Abstract
We previously showed that the 12/15-lipoxygenase (12/15-LO) pathway of arachidonate acid metabolism is involved in multiple events related to diabetic nephropathy (DN), including glomerular hypertrophy and extracellular matrix deposition (Kang SW, Adler SG, Nast CC, LaPage J, Gu JL, Nadler JL, Natarajan R. Kidney Int 59: 1354–1362, 2001; Kang SW, Natarajan R, Shahed A, Nast CC, LaPage J, Mundel P, Kashtan C, Adler SG. J Am Soc Nephrol 14: 3178–3187, 2003; Kim YS, Lanting L, Adler SG, Natarajan R. Kindney Int 64: 1702–1714, 2003; Reddy MA, Adler SG, Kim YS, Lanting L, Rossi JJ, Kang SW, Nadler JL, Shahed A, Natarajan R. Am J Physiol Renal Physiol 283: F985–F994, 2002). In this study, we investigated whether in vivo delivery of small interfering RNAs (siRNAs) targeting 12/15-LO can ameliorate renal injury and DN in a streptozotocin-injected mouse model of type 1 diabetes. To achieve greater in vivo access and siRNA expression in the kidney, we used double-stranded 12/15-LO siRNA oligonucleotides conjugated with cholesterol. Diabetic DBA/2J mice were injected subcutaneously with either cholesterol-tagged 12/15-LO siRNA, mismatched control siRNA, or vehicle alone, twice weekly for 7 wk. Relative to controls, mice that received 12/15-LO siRNA showed significant reduction in albuminuria, kidney-to-body weight ratios, glomerular mesangial matrix expansion, renal structural damage, and monocyte/macrophage infiltration. These effects were associated with lower renal cortical or glomerular levels of profibrotic markers transforming growth factor-β, connective tissue growth factor, type I and type IV collagens, plasminogen activator inhibitor 1, and fibronectin. The diabetes-induced increase in glomerular cyclin-dependent kinase inhibitors that are associated with hypertrophy was also prevented by siRNA administration. Our results show for the first time that systemic delivery of cholesterol-tagged siRNAs targeting 12/15-LO has renoprotective effects under diabetic conditions and therefore could be a novel therapeutic approach for DN.
Keywords: gene delivery
diabetic nephropathy (DN) is associated with end-stage renal disease and hence is a major cause of mortality in patients with diabetes. DN is characterized by structural abnormalities in the kidney, including glomerular and tubular hypertrophy, thickening of glomerular basement membrane, and progressive accumulation of mesangial extracellular matrix (ECM) components (1, 25). Several factors have been implicated in these pathological processes, including hyperglycemia, advanced glycation end products, oxidant stress, oxidized lipids, and growth factors such as ANG II and transforming growth factor-β (TGF-β) (1, 17, 40, 43, 51).
Lipoxygenases (LO) are a family of non-heme iron-containing enzymes that insert molecular oxygen into polyunsaturated fatty acids such as arachidonic and linoleic acids (11, 50). They are classified as 5-, 8-, 12-, or 15-LO according to the carbon atom of arachidonic acid at which the oxygen is inserted. 12-LO activation can lead to the formation of oxidized lipids such as 12(S)-HETE. Human and rabbit 15-LO as well as the leukocyte-type 12-LO have high homology and are classified as 12/15-LO since they can form both 12(S)-HETE and 15(S)-HETE from arachidonic acid (11, 50). Leukocyte-type 12/15-LO is found in various cells and tissues, including adrenal cells, vascular smooth muscle cells (VSMC), brain, kidney, and pancreas (2, 6, 11, 14, 19, 37).
Studies show that 12/15-LO mRNA and protein are increased in glucose-stimulated mesangial cell (MC) and in experimental DN (17). Growth factors relevant to the pathogenesis of DN such as ANG II, platelet-derived growth factor (PDGF), and TGF-β can induce leukocyte-type 12/15-LO activity and expression in VSMC and MC (35, 36, 38, 39). The growth-promoting and matrix-inducing effects of ANG II in MC were blocked by pharmacological 12-LO inhibitors as well as a 12-LO ribozyme (38). Furthermore, 12(S)-HETE could directly induce cellular hypertrophy and expression of the ECM protein fibronectin (FN) in MC and VSMC with similar potency as ANG II (38, 39).
We also demonstrated the potential functional role of 12/15-LO in MC growth and ECM protein expression related to glomerulosclerosis and nephropathy. Compared with wild-type (WT) mice, MC derived from 12/15-LO knockout mice grew more slowly and synthesized less FN under both basal and ANG II-stimulated conditions (23). In another in vivo study with a rat model of DN, a pharmacological 12/15-LO inhibitor showed some improvement in albuminuria, but the protection was not sustained (30). These recent studies demonstrate that 12/15-LO plays a key role in the development of DN.
Recent advancements in biotechnology have led to promising small interfering RNA (siRNA)-based therapeutic approaches to specifically silence disease-related target genes. siRNAs are duplexes of RNA that are 21–23 nucleotides long. One of these strands becomes incorporated into a complex of proteins called RNA-induced silencing complex. This RNA-induced silencing complex component serves as a guide to identify a complementary sequence in a specific target mRNA, thereby leading to cleavage of this mRNA, or inhibition of its translation (10, 15, 22). Efficient delivery of siRNAs into cells is essential for effective downregulation of target gene expression both in vitro and in vivo. While in vitro delivery and gene silencing can now be achieved with high efficiency due to the development of excellent transfection reagents, challenges still remain for in vivo delivery. Chemical modifications and bioconjugation of one or both siRNA strands with lipid and polymers are needed to increase their thermodynamic and nuclease stability, and also to improve their biodistribution and pharmacokinetic profiles in vivo (8, 9, 22). Recent evidence shows that cholesterol conjugation of siRNAs could enhance their cellular uptake and in vivo access without any transfection reagent (5, 42). Significant levels of cholesterol-tagged siRNAs were observed in the liver, heart, kidney, lung, and adipose tissue of mice (5, 42).
In this report, we therefore synthesized cholesterol-tagged siRNAs targeting mouse leukocyte 12/15-LO to improve their in vivo access and determine whether they have protective effects against renal injury and DN in a streptozotocin (STZ)-injected mouse model of type 1 diabetes. We observed for the first time that these siRNAs could reduce 12/15-LO expression in the kidney and confer beneficial effects on diabetes-induced changes in glomerular structure and ECM accumulation. Such modified siRNAs targeting key genes could therefore be developed as novel therapeutic approaches for DN.
MATERIALS AND METHODS
Materials.
SYBR Green PCR Master Mix kits were from Applied Biosystems (Foster City, CA); RNA-STAT60 reagent was from Tel-Test (Friendswood, TX); polyclonal rabbit anti-mouse antibodies specific for type IV collagen and an anti-FN monoclonal antibody (catalog no. sc-8422) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). This FN antibody does not cross-react with soluble, plasma fibronectin; the TGF-β1 monoclonal antibody was from R&D Systems (catalog no. MAB1835, Minneapolis, MN); rat anti-mouse F4/80 antibody from eBioscience (San Diego, CA); horseradish peroxidase-conjugated secondary antibodies were from Cell Signaling (Beverly, MA); the β-actin antibody was from Sigma (St. Louis, MO); Supersignal chemiluminescence reagent was from Pierce (Rockford, IL); urinary albumin and creatinine ELISA kits were from Exocell (Philadelphia, PA).
Synthesis of siRNAs.
The sequences of the siRNAs targeting 12/15-LO and control mismatched (MM) oligonucleotides (oligos) have been described earlier (24, 28). For the current study, to obtain greater in vivo expression in the kidney, we used synthetic methods to attach a cholesterol (chol) tag to these double-stranded siRNA oligos, based on reports showing improved in vivo access and kidney expression of such tagged siRNAs (42). Two siRNA oligos that we recently showed to efficiently target mouse leukocyte 12/15-LO (siRNA 233 and 826) (28) were thus further modified by adding a chol tag (C3 link) as reported (42). Synthesis of these tagged siRNAs (12/15-LO and control MM) was performed at the City of Hope DNA/RNA Synthesis Core. An equimolar mixture of siRNAs 233 and 826 was used to attain maximal target knockdown as seen in vitro (28). The sequences of the siRNA oligos used are the following: chol-12/15LO-siRNA-233: sense 5′-GCAACUGGAUUUCUGUGAAGG*chol-3′, antisense 3′-G*a*cGUUGACCUAAAGACACUUCC-5′; chol-mismatched-siRNA-233: sense 5′-GCUACUGCAUUACUGUGUAGG*chol-3′, antisense 3′-G*a*cGAUGACGUAAUGACACAUCC-5′; chol-12/15LO-siRNA-826: sense 5′-GAAGCGGAUUUCUUCCUUCUG*chol-3′, antisense 3′-A*a*c UUCGCCU AAAGAAGGAAGAC-5′; and chol-mismatched-siRNA-826: sense 5′-GAUGCGGAAUUGUUCCUACUG*chol-3, antisense 3′-A*a*cUACGCCUUAACAAGGAUGAC-5′.
