Abstract
The present study describes two novel compound heterozygous mutations, c.410C>T(p.T137M) (T137M) on the maternal and g.4225_50del on the paternal allele of SLC34A3, in a previously reported male with hereditary hypophosphatemic rickets with hypercalciuria (HHRH) and recurrent kidney stones (Chen C, Carpenter T, Steg N, Baron R, Anast C. Pediatrics 84: 276–280, 1989). For functional analysis in vitro, we generated expression plasmids encoding enhanced green fluorescence protein (EGFP) concatenated to the NH2 terminus of wild-type or mutant human type IIc Na-Pi cotransporter (NaPi-IIc), i.e., EGFP-hNaPi-IIc, EGFP-[M137]hNaPi-IIc, or EGFP-[Stop446]hNaPi-IIc. The V446Stop mutant showed complete loss of expression and function when assayed for apical patch expression in opossum kidney (OK) cells and sodium-dependent 33P uptake into Xenopus laevis oocytes. Conversely, EGFP-[M137]hNaPi-IIc was inserted into apical patches of OK cells and into oocyte membranes. However, when quantified by confocal microscopy, surface fluorescence was reduced to 40% compared with wild-type. After correction for surface expression, the rate of 33P uptake by oocytes mediated by EGFP-[M137]hNaPi-IIc was decreased by an additional 60%. The resulting overall reduction of function of this NaPi-IIc mutant to 16%, taken together with complete loss of expression and function of g.4225_50del(V446Stop), thus appears to be sufficient to explain the phenotype in our patient. Furthermore, the stoichiometric ratio of 22Na and 33P uptake was increased to 7.1 ± 3.65 for EGFP-[M137]hNaPi-IIc compared with wild-type. Two-electrode studies indicate that EGFP-[M137]hNaPi-IIc is nonelectrogenic but displayed a significant phosphate-independent inward-rectified sodium current, which appears to be insensitive to phosphonoformic acid. M137 thus may uncouple sodium-phosphate cotransport, suggesting that this amino acid residue has an important functional role in human NaPi-IIc.
Keywords: SLC34A3, rickets, hypercalciuria, nephrolithiasis
hereditary hypophosphatemic rickets with hypercalciuria (HHRH; OMIM 241530) is an autosomal recessive renal phosphate-wasting disorder that was originally described by Tieder et al. (33, 34, 32). We and others recently reported homozygous or compound heterozygous mutations in SLC34A3, the gene encoding the proximal tubular sodium-phosphate cotransporter NaPi-IIc, as the genetic cause of HHRH (7, 16, 19). Individuals carrying SLC34A3 mutations on both alleles display the full clinical picture of HHRH with an expressivity of ∼70% at least for the homozygous c.228del mutation identified in the original Bedouin kindred (7). Laboratory findings consist of hypophosphatemia due to insufficient renal phosphate retention, leading to rickets or osteomalacia. Compensatory upregulation of 1-α-hydroxylase in the kidney increases circulating 1,25-dihydroxy-vitamin D [1,25(OH)2D] levels, thereby enhancing intestinal absorption of calcium, which then causes hypercalciuria. Parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) levels are generally normal or low (16). Heterozygous carriers of SLC34A3 mutations often exhibit (presumably absorptive) hypercalciuria, and these individuals display hypophosphatemia and elevation of 1,25(OH)2D, albeit in a less pronounced manner than observed in patients with HHRH, and bone changes are generally missing. Renal calcifications, either on presentation, or as the consequence of inappropriate therapy with vitamin D analogs, may be more common in HHRH than originally described (5, 6, 11, 16, 32).
NaPi-IIc belongs to the type II class of sodium-phosphate cotransporters, which is, along with NaPi-IIa (SLC34A1), expressed in the proximal tubule of the kidney (3, 22, 23). In contrast to NaPi-IIa, which is expressed in segments S1–S3, expression of NaPi-IIc appears to be confined to the S1 segment (20). Furthermore, the latter interacts with the sodium-hydrogen exchanger regulator 1 and 3 (NHERF-1 and -3) via determinants in the third intracellular loop (17) and the COOH-terminal tail (35), and this interaction does not appear to involve a COOH-terminal PDZ-binding motif similar to that required for binding of NaPi-IIa to NHERF-1 and -2 (14). Like the expression of NaPi-IIa, the abundance of NaPi-IIc protein in the brush-border membrane is regulated by phosphate, PTH, and FGF-23 (21). However, disappearance of NaPi-IIc from brush-border membrane vesicles in response to the injection of PTH into mice appears to be considerably slower (hours) than the disappearance of NaPi-IIa (minutes) (29). Furthermore, NaPi-IIc protein appears to get reinserted into the membrane (8, 28), whereas NaPi-IIa is internalized via the clathrin-coated pits within minutes and degraded via the endosomal pathway (2). These differences in regulation may explain why NaPi-IIa can only partially compensate for the lack of NaPi-IIc in individuals affected by HHRH and why Npt-2c appears to compensate only partially for the lack of Npt-2a in the mouse (4, 31).
This inability to fully compensate for the lack of one transporter by expressing the respective other may also be related, at least partially, to the finding that Npt-2c is most prominently expressed during development (26). Consequently, adult Npt-2c null mice show no hypophosphatemia (27). Their residual moderate hypercalciuria and elevation of their serum calcium and 1,25(OH)2D levels furthermore indicate that the observed small increase in Npt-2a mRNA and protein is not sufficient to fully normalize phosphate homeostasis (27). Similarly, Npt-2a null animals show renal phosphate wasting, hypercalciuria, nephrocalcinosis, and skeletal abnormalities until weaning. However, older mice lacking Npt-2a have serum phosphate levels that are only slightly lower then those of wild-type littermates, which may be related to the upregulation of Npt-2c expression in these mice (4, 31). Mice that are null for both sodium-phosphate cotransporters appear to have a more severe phenotype than those lacking either cotransporter alone (24), which further supports the hypothesis that each transporter has a partially nonredundant role in the regulation of phosphate homeostasis. The hypophosphatemia in individuals with HHRH persists life-long, which is in contrast to the mild phenotype observed in Npt-2c null mice. It is therefore conceivable that some human NaPi-IIc mutations have a dominant phenotype and thus impair renal phosphate reabsorption more severely than predicted by the findings in the Npt-2c null mouse (24).
Because of these considerations, we sought to explore how two novel human NaPi-IIc mutations affect sodium-phosphate cotransporter function. Besides describing two SLC34A3 mutations, c.410C>T(p.T137M) (T137M) and g.4225_50del, which provide a molecular explanation for clinical and laboratory findings in a previously reported male who is affected by HHRH (11), our analyses indicated that the g.4225_50del mutation in SLC34A3 leads to poor mRNA expression, whereas the T137M mutation, which affects a conserved amino acid residue, appears to impair coupled sodium-phosphate cotransport.
MATERIALS AND METHODS
Experimental subjects.
