Abstract
WNK1 kinase belongs to a family of serine-threonine protein kinases with an atypical placement of the catalytic lysine. Increased expression of WNK1 causes hypertension and hyperkalemia in humans. WNK1 inhibits renal potassium channel ROMK1 by enhancing its endocytosis, likely contributing to hyperkalemia in affected patients. The domains of WNK1 involved in inhibition of ROMK1 have not been completely elucidated. Here, we reported that an NH2-terminal proline-rich domain (N-PRD; amino acids 1-119) is necessary and sufficient for WNK1 inhibition of ROMK1. A region (named “NL” for N-linker; amino acids 120-220) located between N-PRD and the kinase domain of WNK1 (amino acids 220-491) antagonized the inhibition of ROMK1 caused by N-PRD. The WNK1 kinase domain reversed the antagonism of NL on N-PRD. Mutagenesis studies revealed that charge-charge interactions between two conserved catalytic residues (Lys-233 and Asp-368) within the kinase domain (not the kinase activity) are critical for kinase domain to reverse the antagonism of NL domain. The WNK1 autoinhibitory domain (AID; amino acids 491-555) also affected ROMK, presumably by modulating the kinase domain conformation. Mutations of two conserved phenylalanine abolished the ability of AID to modulate ROMK1. Finally, the first coiled-coil domain (CC1; amino acids 555-640) of WNK1 alleviated the effect of AID domain toward kinase domain. Thus, multiple intra- and/or intermolecular interactions of WNK1 domains are at play for regulation of ROMK1 by WNK1.
Keywords: clathrin-mediated endocytosis, protein-protein interaction, proline-rich motifs
with-no-lysine [k] (WNK) kinases are a novel family of large serine-threonine protein kinases with an atypical placement of the catalytic lysine (15). Four members, WNK1-4, have been identified in mammals (11, 14, 15). WNK1 consists of ∼2,100 amino acids and an ∼270 amino acid kinase domain located near the NH2 terminus (15). Amino acids of the kinase domain of WNK1-4 are conserved, sharing 85–90% sequence identity. In addition to the kinase domain, WNKs contain an autoinhibitory domain, 1–2 coiled-coil domains, and multiple PXXP proline-rich motifs for potential protein-protein interaction (19). Little sequence identity exists outside these conserved domains and motifs. The human WNK1 gene consists of 28 exons (3, 14). The full-length WNK1 transcript encoded by all 28 exons is present in every tissue examined. A shorter splice variant of WNK1, encoded by an alternative initiating exon 4A followed by exon 5-28, is highly expressed in kidney and known as kidney-specific WNK1 (3, 20).
Pseudohypoaldosteronism type 2 (PHA2) is a rare autosomal-dominant disease characterized by hypertension and hyperkalemia (4). Positional cloning recently identified mutations of WNK1 and WNK4 as causes for PHA2 (14). PHA2 mutations of the WNK1 gene are large deletions within the first intron that cause the increased expression of unmutated WNK1. PHA2-causing mutations in the WNK4 gene are mis-sense mutations outside the kinase domain (14).
Potassium secretion in kidney occurs mainly in the distal nephron from the distal convoluted tubule (DCT) to the cortical collecting duct (CCD), where the apical renal potassium channel ROMK serves a final common exit pathway (6). ROMK1 channel actively undergoes constitutive clathrin-mediated endocytosis, which is important for hour-to-hour regulation of K+ secretion by dietary K+ intake (24). WNK1 colocalizes with ROMK1 in DCT and CCD (1, 14). In the expression system, WNK1 inhibits ROMK1 channel by increasing its endocytosis (8, 12). The mechanism involves recruitment of an endocytic scaffolding protein intersectin to the clathrin-coated pits (5). Inhibition of K+ secretion through ROMK may contribute to hyperkalemia in patients with PHA2 disease caused by upregulation of WNK1.
Several studies examined the mechanism of regulation of ROMK by WNK1 (2, 5, 8, 12). These studies used truncated fragments of WNK1 and found differences in the regulation of ROMK. Thus, different domains of WNK1 may exert positive or negative effect on endocytosis of ROMK1. In support of this idea, various fragments of WNK1 exhibit differential interactions and abilities to form multimers (9, 16). Furthermore, kidney-specific WNK1, which lacks the kinase domain and preceding NH2-terminal amino acids, does not inhibit ROMK1 by itself but antagonizes the inhibition of ROMK1 caused by full-length WNK1 (8, 12). In the present study, we systematically examine the domain structure of WNK1 in the regulation of ROMK1. Our results suggest that multiple intra- and/or intermolecular interactions of WNK1 domains are at play to regulate ROMK1.
METHODS
Molecular biology.