The MM oligos have four point mutations; each * represents phosphorothioation, upper case letters represent ribonucleotides, and lower case letters 2′O-methyl nucleosides.
Animals and MC culture.
All animal experiments were performed in accordance with a protocol approved by the City of Hope Research Animal Care Committee. Our institution is accredited by the American Association of Laboratory Animal Care. Eight-week-old male DBA/2J mice, weighing 18–22 g (Jackson Laboratory, Bar Harbor, ME), were used based on reports showing that this strain develops more robust and early DN relative to the C57BL6/J strain (4). Mice were housed in a temperature-controlled room and given free access to water and standard laboratory chow. Primary cultures of MC from normal C57BL/6 mice were isolated by differential sieving and cultured as described previously (23, 49).
Efficiency of siRNA delivery and distribution.
Synthetic cholesterol-tagged 12/15-LO siRNAs (chol-siRNAs) or control cholesterol-tagged MM siRNA oligos were dissolved in nuclease-free water and diluted with RNase-free PBS before transfection. Mouse MCs were serum depleted for 24 h, treated with chol-siRNAs-233 and 826 (0.1 μg/ml), and incubated for another 6 h. Cell lysates were collected for 12/15-LO detection. To evaluate the initial efficacy of chol-siRNA delivery in vivo, DBA/2J mice were given one injection (subcutaneous) of 400 μg chol-siRNAs or MM mixture. Seventy-two hours later, the mice were killed and various tissues were harvested, including spleen, liver, kidney, and heart, for analysis of 12/15-LO expression.
Animal model of DN and siRNA injections.
12 DBA/2J mice received 50 mg/kg of STZ intraperitoneally on 5 consecutive days to induce diabetes (glucose >300 mg/dl) (13, 49). Another batch of four mice was injected with vehicle (normal saline; NS) to serve as nondiabetic controls. Synthetic chol-siRNAs-233 and -826 were dissolved in saline (total 400 μg) before injection. One week after the first STZ injection, three diabetic mouse groups (4/group) were injected (subcutaneously) twice weekly per mouse with vehicle, cholesterol-tagged 12/15-LO siRNA mixture, or the MM oligos, and killed 7 wk after the first injection. Blood samples were collected before death. Individual mice were placed in metabolic cages to collect 24-h urine samples at the beginning of siRNA delivery (0 wk), then 3.5 and 7 wk after the first injection. Renal cortical tissues were saved for Western blotting and real-time quantitative RT-PCRs (QPCRs). Glomeruli were isolated from cortical tissues by sequential sieving as described (49) and pooled from four mice in each group. The siRNA dose and injection frequency were based on previous reports (13, 42) and our preliminary study. Body weight and blood glucose were measured monthly, and kidney weight after death. Serum creatinine was measured with an autoanalyzer (Technicon RA-1000, Bayer, Tarrytown, NY). Urinary creatinine was determined with the Creatinine Companion kit (Exocell, Philadelphia, PA). Key physiological parameters of control and diabetic mice are shown in Table 1.
Table 1.
Physiological parameters in experimental groups
| Control+NS | STZ+NS | STZ+MM | STZ+siRNA | |
|---|---|---|---|---|
| Fasting glucose 0 wk, mg/dl | 97.5±3.7 | 343±35.9‡ | 388±24.1‡ | 354±16.9‡ |
| Fasting glucose 7 wk, mg/dl | 119.8±3.9 | 569±28.8‡ | 566±29.8‡ | 558±30.7‡ |
| Serum creatinine 7 wk, mg/dl | 0.19±0.02 | 0.43±0.23* | 0.41±0.17* | 0.40±0.17* |
| Body wt 7 wk, g | 26.25±1.71 | 19.25±0.96‡ | 19.75±0.50‡ | 20.52±0.58‡ |
| Kidney wt 7 wk, g | 0.270±0.01 | 0.212±0.02‡ | 0.222±0.01‡ | 0.205±0.01‡ |
| Kidney wt/body wt 7 wk, % | 1.03±0.04 | 1.10±0.13* | 1.13±0.07* | 1.06±0.05$ |
| Urinary volume 0 wk, ml | 2.90±0.57 | 3.40±0.57 | 2.75±1.06 | 2.75±0.35 |
| Proteinuria 0 wk, mg | 8.58±1.14 | 7.07±3.45 | 7.14±1.35 | 6.37±0.88 |
| Albuminuria 0 wk, μg | 111.6±36.3 | 250.8±96.7 | 180.9±38.1 | 190.8±48.5 |
| Urinary volume 3.5 wk, ml | 2.15±0.21 | 5.1±0.57* | 6.6±1.16* | 6.15±0.36* |
| Proteinuria 3.5 wk, mg | 12.64±0.80 | 15.58±2.78 | 18.01±1.58 | 16.49±0.32 |
| Albuminuria 3.5 wk, μg | 119.2±26.9 | 293.9±45.5* | 246.1±76.5* | 193.7±48.3* |
| Urinary volume 7 wk, ml | 2.53±1.37 | 6.45±0.77* | 6.85±1.62* | 6.65±1.06* |
| Proteinuria 7 wk, mg | 10.92±4.11 | 36.59±5.08† | 42.07±5.03† | 32.07±5.60*§ |
| Albuminuria 7 wk, μg | 120.8±77.9 | 413.4±46.1† | 362.2±21.2† | 200.6±42.5*§ |
Values are means ± SE; n = 4/group. DBA/2J mice were injected with streptozotocin (STZ), and 1 wk later injections were commenced, twice weekly for ≤7 wk with either normal saline (STZ+NS), mismatched oligos (STZ+MM), or cholesterol-tagged 12/15-lipoxygenase (12/15-LO) small interfering RNA (siRNA; STZ+siRNA). A control nondiabetic group of DBA/2J mice was injected with normal saline alone (control+NS) Parameters were first measured soon after establishment of hyperglycemia (1 wk post-STZ injection) at 0 wk before oligo delivery (0 wk), 3.5 wk after the first oligo injections (3.5 wk), and 7 wk after the first oligo injections at the end of the experimental period (7 wk).
P < 0.05,
P < 0.01,
P < 0.001 vs. control+NS.
P < 0.05 vs. STZ+MM.
Urinary albumin and protein assay.
Albumin concentrations in 24-h urine samples were measured with an indirect competitive ELISA kit according to the manufacture's instructions. For the urinary protein assay, all samples were diluted 8× with Milli Q distilled water, and protein concentration was determined with a kit (Bio-Rad Laboratories, Hercules, CA). Albumin and protein excretion was normalized to urinary creatinine concentration.
RNA isolation and QPCR.
Total RNA was isolated from MC and glomeruli in renal cortical tissues as described previously (13, 49). Two micrograms of total RNA was used to synthesize cDNA by using MuLV reverse transcriptase and random hexamers. All QPCRs were performed in triplicate in a final volume of 25 μl with an ABI 7300 real-time PCR thermal cycler (Applied Biosystems). Standard curves were generated for target genes and the internal control 36B4. To confirm single products were amplified in each reaction, dissociation curves were run to detect nonspecific amplification. The quantities of each test gene and internal control 36B4 mRNA were calculated from the standard curves using the Applied Biosystems software as described (21). p27kip1 and p21cip1 primers for QPCR were from Qiagen; the other primers were designed using Invitrogen D-LUX Designer software and obtained from the City of Hope DNA Synthesis Core. Sequences of the primers are summarized in Table 2.
Table 2.