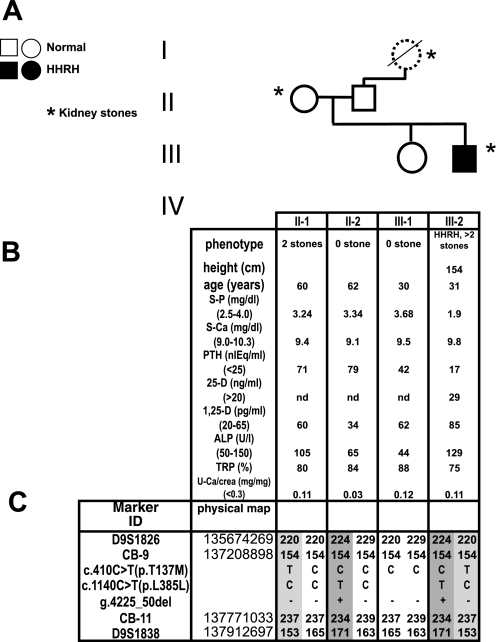
All clinical investigations described in this study was conducted after informed consent in accordance with the ethical standards of the Helsinki Declaration of 1975 (and as revised in 1983) and approved by the institutional review committees of Yale University and Massachusetts General Hospital (Boston, MA). The index case, a now 38-year-old Turkish man, presented at age 2 yr with knee and hip pain, fatigue, and difficulty walking. He developed genua valga and radiological rickets at age 5 yr and received vitamin D (5,000 U/day) for 3 wk, after which he was lost to follow-up. At age 10 yr, he was seen again with severe rickets, but treatment with vitamin D (40,000 U/day) and phosphate (1.1 g/day) again was stopped after 3 wk due to development of flank pain. At age 14 yr, he was admitted for a metabolic ward study, which established the clinical diagnosis of hypophosphatemic rickets with hypercalciuria (11). His height at the time was 148 cm (5th percentile), and his weight was 43 kg (10th percentile). His biochemical analyses showed mild hypophosphatemia (3.0–3.8 mg/dl, normal range 3.5–5.5); elevated 1,25(OH)2D (133–148 pg/ml, normal range 20–80); normal PTH (1.9–2.0 μeq/ml, normal range <25); elevated alkaline phosphatase (23.6–37.9 IU/ml, normal range 5–19); normal calcium (9.8–10.4 mg/dl, normal range 8.5–10.5); normal 1,25(OH)2D (12.6–16.1 ng/ml, normal range 10–40); and normal renal function. His urinary calcium ranged from 5.8 to 6.4 mg·kg−1·day−1 (normal range <4), his TmP/GFR was 2.6–3.2 mg/dl (normal range 2.7–5.6); his radiographical evaluation showed epiphyseal flaring; and the histomorphometric analysis of a tetracyclin-labeled iliac crest bone biopsy was consistent with osteomalacia. On a controlled diet containing 850 mg/day phosphate, 500 mg calcium, and 385 mg magnesium, his phosphate balance was negative. On 1.2–1.4 g elemental phosphate/day, his hypophosphatemia resolved and his urinary calcium excretion dropped to 0.09 mg·kg−1·day−1. He developed flank pain on day 11 of his admission and was found to have a left urethral stone. He remained off phosphate and vitamin D supplements for most of his young adult life, during which time he suffered three additional episodes of renal colic due to stones. His biochemical analysis off therapy at age 31 yr continued to show hypophosphatemia due to renal phosphate losses (see Fig. 2B). He is the son of nonconsanguineous parents; his mother had a renal stone at age 4 yr, requiring surgical removal and a second stone during pregnancy, but had normal serum and urine biochemical findings. His paternal grandmother reportedly had recurrent renal stones, but biochemical analyses were not available; his father, sister, and daughter are clinically and biochemically unaffected (see Fig. 2B).
Fig. 2.
Pedigree and haplotype analysis. A: pedigree. Solid circles or squares indicate individuals who developed rickets during childhood along with renal phosphate-wasting, hypophosphatemia, and hypercalciuria. Open symbols indicate healthy individuals. Samples with dashed lines are from individuals who were unavailable for genotyping. B: clinical and biochemical parameters of the available family members were obtained before therapy at the age indicated. Age-related reference ranges are given in brackets. n.d., Not done. C: haplotypes for chromosomal region 9q34 between markers D9S1826 and D9S1838. Alleles for microsatellite markers are designated as bp or coded; the haplotypes associated with hereditary hypophosphatemic rickets with hypercalciuria (HHRH) are depicted by numbers on a gray background. The mutations were identified by nucleotide sequencing analysis and confirmed by PCR product length or allele-specific restriction enzymatic digest on at least 2 independent PCR products.
Clinical studies.
Current fasting blood samples were taken after informed consent at Yale University or in the Duzen Laboratuvarlari (Ankara, Turkey) and assayed for calcium, phosphate, creatinine, unfractionated alkaline phosphatase, 1,25(OH)2D, and intact PTH using commercially available kits.
Genetic studies.
The entire SLC34A3 gene of the index case (III-2), spanning 5 kb including 800 bp of the 5′-promoter region and all 13 exons and intronic sequences, was amplified by PCR and subjected to nucleotide sequence analysis at the Massachusetts General Hospital DNA Sequencing Core Facility. PCR assays for the confirmation in III-2 and analysis of g.4225_50del and c.410C>T(p.T137M) in the available family members were designed as described (7). In brief, 20 μM of forward primer 50 and reverse primer 53 (see Table 1) were used with reagents from Qiagen (Valencia, CA), 250 μM dNTP at 94°C × 5-min initial denaturation followed by 40 cycles of 94°C × 1 min, 65°C × 1 min, 72°C × 1 min, and 72°C × 10-min final extension to obtain a normal 397-bp and mutant 370-bp PCR product for g.4225_50del. To amplify c.410C>T(p.T137M), primers 32 and 33 were used at 94°C × 5 min for the initial denaturation followed by 40 cycles of 94°C × 1 min, 70°C × 1 min, 72°C × 2 min, and 72°C × 10 min final extension, followed by restriction enzymatic digest with NlaIII to obtain fragments of 377 and 71 bp (wild-type) or 352, 71, and 24 bp (mutant) (Table 2).
Table 1.
List of primers
| Primer | Orientation | Location | Sequence | AT |
|---|---|---|---|---|
| 32 | Forward | Intron 4 | GAGGGCCAGCCAGGGACA | 70°C |
| 33 | Reverse | Exon 13 | TCCAGAGAATGGAGCCAGAC | 70°C |
| 46 | Forward | Exon 12 | CAGGGCTGACCCAGCATC | 63°C |
| 50 | Forward | Exon 13 | CTGGTGACCCCACCTCGT | 65°C |
| 53 | Reverse | Exon 13 | TCCAGAGAATGGAGCCAGAC | 65°C |
| 104 | Reverse | Exon 13 | GGTACCACAGCAGGATGC | NA |
| 105 | Forward | Exon 11 | GCTGGCTCGGCGGCTACC | 65°C |
| 190 | Forward | Exon 5 | CCCTGGTGCAGAGCTCGAGCATGTCCTCCTCCATC | 55°C |
| 191 | Reverse | Exon 5 | GATGGAGGAGGACATGCTCGAGCTCTGCACCAGGG | 55°C |
| 238 | Forward | pEGFP-C1 | ATTTAGGTGACACTATAGAAGCGTGTACGGTGGGAGGTCTA | 60°C |
| 239 | Reverse | Exon 13 | ACAGAAGGGGAAGCAGGTG | 60°C |
| 274 | Reverse | Exon 12 | GCGCTGAGCATCCTGTCT | 63°C |
| 275 | Forward | Exon 12 | CTGGCTCGGCGGCTACCT | NA |
| 276 | Reverse | Exon 13 | CGCCGCTGCAGGACAGTAAC | 65°C |
| 280 | Forward | Exon 12 | ACAGGATGCTCAGCGCCCTGCAGTAAGCTTTGATCCACTTCTTCTTCAACCTG | 55°C |
| 281 | Reverse | Exon 13 | CAGGTTGAAGAAGAAGTGGATCAAAGCTTACTGCAGGGCGCTGAGCATCCTGT | 55°C |
| 282 | Forward | Exon 10 | CAGTCATCAATGCGGACTTC | 65°C |
AT, annealing temperature; EGFP, enhanced green fluorescence protein; NA, not applicable.
