Abstract
Clinical and preclinical studies have demonstrated an important effect of arterial pathobiology on the progressive loss of renal function that occurs in chronic kidney disease. Chronic kidney disease, in turn, promotes alterations in vascular function. A modulating role for dietary salt has been suggested, with the amount of salt intake regulating endothelial cell production of transforming growth factor-β1 (TGF-β1), a fibrogenic growth factor that promotes arteriosclerosis and glomerulosclerosis. The purpose of the present studies was to determine how the interaction between dietary salt intake and vasculature promoted the production of TGF-β1 in rats. Two different vascular tissues, aortic rings and glomeruli, were chosen for study. Dietary salt induced, in a dose-dependent fashion, activation of proline-rich tyrosine kinase-2 (Pyk2) and further identified c-Src as an important binding partner of Pyk2 in these tissues. Use of pharmacological inhibitors and dominant negative strategies confirmed that dietary salt induced complex formation of Pyk2 and c-Src with downstream activation of p38 and p42/44 mitogen-activated protein kinases and generation of TGF-β1. The experiments defined the molecular signaling events that promoted the production of TGF-β1, a key growth factor involved in the vascular response to increased salt intake.
Keywords: proline-rich tyrosine kinase-2, c-Src, aorta, glomerulus, endothelium
an interrelationship between vascular disease and chronic kidney disease is well accepted. In particular, increased blood pressure and decreased vascular compliance correlate with progression of chronic kidney disease and cardiovascular disease (32). The seminal publication by Klag et al. (20) demonstrated a direct correlation between blood pressure and development of end-stage kidney disease, with systolic blood pressure, an indirect reflection of arterial compliance, serving as a strong predictor of subsequent renal disease. Fesler et al. (13) showed that pulse pressure, a marker of arterial stiffening, correlated well with decline in renal function during treatment of patients with essential hypertension. Direct involvement of dietary salt intake in the development of systolic hypertension and decreased conduit artery compliance was established by Gates et al. (15), who demonstrated in a double-blind, placebo-controlled crossover study that reduction in salt intake in patients with untreated systolic hypertension lowered systolic blood pressure and increased carotid arterial compliance. The decrease in systolic blood pressure correlated inversely with the change in arterial compliance in that study. The Trials of Hypertension Prevention studies provided convincing evidence that modest reductions in salt intake promoted a long-term 25% reduction in the risk of cardiovascular events, confirming an important effect of salt intake on cardiovascular function (10). Although changes in renal function were not reported in that study, correlation between dietary salt intake and progression of chronic kidney disease has been observed in patients with advanced chronic kidney disease (9).
Early work by Meneely and colleagues (25, 26) demonstrated that increasing salt intake promoted a dose-dependent decrease in life span of rats; vascular lesions and renal failure were prominent findings in these studies. In animal models of progressive kidney disease, dietary salt also played a role in altering disease progression. Dietary salt restriction has been shown to be as effective as angiotensin-converting enzyme inhibition in preventing glomerular injury (35) and superior to diuretics in reducing glomerular sclerosis in uninephrectomized spontaneously hypertensive rats (5). A high-salt diet increased albuminuria and accelerated progression of kidney injury in a rodent model of chronic allograft nephropathy (31). These effects occurred without an associated increase in blood pressure. Thus human and animal data have provided a need to understand in greater detail the mechanisms by which dietary salt intake affects vascular biology.
The interaction between salt intake and endothelial cell function has been examined in some detail (37, 38, 40–42). The major focus of these studies was transforming growth factor-β1 (TGF-β1), a fibrogenic growth factor that has been shown to play seminal roles in vascular and glomerular physiology and pathophysiology (7, 16, 27, 30, 36). Without increasing blood pressure, salt intake exerted a dose-dependent increase in endothelial cell production of TGF-β1. The mechanism appeared to be mediated through shear forces and mitogen-activated protein kinase (MAPK) activation (37–41). The present study was designed to dissect further the intracellular signaling process involved in salt-induced TGF-β1 production. Two different vascular tissues, aortic rings and isolated glomeruli, were examined. Proline-rich tyrosine kinase-2 (Pyk2) activity was chosen specifically, because this cytoplasmic tyrosine kinase serves as an important nodal point in intracellular signaling events (2, 3, 22) including endothelium (11) and, along with binding partners, participates in activation of the MAPK pathways (2, 6, 24, 29, 33). Pharmacological and dominant negative strategies were used to determine whether the cytoplasmic tyrosine kinases Pyk2 and c-Src (the 60-kDa product of c-src, also known as pp60c-src) participate in the signal transduction mechanism involved in dietary salt-mediated vascular production of TGF-β1. The findings support a seminal role for Pyk2 as a nodal point in the signaling cascade involved in dietary salt-mediated effects on the vasculature.
METHODS
Animal and tissue preparation.