GFP-ROMK1 was described previously (5). WNK1 fragments were amplified by PCR and subcloned into pCMV5-myc vector. Point mutations were generated by site-directed mutagenesis (QuickChange kit; Stratagene) and confirmed by sequencing.
Cell culture and transfection.
HEK293 cells were cultured as described (5). Cells were cotransfected with cDNAs encoding GFP-ROMK1 (0.3 μg per 35-mm well) and myc-tagged full-length or fragments of WNK1 (1.5 μg each construct per well) using a commercial transfection kit (Fugene 6) according to the manufacturer's protocol.
In each experiment, the total amount of DNA for transfection was balanced using an empty vector.
Western blot analysis.
Cells were lysed in PBS containing 0.5% Triton X-100. Equal amounts of lysates (10 μg per sample) were separated by SDS-PAGE and transferred to nitrocellulose membranes for Western blotting. WNK1 constructs on the membrane were detected with a rabbit polyclonal anti-myc antibody (Abcam).
Patch-clamp recording.
About 36 to 48 h after transfection, whole cell currents were recorded using an Axopatch 200B amplifier (Molecular Devices) as previously described (5). Transfected cells were identified using epifluorescent microscopy. The pipette solution contained 140 mM KCl and 10 mM HEPES (pH 7.2); the bath solution contained 140 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES (pH 7.4). Capacitance and access resistance were monitored and 75% compensated. The voltage protocol consisted of 0 mV holding potential and 400-ms steps from −100 to 100 mV in 20-mV increments. Current density was calculated by dividing current at −100 mV (pA; measured at 25°C) by capacitance (pF). Results were shown as means ± SE (n = 5–10). Each experiment (i.e., set of results shown in each panel of a figure) was repeated two to four times.
Statistical analysis.
Statistical comparisons between two groups of data were made using two-tailed unpaired Student's t-test. Multiple comparisons were made using one-way ANOVA followed by two-tailed Student's t-test adjusted for multiple comparisons. P values <0.05 and 0.01 were considered significant for single and multiple comparisons, respectively.
RESULTS
Both WNK1 kinase domain and proline-rich domain are important in the regulation of ROMK1.
WNK1 inhibits ROMK channel by increasing its endocytosis (8, 12). Using small fragments of WNK1, we previously showed that both WNK1 kinase domain and the NH2 terminus of WNK1 containing amino acids 1-119 (named N-PRD for NH2-terminal proline-rich domain) are important for inhibition of ROMK1 (5). Here, we further examined the role of WNK1 kinase domain and N-PRD using full-length WNK1 as the backbone for mutagenesis of the kinase domain and N-PRD. Whole cell ROMK1 current density in transfected HEK293 was measured by ruptured whole cell patch-clamp recording. We showed that whole cell ROMK1 current density correlates well with cell surface abundance of ROMK1 and changes in current density caused by WNK kinases reliably reflecting alteration of endocytosis of the channel (5, 8).
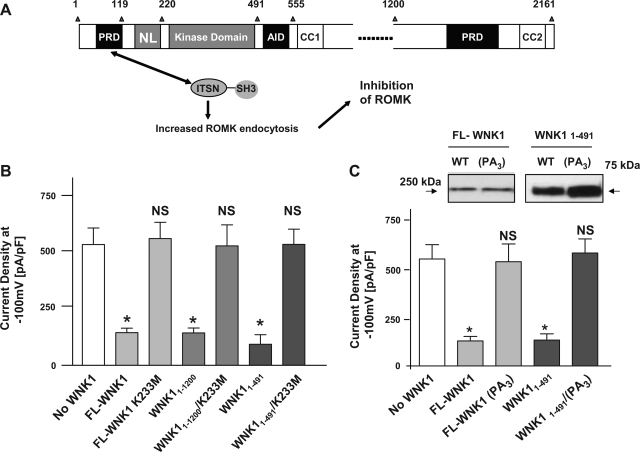
Figure 1A shows the domain structure of WNK1 referenced in the present study and a working model for WNK1 inhibition of ROMK1 by recruitment of intersectin to enhance endocytosis of the channel. As shown, full-length WNK1 (FL-WNK1), a WNK1 fragment containing amino acid 1-1200 (WNK11-1200), or amino acids 1-491 (WNK11-491) each inhibited ROMK1 current density (Fig. 1B, 2nd, 4th, and 6th bar from left, respectively). Consistent with previous reports that WNK1 kinase domain is important for regulation of ROMK1 (5, 8, 12), mutation of the conserved catalytic lysine-233 to methionine (K233M) in FL-WNK1, WNK11-1200, or WNK11-491 abolished their inhibition of ROMK1 (Fig. 1B, 3rd, 5th, and 7th bar, respectively).