Table 2.
PCR assays for SNP/mutations
| SNP/Mutation | Length | Exon | Primers | Enzyme | Bands After Restriction Digest |
|---|---|---|---|---|---|
| c.1140C>T(L385L) | 419 bp | 12 | 46–274 | BstUI | CC: 261,158 CT: 419, 261,158 TT:419 |
| c.410C>T(T->M) | 448 bp | 5 | 32–33 | NlaIII | wt: 377, 71; mut: 352, 71, 24 bp |
| g.4225_50del | 397 bp | 13 | 50–53 | None | wt: 397 bp, mut: 370 bp |
wt, Wild-type; mut, mutation.
Haplotype analysis was performed using the Genotyping Resource Center for Human Genetic Research (Massachusetts General Hospital, Boston, MA) by using microsatellite markers from deCODE Genetics (18), D9S1826 and D9S1838, and the markers CB-9 and CB-11 as described (7). Briefly, all fragments (CB-9, CB-11, D9S1826, and D9S1838) were made in a 7.5-μl PCR reaction that contained 3 ng of DNA, 2.5 mM MgCl2 (Applied Biosystems), 1 × PCR Buffer II (Applied Biosystems), 0.25 mM dNTPs (Amersham), 0.5 pmol of forward (labeled) and reverse primer, and 0.5 U AmpliTaq Gold (Applied Biosystems). The reactions were cycled as follows: 95°C × 12 min for the initial denaturation and then 10 cycles of 94°C × 30 s, 58°C × 30 s, 72°C × 1 min, followed by 20 cycles of 89°C × 30 s, 55°C × 30 s, 72°C × 1 min, and 72°C × 40 min final extension. The products were amplified and run on an Applied Biosystems 3730XL DNA Analyzer along with GeneScan 500Liz as an internal standard. The data were analyzed using Applied Biosystems GeneMapper v3.7 software package. GenBank accession numbers for SLC34A3 are as follows: genomic contig NT_024000.15; cDNA, NM_080877.1; and protein, NP_543153.1.
RT-PCR assay for ectopic expression of mutant NaPi-IIc mRNA in peripheral lymphocytes.
To permit allele assignment of the identified mRNA species, we first performed segregation analysis for the marker SNP c.1140C/T(L385L) using genomic DNA of the index case III-2 and his parents. For this purpose, exon 12 of the SLC34A3 gene was amplified by PCR using a Qiagen PCR kit, containing Q reagent, a final MgCl2 concentration of 3.25 mM and 20 μM of primers 46 and 274 (Table 1) at 94°C for 5 min for the initial denaturation, followed by 94°C × 1 min, 63°C × 1 min, 72°C × 1 min for 40 cycles, and 72°C × 10 min final extension. The PCR product was subjected to restriction endonuclease digestion with BstU1 to obtain the fragments indicated in Table 2. RNA was extracted from peripheral lymphocytes of the index case III-2 using the Pax-Purification system (Qiagen, Valencia, CA) and subjected to RNAse-free DNAse treatment to remove residual genomic DNA followed by cDNA synthesis using a Qiagen Omniscript kit. Using primers 282 and 53, followed by nested PCR with primers 105 and 276, the region encompassing the c.1140C>T and g.4225_50del was amplified (cycler conditions: 94°C × 5 min initial denaturation, followed by 40 cycles at 94°C × 1 min, 65°C or 72°C × 1 min, 72°C × 1 min, and 72°C × 10 min final extension). The nested-PCR product was purified using spin columns (Qiagen, Qiaquick PCR Purification Kit) and subjected to nucleotide sequence analysis with primers 275 and 104 at the Massachusetts General Hospital DNA Sequencing Core Facility.
Generation of pEGFP-[M137]hNaPi-IIc and pEGFP-[446Stop] hNaPi-IIc.
NaPi-IIc tagged with enhanced green fluorescence protein (EGFP) at the NH2 terminus (pEGFP-NaPi-IIc) was generated using BspEII/HindIII restriction sites and standard cloning techniques. A QickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) was then used to introduce into pEGFP-NaPi-IIc the T137M mutation and the V446Stop mutation. The latter mutation truncates NaPi-IIc after amino acid 446, which represents one possible reading frame for g.4225_50del. The mutagenesis primers were designed with the silent site selector DNA mutagenesis program by David Nix, http://rana.lbl.gov/SSS/ (see Table 1). Fifty nanograms pEGFP-NaPi-IIc were then amplified using Pfu-turbo DNA polymerase (Stratagene), Qiagen Q-reagent (5×), a final concentration of MgCl2 of 3.68 mM, and primers 190+191 to introduce M137 or 280+281 to introduce 446Stop, respectively, with the following thermal cycler conditions: initial denaturation at 94°C for 1 min; 16 cycles at 94°C for 30 s, 55°C for 1 min, and 65°C for 12 min; and final extension at 68°C for 10 min. The PCR product was then digested with the methylation-sensitive restriction endonuclease DpnI to selectively remove the original plasmid, followed by transformation into OneShot Top10 Escherichia coli cells. A XhoI or HindIII site engineered into the mutagenesis primers was used to identify the plasmid containing the new mutation, T137M or V446Stop, respectively. The correct sequence of the entire coding region of NaPi-IIc including the introduced mutations and EGFP was subsequently confirmed by nucleotide sequencing analysis at the Massachusetts General Hospital DNA Sequencing Core Facility.
cRNA synthesis.
To permit functional analysis of T137M and V446Stop in vitro, polyadenylated cRNA for injection into Xenopus laevis oocytes was transcribed from PCR templates. PCR templates were generated using the Expand Long Template PCR System (Roche) with buffer 1, 200 μM dNTP and a final MgCl2 concentration of 1.5 mM, forward primer 238 containing a SP6 RNA polymerase promoter, the reverse primer 239 (see Table 1), and the plasmids pEGFP-[M137]hNaPi-IIc or pEGFP-[V446Stop]hNaPi-IIc, pEGFP-hNaPi-IIc, or pEGFP-hNaPi-IIa as templates. Cycler conditions were 94°C × 5 min initial denaturation, followed by 40 cycles of 94°C × 1 min, 94°C 30 s, 60°C × 30 s, 68°C × 6 min, and 68°C × 10 min final extension. cRNA was synthesized using the Message mMachine (Ambion, Austin, TX) followed by addition of a poly-A tail (Ambion) according to the manufacturer's instructions and purified with NucAway spin columns (Ambion). cRNA integrity was verified on 1.3% agarose/TAE electrophoresis gels, and yield was determined by optical density at 260 nm.
Transient expression of mutant and wild-type NaPi-IIc in opossum kidney cells.