Primer sequences for amplification of various transcripts
| Target | Sequence (5′ ∼ 3′) | Product, bp |
|---|---|---|
| 12/15-LO | 139 | |
| Sense | CGCTGGCACTCTGTTTGAAGCG | |
| Antisense | TGGATGGCTATGGGCAAGAG | |
| TGF-β1 | 213 | |
| Sense | AGGAAGGACCTGGGTTGGAAG | |
| Antisense | CGTCTCGACCCACGTAGTAGACG | |
| CTGF | 181 | |
| Sense | GCGAAGCTGACCTGGAGGA | |
| Antisense | CGCACGAGTGGTGGTTCTGTGCG | |
| Type IV α1 collagen | 157 | |
| Sense | CACGAGCTTCCCTGGTAGTCGTG | |
| Antisense | GGACAACCTTTCCTGCCTCA | |
| Type I α1 collagen | 201 | |
| Sense | CGGATAGCAGATTGAGAACATCCG | |
| Antisense | CGGCTGAGTAGGGAACACACA | |
| Type I α2 collagen | 101 | |
| Sense | CAGAACATCACCTACCACTGCAA | |
| Antisense | TTCAACATCGTTGGAACCCTG | |
| Fibronectin | 149 | |
| Sense | CGGCGTATGCTGTCACTGGCCG | |
| Antisense | AAGTTGAAGGCAGCCACCTG | |
| PAI-1 | 169 | |
| Sense | GACGCCTTCATTTGGACGAA | |
| Antisense | CGGACCTTTTCCCTTCAAGAGTCCG | |
| 36B4 | 92 | |
| Sense | GCCCTGCACTCTCGCTTTCT | |
| Antisense | CAACTGGGCACCGAGGCAACAGTTG | |
| MCP-1 | 187 | |
| Sense | CAGCACTTCTTTGGGACACCTGCTG | |
| Antisense | AGGTCCCTGTCATGCTTCTGG | |
| Laminin | 240 | |
| Sense | TCCTGGTGGGAATCCTTGTGAT | |
| Antisense | CGGCCACCTCGTTACACTGCCG | |
| MMP2 | 242 | |
| Sense | GTGATGGCTTCCTCTGGTGCTC | |
| Antisense | CGGGCAGAATCCATACTTCTTATCCCG |
TGF-β1, transforming growth factor-β1; CTGF, connective tissue growth factor; PAI-1, plasminogen activator inhibitor-1.
Western blotting.
MC, glomeruli, and renal cortical tissues were lysed in 1.5× SDS sample buffer, and Western immunoblotting was performed as described (17, 28).
Morphological studies and immunohistochemistry.
All histological studies were performed with nonperfused kidneys. One section of renal cortex per mouse was fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned (5 μm), and stained with periodic acid-Schiff (PAS) reagent. Twenty glomeruli from random regions of renal cortical sections were selected, and then the extent of mesangial matrix identified by PAS-positive material, the glomerular tuft, and mesangial matrix area were measured with image-analysis software (Image-Pro Plus 5.1, Media Cybernetics, Silver Spring, MD). The PAS-positive area in the mesangium was factored by the glomerular tuft area to obtain the fraction of mesangial matrix. The surface areas of glomeruli cut at the vascular pole on the PAS-stained tissue sections were traced along the outline of capillary loops using Image-Pro Plus 5.1 software. Glomeruli with area <1,500 μm2 were excluded from the analysis as described (29).
IHC was performed as described recently (20). Briefly, sections were incubated for 30 min at room temperature with a polyclonal rabbit anti-mouse antibody specific for type IV collagen or monoclonal antibodies against TGF-β or FN diluted 1:100, 1:100, and 1:50 with 2% casein in BSA. For 12/15-LO staining, a polyclonal peptide antibody (1:100) was used as described previously (17). After washing, secondary goat anti-rabbit or anti-mouse antibodies were added for 20 min. For macrophage staining, frozen renal sections (5 μm) were stained with a rat anti-mouse F4/80 antigen antibody as described (27). At least 30 glomeruli in each section were examined using light microscopy at ×400 magnification. Positively stained areas in glomeruli were measured using Image-Pro Plus 5.1. For negative nonimmune control slides, the primary antibody was replaced by IgG.
Statistical analyses.
Data are expressed as means ± SE of multiple experiments. Paired Student's t-tests were used to compare two groups or ANOVA with Dunnett's posttests for multiple groups using PRISM software (Graph Pad, San Diego, CA). Statistical significance was detected at the 0.05 level.
RESULTS
Effects of siRNA delivery on 12/15-LO levels in vitro and in vivo.
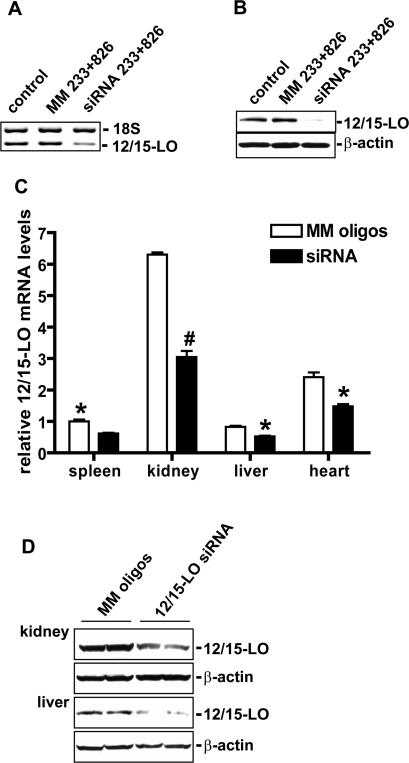
To test the efficacy of synthetic chol-siRNAs to silence endogenous target genes, we first examined effects in vitro and found that chol-siRNA-233 and -826 designed to target 12/15-LO significantly reduced 12/15-LO mRNA and protein expression in mouse MC (Fig. 1, A and B). Next, to determine in vivo efficacy and distribution, we examined 12/15-LO levels in various tissues 72 h after a single injection of cholesterol-tagged 12/15-LO siRNAs or MM oligos in DBA/2J mice. As shown in Fig. 1C, systemic delivery of the siRNAs significantly reduced 12/15-LO mRNA expression in the kidney, spleen, heart, and liver relative to the control MM oligos. Protein levels of 12/15-LO were also clearly decreased in the kidney and liver (Fig. 1D).
Fig. 1.
Efficacy of 12/15-lipoxygenase (12/15-LO) cholesterol-tagged small interfering RNA (chol-siRNA) on 12/15-LO levels in vitro and distribution in vivo. A: murine mesangial cells (MMCs) were transfected with cholesterol-tagged siRNA 233 and 826 mixture or mismatched (MM) oligo mixture, and 12/15-LO gene expression was examined by RT-PCR with 18S internal control. Significant reduction (almost 60%) of 12/15-LO mRNA level was observed. Ctrol, MMC without transfection; MM233+826, mismatched oligo transfection; siRNA233+826, mixture of two 12/15-LO siRNAs. B: Western blot showing that siRNA 233 and 826 mixture inhibited 12/15-LO protein expression in MMC, while MM siRNA had no effect relative to control. C: siRNAs targeting 12/15-LO or MM mixture. Four hundred micrograms were delivered to normal DBA/2J mice. Seventy-two hours later, the mice were killed and 12/15-LO mRNA levels in various tissues were quantified by real-time quantitative PCR (QPCR) as described in materials and methods. Data show that the siRNA significantly decreased 12/15-LO gene expression in the spleen, kidney, liver, and heart, indicating broad tissue biodistribution of the siRNAs.*P < 0.05, #P < 0.01 vs. MM; n = 3. D: representative Western blots showing clear reduction of 12/15-LO protein levels in the renal cortex and liver of mice treated with 12/15-LO siRNA relative to MM.
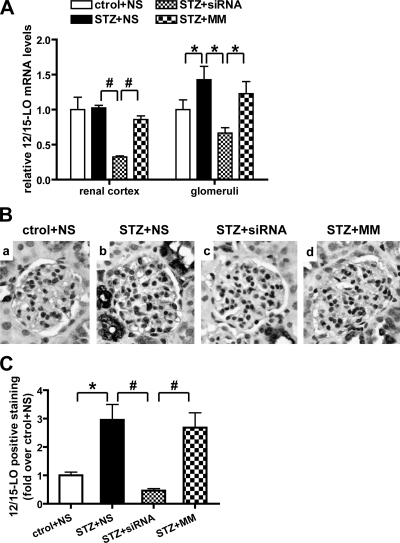
We next examined the efficacy of these siRNAs in diabetic mice. Figure 2A shows that 12/15-LO mRNA levels were significantly increased in glomeruli (but not renal cortex) of STZ-injected diabetic mice, and these were significantly ameliorated by the 12/15-LO siRNAs but not MM control. This was further supported by the IHC results in Fig. 2, B and C, showing increased 12/15-LO protein expression in glomeruli of diabetic mice that was blocked by over 75% by the siRNAs compared with vehicle (NS) or MM (Fig. 2C). Increased 12/15-LO staining in tubular regions was also attenuated by the siRNA (data not shown). These results demonstrate that the chol-siRNAs can reach the target tissues and induce efficient 12/15-LO silencing under basal and diabetic conditions.