Fig. 1.
Role of kinase domain and proline-rich domain in the regulation of ROMK1. A: schematic diagram of rat full-length WNK1 (FL-WNK1). N-PRD, proline-rich domain; NL, N-linker domain; CC1 and 2, coiled-coil domain 1 and 2 [predicted using the COILs program (14)]; AID, autoinhibitory domain; ITSN, intersectin; SH3, Src-homology domain-3. As shown, N-PRD of WNK1 binds ITSN via SH3 domain. This recruitment of ITSN stimulates endocytosis of ROMK1 (5). B: effect of lysine-233 to methionine (K233M) mutation of FL-WNK1, amino acids 1-1200 (WNK11-1200), or amino acids 1-491 of WNK1 (WNK11-491) on the regulation of ROMK1. HEK cells were cotransfected by ROMK1 and indicated WNK1 construct or an empty vector (“No WNK1”). About 36-48 h after transfection, whole cell ROMK1 current density (pA/pF, at −100 mV) was measured. *P < 0.01 vs. control (No WNK1). NS, statistically not significant vs. control. Of note, HEK cells express endogenous FL-WNK1, which exerts inhibition of ROMK1 in the absence of exogenous WNK1 (8). C: effect of triple mutations of proline-94, -103, and -114 (to alanine; labeled as PA3) on WNK1 regulation of ROMK1. Expression of myc-tagged wild-type (WT) and PA3 mutant in transfected HEK cells was examined using anti-myc antibody. *P < 0.01 vs. control (No WNK1). NS, statistically not significant vs. control.
We also reported that N-PRD of WNK1 (amino acid 1-119) is sufficient for inhibition of ROMK1 (5). Mutations of proline-94, -103, and -114 within N-PRD of WNK11-491 disrupt its interaction with intersectin and inhibition of ROMK1 (5). Mutations of other proline residues (proline-13, -18, and -23) did not affect WNK11-491 regulation of ROMK1 (not shown). These results indicate that, within amino acids 1-119 of WNK1, these three proline residues are involved in interaction with intersectin and thus regulation of ROMK1. However, full-length WNK1 contains more than 20 proline-rich domains (15). It is not known whether proline-94, -103, and -114 are the only proline residues of full-length WNK1 essential for inhibition of ROMK1. Here, we found that triple mutations of proline-94, -103, and -114 to alanine (“FL-WNK1/PA3”; “PA” indicates proline-to-alanine mutation) abolished the inhibition of ROMK1 by full-length WNK1 (Fig. 1C, 2nd and 3rd bar from left). Western blot analysis of WNK1 revealed that the loss of inhibition by triple proline mutant of full-length WNK1 was not due to reduced or lack of expression of the protein (Fig. 1C, top). As reported previously (5), triple mutations of proline-94, -103, and -114 to alanine abolished the inhibition of ROMK1 by WNK11-491 (“WNK11-491/PA3”; Fig. 1C, 4th and 5th bar from left). Thus, N-PRD is not only sufficient but also essential for full-length WNK1 to inhibit ROMK1.
Role of amino acids between N-PRD and the kinase domain.
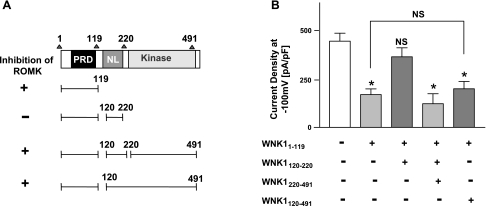
The above results, however, create an apparent contradiction: why K233M mutants of FL-WNK1, WNK11-1200, and WNK11-491 fail to inhibit ROMK1 while they contain the N-PRD domain sufficient for inhibition of the channel? To resolve this conundrum, we hypothesized that the region located between N-PRD and the kinase domain WNK1 [including amino acids 120-220; named N-linker (NL); see Fig. 1A] plays an important role in modulating WNK11-491 regulation of ROMK1. In this hypothesis, NL region antagonizes the inhibition of ROMK1 by N-PRD and WNK1 kinase domain further reverses the antagonism of NL to N-PRD. (The role of amino acids distal to the kinase domain will be addressed in later sections.)