Opossum kidney cells were obtained (12) and maintained in DMEM/F12 (1:1, Invitrogen, Carlsbad, CA), supplemented with 10% FBS (Hyclone, Logan, UT), 100 U of penicillin (base), 100 μg of streptomycin (base), and 0.25 μg of amphotericin B/ml (Invitrogen) at 37°C in 95% humidified air-5% CO2. Ten thousand cells per well were plated into a eight-well slide flask (Nalge Nunc, Naperville, IL), transfected with 0.1 μg plasmid DNA/well using Transfectamine (Qiagen) according to the manufacturer's instructions, and cultured for an additional 3 days before fixation with 4% paraformaldehyde in phosphate-buffered saline containing 0.5 mM CaCl2 and 1 mM MgCl2 for 20 min at room temperature. Epifluorescent images were obtained using a Nikon Eclipse E800 fluorescent microscope (Nikon, Melville, NY) after nuclear counterstaining with propidium iodide.
X. laevis oocyte preparation and injection.
X. laevis maintenance and oocyte harvest procedures were approved by the Subcommittee on Research and Animal Care of the Massachusetts General Hospital. X. laevis oocytes (Nasco, Fort Atkinson, WI) were harvested by a minilaparotomy using sterile techniques, defolliculated in OR2 (170 mM NaCl, 5 mM KCl, 2 mM MgCl2, 10 mM HEPES, pH 7.5) with 1 mg/ml collagenase I from Clostridium histolyticum (C-9891, Sigma, St. Louis, MO, or CLS1, no. 4197, Worthington, Lakewood, NJ) rocking for 2 × 90 min at room temperature, rinsed several times with OR2, and then cultured in ND96+++ [96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM HEPES (pH 7.5), penicillin G sodium (10,000 U/ml), and streptomycin sulfate (10,000 μg/ml)] at 18°C as previously described (25). Oocyte injections were performed within the first 24 h of preparation with 100 nl of RNAse-free water containing 0–50 ng of wild-type and mutant cRNAs using a positive displacement micropipette. The medium was changed every day. Uptake and voltage-clamp experiments were done on day 3 after injection.
33P/22Na isotope uptake studies.
Single or dual isotope sodium-phosphate uptake experiments were performed as previously described (1). [33P]orthophosphoric acid (PerkinElmer NEX080) and [22Na]sodium Cl (PerkinElmer NEZ081) were used at 0.1 mCi 33P/ml and 0.02 mCi 22Na/ml for the uptake experiments. On day 3 after injection, oocytes were washed three times with ND40+0.3 mM Pi, containing 40 mM NaCl, 60 mM choline chloride, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM HEPES-Tris (pH 7.4), and 0.3 mM K2HPO4/KH2PO4 followed by incubation with ND40+0.3 mM Pi containing 33P and/or 22Na in a total volume of 200 μl for 60 min. Uptake was stopped with ND0, in which NaCl is replaced by 100 mM choline chloride, and radionucleotide uptake of single oocytes was determined after lysis with 10% SDS and addition of 3 ml of scintillation fluid in a Beckman LS3801 counter using single-isotope mode or dual-isotope mode with quench-curve correction. Recounting after permitting decay of 33P for 3 mo and single-isotope experiments were used to independently confirm the dual-isotope uptake results. Saturation experiments were performed in ND100 (100 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM HEPES-Tris, pH 7.4) supplemented with 0.001, 0.1, 0.3, 1, and 10 mM Pi and 0.01 mCi 33P/ml in a total volume of 200 μl. For the time course experiments, oocytes were incubated with ND100+2 mM Pi+0.01 mCi 33P/ml in a total volume of 200 μl for 10, 20, 30, 40, 60, and 90 min. An incubation time of 60 min was used for the dose-response curve and other uptake experiments. After 60 min, radioactive buffer was replaced by ND0 and incubation continued for 60 min at room temperature. 22Na efflux was tested after loading the oocytes expressing mutant and wild-type EGFP-hNaPi-IIc with ND0 containing 22Na. To stop the uptake, the oocytes were washed four times with ND0+2 mM Pi (time course) or ND0 (dose-response-efflux) and counted individually using the wide window 0–1,000 of a Beckman LS3801 in single-isotope mode.
Surface expression.
Membrane fluorescence was appreciated as a bright rim in oocytes expressing wild-type and mutant EGFP-hNaPi-IIc when live-cell epifluorescence images obtained using a Nikon Eclipse E800 fluorescent microscope (Nikon) (Fig. 3D, e–g). However, significant intracellular accumulation of the cotransporters was observed, using this technique. We, therefore, decided to use an Axioplan (Zeiss, Thornwood, NY) Radiance 2000 (Bio-Rad, Hercules, CA) confocal microscopic system to selectively and more accurately measure the membrane section of live oocytes to quantify surface expression, as shown in Fig. 3E (9). Images were obtained with Lasersharp 2000 (version 6.0) software using constant laser settings (intensity 30%, aperture 10%, gain 10%). Images were saved as TIF files and imported into IPLab 3.9.5 r2 for quantification using a personal computer. With this technique, the relative membrane fluorescence was linear over a wide range of cRNA injected into the oocyte and permitted detection of as little as 20% difference in surface expression with a coefficient of variation of <10%, as shown in Fig. 3B, which cannot be achieved by Western blot-based methods.
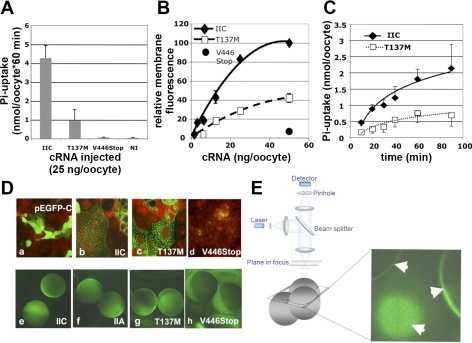
Fig. 3.
A: 33P uptake into Xenopus laevis oocytes expressing enhanced green fluorescent protein (EGFP)-[446Stop]- and EGFP-[M137]hNaPi-IIc. Twenty-five nanograms of polyadenylated cRNA transcribed from the appropriate plasmids encoding for wild-type human type IIc Na-Pi cotransporter (NaPi-IIc), human NaPi-IIa, and the mutants T137M and V446Stop were injected into X. laevis oocytes, followed by incubation at 18°C until day 3, when 33P uptake was measured over 60 min in ND100+1 mM Pi. Values are means ± SE of 3 independent batches of 4 oocytes each. B: surface expression of EGFP-[446Stop]-, EGFP-[M137]hNaPi-IIc, and wild-type EGFP-hNaPi-IIc. Polyadenylated cRNA encoding for EGFP-[446Stop]-, EGFP-[M137]hNaPi-IIc, or wild-type EGFP-hNaPi-IIc (125–50 ng/oocyte) were injected into X. laevis oocytes, followed by incubation at 18°C for 3 days. Surface expression was determined by confocal microscopy as described in materials and methods and in E. C: time course of 33P uptake into X. laevis oocytes expressing EGFP-[M137]hNaPi-IIc and wild-type EGFP-hNaPi-IIc. X. laevis oocytes were injected with 50 ng of polyadenylated cRNA encoding for wild-type (□) and EGFP-[M137]hNaPi-IIc (⧫), followed after culture for 3 days by 33P uptake in ND100+2 mM Pi corrected for surface expression using confocal microscopy. Values are means ± SE of 4 independent batches of 4 oocytes each. D: expression of EGFP-[446Stop]-, EGFP-[M137]hNaPi-IIc, wild-type hNaPi-IIc, and hNaPi-IIa in opossum kidney cells and X. laevis oocytes. Opossum kidney cells were transfected with plasmid DNA of pEGFP-C1 encoding for EGFP (a), wild-type EGFP-hNaPi-IIc (b), EGFP-[M137]hNaPi-IIc (c), or EGFP-[446Stop]-hNaPi-IIc (d), incubated for 3 days at 37°C, followed by fixation with 4% paraformaldehyde/PBS (+Ca+Mg) and microphotography (×63) with propidium iodide as the counterstain. For the following panels, 6.25 (e and f) or 50 ng (g and h) polyadenylated cRNA were injected into X. laevis oocytes. After culture at 18°C for 3 days, live-cell epifluorescent microscopy (×10) was used to qualitatively demonstrate membrane (bright rim) and intracellular expression of wild-type EGFP-hNaPi-IIc (e), wild-type EGFP-hNaPi-IIa (f), and EGFP-[M137]hNaPi-IIc (g), while EGFP-[446Stop]-hNaPi-IIc (h) was only expressed intracellularly as indicated by absence of the bright rim. E: confocal images of EGFP-[M137]hNaPi-IIc surface expression in X. laevis oocytes. For better quantification of surface expression, relative membrane fluorescence was assessed using an Axioplan Radiance 2000 confocal microscopy system. Images from tangential and/or across membrane sections (white arrows) were saved as TIF files and imported into IPLab 3.9.5 r2 for quantification using a personal computer.