The Institutional Animal Care and Use Committee at the University of Alabama at Birmingham approved the project. Studies were conducted using male Sprague-Dawley (SD) rats (Harlan Sprague Dawley, Indianapolis, IN) and were 28 days of age at the start of study. The protocol that was followed has been standardized in our laboratory (37, 38, 40). The rats were housed under standard conditions and given formulated diets (AIN-76A; Dyets, Bethlehem, PA) that contained 0.3, 1.0, 3.0, or 8.0% NaCl. These diets were prepared specifically to be identical in protein composition and differed only in NaCl and sucrose content. On the fourth day of the diets, the rats were anesthetized by intraperitoneal injection of pentobarbital sodium (50 mg/kg body wt; Abbott Laboratories, North Chicago, IL), and aorta and kidneys were perfused in situ with cold, isotonic, heparinized perfusion solution that contained 90 mM NaCl, 50 mM NaF, 1 mM Na3VO4, and 10 mM sodium pyrophosphate until blanched (50–60 ml over 2 min). Glomeruli and aortic rings for incubation studies and immunoblot analyses were isolated under sterile conditions as performed previously (37–42).
Lysates were produced by incubating isolated glomeruli and aortic tissue in modified radioimmunoprecipitation assay (RIPA) buffer that contained 10 mM Tris·HCl, pH 7.4, 100 mm NaCl, 1 mM EDTA, 1 mM EGTA, 0.5% sodium deoxycholate, 1% Triton X-100, 10% glycerol, 0.1% SDS, 20 mM sodium pyrophosphate, 2 mM Na3VO4, 1 mM NaF, 1 mM PMSF, and protease inhibitor cocktail. To obtain isolated endothelial cell lysates, we removed the aorta and opened it with a longitudinal incision. The intimal surface was very gently scraped with a curved scalpel blade, and the endothelial cells that built up on the edge of the blade were rinsed into a centrifuge tube that contained serum-free medium (RPMI 1640; Invitrogen, Carlsbad, CA). Each area was only scraped once, avoiding scraping close to any cut edge. The cell suspension was centrifuged at 500 g for 5 min, and the supernatant was discarded. The cell pellet was resuspended in 500 μl of modified RIPA buffer. Total protein concentration was determined using a kit (Micro protein assay reagent kit; Pierce Biotechnology, Rockford, IL), and the samples were processed for Western blotting and kinase activity assays as we have performed previously (39, 41). For Western blotting, samples containing 20–60 μg of total protein were used. The primary antibodies were diluted 1:1,000 and specifically recognized total Pyk2 (Cell Signaling Technology, Beverly, MA), phospho-Pyk2(Y402) (Cell signaling Technology), phospho-Pyk2(Y579/580) (Invitrogen), phospho-Pyk2(pY881) (Invitrogen), total c-Src (Cell Signaling Technology), and phospho-c-Src(Y416) (Cell Signaling Technology).
Kinase assays.
Activity of Pyk2 was determined using a kit (Pyk2 kinase assay kit; CycLex, Nagano, Japan), following the protocol provided by the manufacturer. Standards were performed using recombinant Pyk2 as a positive control to construct standard curves from which the activities of the samples were determined. Activities of p38 MAPK and p42/44 MAPK were determined by immunoprecipitation of the MAPK of interest followed by in vitro kinase assays using kits (Cell Signaling Technology) as performed previously (39, 41).
Coimmunoprecipitation assays of Pyk2 and c-Src.
Coimmunoprecipitation studies to determine the interaction of Pyk2 or c-Src with phospho-Pyk2(Y402) or phospho-Src(Y416) were performed on aortic tissue lysates (500 μg of total protein) that were incubated with either 2 μg of an anti-Pyk2 polyclonal antibody or an anti-c-Src polyclonal antibody (Dako) at 4°C for 2 h, followed by addition of 30 μl of protein A-Sepharose and overnight incubation. Immune pellets were washed three times with ice-cold RIPA buffer and then boiled in SDS sample buffer containing dithiothreitol (6.0 mg/ml). The proteins were resolved on 7.5% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes. The membranes were probed with phospho-Pyk2(Y402) or phospho-c-Src(Y416) polyclonal antibody. Immunoreactive bands were visualized with the use of enhanced chemiluminescence reagent and X-Omat film (Hawkins X-Ray Supply, Oneonta, AL).
In vitro incubation studies.
After removal of adherent fat and connective tissue, the aorta was cut into 3-mm ring segments and placed in 48-well plates. Isolated glomeruli (5 × 103 glomeruli/ml), which were obtained by sieving renal cortical tissue, and aortic ring preparations were washed with cold PBS. Pelleted glomeruli and aortic ring segments were resuspended in serum-free medium (RPMI 1640; Invitrogen) that contained vehicle alone, 5 μM tyrphostin A9 (EMD Biosciences, La Jolla, CA), or 10 μM PP2 {4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; EMD Biosciences}. Tyrphostin A9 served as a relatively specific inhibitor of Pyk2 (14), and PP2 was a potent, cell-permeable, Src family-selective tyrosine kinase inhibitor (18).