In support of the hypothesis that NL domain plays a role, we previously showed that, while WNK11-119 and WNK11-491 cause inhibition of ROMK1, WNK11-220 has no effect (5). Moreover, WNK1120-220 (namely, NL region) by itself has no effect on ROMK1 but reverses WNK11-119 (namely, N-PRD)-mediated inhibition of the channel (5) (see Fig. 2A for summary of these results). To further support the above hypothesis that kinase domain reverses the function of NL domain, we compared in this study the effects of WNK1120-220 and WNK1120-491 on WNK11-119-mediated inhibition of ROMK1. WNK1120-491 differs from WNK1120-220 by having kinase domain in addition to NL domain. As in our previous results (Ref. 5 and summarized in Fig. 2A), WNK1120-220 reversed WNK11-119-mediated inhibition of ROMK1 (Fig. 2B, 2nd and 3rd bar from left). In contrast, WNK1120-491 did not reverse the inhibition caused by WNK11-119 (Fig. 2B, compare 2nd and 5th bar), supporting that WNK1 kinase domain neutralizes the function of WNK1120-220 on WNK11-119-mediated inhibition of ROMK1. Moreover, coexpression of WNK11-119, WNK1120-220, and WNK1220-491 (each as an independent peptide) inhibited ROMK1 (Fig. 2B, 4th bar). Western blot analysis confirmed that all three domains could express as independent peptides in HEK293 cells (data not shown). Together, these results support the hypothesis that N-PRD inhibits ROMK1, NL domain reverses N-PRD-mediated inhibition of the channel, and that kinase domain neutralizes the function of NL domain.
Fig. 2.
Role of amino acids between NH2-terminal PRD and kinase domain of WNK1 in the regulation of ROMK1. A: amino acids 1-491 of WNK1 include NH2-terminal PRD (amino acids 1-119), NL (amino acids 120-220), and kinase domain (amino acids 221-491). Effects of these domains on ROMK1 (“inhibition of ROMK1”) from a previous study (5) and the present study shown in B are summarized. + And − indicate inhibition and no inhibition of ROMK1, respectively. B: role of NL domain in the regulation of ROMK1. HEK cells were cotransfected with ROMK1 plus various combinations of WNK1 constructs as indicated. *P < 0.01 vs. control (no WNK1; white bar). NS, statistically not significant vs. control or between indicated groups.
WNK1 kinase domain conformation rather than kinase activity is critical for inhibition of ROMK1.
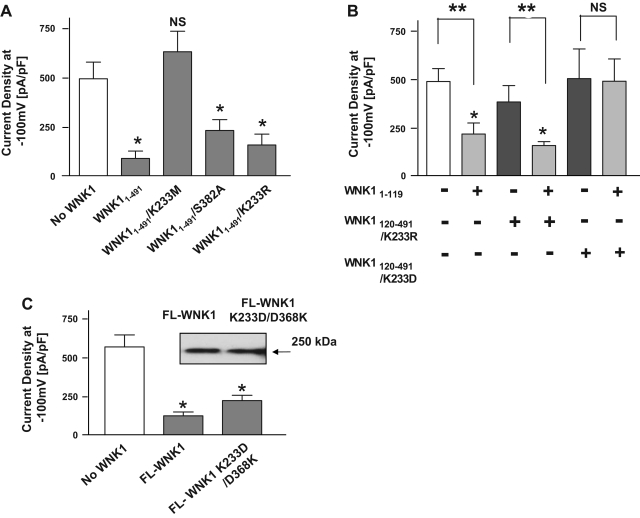
Autophosphorylation of Ser-382 in the activation loop of WNK1 is critical for its kinase activity (16, 23). Mutation of the serine residue abolishes kinase activity (16). We used Ser-382 to alanine mutant of WNK11-491 (WNK11-491/S382A) to further test the role of kinase activity in the regulation of ROMK1. We found that WNK11-491/S382A inhibited ROMK1 similarly as wild-type WNK11-491 (Fig. 3A, 2nd and 4th bar from left). For comparison, WNK11-491/K233M did not inhibit ROMK1 (Fig. 3A, 3rd bar). These results provide further support for the idea that WNK1 kinase activity is not necessary for regulation of ROMK1. The crystal structure of kinase domain of WNK1 reveals that Lys-233 and Asp-368 are in close proximity and may form a salt bridge (10). Previously, we hypothesized that an electrostatic interaction between Lys-233 and Asp-368 is critical for maintaining a structure of kinase domain for inhibition of ROMK1 (5). In support of this hypothesis, we showed that a double mutant of WNK11-491 carrying charge-reversal double mutations of Lys-233 (K233) and Asp-368 (D368; namely, double mutations of K233 to Asp and of Asp-368 to Lys; K233D/D368K) remains capable of inhibiting ROMK1 while possessing no kinase activity (5). Here, we further showed that WNK11-491/K233R, which carries a charge conservation mutation of Lys-233 to arginine, inhibited ROMK1 similarly as the wild-type (Fig. 3A, 5th bar).
Fig. 3.