Electrophysiology.
Electrophysiological recordings using the two-microelectrode oocyte voltage-clamp technique were performed at room temperature (22°C). Oocytes were placed in a 0.02-ml flow chamber, impaled with borosilicate pipettes filled with 3 M KCl (0.5–2 M[OMEGA]), and voltage clamped at −50 mV (model OC-725C, Warner Instrument, Hamden, CT). Cells were superfused at a rate of 2–3 ml/min with ND100 recording solution from glass syringe reservoirs via a polytetrafluoroethylene valve and tubing system. Delivery of superfusion solutions from reservoirs to the flow chamber was controlled by computer-activated solenoid valves. Currents were digitized at 200 Hz (Digidata 1200, Axon Instruments, Foster City, CA) and recorded on a personal computer running commercial software (Clampex8.2, Axon Instruments) as previously described (25).
Baseline current was determined at −50 mV with ND100. Oocyte viability was confirmed by resting membrane potentials below −18 mV in ND100 and by epifluorescence microscopy to confirm expression of EGFP-hNaPi-IIc. For intervals of 25 s, the solution then was changed to ND0, ND100+0.3 mM Pi, or ND100+0.3 mM Pi+3 mM phosphonoformic acid (PFA). Current-voltage (I-V) plots of sodium-dependent currents were obtained either by sequential runs in ND100 and ND0 for 20 s in 20-mV steps from −120 to +40 mV, following 5-s equilibration at the new membrane potential, or using a ramp from −120 to +40 mV over 1,600 ms following 15-s equilibration in either ND100 or ND0 and 5-s adjustment to 120 mV. Sodium-dependent currents were calculated by subtracting the currents obtained in ND0 from those obtained in ND100.
Statistical and data analysis.
Radioactive isotope uptake and electrophysiological experiments were repeated with oocytes from at least three donor frogs, four to five oocytes/experimental condition. The electrophysiological data were recorded with Clampex8.2 (Axon Instruments) and analyzed with Campfit8.2 (Axon Instruments) using a personal computer. Data are expressed as means ± SE.
RESULTS
Sequencing and haplotype analysis.
Lymphocyte DNA of the index case III-2 was used to generate four overlapping PCR products spanning 5 kb of genomic DNA, including 800 bp of the 5′-promoter region and all 13 exons and intronic sequences of SLC34A3, followed by nucleotide sequence analysis as described previously (7). Two novel heterozygous mutations, c.410C>T(p.T137M) and g.4225_50del, were identified (Fig. 1), which were absent in 94 and 124 control alleles, respectively. Furthermore, several known or novel polymorphisms were detected: hom. c.200G>A(R67H), het. c.496G>T(G166C), het. c.558G>A(Q186Q), hom. g.2704T>A, het. c.1140C>T(L385L), hom. g.3296C>G, hom. g.3701G>C, hom. g.3736C>T, hom. g.4107T>C, and hom. c.1538A>T(V513E) (7, 14, 19). Haplotype analysis indicated that c.410C>T(p.T137M) was inherited from the mother II-1, while g.4225_50del was inherited from the father II-2 (Fig. 2C).
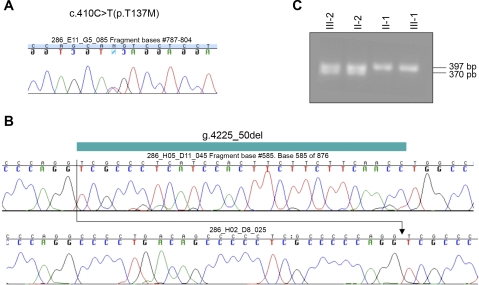
Fig. 1.
Mutational analysis. A: chromatogram of the nucleotide sequence of a PCR product using primers 32 and 33 and genomic DNA from the index case III-2, showing the heterozygous mutation c.410C>T(p.T137M). B: chromatogram of the nucleotide sequence of a PCR product using primers 50 and 53 and genomic DNA from the index case III-2. Presumably due to preferred amplification of the shorter sequence containing g.4225_50del, the chromatogram fails to show the wild-type allele, which subsequently was successfully amplified using primers 50 and 53, as can be seen in C, after ethidium-bromide staining of PCR products which were run on 2% agarose/TAE for index case III-2 and his relatives.
RT-PCR analysis of SLC34A3 transcripts in lymphoblastoid cells.
g.4225_50del deletes the last 25 nucleotides of intron 12 and the first nucleotide of exon 13 on the paternal allele. To determine whether this allele is transcribed, first-strand cDNA was synthesized from total RNA of peripheral lymphocytes of the index case III-2, followed by RT-PCR to amplify the region flanking the splice junction between exons 12 and 13, which is altered by g.4225_50del. The sequence obtained was assigned to one of the parental alleles using the single nucleotide polymorphism c.1140C>T(L385L) in exon 12, which is homozygous for cytidine in the mother and heterozygous for cytidine and thymidine in III-2 and the father (Fig. 2C). A single 499-bp PCR fragment was obtained which was homozygous for cytidine at position c.1140, indicating that only the maternal allele had been amplified. The mutant transcript was likewise absent from lymphoblastoid cells of an unrelated individual affected by HHRH carrying g.4225_50del (5, 6). These findings suggest that transcripts containing g.4225_50del are either unstable (nonsense-mediated decay) or lack the annealing sites for primers 105 and 276 due to alternative splicing and deletion of exon 13.
Functional analysis of V446Stop.