Fig. 2.
Chol-siRNA targeting 12/15-LO attenuated 12/15-LO expression in the kidneys of type 1 diabetic mice. A: a significant increase in 12/15-LO mRNA expression was detected in the glomeruli, but not renal cortex, of diabetic mice. Biweekly injections of 12/15-LO siRNA for 7 wk reduced 12/15-LO mRNA levels by ∼70% in the renal cortex and 50% in glomeruli. Ctrol+NS, nondiabetic mice injected with normal saline; STZ+NS, diabetic mice with normal saline; STZ+siRNA, chol-siRNA mixture injection; STZ+MM, MM oligo injection. *P < 0.05. #P < 0.01. B: representative immunohistochemistry (IHC) for 12/15-LO. A clear increase in staining was observed in diabetic glomeruli (STZ+NS) compared with control (ctrol+NS). Staining was also seen in tubules. This increase was diminished by 12/15-LO siRNA injection (STZ+siRNA) but not mismatched oligos (STZ+MM). C: bar graph showing quantification of immunostaining in glomerulus, *P < 0.05. #P < 0.01.
Effect of 12/15-LO siRNA on physiological parameters of diabetic mice.
As shown in Table 1, all diabetic STZ mice in the three treated groups (siRNA, MM, or vehicle) remained hyperglycemic throughout the experimental period. As expected, urine volumes were markedly increased in diabetic groups due to glycosuria. Thus the 12/15-LO siRNAs did not influence blood sugar or urinary volume. Although the diabetic mice clearly had reduced body and kidney weights, there was still a significant increase in the kidney weight-to-body weight ratios in the vehicle or MM-treated diabetic mice relative to nondiabetic animals, demonstrating the development of renal hypertrophy characteristic of DN. Furthermore, the siRNAs significantly decreased the kidney weight-to-body weight ratio (Table 1, row 6, rightmost column) compared with MM or vehicle controls (P < 0.05), suggesting that diabetes-induced renal hypertrophy can be attenuated by 12/15-LO siRNAs.
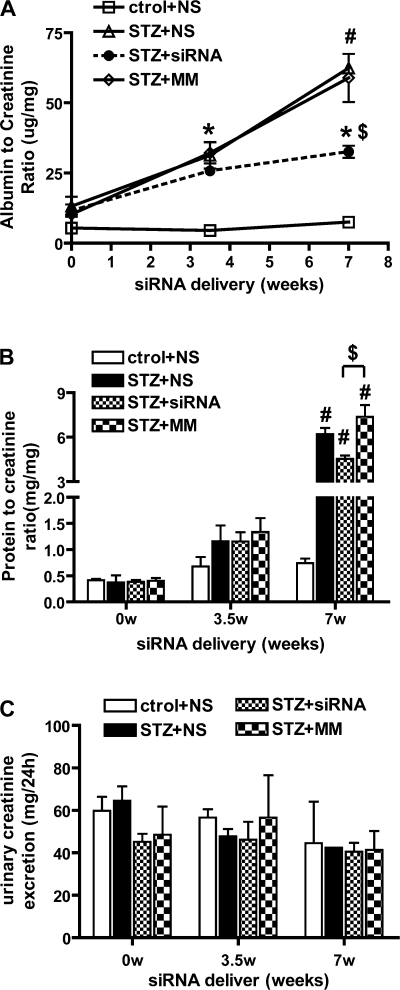
We next examined whether the 12/15-LO siRNAs could affect urinary albumin excretion and proteinuria, key pathological features of DN. Table 1 shows an increase in urinary albumin levels at 1 wk after the induction of diabetes and just before the initiation of the oligo injections (0 wk). This increase was statistically significant by 3.5 wk (P < 0.05 vs. control) in all three diabetic groups. The albuminuria progressively increased up until the end of the study (7 wk, P < 0.001 vs. control), but this was significantly attenuated by the siRNA at 7 wk (last row, rightmost column, Table 1). To minimize errors due to incomplete urine collection, urinary albumin excretion was calculated as relative ratio to creatinine excretion. The 12/15-LO siRNA-treated mice showed decreases in the albumin-to-creatinine ratio starting from 3.5 wk, and this protection was statistically significant by the end of the study (7 wk, 40% reduction) (Table 1 and Fig. 3A). We also noted that the urinary protein-to-creatinine ratio (Fig. 3B) was significantly enhanced only at 7 wk in the three diabetic groups, and this was partially but significantly attenuated in the siRNA-treated group relative to the two other STZ groups (P < 0.05 vs. MM) (Fig. 3B). Serum and urine creatinines were also examined as key parameters of kidney function. Serum creatinine was elevated in diabetic mice, but not urine creatinine. siRNA treatment did not significant alter either serum creatinine (Table 1) or urine creatinine excretion (Fig. 3C) in diabetic mice.
Fig. 3.
12/15-LO siRNA injection reduced urinary albumin and protein excretion in diabetic mice. A: albumin to creatinine ratio (ACR) was determined to evaluate 24-h urinary albumin excretion. Increased excretion of albumin in diabetic mice was detected from 3.5 wk until the end of the study. Seven weeks of repeated 12/15-LO chol-siRNA administration could attenuate this increase (STZ+siRNA) but not MM oligos (STZ+MM). B: significant decrease in urinary protein was detected at 7 wk after chol-siRNA injection whereas no difference was seen at 3.5 wk. Values are means ± SE; n = 4. *P < 0.05, #P < 0.01 vs. control+NS. $P < 0.05 vs. STZ+MM. C: urinary creatinine measured by specific ELISA shows no change.
12/15-LO siRNAs can attenuate glomerular hypertrophy and mesangial matrix expansion in diabetic mice.
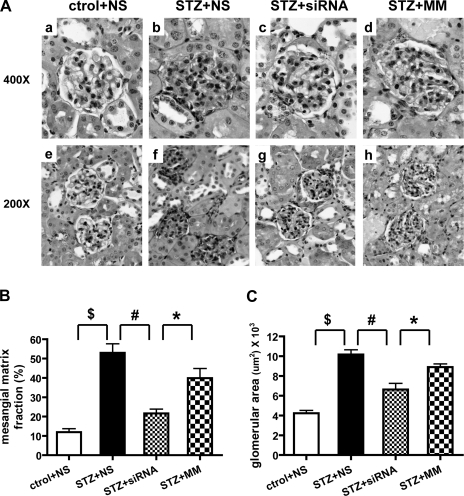
We next performed histological evaluation of diabetes-induced matrix expansion, hypertrophy, and glomerulosclerosis. Figure 4A shows cortical sections with glomeruli from each experimental group. The diabetic groups show diffuse mesangial expansion characterized by an increase in the accumulation of PAS-positive matrix and increased glomerular area (Fig. 4A). Morphometrical analyses in Fig. 4B show that glomeruli of diabetic mice in the vehicle and MM groups have significant mesangial matrix expansion compared with the NS control nondiabetic group (P < 0.001). Furthermore, 12/15-LO siRNA treatment reduced both glomerular cross-sectional area (Fig. 4A, c and g) and the mesangial matrix index (ratio of mesangial matrix area divided by the tuft area) (Fig. 4B) compared with the vehicle or MM diabetic groups. Glomerular area measurements (Fig. 4C) show that siRNA treatment also significantly blocked glomerular enlargement induced by diabetes.
Fig. 4.
12/15-LO siRNA administration reduces glomerular hypertrophy and mesangial matrix expansion in diabetic mice. A: representative photographs of periodic acid-Schiff (PAS)-stained kidney sections from nondiabetic mice with vehicle (a and e), diabetic mice treated with vehicle (b and f), siRNAs (c and g), or MM oligos (d and h). Quantitative measurements of glomerular area and mesangial matrix index, i.e., PAS-positive mesangial matrix area per total glomerular tuft cross-sectional area, are shown in B and C. A significant decrease in mesangial matrix fraction and glomerular area were observed in siRNA-injected mice relative to control groups. Values are means ± SE. *P < 0.05. #P < 0.01. $P < 0.001.
Increased mRNA expression of profibrotic and ECM genes in the diabetic kidneys were reduced by 12/15-LO siRNA.