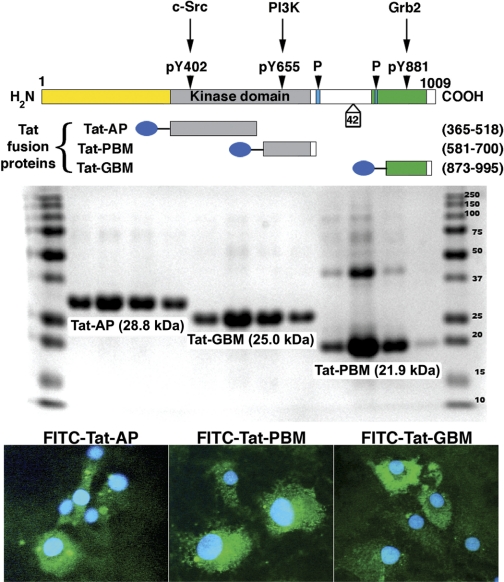
Site-specific inhibitors of Pyk2 were also used. Tat-AP was a fusion protein that consisted of the Tat peptide fused to amino acid residues 365–518 of the kinase domain of Pyk2 and included the autophosphorylation site (Y402) in the recognition domain for the SH2 group of c-Src. Tat-PBM consisted of the Tat peptide fused to amino acid residues 581–700 of the kinase domain of Pyk2 and included the purported binding site (YTLM) for the SH2 domain of phosphatidylinositol 3-kinase (PI3K). Tat-GBM consisted of the Tat peptide fused to amino acid residues 873–995 of Pyk2 and contained the Grb2-binding motif that includes Y881. These peptides served as cell-permeant, site-specific, dominant negative inhibitors of Pyk2 interaction and activation (17). The constructs in the pTatHA bacterial expression vector were generous gifts from Dr. Carl Nathan (Weill Medical College of Cornell University, New York, NY).
Protein transduction has been shown to occur within 10 min of addition of Tat fusion peptides to the medium and affects nearly 100% of cells regardless of cell type (28). Published detailed methodology regarding Tat-mediated protein transduction into mammalian cells was followed (4). Each fusion protein was used at 500 nM, since this dose was shown to be efficacious by other investigators (17). After synthesis and purification of the fusion proteins, transduction into endothelial cells was demonstrated by labeling each peptide with a fluorescent tag using a kit (EZ-Label FITC protein labeling kit; Pierce Biotechnology). Primary cultures of endothelial cells were prepared from the aorta of SD rats using a lumen digest technique (8). These cells, which were maintained on 0.1% gelatin-coated plates (BD Biosciences) in endothelium basal cell medium (Lonza Walkersville, Walkersville, MD) containing 10% fetal calf serum and endothelial cell growth supplement, expressed CD31 and VE-cadherin (data not shown) and were used between passages 4 and 14. Cells were incubated with the FITC-labeled fusion proteins for 1 h before study.
Isolated glomeruli and aortic ring segments were incubated in serum-free medium (RPMI 1640; Invitrogen) that contained vehicle alone or 500 nM of peptides that included Tat-AP, Tat-GBM, or Tat-PBM. After a 30-min incubation period, the medium was replaced and incubation continued at 37°C for 24 h. The conditioned medium was harvested, centrifuged at 300 g for 10 min at 4°C to remove cell debris, and then stored at −80°C until assayed for total and active TGF-β using enzyme-linked immunoassay (TGF-β Emax ImmunoAssay System; Promega) as described previously (37, 38, 40–42), and the results were factored by wet weight (for aortic tissue) or total protein (for glomeruli). Experiments using lysates of endothelial cells and isolated glomeruli from rats on the high-salt diet confirmed the inhibitory effects of tyrphostin A9, Tat-AP, and Tat-PBM on salt-induced Pyk2 activity (data not shown). Tat-GBM had no effect on Pyk2 activity in these studies and was used as an additional control in those experiments that used the Tat peptides.
In vivo studies.
On the third day on either the 0.3 or 8.0% NaCl diet, rats received a single intravenous bolus of 0.5 ml of PBS that contained vehicle alone or 2.5 μM Tat-AP, Tat-GBM, or Tat-PBM. On the following day, the rats were anesthetized and aortic tissue and glomeruli were obtained to generate lysates, as described above, for Western blotting and immunoprecipitation experiments or in vitro incubation experiments. At the time of tissue harvesting, urine was collected from the bladder to determine TGF-β and creatinine levels, which were assayed using an autoanalyzer (Creatinine Analyzer 2; Beckman Coulter, Fullerton, CA). In these studies, TGF-β was determined using a bioassay (1). Briefly, mink lung epithelial cells (MLEC-clone 32) stably transfected with a construct that consisted of a truncated 800-bp fragment of the human plasminogen activator inhibitor-1 promoter fused to the firefly luciferase reporter gene in a p19LUC-based vector (originally provided as a generous gift from Dr. Daniel B. Rifkin, New York University Medical Center, New York, NY) were plated in 24-well plates at a density of 2 × 105 cells/well in DMEM containing 10% FBS, 2 mM l-glutamine, 1% penicillin-streptomycin, and 200 μg/ml G418. Cells were allowed to attach for 4 h and then washed with serum-free medium. For measurement of urinary TGF-β activity, urine samples were diluted 1:5 in a total volume of 1 ml of serum-free medium and directly added to each well. For assay of total TGF-β, urine samples were heated for 5 min at 100°C, diluted 1:20 in a total volume of 1 ml of serum-free medium, and incubated with reporter cells for 18 h at 37°C. Mink lung epithelial cell lysates from each well were prepared using reporter lysis buffer (Promega). Luciferase activity was determined as relative light units using a microplate luminometer (Clarity Luminescence Microplate Reader; BioTek Instruments, Winooski, VT) and converted to TGF-β (pg) using a standard curve generated using human recombinant TGF-β1. Assays were performed in triplicate and averaged; values were normalized using the creatinine concentration obtained in each sample.
Statistical analysis.