Electrostatic interaction between lysine-233 and aspartate-368 of kinase domain of WNK1 is critical for inhibition of ROMK1. A: effect of serine-382 to alanine (S382A), lysine-233 to methionine (K233M), or lysine-233 to arginine (K233R) mutation. *P < 0.01 vs. control (No WNK1). NS, statistically not significant vs. control. B: effect of charge conservation (K233R) and charge disruption mutation (lysine-233 to aspartate; K233D). *P < 0.01 vs. control (white bar). **P < 0.001 between indicated groups. NS, statistically not significant between indicated groups. C: effect of charge reversal mutation between K233 and D368 [i.e., double mutations of lysine-233 (K233) to aspartate and of aspartate-368 (D368) to lysine; K233D/D368K] of FL-WNK1. *P < 0.01 vs. control (No WNK1). Expression of myc-tagged wild-type and mutant WNK1 in transfected HEK cells was examined using anti-myc antibody.
The results of Fig. 2 above suggest that multiple domain interactions are important for WNK1 inhibition of ROMK1. Among these is the result that WNK1120-220 antagonized WNK11-119-mediated inhibition of ROMK1 while WNK1120-491 did not antagonize the inhibition. To know whether the electrostatic interaction between Lys-233 and Asp-368 on regulation of ROMK1 is related to these interactions, we examined the effects of mutation of Lys-233 using WNK1120-491 as a backbone. We found that WNK1120-491 carrying the mutation of Lys-233 to aspartate (WNK1120-491/K233D) antagonized WNK11-119-mediated inhibition of ROMK1 (Fig. 3B, 6th bar from left), in contrast to its wild-type counterpart (see Fig. 2B, 5th bar). By itself, WNK1120-491/K233D had no effect on ROMK1 (Fig. 3B, 5th bar). These results indicate that the mutant kinase domain no longer neutralizes the function of NL domain toward N-PRD. For comparison, a charge conservation mutation of WNK1120-491 that preserves the electrostatic interaction (K233 to arginine; WNK1120-491/K233R) did not antagonize N-PRD-mediated inhibition of ROMK1 (Fig. 3B, 4th bar). By itself, WNK1120-491/K233R had no effect on ROMK1 (Fig. 3B, 3rd bar). Thus, the ability of kinase domain to antagonize NL domain depends on the charge interaction between K233 and D368 in the kinase domain. We next examined the role of electrostatic interaction of the kinase domain for full-length WNK1. Full-length WNK1 bearing K233D/D368K double mutations inhibited ROMK1 (Fig. 3C). As reported previously, WNK11-491 carrying the same double mutation inhibited ROMK1 (5). Thus, the electrostatic interaction between Lys-233 and Asp-368 is important for inhibition of ROMK1 by full-length WNK1.
Role of the autoinhibitory domain.
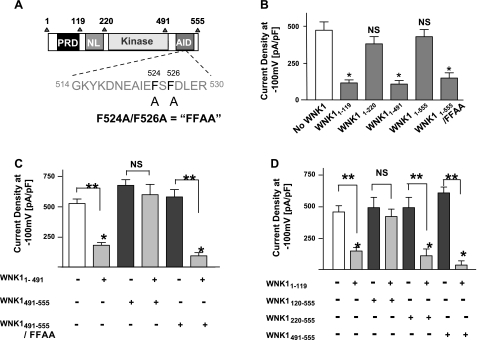
The autoinhibitory domain (AID; amino acids 491-555; see Fig. 4A) of WNK1 negatively regulates its kinase activity (16). A WNK1 fragment containing the AID domain in addition to kinase domain (WNK11-555, consisting of amino acids 1-555) does not exhibit kinase activity (16). We examined the role of WNK1 AID domain in the regulation of ROMK1. We found that WNK11-555 had no effect on ROMK1 (Fig. 4B, 5th bar from left). As a control, WNK11-491 inhibited ROMK1 (Fig. 4B, 4th bar). Two phenylalanine residues (Phe-524 and -526; Fig. 4A) within AID domain of WNK1 are critical for inhibition of the kinase activity; mutation of both residues abrogates the function of AID domain to inhibit kinase activity (16). We found that WNK11-555 carrying double mutations of phenylalanine to alanine (WNK11-555/FFAA) inhibited ROMK1 (Fig. 4B, 6th bar), confirming that a functional AID domain is necessary for antagonism toward WNK11-491. When expressed as a separate peptide from WNK11-491, AID domain (WNK1491-555) still prevented inhibition of ROMK1 by WNK11-491 (Fig. 4C, 4th bar from left). In contrast, WNK1491-555/FFAA did not prevent inhibition of ROMK1 by WNK11-491 (Fig. 4C, 6th bar). Neither WNK1491-555 nor WNK1491-555/FFAA affected ROMK1 in the absence of WNK11-491 (Fig. 4C, 3rd and 5th bar).
Fig. 4.