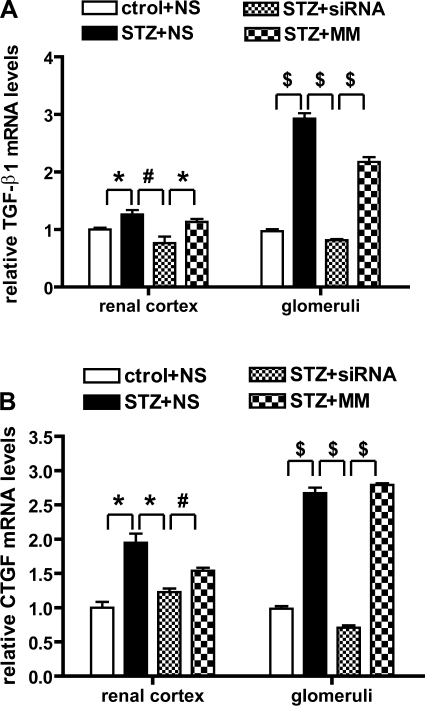
We recently demonstrated that TGF-β and 12/15-LO are upregulated in the glomeruli of diabetic mice (21) and that 12/15-LO induces TGF-β expression in MMC and vice versa (24). These results suggest that 12/15-LO may regulate TGF-β and subsequent downstream fibrotic genes. Therefore, we next examined whether 12/15-LO siRNA could serve as a good tool block TGF-β mRNA levels in renal cortex and glomeruli of diabetic mice. Compared with kidneys from normal mice, TGF-β mRNA levels were significantly enhanced in kidneys of diabetic mice, particular in the glomeruli (Fig. 5A). Furthermore, this increase was significantly attenuated in the 12/15-LO siRNA-treated mice relative to control groups. Connective tissue growth factor (CTGF) is a prosclerotic cytokine, acting downstream of TGF-β and transcriptionally controlled by TGF-β (13). We noted that, along with TGF-β, there was also a significant increment in CTGF mRNA expression in the diabetic kidneys and that the siRNA treatment could also attenuate this relative to vehicle or MM (Fig. 5B).
Fig. 5.
Expression of profibrotic genes transforming growth factor (TGF)-β1 (A) and connective tissue growth factor (CTGF; B) in diabetic mice was reduced by siRNA treatment. The mRNA levels of TGF-β1 and CTGF in renal cortex and glomeruli were quantified by QPCR and normalized to 36B4 mRNA levels. 12/15-LO siRNAs significantly inhibited the increased expression of these genes in renal cortex or glomeruli of streptozotocin (STZ)-treated diabetic mice. Values are means ± SE (n = 3). *P < 0.05. #P < 0.01. $P < 0.001.
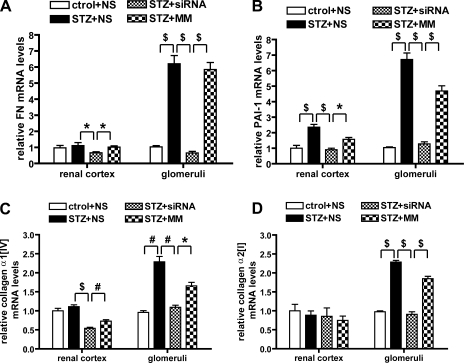
To further explore the mechanisms by which the 12/15-LO siRNA was exerting its protective effects against matrix expansion under diabetic conditions, we next evaluated mRNA levels of ECM or basement membrane components, including FN, laminin, type I and type IV collagens, associated matrix proteinases, and their inhibitors, in the renal cortex and glomeruli. Compared with nondiabetic mice, there was significant enhancement of FN, plasminogen activator inhibitor-1 (PAI-1), collagen α2(I), and collagen α1(IV) in the glomeruli of vehicle- or MM-treated diabetic mice. 12/15-LO siRNA treatment resulted in a significant reduction of FN, PAI-1, and collagen α1(IV) and collagen α2(I) mRNA levels in diabetic glomeruli compared with MM- or vehicle-treated diabetic groups (Fig. 6, A–D). Changes in the renal cortical tissues were not as significant as in the isolated glomeruli (Fig. 6. A–D, left). Other components such as collagen α1(I) and laminin were increased in diabetic glomeruli but did not show significant changes in the siRNA-treated group, while matrix metalloproteinase 2 (MMP-2) did not show significant changes in the diabetic groups with or without siRNA treatment (data not shown). Thus the siRNAs specifically attenuated some but not all ECM component genes.
Fig. 6.
Effects of 12/15-LO siRNA on the expression of ECM or basement membrane components in the diabetic kidney. mRNA levels of fibronectin (FN; A), plasminogen activator inhibitor-1 (PAI-1; B), collagen α1(IV) (C), and collagen α2(I) (D) genes in renal cortex and glomeruli were determined by QPCR. The diabetes-induced increased expression of these genes in glomeruli was clearly attenuated by siRNA injection. On the other hand, the changes in the renal cortex were relatively more modest, although statistically significant. Values are means ± SE of 3 independent experiments. *P < 0.05. #P < 0.01. $P < 0.001.
Effects of 12/15-LO siRNA on glomerular expression of profibrotic proteins.
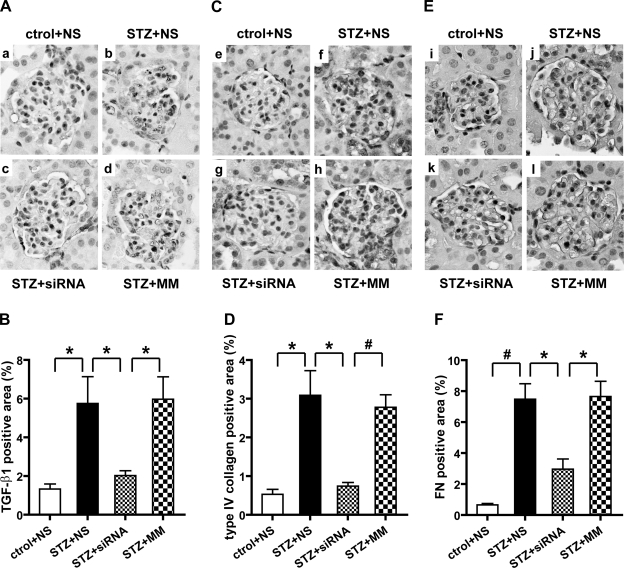
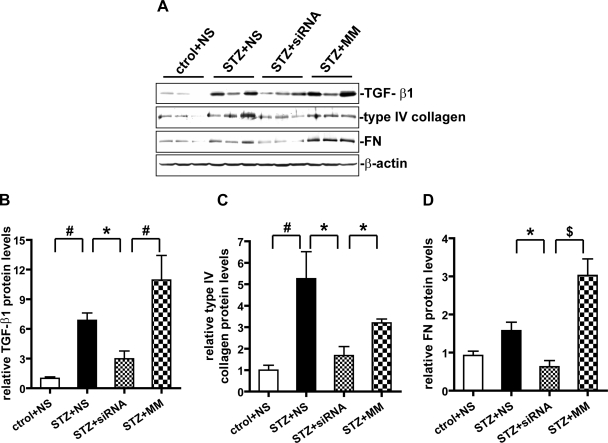
We next examined whether, apart from mRNA, the protein levels of profibrotic and ECM genes were also attenuated by the 12/15-LO siRNA treatment of diabetic mice. As seen in the IHC data in Fig. 7A, strong staining of TGF-β was seen in the glomeruli of control diabetic groups namely NS and MM (Fig. 7A, b and d), but this was much weaker in the glomeruli from 12/15-LO siRNA-treated diabetic mice (c). Quantification of the TGF-β-positive staining area in Fig. 7B showed that diabetes-induced increases in the glomeruli were significantly reduced by siRNA treatment (P < 0.05). Western blots of renal cortical lysates also showed marked reduction in TGF-β protein expression in siRNA-treated (STZ+siRNA) relative to control diabetic mice (STZ+NS or STZ+MM) (Fig. 8, A and B). These results further support the cross talk between TGF-β expression and the 12/15-LO metabolic pathway under diabetic conditions and that the 12/15-LO pathway may be essential, at least in part, for TGF-β induction under diabetic conditions.
Fig. 7.
IHC showing effects of siRNA on the expression of TGF-β1, type IV collagen, and FN in glomeruli. Renal cortical sections were immunostained with antibodies specific for TGF-β1 (A), type IV collagen (C), and FN (E) to evaluate glomerular expression. 12/15-LO siRNAs significantly reduced TGF-β positive area in diabetic glomeruli (c) and also type IV collagen (g) and FN (k). Values are means ± SE. Magnification, ×400 (A, C, and E). Bar graph quantifications of immunostaining are shown in B, D, and F. *P < 0.05. #P < 0.01.
