawal threshold occurred (time of test * ropivacaine vs. vehicle * time of injection into NRM) ipsilaterally (F1, 22 = 11.083 and p= 0.003) and contralaterally (F1, 22 = 18.401 and p= 0.0001) with the group injected with ropivacaine into the NRM significantly greater than the group receiving vehicle or the group receiving ropivacaine prior to first intramuscular injection of acidic saline (p≤ 0.001) (Fig. 1E).
Similarly, for intra-Gi ropivacaine treatment, changes in paw withdrawal threshold were significantly greater 24 h after the second intramuscular acid injection (ipsilateral paw: χ2= 5.073, p= 0.002; contralateral paw: χ2= 15.404, p= 0.001) compared with counterparts that received intra-Gi vehicle prior to the second intramuscular acid injection or those receiving intra-Gi ropivacaine prior to the first intramuscular injection of acidic saline (p ≤ 0.002) (Fig. 2D). For changes in the muscle withdrawal threshold after intra-Gi injections, an overall effect for changes in muscle withdrawal threshold occurred (time of test * ropivacaine vs. vehicle * time of injection into Gi) ipsilaterally (F1, 22 = 35.838 and p= 0.0001) and contralaterally (F1, 20 = 33.84 and p= 0.0001) with the group injected with ropivacaine into the Gi significantly greater than the group receiving vehicle or group receiving ropivacaine prior to first intramuscular injection of acidic saline (p ≤ 0.0001) (Fig. 2E).
To assess the specificity of injection site, we deliberately misplaced cannulae outside the RVM (later located in the facial nucleus, perifacial zone or the intermediate reticular nucleus). In the group with cannulae outside the RVM (n=4), ropivacaine was injected prior to the second intramuscular acid injection and withdrawal threshold measured 24 h later. The withdrawal thresholds of the paws and muscle decreased 24 h after the second acid injection (Fig. 3A, Fig. 3B) and were significantly less than the group that received ropivacaine into the NRM prior to the second intramuscular acid injection (ipsilateral paw: p=0.0001; contralateral paw: 0.0001) or Gi (ipsilateral paw: p=0.0001; contralateral paw: 0.0001).
Figure 3.
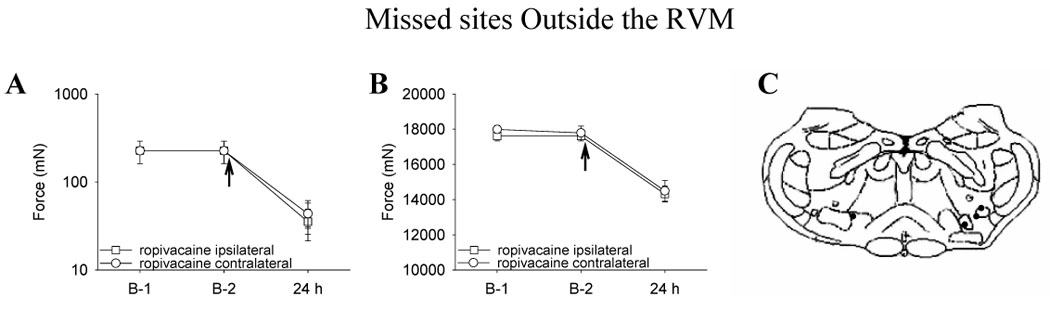
Line graphs representing mechanical withdrawal thresholds of the paw (A) and of the gastrocnemius muscle (B) from animals injected intramuscularly with ropivacaine outside the NRM or Gi prior to injection 2 of intramuscular acidic saline. Ropivacaine microinjected outside the NRM or Gi before the second injection of acidic saline did not prevent the decrease in withdrawal thresholds of the paw and muscle. The arrow represents the time when the drug was delivered, i.e. after the baseline testing and 15 min before the second intramuscular acid injection. The mechanical withdrawal thresholds of the paw are represented as the median with 25th and 75th percentiles and the compression withdrawal thresholds of the muscle are represented as the mean ± SEM. C. Sites of injection in the medulla.