Because it cannot be definitively concluded from the absence of ectopic transcripts in peripheral lymphocytes or lymphoblastoid cells that truncated transcripts from the paternal allele are missing in the patient's kidney, we next functionally evaluated this mutation in opossum kidney cells and in X. laevis oocytes. For this purpose, we generated an expression plasmid containing the cDNA encoding for EGFP concatenated to the NH2 terminus of wild-type and mutant forms of human NaPi-IIc (pEGFP-hNaPi-IIc). This NH2-terminal EGFP tag has been successfully used for the functional evaluation of NaPi-IIa in the past (15) and does not affect the function of unmodified NaPi-IIc when identical amounts of cRNA are injected into oocytes and assayed for sodium-dependent phosphate uptake (data not shown). The EGFP tag permits the observation of membrane fluorescence as a measure of surface expression of the cotransporters using confocal fluorescent microscopy (Fig. 3E) (10). Images taken with constant laser settings were analyzed to obtain the relative membrane fluorescence, which was linear over a wide range of cRNA injected into the oocyte and permitted detection of as little as 20% difference in surface expression with a coefficient of variation of <10%, as shown in Fig. 3B. Using the Stratagene Qickchange method, a stop codon was introduced into pEGFP-hNaPi-IIc in place of the first codon of exon 13 (V446Stop). This mutation truncates the NaPi-IIc protein after the fifth membrane-spanning domain, the site predicted from an alternatively spliced hypothetical paternal transcript containing g.4225_50del. X. laevis oocytes injected with 25 ng/oocyte polyadenylated cRNA encoding for EGFP-[446Stop]hNaPi-IIc failed to show sodium-dependent 33P uptake (Fig. 3A) or measurable surface expression (Fig. 3B) compared with oocytes injected with 25 ng polyadenylated cRNA encoding for EGFP-hNaPi-IIc. Furthermore, EGFP-[446Stop]hNaPi-IIc accumulated intracellularly and failed to insert into apical patches of opossum kidney cells, the brush border equivalent of this cell line (10), when transiently transfected with pEGFP-[446Stop]hNaPi-IIc (Fig. 3D).
Functional analysis of T137M.
We next evaluated the impact of T137M on NaPi-IIc function. When 25 ng/oocyte of polyadenylated cRNA encoding for EGFP-[M137]hNaPi-IIc were injected into X. laevis oocytes, a significant reduction of sodium-dependent 33P uptake to 20% was observed compared with wild-type (Fig. 3A). However, unlike V446Stop/g.4225_50del, surface expression of T137M was only reduced to 40% of wild-type and did not seem to fully explain this lack of function (Fig. 3B). Furthermore, after transient transfection into opossum kidney cells, the mutant cotransporter was inserted into apical patches, the brush border equivalent in this cell line (10) (Fig. 3D). To better understand how T137M affects cotransport, we first evaluated the rate of 33P uptake after correction for surface expression using confocal live-cell fluorescent microscopy and quantitative image analysis of membrane sections. This experiment indicated that the rate of 33P uptake was reduced to 40% compared with cells expressing wild-type NaPi-IIc (Fig. 3C). To allow for similar levels of surface expression, all subsequent experiments were performed with X. laevis oocytes injected with 50 ng of polyadenylated cRNA encoding for EGFP-[M137]hNaPi-IIc or 6.25 ng of polyadenylated cRNA encoding for EGFP-hNaPi-IIc, followed by 33P and 22Na isotope uptake experiments (13). 33P uptake by oocytes expressing mutant and wild-type transporters was, as expected, sodium dependent (data not shown). Furthermore, the half-maximal inhibitory concentrations for phosphate were similar for EGFP-[M137]hNaPi-IIc (0.14 ± 0.07 mM) and wild-type EGFP-hNaPi-IIc (0.43 ± 0.23 mM), and thus neither assay could explain the observed reduction in the rate of phosphate uptake. However, we observed a significantly increased Na conductance for EGFP-[M137]hNaPi-IIc, which resulted in an increased 22Na/33P ratio of 7.1 ± 3.65 for the mutant cotransporter (compared with 2.3 ± 0.4 for wild-type) (Fig. 4C). 22Na uptake was independent of extracellular phosphate and resistant to treatment with PFA, a known inhibitor of type II sodium-phosphate cotransporters (15) (data not shown). After the oocytes were loaded with 22Na in ND0, no 22Na efflux was observed after incubation of the oocytes in nonradioactive ND0 for 60 min at room temperature, which argues against recycling of sodium and makes nonspecific toxic effects caused by the mutant cotransporter unlikely (data not shown).
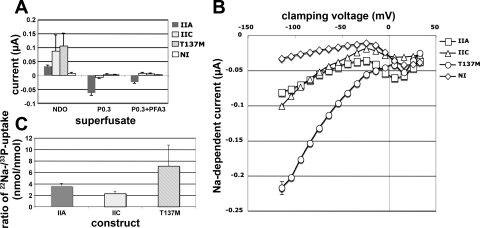
Fig. 4.
Voltage-clamp analysis and 33P/22Na uptake in X. laevis oocytes expressing EGFP-[M137]hNaPi-IIc. X. laevis oocytes were injected with 6.25 ng cRNA encoding for wild-type EGFP-hNaPi-IIa or wild-type EGFP-NaPi-IIc or with 50 ng cRNA encoding for EGFP-[M137]hNaPi-IIc to obtain similar surface expression. A: after 3-day incubation at 18°C, 4 oocytes/construct and condition were subjected to isotope uptake for 60 min in the presence of ND40+0.3 mM Pi using 0.01 mCi/ml [33P]orthophosphoric acid or 0.02 mCi/ml [22Na]-labeled chloride. B: dual-electrode studies obtained at a −50-mV clamp indicating the relative positive (reduction) or negative (increase) inward rectified current induced by changing the superfusate from ND100 to ND0, ND100+0.3 mM Pi, or ND100+0.3 mM Pi+3 mM phosphonoformic acid (PFA). C: voltage dependence of the sodium-dependent inward current (current-voltage plot). Oocytes expressing the 3 cotransporters were clamped at voltages ranging from −120 to +40 mV using ND100 and ND0 as superfusate. Sodium-dependent inward current was determined by subtracting the currents measured with ND0 from the current measured with ND100. Data are corrected for surface expression determined by confocal laser microscopy and represent means ± SE of 3 independent batches of oocytes.
To further characterize the nature of the increased sodium conductance, we performed dual electrode voltage-clamp studies, which indicated that EGFP-[M137]hNaPi-IIc was nonelectrogenic (Fig. 4A). The baseline leak current was reversed when sodium was removed from the superfusate but was unaffected by removal of potassium, calcium, or magnesium from the superfusate, or by replacement of the superfusate by LD100 (containing 100 mM LiCl in place of NaCl) (data not shown), indicating that it was selective for sodium ions. The hyperbolic shape of the I-V plot for EGFP-[M137]hNaPi-IIc furthermore indicates that this sodium-dependent conductance is inward rectified (Fig. 4B). Also, in this experimental setup no effect on sodium intake was observed with PFA and phosphate (Fig. 4A), indicating that the increase in the 22Na/33P ratio is independent and different from coupled sodium-phosphate cotransport.
DISCUSSION
Compound heterozygous mutations in SLC34A3.
Analysis of genomic DNA from the affected male III-2 revealed a compound heterozygous mutation in SLC34A3, which was absent in DNA from healthy controls. The maternal allele carries a missense mutation affecting a highly conserved amino acid residue (T137M), which, as shown here, may be an important determinant of sodium-phosphate cotransport. The paternal allele carries a deletion mutation (g.4225_50del), which abrogates ectopic transcription in peripheral lymphocytes of the index case and predicts truncation of the NaPi-IIc cotransporter after the fifth membrane-spanning domain. One possible transcript for g.4225_50del, [446Stop]hNaPi-IIc, furthermore, does not appear to be expressed in X. laevis oocytes. Thus the combined sequence alterations of SLC34A3 likely represent disease-causing mutations and lead to the development of HHRH in III-2.
Compound heterozygous SCL34A3 mutations are relatively frequent in HHRH, and in 10 of the 14 independent kindreds described to date, the affected patients carry such mutations (7, 16, 19). This may indicate a high mutational rate of SLC34A3 and/or a relatively high frequency of heterozygous SLC34A3 mutations in the general population. Carriers of SLC34A3 mutations on one allele often present with hypercalciuria; however, no biochemical abnormalities were observed in the heterozygous parents II-1 and II-2 investigated in this study.