Role of AID domain of WNK1 in the regulation of ROMK1. A: diagram of WNK1 domains in the study. Amino acids of AID surrounding phenyalanine-524 and -526 critical for the function of AID domain are shown. Double mutations of phenylalanine to alanine is abbreviated as “FFAA.” B: HEK cells were cotransfected with ROMK1 plus indicated construct or no WNK1 (control). *P < 0.01 vs. control (No WNK1). C: cells were cotransfected with ROMK1 plus indicated construct. *P < 0.01 vs. control (white bar). **P < 0.001 between indicated groups. NS, statistically not significant between indicated groups. D: cells were cotransfected with ROMK1 plus indicated construct. *P < 0.01 vs. control (white bar). **P < 0.001 between indicated groups. NS, statistically not significant between indicated groups.
In our model of multiple domain interactions for WNK1 regulation of ROMK1, the effect of NL domain to reverse N-PRD-mediated inhibition of ROMK1 is the first and essential step. We next examined the role of NL domain (amino acids 120-220) in regulation of ROMK1 by the AID domain. In these experiments, we made WNK1 constructs containing amino acids 120-555 (WNK1120-555) and amino acids 220-555 (WNK1220-555) and compared their effects on WNK11-119-mediated inhibition of ROMK1. As expected from the results by using a continuous peptide WNK11-555 (Fig. 4B, 5th bar), we found that WNK1120-555 reversed WNK11-119-mediated inhibition of ROMK1 (Fig. 4D, 4th bar from left). WNK1120-555 had no effect on ROMK1 in the absence of WNK11-119 (Fig. 4D, 3rd bar). In contrast, WNK1220-550 did not reverse WNK11-119-mediated inhibition of ROMK1 (Fig. 4D, 6th bar), indicating that amino acids 120-220 are necessary for the effect of AID domain through kinase domain. WNK1220-555 had no effect on ROMK1 in the absence of WNK11-119 (Fig. 4D, 5th bar). Moreover, we found that AID domain (WNK1491-555) by itself (i.e., without NL and kinase domain) did not cause inhibition of ROMK1 nor did it affect WNK11-119-mediated inhibition of ROMK1 (Fig. 4D, 7th and 8th bar from left). Taken together, these results suggest that WNK1 AID domain regulates the functional coupling between kinase domain and NL domain, thus influencing the functionality of the N-PRD domain to cause endocytosis of ROMK1.
Role of amino acids COOH-terminal to AID domain.
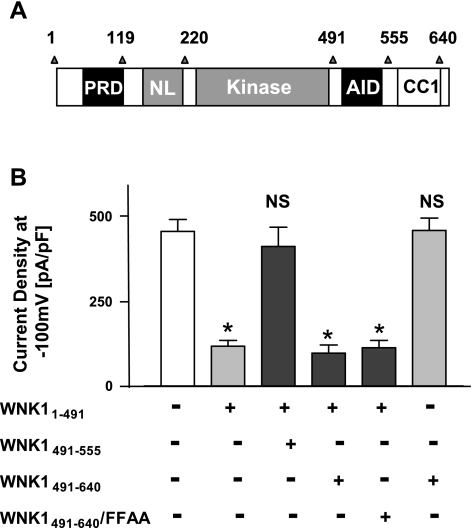
One of the two coiled-coil domains of WNK1 (labeled CC1 for 1st coiled-coil domain; located within amino acids 555-640) is localized adjacent to the AID domain (Fig. 5A). In a kinase activity assay, the CC1 domain antagonizes the inhibitory effect of AID domain on kinase domain (16). We hypothesized that CC1 domain may also be important in modulating the effect of AID domain on the kinase domain with respect to the regulation of ROMK1. The hypothesis may explain why full-length WNK1 and WNK11-1200 each inhibited ROMK1 (Fig. 1B) while WNK11-555 did not (Fig. 4B). We tested this by addition of CC1 to AID domain and examined ability of the construct to reverse inhibition of ROMK1 by WNK11-491. We found that WNK1491-640, which contains both AID and CC1 domains, did not reverse WNK11-491-mediated inhibition of ROMK1 (Fig. 5B, 4th bar from left). By itself, WNK1491-640 had no effect on ROMK1 (Fig. 5B, 6th bar). In contrast, WNK1491-550 (which contains only AID domain) was capable of reversing the inhibition of ROMK1 by WNK11-491 (Fig. 5B, 3rd bar). As a control, WNK1491-640 carrying double phenylalanine mutation (WNK1491-640/FFAA) had no effect on the inhibition of ROMK1 by WNK11-491 (Fig. 5B, 5th bar), suggesting that (without an intact AID domain) CC1 domain did not exert nonspecific effect on ROMK1 and/or WNK11-491.