3.3. Effects of ropivacaine administration 24 h after second intramuscular injection
In both the intra-NRM and intra-Gi saline treatment groups there was a significant decrease bilaterally in withdrawal thresholds 24 h after the second intramuscular injection of acidic saline. Injection of ropivacaine into either the NRM or Gi 24 h after the second intramuscular injection of pH 4.0 saline reversed the bilateral decrease in paw and muscle withdrawal thresholds. An overall effect for changes in paw withdrawal thresholds was observed 30 min (ipsilateral paw: χ2= 16.990, p= 0.001; contralateral paw: χ2= 11.143, p= 0.001) and 1 h (ipsilateral paw: χ2= 10.993, p= 0.001; contralateral paw: χ2= 11.143, p= 0.001) after the injection of ropivacaine into the NRM. That is, paw withdrawal thresholds were significantly greater bilaterally in the group injected with ropivacaine than the group receiving vehicle, which exhibited persistent hyperalgesia as previously documented (Fig. 1G). Muscle withdrawal thresholds were also significantly increased 1 hr after the intra-NRM injection of ropivacaine (time of test * ropivacaine vs. vehicle * time of injection into NRM) both ipsilaterally (F1, 11 = 12.664 and p= 0.004) and contralaterally (F1, 11 = 46.95 and p= 0.0001) compared with the group receiving intra-NRM vehicle (p ≤ 0.0001) (Fig. 1H). However, withdrawal thresholds for paws and muscle 2 h after intra-NRM injection of ropivacaine were no different than withdrawal threshold 2 hr after intra-NRM injection of vehicle.
Similarly, an overall significant effect for changes in paw withdrawal thresholds was observed 30 min (ipsilateral paw: χ2= 9.90, p= 0.002; contralateral paw: χ2= 8.89, p= 0.003) and 1 h (ipsilateral paw: χ2= 8.8, p= 0.003; contralateral paw: χ2= 9.659, p= 0.002) after injection of ropivacaine into the Gi. Again, paw withdrawal thresholds were significantly greater bilaterally in the group injected with ropivacaine than the group receiving vehicle (Fig. 2G). An overall effect for changes in muscle withdrawal threshold occurred (time of test * ropivacaine vs. vehicle * time of injection into Gi) both ipsilaterally (F1, 10 = 25.709 and p= 0.0001) and contralaterally (F1, 10 = 13.482 and p= 0.004) with muscle withdrawal thresholds greater bilaterally 1 h after the injection of ropivacaine when compared to the group receiving vehicle (Fig. 2H). However, when tested 2 h after intra-Gi ropivacaine, withdrawal thresholds for paw and muscle were no different from withdrawal thresholds in vehicle controls.
4. Discussion
The principal finding of the present study is that reversible blockage (by ropivacaine) of either the NRM or Gi bilaterally prior to the second, hyperalgesia-producing intramuscular injection of pH 4.0 saline prevents development of both primary muscle and secondary cutaneous mechanical hyperalgesia. Further, if the hyperalgesia is permitted to develop, intra-RVM ropivacaine is able to reverse the fully developed primary (muscle) and secondary (paw) mechanical hyperalgesia. Thus, it can be concluded that cells in the NRM and Gi (or axons passing through these RVM cell groups) are critical for both the development and maintenance of mechanical hyperalgesia after muscle insult.