Despite the presence of hypercalciuria in homozygous or heterozygous carriers of SLC34A3 mutations, renal calcifications have been underappreciated in the original clinical descriptions of patients with HHRH and were generally attributed to the inappropriate treatment with vitamin D analogs (11, 32). Recently, however, stone formation was noticed as part of the initial presentation in two kindreds, in which carriers were heterozygous for c.586G>A (G196R), g.2259_359del, or c.1402C>T (R468W) (7). Furthermore, recurrent nephrolithiasis was described in heterozygous and homozygous carriers of g.2259_2359del (14) and in homozygous carriers of c.586G>A (G196R) (5, 6). Thus renal stones may be more common in HHRH but may have previously been missed, if patients were asymptomatic and appropriate imaging studies were not done.
Renal stones were also observed in the present case and in some of his first-degree relatives. g.4225_50del was found in an unrelated case with HHRH, and multiple heterozygous carriers of the deletion in this kindred also suffer from recurrent renal stones (5). However, given the small number of kindreds and since stone disease is a common condition in the general population, it cannot be excluded at the present time that the observed association of stones with SLC34A3 mutations occurred by chance alone.
Loss of function of both SLC34A3 alleles explains renal phosphate wasting.
Opossum kidney cells and X. laevis oocytes are well-established cell systems used to study sodium-phosphate cotransporters (23, 26). When visualized in vivo with EGFP, we found that membrane expression in X. laevis oocytes and opossum kidney cells is markedly reduced for T137M and virtually absent after expression of V446Stop, the predicted transcript for g.4225_50del. Amino acid 137 is highly conserved in the known NaPi-IIc species, and T137M changes a polar residue to a hydrophobic residue, which may extend further the first membrane-spanning region and thus lead to abnormal folding or membrane insertion, thereby explaining the observed 40% reduction in surface expression with this mutation. Truncation of the NaPi-IIc protein after the fifth membrane-spanning domain by V446Stop deletes determinants of surface expression in the third intracellular loop of NaPi-IIc (17) and the COOH-terminal intracellular tail, which binds to NHERF-1 and -3 (35). Since ectopic transcripts of the paternal SLC34A3 allele were absent from lymphoblastoid cells of the affected individual III-2, nonsense-mediated decay may further impair expression of the paternal allele.
Interestingly, the father, II-2, who carries g.4225_50del on one of his SLC34A3 alleles, is biochemically normal and has no history of childhood rickets, hypercalciuria, or renal stones despite a predicted 50% reduction of NaPi-IIc function. By comparison, 70% of the heterozygous carriers of another previously reported null-allele, c.228del, displayed hypercalciuria (7). Alimentary modifiers including the intake of vitamin D (5, 6) and genetic factors may explain the reduced penetrance of hypercalciuria for g.4225_50del in the present kindred.
The relatively low abundance of NaPi-IIc in the proximal tubules, at least in adult rodents (26), and the lack of a hyphophosphatemic phenotype in the murine knockout of Npt-2c (27) raise the possibility that human NaPi-IIc mutations result in dominant effects. However, the presence of deletion and missense mutations throughout the cotransporter molecule rather than a few mutational hot spots is generally considered more suggestive for loss-of-function mutations (30), and our finding that both mutations significantly reduce surface expression continue to support the previous proposal that HHRH is an autosomal recessive disorder caused by loss-of-function mutations in NaPi-IIc.
T137 is a key determinant of sodium-phosphate cotransport in NaPi-IIc.
After correction for surface expression, the rate of phosphate uptake in X. laevis oocytes injected with cRNA encoding for EGFP-[M137]-hNaPi-IIc was 40% of wild-type. Thus, in addition to affecting membrane expression as discussed above, T137 appears to be an important determinant of cotransport in NaPi-IIc. Because the apparent affinity constant for phosphate remained unchanged, it appears unlikely that T137 is involved in substrate binding. T137 is located in the first intracellular loop, which contains residues that appear to be important determinants of sodium-phosphate cotransport and stoichiometry in the related cotransporter NaPi-IIa (1). Therefore, we explored the possibility that this mutation affects Na:P stoichiometry. Compared with wild-type NaPi-IIc, EGFP-[M137]-hNaPi-IIc permitted uptake of 7.1 ± 3.65 sodium ions for each phosphate ion (Fig. 4C). Under the assumption that divalent phosphate, in agreement with previous reports for NaPi-IIa, is the preferred substrate also for NaPi-IIc (13), this may be due to cotransport of five Na+ ions in the presence of phosphate. Electrophysiological evaluation of EGFP-[M137]-hNaPi-IIc by dual-electrode voltage-clamp studies, however, suggested that the Na:P cotransport stoichiometry is unaltered since EGFP-[M137]-hNaPi-IIc remains nonelectrogenic (Fig. 4A). Instead, we noticed that the phosphate-independent rectified sodium conductance of EGFP-[M137]-hNaPi-IIc is significantly increased and unaffected by other cations (Fig. 4B). Mutation of T137 to methionine may thus lead to an uncoupling of cotransport in NaPi-IIc, and this uncoupling may render cotransport and thus the rate of phosphate uptake less efficient. This finding is consistent with the observed unchanged apparent Kd, but decreased Vmax of the mutant transporter for phosphate. Increased sodium uptake may furthermore reduce the sodium gradient across the cell membrane, which could lead to a certain degree of toxicity in the proximal tubular cell and could conceivably cause internalization and reduction of surface expression of the mutant cotransporter.
In summary, we here describe two novel SLC34A3 mutations, c.410C>T(p.T137M) on the maternal allele and g.4225_50del on the paternal allele, of a previously reported male affected by HHRH and recurrent nephrolithiasis (11). Functional analysis led to the conclusion that the paternal transcript carrying g.4225_50del is absent at least in peripheral lymphocytes of the index case and that the maternal mutation T137M affects a conserved amino acid residue, which appears to be important for coupled sodium-phosphate cotransport in NaPi-IIc.