Data are means ± SE. Significant differences among data sets were determined using analysis of variance with post hoc testing (Fisher's protected least significant difference, Statview, version 5.0; SAS Institute, Cary, NC). A P value <0.05 was assigned statistical significance.
RESULTS
Pyk2 integrally participated in dietary salt-mediated vascular production of TGF-β1.
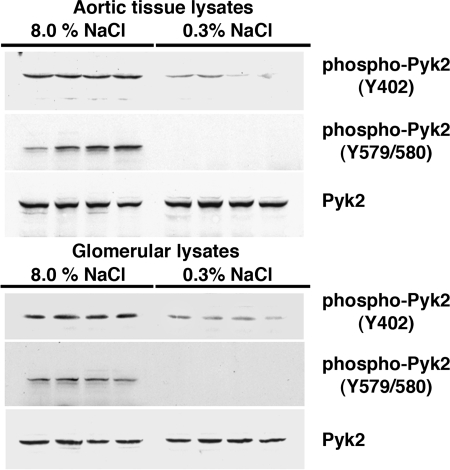
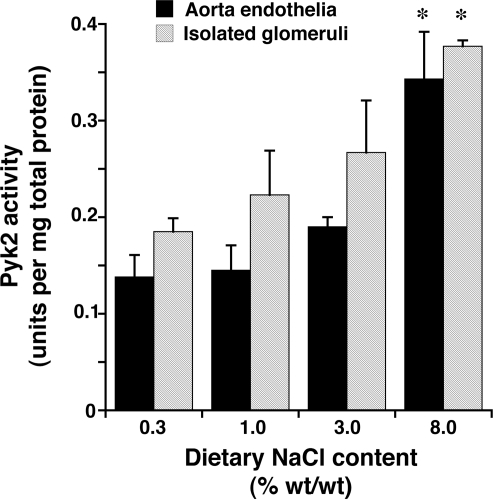
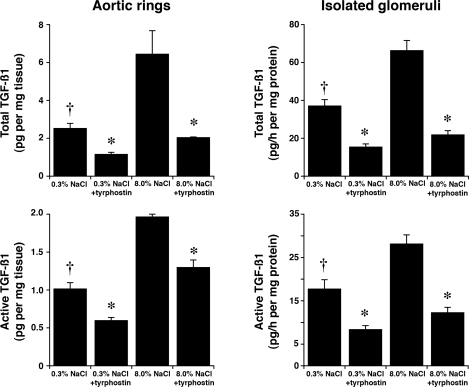
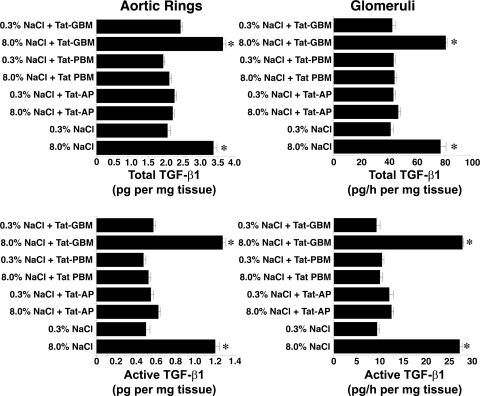
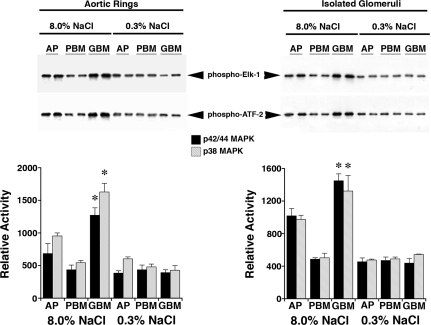
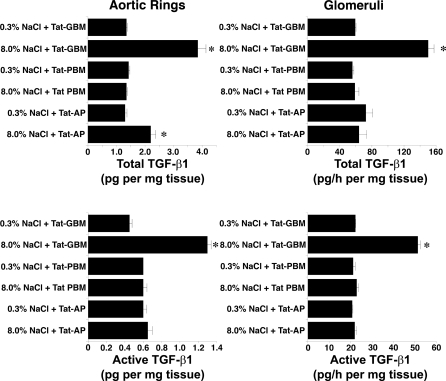
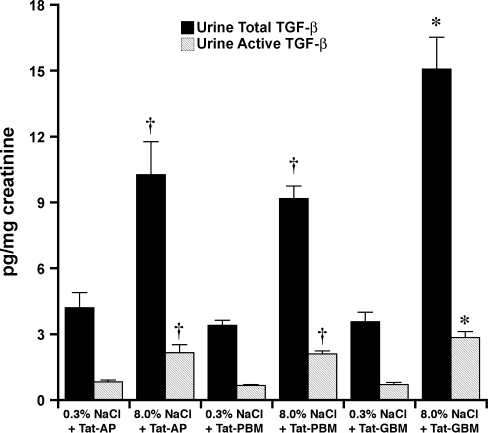
Four days after initiation of the diets, rats on 0.3 and 8.0% NaCl diets were anesthetized and lysates of isolated glomeruli and aortic tissue were produced. Although total levels of Pyk2 did not differ between the two groups, lysates from rats on the high-salt diet demonstrated increased amounts of phospho-Pyk2 at Y402 and Y579/80 (Fig. 1), indicating activation of Pyk2 (2). The phosphorylation state of Pyk2 at Y881 was also examined, but a signal was not observed in lysates of aortic tissue or glomeruli from rats on either diet (data not shown). In other experiments, Pyk2 activity was quantified in lysates from aortic endothelial cells and isolated glomeruli. An increase in dietary salt intake promoted a dose-dependent increase in Pyk2 activity (Fig. 2). The effect on Pyk2 activity was not linear, since increased activation did not occur until the diet contained >1% (wt/wt) NaCl. As demonstrated previously (37, 38, 40–42), an increase in salt intake increased production of total and active TGF-β1 by preparations of aortic rings and isolated glomeruli from rats on the 8.0% NaCl diet compared with preparations from rats on the 0.3% NaCl diet (Fig. 3). To determine whether Pyk2 participated in TGF-β1 production, we initially employed tyrphostin A9, a potent and selective inhibitor of Pyk2 (14), in experiments. Addition of tyrphostin A9 inhibited both total and active TGF-β1 production induced by the high-salt diet (Fig. 3). Pyk2 activity is regulated by multiple processes and has several potential binding partners (Fig. 4). Employing the Tat fusion proteins, which served as dominant negative inhibitors of specific actions of Pyk2, permitted a dissection of potential interactions among candidate proteins. These purified Tat fusion proteins readily transduced rat endothelial cells in culture (Fig. 4, bottom). Incubation of aortic rings and isolated glomeruli with Tat-AP and Tat PBM, but not with Tat-GBM, inhibited the salt-mediated increase in total and active TGF-β1 (Fig. 5). None of the fusion proteins altered TGF-β1 production by tissues from rats on the 0.3% NaCl diet. In additional experiments, the fusion proteins were administered intravenously, and tissues were harvested for study on the following day. Lysates from aorta and isolated glomeruli were obtained