The RVM is an important supraspinal site for the integration of ascending nociceptive signals from the spinal cord and other supraspinal sites such as the cortex and periaqueductal grey matter (PAG) (Fields and Basbaum 1999). The NRM and the Gi are two nuclei within the RVM that prior studies demonstrate can facilitate spinal nociceptive transmission and maintain hyperalgesia associated with tissue injury (Morgan and Fields 1994;Zhuo and Gebhart 1997;Urban et al. 1999;Kovelowski et al. 2000;Porreca et al. 2001;Terayama et al. 2002;Burgess et al. 2002;Vera-Portocarrero et al. 2006a). Previous literature shows that neuron lesions (ibotenic acid) of the Gi prevent secondary hyperalgesia of the ipsilateral paw induced by joint inflammation or mustard oil (Urban et al. 1999), but have no effect on the development of primary hyperalgesia induced by paw inflammation (Urban et al. 1999). Similarly, following spinal nerve ligation, mechanical and heat hyperalgesia of the paw is reversed by anesthetic blockade of Gi with lidocaine or selective lesion of RVM neurons expressing µ-opioid receptors (Porreca et al. 2001;Vanderah et al. 2001;Burgess et al. 2002). Further, blockade of NMDA receptors in the NRM reverses visceral hyperalgesia that occurs after colon inflammation (Coutinho et al. 1998). Increased facilitation after cutaneous paw inflammation occurs not only for the inflamed paw but also the non-inflamed paw and tail (Terayama et al. 2002). Descending projections from the RVM are bilateral (Zemlan et al. 1984;Antal et al. 1996) and when stimulated electrically or chemically, changes occur bilaterally in both hindpaws (Hurley and Hammond 2000;Terayama et al. 2002). The present results suggest that bilateral cutaneous secondary hyperalgesia, as observed after muscle insult, likely involves facilitatory influences descending from the RVM. We extend prior studies by showing that bilateral primary muscle hyperalgesia is also prevented or reversed by anesthetic blockade of the Gi or NRM. Although we refer to the muscle hyperalgesia as primary, it may also be secondary since there is no observed tissue injury in this model, and the hyperalgesia is maintained independent of the afferent input (Sluka et al. 2001). This issue remains to be resolved, however.
The effects of intra-RVM local anesthetic described here may be due to either a shift in the normally pre-potent inhibitory influence on spinal nociceptive transmission to activation and dominance of facilitatory influences, masking (or inhibition) of descending inhibitory influences (i.e., disinhibition), or a combination of both. After tissue injury, time dependent changes in the balance between facilitation and inhibition are observed. For example, lesions of the Gi reduce the intensity of stimulating current in the NRM needed to inhibit nociception 3 h, but not 24 h after a cutaneous inflammatory insult, when compared to controls with intact Gi. These data suggest that the Gi may be involved in early pain facilitation through the NRM.
Similarly, NMDA receptor antagonists also reduce the stimulating current needed to produce inhibition at 3 h, also suggesting a role for NMDA receptors in descending facilitation in a model of cutaneous inflammatory pain (Terayama et al. 2002;Guan et al. 2002). These studies further show that at 24 h there is an increase in inhibition (Terayama et al. 2002;Guan et al. 2002). Additionally, reversal of secondary hyperalgesia occurs within 4 h after induction of deep tissue inflammation (viscera and joint) (Coutinho et al. 1998;Urban et al. 1999). Time dependent activation of descending facilitatory pathways is also observed in a neuropathic pain model. However, in this neuropathic pain model descending facilitation is not detectable until 6 days after injury (Burgess et al. 2002). Different models, inflammatory and neuropathic, result in a different pattern of descending facilitation that may be unique to the site of the injury (e.g., nerve, viscera, muscle, skin/subcutaneous). In support, formalin injected into the muscle increases c-fos expression predominately in the ventrolateral PAG with some expression in the lateral PAG. Whereas formalin injection into skin increases c-fos predominately in the lateral PAG with some expression in the ventrolateral PAG. Together these data suggest differential supraspinal patterns of nociceptive transmission from muscle and skin (Keay and Bandler 1993). In the present study, injection of ropivacaine into the RVM prior to the second intramuscular injection of acidic saline prevents the development of cutaneous and muscle hyperalgesia, and reverses the hyperalgesia if administered 24 h later. These outcomes permit the conclusion that descending facilitation associated with deep tissue injury (muscle, joint, viscera) predominates over descending inhibition at early time points and is critical for full development of hyperalgesia after muscle insult.