Fig. 8.
Protein expression of TGF-β1, type IV collagen, and FN in renal cortical tissues. A: representative Western blots showing relative protein expression levels in renal cortical tissue lysates. B–D: quantitative analyses of relative protein abundance were performed as described in materials and methods, and values are means ± SE of 3 independent experiments. STZ treatment showed significant increase in protein levels of TGF-β1, type IV collagen, and FN. These increases were attenuated by siRNA treatment. *P < 0.05. #P < 0.01. $P < 0.001.
We next performed IHC with specific antibodies to evaluate type IV collagen and FN expression. Similar to the effects on mRNA levels, siRNA also significantly attenuated the increased expression of these two key ECM proteins in the glomeruli of diabetic mice (Figs. 7, C–F). Western blotting of cortical tissues also showed similar trends (Fig. 8, A, C, and D), although some augmentation of TGF-β and FN protein levels in these tissues by the MM was noted. The reason for this effect is unclear and might be due to unrelated effects of the MM on other cells in these heterogeneous cortical tissues. Overall, since TGF-β is a major regulator of many of these ECM molecules, it is possible that the siRNA-induced attenuation of ECM accrual and mesangial expansion may be mediated, at least in part, via TGF-β inhibition.
Expression of cyclin-dependent kinase inhibitors was reversed by 12/15-LO siRNA.
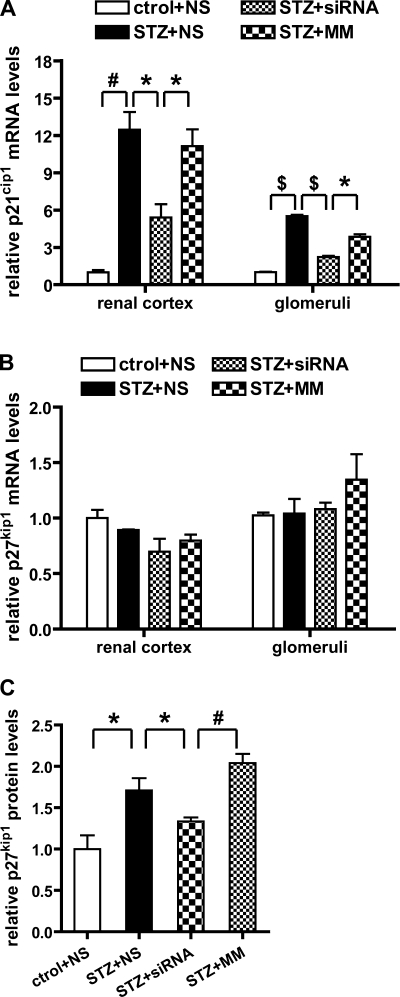
Cyclin-dependent kinase inhibitors (CDKIs) can inhibit cyclin-dependent kinase activity and induce G1 cell cycle arrest, and this is associated with glomerular hypertrophy in the early stages of DN (26, 47). Since we observed that systemic 12/15-LO siRNA delivery could attenuate diabetes-induced glomerular and kidney enlargement (Table 1), to further explore the mechanism, we examined the expression of CDKIs such as p21cip1 and p27kip1 that are closely associated with renal hypertrophy in DN. As shown in Fig. 9A, p21cip1 mRNA expression was increased in diabetic kidneys (cortex and glomeruli) compared with normal kidney and this was attenuated by >60% by the siRNA compared with MM. On the other hand. there was no statistically significant change in p27kip1 mRNA levels either in the diabetic mice relative to control or after siRNA treatment (Fig. 9B). Since p27kip1 is usually regulated at the posttranscriptional or translational level, we therefore examined the glomerular expression levels of p27kip1 protein by Western blotting. The bar graph in Fig. 9C shows that p27kip1 protein expression was enhanced in diabetic glomeruli and that this was attenuated by the 12/15-LO siRNA. Taken together, these results show that 12/15-LO siRNA treatment can attenuate diabetes-induced increases in CDKIs and that this effect may also be via TGF-β inhibition.
Fig. 9.
Increased expressions of cyclin-dependent kinase inhibitors (CDKIs) in the diabetic kidney are reduced by siRNA injection. A and B: relative CDKI mRNA levels in renal cortex and glomeruli were examined by QPCRs. Significant increase of p21cip1 mRNA levels was observed in diabetic kidneys, and the siRNA inhibited this increase in both cortex and glomeruli. No significant change in p27kip1 mRNA level was detected. C: expression of p27kip1 protein in glomeruli was detected by Western blot and quantitative analysis showed that 12/15-LO siRNA attenuated the diabetes-induced increase in p27kip1 protein expression in glomeruli. Values are means ± SE. *P < 0.05. #P < 0.01. $P < 0.001.
12/15-LO chol-siRNA can attenuate macrophage infiltration in diabetic glomeruli.
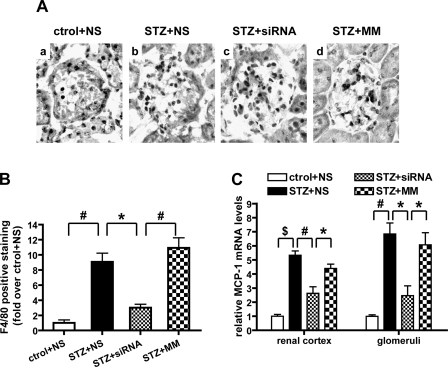
Evidence now suggests that renal macrophage infiltration and inflammation can occur during the progression of DN (3, 27). We therefore assessed F4/80-positive cells (marker for macrophages) in the renal sections. There was almost no staining in sections from nondiabetic mice, indicating the absence of macrophage infiltration (Fig. 10A). On the other hand, there was increased glomerular macrophage infiltration in all three diabetic groups. siRNA, but not MM, significantly decreased macrophage infiltration in the glomeruli of diabetic mice (Fig. 10, A and B). Monocyte chemoattractant protein-1 (MCP-1) is an inflammatory chemokine implicated in recruiting and activating monocyte/macrophages to the glomerulus and implicated in the pathology of DN (3). QPCR results demonstrated that diabetes significantly enhanced MCP-1 gene expression in both cortical tissues and isolated glomeruli and that this was significantly attenuated by 12/15-LO siRNA but not MM (Fig. 10C). These results suggest that 12/15-LO and its products can induce inflammatory genes such as MCP-1 in glomerular cells and thereby promote macrophage activation in DN. This is supported by data in VSMC and macrophages showing that 12/15-LO products can enhance MCP-1 expression (28, 46).
Fig. 10.
MCP-1 expression and monocyte/macrophage infiltration in glomeruli. A: IHC staining of F4/80 in mouse renal sections. B: quantification of F4/80-positive macrophage staining in glomeruli. A significant increase in staining was observed in the diabetic glomeruli, and this was reduced by the 12/15-LO siRNAs compared with MM. Values are means ± SE. *P < 0.05. #P < 0.01. C: mRNA levels of MCP-1 in renal cortex and glomeruli were quantified by QPCR. Values are means ± SE of 3 experiments; *P < 0.05. #P < 0.01. $P < 0.001.
DISCUSSION
12/15-LO and its oxidized lipid products have physiological and pathological effects, including the stimulation of cell growth, hypertrophy, ECM production, and inflammatory and profibrotic gene expression (18, 24, 34, 38, 39). Evidence suggests that high-glucose culture mimicking diabetic conditions can increase 12/15-LO activity and expression in MC, VSMC, and endothelial cells (17, 34, 37) and that animal models of diabetes show increased 12/15-LO expression in the kidney (17, 18, 45, 49). Studies with 12/15-LO gene knockout mice and 12/15-LO inhibitors have supported the role of 12/15-LO in the pathogenesis of renal disease and DN (23, 30). A recent in vivo study examining the effects of a nonselective pharmacological inhibitor in diabetic rats showed early renal protection from albuminuria that did not persist at later time periods (30). Therefore, with an objective to evaluate more specific modalities to block 12/15-LO, in this study we developed a new generation of cholesterol-conjugated siRNAs to silence 12/15-LO in vivo and examined their functional effects on the development of DN in mice. Since 12/15-LO is upregulated by key diabetic and prosclerotic stimuli such as TGF-β, HG, and ANG II, we reasoned that it would be a better target for intervention.