to determine activity of p38 and p42/44 MAPK. Compared with tissues obtained from rats fed the 8.0% NaCl diet and injected with Tat-GBM, intravenous injection of Tat-AP and Tat-PBM reduced the activities of p38 and p42/44 MAPK (Fig. 6). MAPK activities from rats fed the 0.3% NaCl diet and given the fusion proteins parenterally did not differ. Compared with tissues from rats that were fed the 8.0% NaCl diet and received Tat-GBM intravenously, both Tat-AP and Tat-PBM reduced the production of total and active TGF-β1 by aortic ring and glomerular preparations from rats on the 8.0% NaCl diet (Fig. 7). Urine samples from these animals were obtained the morning after the injection of the fusion proteins, and total and active TGF-β production were determined and factored by creatinine concentration. Compared with Tat-GBM, both Tat-AP and Tat-PBM reduced urinary excretion of total and active TGF-β by rats fed the 8.0% NaCl diet (Fig. 8).
Fig. 1.
Western analyses of the effect of salt intake on the phosphorylation state of proline-rich tyrosine kinase-2 (Pyk2) in lysates from aortic tissue (top) and isolated glomeruli (bottom). Although total levels of Pyk2 did not differ between the 2 groups, compared with rats on the 0.3% NaCl diet, rats on the 8.0% NaCl diet demonstrated increased levels of phospho-Pyk2(Y402), represented as the ratio of density of phospho-Pyk2(Y402) to the density of total Pyk2 in lysates of aortic tissue (0.63 ± 0.03 vs. 0.14 ± 0.02; P < 0.05) and isolated glomeruli (0.708 ± 0.10 vs. 0.06 ± 0.04; P < 0.05). Levels of phospho-Pyk2(Y579/80)/total Pyk2 in both aortic tissue lysates (0.56 ± 0.11 vs. undetectable; top) and glomerular lysates (0.58 ± 0.08 vs. undetectable; bottom) were also dramatically increased in rats on the 8.0% NaCl diet. Immunoblot analysis of phospho-Pyk2(Y881) was performed but was not observed in either group (data not shown). Each column represents data from the same rat (n = 4 rats/group).
Fig. 2.
Increasing dietary salt intake produced a dose-dependent increase in Pyk2 activity. Pyk2 activity assays were performed using lysates of aortic endothelia and isolated glomeruli obtained from groups of rats (n = 4 in each group) that had been fed 0.3, 1.0, 3.0, and 8.0% NaCl diets for 4 days before the study. As dietary salt intake increased, Pyk2 activity in aortic endothelia and glomeruli increased. *P < 0.05 compared with values obtained from rats on the other 3 diets.
Fig. 3.
Effect of tyrphostin A9, an inhibitor of Pyk2, on production of total and active transforming growth factor-β1 (TGF-β1) by aortic rings and isolated glomeruli from rats (n = 6 in each group) on 0.3 and 8.0% NaCl diets. The augmented production of total and active TGF-β1 observed in both tissues with the increase in salt intake was inhibited by 2 μM Pyk2. *P < 0.05 compared with values obtained from rats on the 8.0% NaCl diet. †P < 0.05 compared with untreated tissues from rats on the same diet.
Fig. 4.