Fig. 5.
Role of the first coiled-coil (CC1) domain of WNK1 in the regulation of ROMK1. A: diagram of WNK1 domains in the study. B: HEK cells were cotransfected with ROMK1 plus various combinations of WNK1 constructs as indicated. *P < 0.01 vs. control (no WNK1 constructs; white bar). NS, statistically not significant vs. control.
DISCUSSION
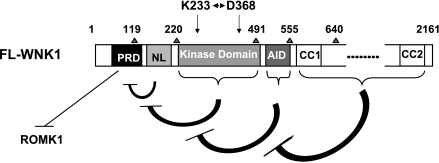
WNK1 inhibits ROMK1 by stimulating clathrin-mediated endocytosis of the channel (8, 12). We recently reported that specific proline-rich motifs in the N-PRD of WNK1 are important for this process (5). N-PRD binds and recruits the endocytic scaffold protein intersectin to enhance endocytosis of the channel. In the present study, we further examined the role of the entire domain structure of WNK1 in the regulation of ROMK1. Although we cannot exclude the possibility that some of the expressed short peptide domains may act differently from the full-length protein, the totality of our results in this and previous studies is consistent and allows us to propose the following working model for WNK1 regulation of ROMK1 (Fig. 6). The N-PRD domain functions (through recruitment of intersectin, not shown in Fig. 6) to inhibit ROMK1. The NL domain inhibits the function of N-PRD domain. WNK1 kinase domain antagonizes the function of NL domain toward N- PRD domain. The AID domain further modulates the coupling between WNK1 kinase domain and NL domain and indirectly regulates the function of N-PRD domain. Finally, the first COOH-terminal coiled-coil domain (CC1) prevents AID from exerting inhibition on the kinase domain.
Fig. 6.
Model of functions of different WNK1 domains in regulating ROMK1.
The role of WNK1 kinase domain deserves further discussion. Previously, we and others reported that kinase-dead WNK1 caused by charge neutralization mutations of the catalytic lysine or a conserved aspartate fails to regulate ROMK1 (8, 12). In a more recent study, we suggested that the role of WNK1 kinase domain in regulating ROMK1 requires a properly folded kinase domain maintained by charge-charge interactions between the catalytic lysine (L233) and the conserved aspartate (D368) (5). Thus, charge neutralization mutations of the catalytic lysine or the conserved aspartate disrupt WNK1 regulation of ROMK1 by affecting the structure, not the catalytic activity, of the kinase domain (5). In the present study, we employed additional mutants of WNK1 with preserved electrostatic interactions but lack kinase activity and found that they could still inhibit ROMK1. These results provide further support for the above working model.
To understand how the kinase domain contributes to the recruitment of intersectin mediated by the N-PRD, we identified an NL domain between N-PRD and kinase domain. The NL domain inhibits the function of N-PRD domain and the inhibition can be reversed by a properly folded kinase domain. The AID domain of WNK1 inhibits catalytic activity of the kinase domain, presumably by direct binding to the kinase domain (9, 16). In our model, AID domain also affects the function of kinase domain in the regulation of ROMK1. The first coiled-coil domain (CC1) following AID domain, in turn, interferes with the inhibitory effect of AID domain toward kinase domain. This effect of CC1 on AID domain may underscore that full-length WNK1 or constructs that contain the region of amino acids 1 through 640 of WNK1 (such as WNK11-1200 and WNK11-555 plus WNK1555-640) inhibit ROMK1.
One interesting feature of the biology of WNK kinases is that many processes regulated by WNKs do not require the catalytic activity (17, 18, 21). As shown in the case of regulation of ROMK1, the noncatalytic mechanism of regulation by WNK1 may involve the kinase domain. This noncatalytic, but kinase domain-dependent mechanism of regulation may be applicable to other targets and/or by other WNKs. For example, charge neutralization of the conserved aspartate of WNK4 prevents its inhibition of the thiazide-sensitive sodium-chloride cotransporter NCC (13). Yet, a COOH-terminal region of WNK4 not containing the kinase domain is capable of inhibiting NCC (21).
WNK kinases are large proteins with multiple domains for protein-protein interactions (19). Xu et al. (16) and Lenertz et al. (9) reported that WNK1 forms multimers and exhibits extensive biochemical interactions between NH2-terminal amino acids 1-220, kinase domain, and the AID and CC1 domain in vitro. The functional significance of these interactions was not known. Our data provide a scenario where these different domains of WNK1 are coupled through physical interactions to regulate clathrin-mediated endocytosis of target proteins. Since WNK1 forms multimers, the multiple domain interactions may occur between subunits as well as within subunits. Moreover, it may occur between different WNK kinases (9, 21, 22). These interactions among domains of WNKs, inter- or intramolecularly, may be facilitated by interactions with other proteins, such as intersectin. In the end, they provide additional noncatalytic mechanisms of regulation of target proteins by WNKs.