The results described here specifically address two distinct areas within the RVM that have been implicated in several studies of descending inhibition. Blockade of either of the NRM or Gi appears to completely reverse hyperalgesia, suggesting that both nuclei are critical to the maintenance of the bilateral hyperalgesia and, further, that the Gi and the NRM may be functionally interconnected. Lesions of the Gi reduce hyperalgesia and spinal Fos expression produced by hindpaw inflammation; additional ablation of the NRM along with the Gi prevented the anti-hyperalgesic effect produced by lesioning the Gi (Wei et al. 1999). Further, lesions of the Gi reduce the intensity of current in the NRM needed to inhibit hyperalgesia 3 h after inflammation (Terayama et al. 2002). Together these outcomes suggest that the Gi drives facilitation from the NRM. Efferent projections from the Gi to the spinal cord terminate in the intermediate zones of the spinal cord, laminae VII and VIII bilaterally (Zemlan et al. 1984) and those of the NRM terminate in dorsal horn laminae I, II, and V (Basbaum et al. 1986;Jones and Light 1990). Interestingly, however, there are interconnections between most nuclei in the RVM, including projections between the NRM and the paragigantocellularis lateralis, and between the Gi and the NRM (Matsuyama et al. 1988;Zagon 1993;Zagon 1995).Alternatively, the Gi could project rostrally to areas involved in nociception that project back to the NRM. For example, the Gi projects to the periaqueductal gray matter and the dorsolateral pontine nuclei, which in turn project directly to the NRM (Abols and Basbaum 1981;Zemlan et al. 1984;Sim and Joseph 1992;Vertes and Kocsis 1994;Cameron et al. 1995). Together these studies suggest that the Gi interacts with the NRM to enhance nociception and that both the NRM and the Gi directly inhibit nociception at the level of the spinal cord. Thus, future studies should be carried out to understand the interaction between these two nuclei.
In summary, descending facilitatory input from the RVM initiates and maintains cutaneous and muscle hyperalgesia associated with chronic muscle pain. Clinical muscle studies show that deficits in the descending pain inhibitory systems may contribute to chronic conditions like fibromyalgia (Julien et al. 2005). Thus, pain in people with fibromyalgia may be due to a shift in the balance between endogenous inhibitory and facilitatory influences, representing a dysfunction within endogenous systems of modulation.
Acknowledgements
We wish to thank Ms. Tammy Lisi, Ms. Lynn Burnes, Ms. Jing Danielson, Dr. Yumi Maeda and Dr. Takeshi Yokoyama for technical assistance. Supported by National Institutes of Health awards AR052316, AR02201 and DA02879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abols IA, Basbaum AI. Afferent connections of the rostral medulla of the cat: a neural substrate for midbrain-medullary interactions in the modulation of pain. J Comp Neurol. 1981;201:285–297. doi: 10.1002/cne.902010211. [DOI] [PubMed] [Google Scholar]
- Antal M, Petko M, Polgar E, Heizmann CW, StormMathisen J. Direct evidence of an extensive GABAergic innervation of the spinal dorsal horn by fibres descending from the rostral ventromedial medulla. Neruosci. 1996;73:509–518. doi: 10.1016/0306-4522(96)00063-2. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Ralston DD, Ralston HJI. Bulbospinal projections in the primate: a light and electron microscopic study of a pain modulating system. J Comp Neurol. 1986;250:311–323. doi: 10.1002/cne.902500305. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: A Phaseoleus vulgaris-leucoagglutinin study. II. Descending projections. J Comp Neurol. 1995;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Urban MO, Gebhart GF. Role of glutamate receptors and nitric oxide in the rostral ventromedial medulla in visceral hyperalgesia. Pain. 1998;78:59–69. doi: 10.1016/S0304-3959(98)00137-7. [DOI] [PubMed] [Google Scholar]
- Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Textbook of Pain. New York: Churchill Livingstone; 1999. pp. 243–257. [Google Scholar]
- Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity and pulse duration of TENS on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81:984–990. doi: 10.1053/apmr.2000.5576. [DOI] [PubMed] [Google Scholar]