GRANTS
This work was supported by the National Institutes of Health (PO1-DK-11794, K24-HD-01288 to H. Jüppner), a Young Investigator Award by the National Kidney Foundation, and the American Association for Clinical Investigation (C. Bergwitz).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bacconi A, Virkki LV, Biber J, Murer H, Forster IC. Renouncing electroneutrality is not free of charge: switching on electrogenicity in a Na+-coupled phosphate cotransporter. Proc Natl Acad Sci USA 102: 12606–12611, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacic D, Lehir M, Biber J, Kaissling B, Murer H, Wagner CA. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int 69: 495–503, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Bacic D, Wagner CA, Hernando N, Kaissling B, Biber J, Murer H. Novel aspects in regulated expression of the renal type IIa Na/Pi-cotransporter. Kidney Int 91, Suppl: S5–S12, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA 95: 5372–5377, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergwitz C, Kremke B, Bounoutas G, Hiort O, Insogna K, Jüppner H. Hypophosphatemic rickets with hypercalciuria (HHRH) can be associated with renal stones (Abstract). J Bone Miner Res 21, Suppl 1: S312, 2006. [Google Scholar]
- 6.Bergwitz C, Kremke B, Phulwani P, Bounoutas G, Hiort O, Insogna K, Carpenter TO, Estrada E, Jüppner H. Novel NaPi-IIc mutations as the cause of hypophosphatemic rickets with hypercalciuria (HHRH) and renal stones (Abstract). J Am Soc Nephrol 17: 526A, 2006. [Google Scholar]
- 7.Bergwitz C, Roslin NM, Tieder M, Loredo-Osti JC, Bastepe M, Abu-Zahra H, Frappier D, Burkett K, Carpenter TO, Anderson D, Garabedian M, Sermet I, Fujiwara TM, Morgan K, Tenenhouse HS, Jüppner H. SLC34A3 mutations in patients with hereditary hypophosphatemic rickets with hypercalciuria predict a key role for the sodium-phosphate cotransporter NaPi-IIc in maintaining phosphate homeostasis. Am J Hum Genet 78: 179–192, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaine J, Breusegem SY, Giral H, Barry NP, Levi M. Differential regulation of renal NaPiIIa and NaPi-IIc trafficking by PTH (Abstract). J Am Soc Nephrol 18: 56A, 2007. [Google Scholar]
- 9.Bueno OF, Robinson LC, Alvarez-Hernandez X, Leidenheimer NJ. Functional characterization and visualization of a GABAA receptor-GFP chimera expressed in Xenopus oocytes. Brain Res Mol Brain Res 59: 165–177, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Caverzasio J, Rizzoli R, Bonjour JV. Sodium-dependent phosphate transport inhibited by parathyroid hormone and cyclic AMP stimulation in an opossum kidney cell line. J Biol Chem 261: 3233–3237, 1986. [PubMed] [Google Scholar]
- 11.Chen C, Carpenter T, Steg N, Baron R, Anast C. Hypercalciuric hypophosphatemic rickets, mineral balance, bone histomorphometry, and therapeutic implications of hypercalciuria. Pediatrics 84: 276–280, 1989. [PubMed] [Google Scholar]
- 12.Cole JA, Forte LR, Krause WJ, Thorne PK. Clonal sublines that are morphologically and functionally distinct from parental OK cells. Am J Physiol Renal Fluid Electrolyte Physiol 256: F672–F679, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Forster IC, Loo DD, Eskandari S. Stoichiometry and Na+ binding cooperativity of rat and flounder renal type II Na+-Pi cotransporters. Am J Physiol Renal Physiol 276: F644–F649, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Hernando N, Deliot N, Gisler SM, Lederer E, Weinman EJ, Biber J, Murer H. PDZ-domain interactions and apical expression of type IIa Na/Pi cotransporters. Proc Natl Acad Sci USA 99: 11957–11962, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernando N, Forgo J, Biber J, Murer H. PTH-Induced downregulation of the type IIa Na/Pi-cotransporter is independent of known endocytic motifs. J Am Soc Nephrol 11: 1961–1968, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Ichikawa S, Sorenson AH, Imel EA, Friedman NE, Gertner JM, Econs MJ. Intronic deletions in the SLC34A3 gene cause hereditary hypophosphatemic rickets with hypercalciuria. J Clin Endocrinol Metab 91: 4022–4027, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Ito M, Segawa H, Sakurai A, Uehata Y, Tasumi S, Kuwahata M, Miyamoto K. The last intracellular loop of NaPi-IIc contains the apical localization signal for renal epithelial cells (Abstract). J Am Soc Nephrol 17: 3A, 2006. [Google Scholar]
- 18.Kong A, Gudbjartsson DF, Sainz J, Jonsdottir GM, Gudjonsson SA, Richardsson B, Sigurdardottir S, Barnard J, Hallbeck B, Masson G, Shlien A, Palsson ST, Frigge ML, Thorgeirsson TE, Gulcher JR, Stefansson K. A high-resolution recombination map of the human genome. Nat Genet 31: 241–247, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM. Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78: 193–201, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madjdpour C, Bacic D, Kaissling B, Murer H, Biber J. Segment-specific expression of sodium-phosphate cotransporters NaPi-IIa and -IIc and interacting proteins in mouse renal proximal tubules. Pflügers Arch 448: 402–410, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto K, Segawa H, Ito M, Kuwahata M. Physiological regulation of renal sodium-dependent phosphate cotransporters. Jpn J Physiol 54: 93–102, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Miyamoto KI, Ito M, Tatsumi S, Kuwahata M, Segawa H. New aspect of renal phosphate reabsorption: the type IIc sodium-dependent phosphate transporter. Am J Nephrol 27: 503–515, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflügers Arch 447: 763–767, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Ohkido I, Hara S, Segawa H, Yokoyama K, Yamamoto H, Miyamoto K, Kawaguchi H, Hosoya T. Localization of sodium-phosphate cotransporter NaPi-IIa and -IIc in human proximal renal and distal tubules (Abstract). J Am Soc Nephrol 18: 739A, 2007. [Google Scholar]
- 25.Rusch D, Forman SA. Classic benzodiazepines modulate the open-close equilibrium in alpha1beta2gamma2L gamma-aminobutyric acid type A receptors. Anesthesiology 102: 783–792, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Segawa H, Kaneko I, Takahashi A, Kuwahata M, Ito M, Ohkido I, Tatsumi S, Miyamoto K. Growth-related renal type II Na/Pi cotransporter. J Biol Chem 277: 19665–19672, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Segawa H, Onitsuka A, Aranami F, Tomoe Y, Kaneko I, Furutani J, Ito M, Matsumoto M, Li M, Amizuka N, Kuwahata M, Miyamoto K. Npt2a and Npt2c in mice play distinct and synergistic roles in inorganic phosphate metabolism and skeletal development (Abstract). J Am Soc Nephrol 18: 56A, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Segawa H, Yamanaka S, Ito M, Kuwahata M, Shono M, Yamamoto T, Miyamoto K. Internalization of renal type IIc Na-Pi cotransporter in response to a high-phosphate diet. Am J Physiol Renal Physiol 288: F587–F596, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Segawa H, Yamanaka S, Onitsuka A, Tomoe Y, Kuwahata M, Ito M, Taketani Y, Miyamoto K. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol 292: F395–F403, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Strachan T, Read AP. Human Molecular Genetics 2. New York: Wiley, 1999.
- 31.Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto K. Differential effects of Npt2a gene ablation and X-linked Hyp mutation on renal expression of Npt2c. Am J Physiol Renal Physiol 285: F1271–F1278, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Tieder M, Blonder J, Strauss S, Shaked U, Maor J, Gabizon D, Manor H, Sela BA. Hyperoxaluria is not a cause of nephrocalcinosis in phosphate-treated patients with hereditary hypophosphatemic rickets. Nephron 64: 526–531, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Tieder M, Modai D, Samuel R, Arie R, Halabe A, Bab I, Gabizon D, Liberman UA. Hereditary hypophosphatemic rickets with hypercalciuria. N Engl J Med 312: 611–617, 1985. [DOI] [PubMed] [Google Scholar]
- 34.Tieder M, Modai D, Shaked U, Samuel R, Arie R, Halabe A, Maor J, Weissgarten J, Averbukh Z, Cohen N, Edelstein S, Lieberman UA. “Idiopathic” hypercalciuria and hereditary hypophosphatemic rickets. Two phenotypical expressions of a common genetic defect. N Engl J Med 316: 125–129, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Villa-Bellosta R, Barac-Nieto M, Breusegem SY, Barry NP, Levi M, Sorribas V. Interactions of the growth-related, type IIc renal sodium/phosphate cotransporter with PDZ proteins. Kidney Int 73: 456–64, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]