GRANTS
This work was supported by National Institutes of Health Grant DK-59530 and an EIA award from American Heart Association (0440019N).
DISCLOSURES
These experiments were performed by Hao-Ran Wang in partial fulfillment of the requirements of the Ph.D. degree at the University of Texas Southwestern Medical Center at Dallas.
Acknowledgments
We thank committee members Drs. J. Albanesi, M. Kuro-o, and S. Muallem for input and comment.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Choate KA, Kahle KT, Wilson FH, Nelson-Williams C, Lifton RP. WNK1, a kinase mutated in inherited hypertension with hyperkalemia, localizes to diverse Cl− transporting epithelia. Proc Natl Acad Sci USA 10: 663–668, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cope G, Murthy M, Golbang AP, Hamad A, Liu CH, Cuthbert AW, O'Shaughnessy KM. WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol 17: 1867–1874, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Delaloy C, Lu J, Houot AM, Disse-Nicodeme S, Gasc JM, Corvol P, Jeunemaitre X. Multiple promoters in the WNK1 gene: one controls expression of a kidney-specific kinase-defective isoform. Mol Cell Biol 23: 9208–9221, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon RD Syndrome of hypertension and hyperkalemia with normal glomerular filtration rate. Hypertension 8: 93–102, 1986. [DOI] [PubMed] [Google Scholar]
- 5.He G, Wang HR, Huang SK, Huang CL. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest 117: 1078–1087, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahle KT, Wilson FH, Leng Q, Lalioti MD, O'Connell AD, Dong K, Rapson AK, MacGregor GG, Giebisch G, Hebert SC, Lifton RP. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet 35: 372–376, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenertz LY, Lee BH, Min X, Xu BE, Wedin K, Earnest S, Goldsmith EJ, Cobb MH. Properties of WNK1 and implications for other family members. J Biol Chem 280: 26653–26658, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Min X, Lee BH, Cobb MH, Goldsmith EJ. Crystal structure of the kinase domain of WNK1, a kinase that causes a hereditary form of hypertension. Structure 12: 1303–1311, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Verissimo F, Jordan P. WNK kinases, a novel protein kinase subfamily in multi-cellular organisms. Oncogene 20: 5562–5569, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Wade JB, Fang L, Liu J, Li D, Yang CL, Subramanya AR, Maouyo D, Mason A, Ellison DH, Welling PA. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci USA 103: 8558–8563, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson FH, Kahle KT, Sabath E, Lalioti MD, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na-Cl cotransporter is inhibited by wild-type but not mutant WNK4. Proc Natl Acad Sci USA 100: 680–684, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson FH, Disse-Nicodème S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 275: 16795–16801, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Xu BE, Min X, Stippec S, Lee BH, Goldsmith EJ, Cobb MH. Regulation of WNK1 by an autoinhibitory domain and autophosphorylation. J Biol Chem 277: 48456–48462, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Xu BE, Stippec S, Lazrak A, Huang CL, Cobb MH. WNK1 activates SGK1 by a phosphatidylinositol 3-kinase-dependent and noncatalytic mechanism. J Biol Chem 280: 34218–34223, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, Cobb MH. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA 102: 10315–10320, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu BE, Lee BH, Min X, Lenertz L, Heise CJ, Stippec S, Goldsmith EJ, Cobb MH. WNK1: analysis of protein kinase structure, downstream targets, and potential roles in hypertension. Cell Res 15: 6–10, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Xu Q, Modrek B, Lee C. Genome-wide detection of tissue-specific alternative splicing in the human transcriptome. Nucleic Acids Res 30: 3754–3766, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na-Cl cotransport. J Clin Invest 111: 1039–1044, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang CL, Zhu X, Ellison DH. The thiazide-sensitive Na-Cl cotransporter is regulated by a WNK kinase signaling complex. J Clin Invest 117: 3403–3411, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zagórska A, Pozo-Guisado E, Boudeau J, Vitari AC, Rafiqi FH, Thastrup J, Deak M, Campbell DG, Morrice NA, Prescott AR, Alessi DR. Regulation of activity and localization of the WNK1 protein kinase by hyperosmotic stress. J Cell Biol 176: 89–100, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng WZ, Babich V, Ortega B, Quigley R, White SJ, Welling PA, Huang CL. Evidence for endocytosis of ROMK potassium channel via clathrin-coated vesicles. Am J Physiol Renal Physiol 283: F630–F639, 2002. [DOI] [PubMed] [Google Scholar]