- Gran JT. The epidemiology of chronic generalized musculoskeletal pain. Best Practice Res Clinical Rheumatol. 2003;17:547–561. doi: 10.1016/s1521-6942(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Granges G, Littlejohn G. Pressure pain threshold and pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis Rheum. 1993;36:641–646. doi: 10.1002/art.1780360510. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T, Aspegren KS, Henriksson KG, Bengtsson M, Sorensen J, Johnson A, Gerdle B, rendt-Nielsen L. Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain. 2000;85:483–491. doi: 10.1016/S0304-3959(99)00308-5. [DOI] [PubMed] [Google Scholar]
- Guan Y, Terayama R, Dubner R, Ren K. Plasticity in excitatory amino acid receptor-mediated descending pain modulation after inflammation. J Pharmacol Exp Ther. 2002;300:513–520. doi: 10.1124/jpet.300.2.513. [DOI] [PubMed] [Google Scholar]
- Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci. 2003;23:5437–5445. doi: 10.1523/JNEUROSCI.23-13-05437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley RW, Hammond DL. The analgesic effects of supraspinal mu and delta opioid receptor agonists are potentiated during persistent inflammation. J Neurosci. 2000;20:1249–1259. doi: 10.1523/JNEUROSCI.20-03-01249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SL, Light AR. Termination patterns of serotoninergic medullary raphespinal fibers in the rat lumbar spinal cord: an anterograde immunohistochemical study. J Comp Neurol. 1990;297:267–282. doi: 10.1002/cne.902970209. [DOI] [PubMed] [Google Scholar]
- Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114:295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Keay KA, Bandler R. Deep and superficial noxious stimulation increases Fos-like immunoreactivity in different regions of the midbrain periaqueductal gray of the rat. Neurosci Lett. 1993;154:23–26. doi: 10.1016/0304-3940(93)90162-e. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Trempe TM, Hargreaves KM. A new animal model for assessing mechanisms and management of muscle hyperalgesia. Pain. 2000;85:333–343. doi: 10.1016/S0304-3959(99)00282-1. [DOI] [PubMed] [Google Scholar]
- Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70:41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- Kovelowski CJ, Ossipov MH, Sun H, Lai J, Malan TP, Porreca F. Supraspinal cholecystokinin may drive tonic descending facilitation mechanisms to maintain neuropathic pain in the rat. Pain. 2000;87:265–273. doi: 10.1016/S0304-3959(00)00290-6. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clinical Journal of Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Ohta Y, Mori S. Ascending and descending projections of the nucleus reticularis gigantocellularis in the cat demonstrated by the anterograde neural tracer, Phaseolus vulgaris leucoagglutinin (PHA-L) Brain Res. 1988;460:124–141. doi: 10.1016/0006-8993(88)91212-7. [DOI] [PubMed] [Google Scholar]
- Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Fields HL. Pronounced changes in the activity of nociceptive modulatory neurons in the rostral ventromedial medulla in response to prolonged thermal noxious stimuli. J Neurophysiol. 1994;72:1161–1170. doi: 10.1152/jn.1994.72.3.1161. [DOI] [PubMed] [Google Scholar]
- Porreca F, Burgess SE, Gardell LR, Vanderah TW, Malan TP, Ossipov MH, Lappi DA, Lai J. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Quimby L, Block S, Gratwich G. Fibromyalgia: generalized pain intolerance and manifold symptom reporting. J Rheumatol. 1988;15:1264–1270. [PubMed] [Google Scholar]
- Sim LJ, Joseph SA. Efferent projections of the nucleus raphe magnus. Brain Res Bull. 1992;28:679–682. doi: 10.1016/0361-9230(92)90246-t. [DOI] [PubMed] [Google Scholar]
- Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98:69–78. doi: 10.1016/s0304-3959(01)00471-7. [DOI] [PubMed] [Google Scholar]