Top: a schematic representation of Pyk2 with sites along the molecule that potentially permit binding of SH2 domains of c-Src, phosphatidylinositol 3-kinase (PI3K), and Grb2. Proline-rich sites (P) and a potential alternative-splicing site that removes 42 amino acids also are shown. The Tat fusion proteins Tat-AP, Tat-PBM, and Tat-GBM served as dominant negative constructs that inhibited the respective binding of c-Src, PI3K, and Grb2, respectively, to Pyk2. Middle: a Coomassie-stained gel demonstrating that 4 different preparations of the purified Tat fusion proteins were the expected size. Bottom: the 3 FITC-labeled Tat fusion proteins readily transduced rat endothelial cells in culture. Cells were counterstained with Hoechst to label the nuclei.
Fig. 5.
Administration of 500 nM Tat-AP and Tat-PBM, but not Tat-GBM, inhibited the dietary salt-induced increase in TGF-β1 by aortic rings and isolated glomeruli. Preparations of aortic rings and isolated glomeruli were obtained from rats (n = 4 rats in each group) on 0.3 and 8.0% NaCl diets for 4 days. *P < 0.05 compared with corresponding values obtained from rats on the 0.3% NaCl diet.
Fig. 6.
MAPK activity assays were performed in standard fashion by immunoprecipitation of the MAPK of interest and in vitro incubation with substrate (Elk-1 for p42/44 MAPK and ATF-2 for p38 MAPK). Lysates were obtained from aortic tissue and isolated glomeruli from rats (n = 4 rats in each group) on 0.3 and 8.0% NaCl diets for 4 days and injected intravenously with 2.5 μM Tat-AP, Tat-PBM, or Tat-GBM. Top: a representative experiment using 2 rats in each group. Bottom: graphs summarize the data from the 8 rats used in these studies. Both Tat-AP and Tat-PBM inhibited (P < 0.05) the activities of p38 and p42/44 MAPK. Although the effect was less for Tat-AP, it was sufficient to inhibit the production of TGF-β1. *P < 0.05 compared with the other 5 groups.
Fig. 7.
Intravenous administration of 2.5 μM Tat-AP and Tat-PBM, but not Tat-GBM, the day before tissue harvest inhibited the dietary salt-induced increase in TGF-β1 by aortic rings and isolated glomeruli (n = 4 rats in each group). *P < 0.05 compared with corresponding values obtained from rats on the 0.3% NaCl diet.
Fig. 8.
Effect of a single intravenous injection of the Tat fusion proteins (2.5 μM) on subsequent urinary excretion of total TGF-β and active TGF-β. Urine was obtained the day after the intravenous injection. Although the excretion rates did not fall to levels observed in rats on the 0.3% NaCl diet, the increases in urinary total and active TGF-β that occurred in response to increased salt intake were inhibited by intravenous injection of Tat-AP and Tat-PBM (n = 4 rats in each group). *P < 0.05 compared with the other 5 groups. †P < 0.05 compared with the groups of rats that received the 3% NaCl diet and the Tat fusion proteins.
c-Src participated in salt-induced vascular production of TGF-β1.
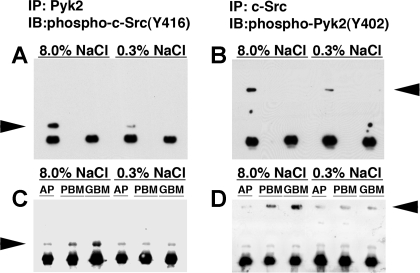
Coimmunoprecipitation assays were performed to examine the interactions between Pyk2 and c-Src in lysates of aortic tissue harvested from rats on 0.3 or 8.0% NaCl diets for 4 days. Increased salt intake promoted complex formation between Pyk2 and c-Src (Fig. 9, A and B). Assays were also performed using lysates of aortic tissue from rats on either the high- or low-salt diet treated on the day before harvest with Tat-AP, Tat-PBM, or Tat-GBM (Fig. 9, C and D). As anticipated, interaction of phospho-c-Src(Y416) with Pyk2 was inhibited by Tat-AP, but complex formation was also reduced by Tat-PBM.
Fig. 9.
Coimmunoprecipitation assays examining the interactions between Pyk2 and c-Src were performed by immunoprecipitation of Pyk2 or c-Src from aortic lysates harvested from rats on 0.3 or 8.0% NaCl diets for 4 days. A: immunoblot (IB) analysis of the immunoprecipitate (IP) generated by using an antibody to Pyk2 showed interaction of phospho-c-Src(Y416) with Pyk2, particularly in rats on the 8.0% NaCl diet. A nonspecific IgG was used in the immunoprecipitation step in the 2nd and 4th lanes. B: IB analysis of the IP generated by using an antibody to c-Src showed interaction with phospho-Pyk2(Y402). A nonspecific IgG was used in the immunoprecipitation step in the 2nd and 4th lanes. C: pull-down assays were performed using the anti-Pyk2 antibody and lysates of aortic tissue from rats on either the high- or low-salt diet treated on the day before harvest with Tat-AP, Tat-PBM, or Tat-GBM. As anticipated, interaction of phospho-c-Src(Y416) with Pyk2 was inhibited by Tat-AP, but interaction was also reduced by Tat-PBM. D: pull-down assays were performed using the anti-c-Src antibody and lysates of aortic tissue from rats on either the high- or low-salt diet treated on the day before harvest with Tat-AP, Tat-PBM, or Tat-GBM. The findings were similar to those shown in C and showed that interaction of phospho-Pyk2(Y402) with c-Src was inhibited by Tat-AP, but interaction was also reduced by Tat-PBM. Arrowheads indicate c-Src in A and C and Pyk2 in B and D.