RNA interference (RNAi) with double-stranded siRNAs is generally more efficient, longer lasting than other mRNA-targeting strategies for gene silencing. The tremendous recent improvements in transfection reagents and delivery vectors have greatly enhanced the utility of siRNAs especially in vitro. The use of siRNAs as therapeutic agents has attracted great attention as a novel approach for treating several diseases. However, effective RNAi-based therapies are still limited by the need for efficient cell-specific delivery of siRNAs in vivo. Duplex siRNAs do not easily penetrate hydrophobic cellular membranes and are also rapidly degraded by serum RNases without proper carriers or chemical modifications to improve stability, pharmacokinetic profiles, and reduce nonspecific effects without affecting biological activity. Most common modifications include phosphorothioate (PS) linkage and 2′-O-methyl or 2′-deoxy-2′-fluoro modifications that increase siRNA stability by providing resistance to RNase activity in serum (7, 9). Our recent study showed the effectiveness of chimeric 2′-O-methoxyethyl-modified antisense oligos targeting CTGF in vivo in mouse models of DN (13). Recent studies showed that conjugation of siRNAs with cholesterol can increase uptake in human liver cells without the use of any transfection reagent (5, 42). This could improve the in vivo pharmacological properties of siRNAs and, interestingly, there was also greater expression in the kidney compared with that attained by unconjugated siRNAs (42). Therefore, effective systemic siRNA delivery can be achieved by administering chemically modified siRNAs (2′-O-methyl) either conjugated to the cholesterol group or packaged into protective liposomal particles (31).
In the current study, we used a combination of two siRNAs, M233 and M826, which were effective against the mouse leukocyte 12/15-LO sequence (28). A cholesterol tag was conjugated to the 3′-end of the sense strand of these siRNAs, which also had PS modifications for improved in vivo stability and renal access. We confirmed that these modified 12/15-LO siRNAs, but not control MM oligos, could downregulate 12/15-LO gene and protein expression efficiently in vitro in mouse MC and in vivo in DBA/2J mice kidneys.
We then examined the therapeutic potential of cholesterol-tagged 12/15-LO siRNAs to prevent or slow down the progression of DN in a mouse model of type 1 DN. We observed that, along with decreased 12/15-LO expression in the kidney and especially glomeruli, 7 wk of twice weekly 12/15-LO siRNA injections resulted in a significant reduction of kidney damage induced by diabetes. Diabetes-induced increases in urinary protein excretion, albuminuria, glomerular and renal hypertrophy, mesangial matrix expansion, and macrophage infiltration were all significantly attenuated by the 12/15-LO siRNA. These results demonstrate the in vivo efficacy of these modified siRNAs and also show that the downregulation of 12/15-LO and its oxidized lipid products can effectively ameliorate key structural and functional parameters associated with renal injury in type 1 diabetic mouse even without a reduction in hyperglycemia. Protective changes in the glomeruli were more consistent than in cortical lysates, possibly due to the more heterogeneous nature of the latter. Furthermore, since creatinine levels did not show any change after siRNA treatment, it is likely that longer treatments are needed to obtain additional or more complete protection.
The pathological hallmark of DN is glomerulosclerosis due to ECM accumulation in the glomerular mesangium and tubulointerstitial fibrosis. It is widely accepted that increased TGF-β expression is a key factor responsible for such renal hypertrophy, fibrosis, and scarring observed in diabetic renal injury (21, 41). CTGF has been identified as prosclerotic cytokine, acting downstream of TGF-β and shown to be involved in ECM accumulation and glomerular hypertrophy (1, 33). We recently showed that 12/15-LO and TGF-β pathways can cross talk and activate each other (24). We also showed that antisense-mediated inhibition of CTGF in vivo in diabetic mice can also block 12/15-LO expression. In the current study, we observed that 12/15-LO siRNAs could block the increased expression of both TGF-β and CTGF in the kidneys of diabetic mice compared with vehicle or MM oligos. These data indicate that 12/15-LO and its metabolic products may be initiators as well as propagators of renal injury by upregulating TGF-β and CTGF expression under diabetic conditions.
Mesangial matrix expansion and glomerular basement membrane thickening in DN can occur via increased expression of ECM proteins such as FN and collagen, inhibitors of ECM degradation such as PAI-1, or a decrease in ECM-degrading proteins such as MMPs. Evidence shows that several ECM proteins such as type I and type IV collagens, laminin, and FN are associated with TGF-β activation (21, 25, 41). TGF-β can also decrease matrix degradation by inhibiting proteases such as MMPs, as well as by activating protease inhibitors (e.g., PAI-1) (40, 44). In our study, we noted that the increased syntheses of key ECM proteins such as FN, collagen α2(I), and collagen α1(IV) in diabetic mice were significantly attenuated by the 12/15-LO siRNAs. In addition, elevated PAI-1 expression in diabetes kidney was also abrogated by siRNAs, while there was no effect on laminin or MMP-2. Overall, the administration of 12/15-LO chol-siRNA resulted in a coordinate decrease in diabetes-induced expression of genes involved in matrix accumulation and inhibition of matrix degradation in the kidney, particularly in glomeruli.
12/15-LO and its products are associated with MC and VSMC hypertrophy (34, 36, 38, 39). 12/15-LO and its product, 12(S)-HETE, can mediate growth and ECM-producing effects of ANG II and TGF-β in MC (24, 38). The involvement of 12/15-LO in cellular hypertrophy in DN is further supported by the current study showing that siRNAs targeting 12/15-LO in vivo significantly attenuated diabetes-induced renal enlargement and glomerular hypertrophy as well as increases in glomerular p21cip1 and p27kip1 expression. High glucose-induced glomerular hypertrophy is an active cell cycle-dependent process regulated by CDKIs, TGF-β synthesis, and activation (48). Therefore, it is likely that silencing of 12/15-LO could reverse glomerular hypertrophy in DN by decreasing TGF-β, p21cip1, and p27kip1 CDKIs.
Similar to other glomerulonephropathies, infiltration and activation of monocytes/macrophages in the glomerulus and associated inflammation have been implicated in the pathogenesis of DN. MCP-1, a chemokine that recruits activated monocytes to sites of injury and inflammation, is enhanced in diabetic glomeruli and contributes to FN deposition and glomerulosclerosis in DN (12, 16). Evidence shows that 12/15-LO and its products can induce inflammatory genes, including MCP-1 (28, 46). The 12/15-LO chol-siRNAs significantly decreased MCP-1 expression and macrophage infiltration in the diabetic kidney, thereby showing that they can confer additional protection against inflammation associated with DN.
In this study, although the 12/15-LO siRNAs significantly attenuated ECM expansion and albuminuria, renal protection was not complete since creatinine levels did not change. It is possible that longer time periods of siRNA administration could lead to even better outcomes. Evidence also shows that 15-LO has a protective anti-inflammatory role in experimental glomerulonephritis (32), suggesting that 12/15-LO products can have both protective and detrimental effects in the kidney. In summary, the current studies show for the first time that cholesterol-tagged siRNAs targeting 12/15-LO have protective effects against renal disease in a mouse model of type 1 diabetes. This in vivo study not only reinforces the mediatory role of 12/15-LO in the pathology of DN but also demonstrates the therapeutic utility of modified siRNAs for renal disease.
GRANTS
These studies were supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-058191 and R21-DK-076669 to R. Natarajan.