- Skyba DA, Lisi TL, Sluka KA. Excitatory amino acid concentrations increase in the spinal cord dorsal horn after repeated intramuscular injection of acidic saline. Pain. 2005a;119:142–149. doi: 10.1016/j.pain.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Skyba DA, Radhakrishnan R, Sluka KA. Characterization of a method for measuring primary hyperalgesia of deep somatic tissue. Journal of Pain. 2005b;6:41–47. doi: 10.1016/j.jpain.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle & Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–139. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Sorensen J, Graven-Nielsen T, Henriksson KG, Bengtsson M, Arendt-Nielsen L. Hyperexcitability in fibromyalgia. J Rheumatol. 1998;25:152–155. [PubMed] [Google Scholar]
- Staud R, Carl KE, Vierck CJ, Price DD, Robinson ME, Cannon RL, Mauderli AP. Repetitive muscle stimuli result in enhanced wind-up of fibromyalgia patients. Arthritis Rheum. 2001a;44:S395. [Google Scholar]
- Staud R, Robinson ME, Mauderli AP, Cannon RL, Vierck CJ, Price DD. Opioids modulate the enhanced temporal summation of second pain of fibromyalgia patients. Arthritis Rheum. 2002;46:S396. doi: 10.1016/s0304-3959(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD. Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 2001b;91:165–175. doi: 10.1016/s0304-3959(00)00432-2. [DOI] [PubMed] [Google Scholar]
- Terayama R, Dubner R, Ren K. The roles of NMDA receptor activation and nucleus reticularis gigantocellularis in the time-dependent changes in descending inhibition after inflammation. Pain. 2002;97:171–181. doi: 10.1016/s0304-3959(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Tunks E, Crook J, Norman G, Kalaher S. Tender points in fibromyalgia. Pain. 1988;34:11–19. doi: 10.1016/0304-3959(88)90176-5. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban MO, Zahn PK, Gebhart GF. Descending facilitatory influences from the rostral medial medulla mediate secondary, but not primary hyperalgesia in the rat. Neurosci. 1999;90:349–352. doi: 10.1016/s0306-4522(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Suenaga NMH, Ossipov MH, Malan TP, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–286. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Xie JY, Kowal J, Ossipov MH, King T, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains visceral pain in rats with experimental pancreatitis. Gastroenterology. 2006a;130:2155–2164. doi: 10.1053/j.gastro.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Vera-Portocarrero LP, Zhang ET, Ossipov MH, Xie JY, King T, Lai J, Porreca F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Nerusoci. 2006b;140:1311–1320. doi: 10.1016/j.neuroscience.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Projections of the dorsal raphe nucleus to the brainstem: PHA-L analysis in the rat. J Comp Neurol. 1994;340:11–26. doi: 10.1002/cne.903400103. [DOI] [PubMed] [Google Scholar]
- Wei F, Dubner R, Ren K. Nucleus reticularis gigantocellularis and nucleus raphe magnus in the brain stem exert opposite effects on behavioral hyperalgesia and spinal Fos protein expression after peripheral inflammation. Pain. 1999;80:127–141. doi: 10.1016/s0304-3959(98)00212-7. [DOI] [PubMed] [Google Scholar]
- Willis WD, Sluka KA, Rees H, Westlund KN. Cooperative mechanisms of neurotransmitter action in central nervous sensitization. Prog Brain Res. 1996;110:151–166. doi: 10.1016/s0079-6123(08)62572-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007 doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Zagon A. Innervation of serotonergic medullary raphe neurons from cells of the rostral ventrolateral medulla in rats. Neruosci. 1993;55:849–867. doi: 10.1016/0306-4522(93)90446-m. [DOI] [PubMed] [Google Scholar]
- Zagon A. Internal connections in the rostral ventromedial medulla of the rat. J Auton Nerv Syst. 1995;53:43–56. doi: 10.1016/0165-1838(94)00164-f. [DOI] [PubMed] [Google Scholar]
- Zemlan FP, Behbehani MM, Beckstead RM. Ascending and descending projections from nucleus reticularis magnocellularis and nucleus reticularis gigantocellularis - an autoradiographic and horseradish peroxidase study in the rat. Brain Res. 1984;292:207–220. doi: 10.1016/0006-8993(84)90757-1. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Characterization of descending inhibition and facilitation from the nucleus reticularis gigantocellularis and nucleus reticularis gigantocellularis pars alpha in the rat. Pain. 1990;42:337–350. doi: 10.1016/0304-3959(90)91147-B. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol. 1997;78:746–758. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]