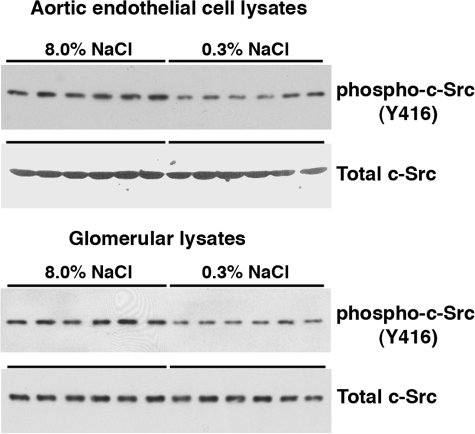
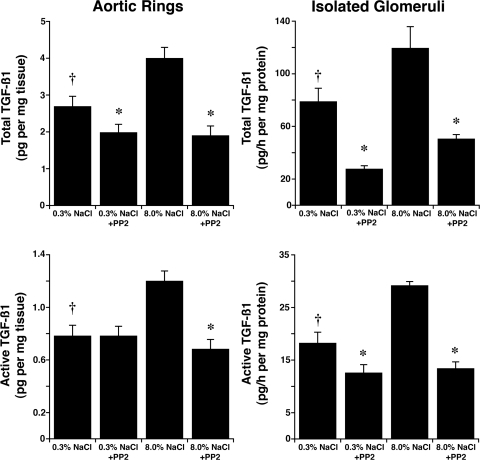
Aortic endothelial cell lysates and isolated glomerular lysates were used to determine phospho-c-Src(Y416) levels, an indicator of active c-Src (19). Compared with lysates from rats fed the 0.3% NaCl diet, without altering total c-Src, an increase in salt intake enhanced relative levels of phospho-c-Src(Y416) in lysates from both aortic endothelial cells (0.16 ± 0.02 vs. 0.62 ± 0.07; P < 0.05) and isolated glomeruli (0.856 ± 0.046 vs. 0.410 ± 0.055; P < 0.05) (Fig. 10). Addition of the c-Src inhibitor PP2 inhibited both total and active TGF-β1 production induced by the high-salt diet (Fig. 11).
Fig. 10.
Activity of c-Src was determined by IB analysis of phospho-c-Src(Y416) in lysates of aortic endothelial cells and isolated glomeruli. Compared with lysates from rats on the 0.3% NaCl diet, phospho-c-Src(Y416) levels relative to total c-Src levels were greater (0.62 ± 0.07 vs. 0.16 ± 0.02; P < 0.05) in endothelial lysates and greater (0.856 ± 0.046 vs. 0.410 ± 0.055; P < 0.05) in glomerular lysates from rats on the 8.0% NaCl diet. Each column represents data from a single rat (n = 6 rats in each group).
Fig. 11.
Effect of pharmacological inhibition of c-Src on total (top) and active (bottom) TGF-β1 was determined using PP2, administered in the medium following harvest of tissues from rats on either 0.3 or 8.0% NaCl for 4 days (n = 12 rats in each group). Addition of PP2 inhibited production of both total and active TGF-β1. *P < 0.05 compared with untreated tissues from rats on the same diet. †P < 0.05 compared with untreated tissues from rats on the 8.0% NaCl diet.
DISCUSSION
TGF-β1 is a locally acting growth factor that plays an integral role in the development of vascular and glomerular fibrosis (7, 27, 30, 36, 44). In addition, TGF-β1 appears to promote the development of hypertension, since mice lacking emilin1, an inhibitor of TGF-β1 activation, demonstrated peripheral vasoconstriction and arterial hypertension that was prevented by inactivation of one TGF-β1 allele (45). Factors that regulate TGF-β1 production in the vasculature therefore merit investigation. The present study demonstrated that dietary salt induced, in a dose-dependent fashion, the activation of Pyk2 and further identified c-Src as an important binding partner of Pyk2 in dietary salt-mediated production of TGF-β1.
Pyk2 is a non-receptor tyrosine kinase that is related to focal adhesion kinase (3, 22). This important signaling molecule is activated by a variety of extracellular stimuli that include hormones, agonists of G protein-coupled receptors including angiotensin II, and shear stress (12, 22, 34, 43). An early intracellular event in common with these stimuli is an increase in intracellular Ca2+, which rapidly activates Pyk2 (22). Although the precise mechanism of activation of Pyk2 was not explored in this report, previous findings have been consistent with a shear-mediated effect of increased salt intake on endothelium (37–41). Although increased intracellular Ca2+ concentration may activate Pyk2 in this setting, other mediators, including reactive oxygen species generated during shear stress, could play a role in activation of Pyk2 (34). Recently, Kohno, et al. (21) demonstrated that Ca2+/calmodulin binding to Pyk2 released the kinase domain from autoinhibition by forming a dimer, permitting phosphorylation at Y402 and activation of the kinase. Kinase activation promotes the subsequent recruitment of other binding partners including c-Src (12). Consistent with this known interaction with c-Src, increased salt intake increased phosphorylation at Y402 and complex formation between Pyk2 and c-Src. Activation of c-Src permitted phosphorylation at Y579/80, which appeared to be required for full Pyk2 activation (23) and was a prominent observation in the present study (Fig. 1).