Acknowledgments
The authors are grateful to Mei Wang for technical help.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adler S Diabetic nephropathy: linking histology, cell biology, genetics. Kidney Int 66: 2095–2106, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Ardaillou R, Baud L, Sraer J. Lipoxygenase products and their functions in glomeruli. Adv Exp Med Biol 259: 49–74, 1989. [DOI] [PubMed] [Google Scholar]
- 3.Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int 58: 684–690, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Breyer MD, Bottinger E, Brosius 3rd FC, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Cheng K, Ye Z, Guntaka RV, Mahato RI. Enhanced hepatic uptake and bioactivity of type α1(I) collagen gene promoter-specific triplex-forming oligonucleotides after conjugation with cholesterol. J Pharmacol Exp Ther 317: 797–805, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia 48: 486–495, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Czauderna F, Fechtner M, Dames S, Aygün H, Klippel A, Pronk GJ, Giese K, Kaufmann J. Structural variations and stabilizing modifications of synthetic siRNAs in mammalian cells. Nucleic Acids Res 31: 2705–2716, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Fougerolles A, Vornlocher HP, Maraganore J, Lieberman J. Interfering with disease: a progress report on siRNA-based therapeutics. Nat Rev Drug Discov 6: 443–453, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De PD, Bentley MV, Mahato RI. Hydrophobization and bioconjugation for enhanced siRNA delivery and targeting. RNA 13: 431–456, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Funk CD The molecular biology of mammalian lipoxygenase and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta 1304: 65–68, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Giunti S, Tesch GH, Pinach S, Burt DJ, Cooper ME, Cavallo-Perin P, Camussi G, Gruden G. Monocyte chemoattractant protein-1 has prosclerotic effects both in a mouse model of experimental diabetes and in vitro in human mesangial cells. Diabetologia 51: 198–207, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Guha M, Xu ZG, Tung D, Lanting L, Natarajan R. Specific down-regulation of connective tissue growth factor attenuates progression of nephropathy in mouse models of type 1 and type 2 diabetes. FASEB J 21: 3355–3368, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Gu JL, Natarajan R, Ben-Ezra J, Valente G, Scott S, Yoshimoto T, Yamamoto S, Rossi JJ, Nadler JL. Evidence that a porcine mononuclear type of 12-lipoxygenase is expressed and regulated in human adrenal glomerulosa cells. Endocrinology 134: 70–77, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Hannon GJ, Rossi JJ. Unlocking the potential of the human genome with RNA interference. Nature 431: 371–378, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Kanamori H, Matsubara T, Mima A, Sumi E, Nagai K, Takahashi T, Abe H, Iehara N, Fukatsu A, Okamoto H, Kita T, Doi T, Arai H. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun 360: 772–777, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Kang SW, Adler SG, Nast CC, LaPage J, Gu JL, Nadler JL, Natarajan R. 12-Lipoxygenase is increased in glucose-stimulated mesangial cells and in experimental diabetic nephropathy. Kidney Int 59: 1354–1362, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Kang SW, Natarajan R, Shahed A, Nast CC, LaPage J, Mundel P, Kashtan C, Adler SG. Role of 12-lipoxygenase in the stimulation of p38 mitogen-activated protein kinase and collagen α5 (IV) in experimental diabetic nephropathy and in glucose-stimulated podocytes. J Am Soc Nephrol 14: 3178–3187, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Katoh T, Lakkis FG, Makita N, Badr KF. Co-regulated expression of glomerular 12/15-lipoxygenase and interleukin-4 mRNAs in rat nephrotoxic nephritis. Kidney Int 46: 341–349, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Kato M, Yuan H, Xu ZG, Lanting L, Li SL, Wang M, Hu MC, Reddy MA, Natarajan R. Role of the Akt/FoxO3a pathway in TGF-beta1-mediated mesangial cell dysfunction: a novel mechanism related to diabetic kidney disease. J Am Soc Nephrol 17: 3325–3335, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA 104: 3432–3437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet 8: 173–184, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Lanting L, Adler SG, Natarajan R. Differential behavior of mesangial cells derived from 12/15-lipoxygenase knockout mice relative to control mice. Kidney Int 64: 1702–1714, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Kim YS, Xu ZG, Reddy MA, Li SL, Lanting L, Sharma K, Adler SG, Natarajan R. Novel interactions between TGF-β1 actions and the 12/15-lipoxygenase pathway in mesangial cells. J Am Soc Nephrol 16: 352–362, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Kreisberg JI, Ayo SH. The glomerular mesangium in diabetes mellitus. Kidney Int 43: 109–111, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Kuan CJ, Al-Douahji M, Shankland SJ. The cyclin kinase inhibitor p21WAF1, CIP1 is increased in experimental diabetic nephropathy: potential role in glomerular hypertrophy. J Am Soc Nephrol 9: 986–993, 1998. [DOI] [PubMed] [Google Scholar]
- 27.Lassila M, Seah KK, Allen TJ, Thallas V, Thomas MC, Candido R, Burns WC, Forbes JM, Calkin AC, Cooper ME, Jandeleit-Dahm KA. Accelerated nephropathy in diabetic apolipoprotein e-knockout mouse: role of advanced glycation end products. J Am Soc Nephrol 15: 2125–2138, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Li SL, Dwarakanath RS, Cai Q, Lanting L, Natarajan R. Effects of silencing leukocyte-type 12/15-lipoxygenase using short interfering RNAs. J Lipid Res 46: 220–229, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Matsusaka T, Xin J, Niwa S, Kobayashi K, Akatsuka A, Hashizume H, Wang QC, Pastan I, Fogo AB, Ichikawa I. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J Am Soc Nephrol 16: 1013–1023, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Ma J, Natarajan R, LaPage J, Lanting L, Kim N, Becerra D, Clemmons B, Nast CC, Prakash GKS, Mandal M, Adler SG. 12/15-Lipoxygenase inhibitors in diabetic nephropathy in the rat. Prostaglandins Leukot Essent Fatty Acids 72: 13–20, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol 23: 1002–1007, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Munger KA, Montero A, Fukunaga M, Uda S, Yura T, Imai E, Kaneda Y, Valdivielso JM, Badr K. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function experimental glomerulonephritis. Proc Natl Acad Sci USA 96: 13375–13380, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadia AW, Benjamin S, Terry R, Roger MM. Connective tissue growth factor and regulation of the mesangial cell cycle: role in cellular hypertrophy. J Am Soc Nephrol 13: 2437–2445, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol 24: 1542–1548, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan R, Bai W, Rangarajan V, Gonzales N, Gu JL, Lanting L, Nadler JL. Platelet-derived growth factor BB mediated regulation of 12-lipoxygenase in porcine aortic smooth muscle cells. J Cell Physiol 169: 391–400, 1996. [DOI] [PubMed] [Google Scholar]
- 36.Natarajan R, Gonzales N, Lanting L, Nadler J. Role of the lipoxygenase pathway in angiotensin II-induced vascular smooth muscle cell hypertrophy. Hypertension 23: 142–147, 1994. [DOI] [PubMed] [Google Scholar]
- 37.Natarajan R, Gu JL, Rossi J, Gonzales N, Lanting L, Xu L, Nadler J. Elevated glucose and angiotensin II increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci USA 90: 4947–4951, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reddy MA, Adler SG, Kim YS, Lanting L, Rossi JJ, Kang SW, Nadler JL, Shahed A, Natarajan R. Interaction of MAPK and 12-lipoxygenase pathways in growth and matrix protein expression in mesangial cells. Am J Physiol Renal Physiol 283: F985–F994, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Reddy MA, Thimmalapura PR, Lanting L, Nadler JL, Fatima S, Natarajan R. The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element binding protein activation. Mediation of angiotensin II effects. J Biol Chem 277: 9920–9928, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Rincon-Choles H, Kasinath BS, Gorin Y, Abboud HE. Angiotensin II and growth factors in the pathogenesis of diabetic nephropathy. Kidney Int Suppl 82: 8–11, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Sharma K, Ziyadeh FN. Hyperglycemia and diabetic kidney disease: the case for transforming growth factor-beta as a key mediator. Diabetes 44: 1139–1146, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432: 173–178, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes 57: 460–469, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Tsuchida K, Zhu Y, Siva S, Dunn SR, Sharma K. Role of Smad4 on TGF-beta-induced extracellular matrix stimulation in mesangial cells. Kidney Int 63: 2000–2009, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Vaziri ND, Xu ZG, Shahkarami A, Huang KT, Rodríguez-Iturbe B, Natarajan R. Role of AT-1 receptor in regulation of vascular MCP-1, IL-6, PAI-1, MAP kinase, and matrix expressions in obesity. Kidney Int 68: 2787–2793, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Wen Y, Gu J, Vandenhoff GE, Liu X, Nadler JL. The role of 12/15-lipoxygenase in the expression of MCP-1 in mouse macrophages. Am J Physiol Heart Circ Physiol 294: H1933–H1938, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Wolf G, Schroeder R, Zahner G, Stahl RAK, Shankland SJ. High glucose-induced hypertrophy of mesangial cells requires p27Kip1, an inhibitor of cyclin-dependent kinases. Am J Pathol 158: 1091–1100, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh FN. High glucose-induced proliferation in mesangial cells is reversed by autocrine TGF-β. Kidney Int 42: 647–656, 1992. [DOI] [PubMed] [Google Scholar]
- 49.Xu ZG, Li SL, Lantin L, Kim YS, Shanmugam N, Reddy MA, Natarajan R. Relationship between 12/15-lipoxygenase and COX-2 in mesangial cells: potential role in diabetic nephropathy. Kidney Int 69: 512–519, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto S Mammalian lipoxygenase: molecular structures and functions. Biochim Biophys Acta 1128: 117–131, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Ziyadeh FN, Sharma K. Role of transforming growth factor-beta in diabetic glomerulosclerosis and renal hypertrophy. Kidney Int Suppl 51: S34–S36, 1995. [PubMed] [Google Scholar]