An important role for Pyk2 and c-Src in TGF-β1 production was identified by the use of tyrphostin A9 and PP2, respectively. To tease out the interactions further, we employed a dominant negative approach designed to interfere with specific interactions between Pyk2 and potential binding partners by using peptide fragments of Pyk2. Transduction of these peptides was accomplished through the generation of fusion proteins that consisted of the peptide segments of Pyk2 and the minimal transduction domain of human immunodeficiency virus (HIV), termed Tat (4) (Fig. 4). The fusion peptides served as cell-permeant, site-specific, dominant negative inhibitors of Pyk2 interaction and activation (17). Tat-AP was a fusion protein that consisted of the Tat peptide fused to a portion of the kinase domain of Pyk2 and contained the autophosphorylation site (Y402) in the recognition domain for the SH2 group of c-Src. Tat-PBM consisted of the Tat peptide fused to amino acid residues 581–700 of the kinase domain of Pyk2 and included the purported binding site (YTLM) for the SH2 domain of PI3K. Tat-GBM consisted of the Tat peptide fused to a peptide fragment that corresponded to a carboxyl terminal portion of Pyk2 and contained the Grb2-binding motif that includes Y881. In the present study, both Tat-AP and Tat-PBM inhibited the salt-mediated increase in TGF-β1 in the vasculature, indicating an essential interaction between c-Src and, likely, PI3K in this process. Experiments that used Tat-GBM demonstrated no effect on salt-induced TGF-β1 production and were consistent with the absence of a detectable increase in the phosphorylation state of Pyk2 at Y881. In some experiments, the Tat fusion proteins were injected intravenously, with identical findings observed in subsequent analyses. Urinary TGF-β levels of rats on the 8.0% NaCl diet fell with the injection of Tat-AP and Tat-PBM but did not reach levels observed in the animals on the 0.3% NaCl diet. Because salt-induced production of TGF-β1 was inhibited in aortic rings and isolated glomerular preparations from these animals, the findings further suggested that the source of urinary TGF-β was not only the vasculature but also another intrarenal mechanism that was unique from that observed in the vasculature and glomerulus. Although a site of action of increased salt intake was confirmed to be the endothelium, a potential limitation with the use of isolated glomerular tissue is the observation that multiple cell types are present and the possibility that mesangial and epithelial cells, in addition to endothelial cells, are also affected by dietary salt intake. In addition, although the high efficiency of transduction was demonstrated in vitro, the relative ability to transduce cell types other than endothelium may differ in vivo.
The combined studies showed that through a series of phosphorylation events, dietary salt induced in vascular tissues the formation of a molecular complex that contained c-Src and Pyk2. Pyk2 is a major upstream regulator of MAPK pathways (2, 6, 24, 29, 33), and these signaling events were demonstrated to regulate the activities of p38 MAPK and p42/44 MAPK in the present study. These MAPK pathways have been shown to promote the production of TGF-β1 in response to dietary salt intake (37–41). The data supported the hypothesis that activation of Pyk2 recruited and activated c-Src and that this complex participated integrally in the vascular production of TGF-β1 in response to dietary salt in the rat. Although these findings require confirmation in humans, this mechanism of intravascular production of TGF-β1 might be of seminal importance in the cardiovascular response and pathophysiology that develops in response to chronically increased salt intake.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK46199 and the Office of Research and Development, Medical Research Service, Department of Veterans Affairs.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abe M, Harpel JG, Metz CN, Nunes I, Loskutoff DJ, Rifkin DB. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem 216: 276–284, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal 12: 123–133, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor LM, White RA, Groopman JE, Avraham H. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem 270: 27742–27751, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods 24: 247–256, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Benstein JA, Feiner HD, Parker M, Dworkin LD. Superiority of salt restriction over diuretics in reducing renal hypertrophy and injury in uninephrectomized SHR. Am J Physiol Renal Fluid Electrolyte Physiol 258: F1675–F1681, 1990. [DOI] [PubMed] [Google Scholar]
- 6.Blaukat A, Ivankovic-Dikic I, Gronroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. Adaptor proteins Grb2 and Crk couple Pyk2 with activation of specific mitogen-activated protein kinase cascades. J Biol Chem 274: 14893–14901, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β1. Nature 346: 371–374, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Sega M, Agarwal A. “Lumen digestion” technique for isolation of aortic endothelial cells from heme oxygenase-1 knockout mice. Biotechniques 37: 84–86, 88–89, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cianciaruso B, Bellizzi V, Minutolo R, Tavera A, Capuano A, Conte G, De Nicola L. Salt intake and renal outcome in patients with progressive renal disease. Miner Electrolyte Metab 24: 296–301, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 334: 885, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies PF Multiple signaling pathways in flow-mediated endothelial mechanotransduction: PYK-ing the right location. Arterioscler Thromb Vasc Biol 22: 1755–1757, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Dikic I, Tokiwa G, Lev S, Courtneidge SA, Schlessinger J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 383: 547–550, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Fesler P, Safar ME, du Cailar G, Ribstein J, Mimran A. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 25: 1915–1920, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Fuortes M, Melchior M, Han H, Lyon GJ, Nathan C. Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest 104: 327–335, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 44: 35–41, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia Autocrine transforming growth factor-β1 expression determines growth response to angiotensin II. J Clin Invest 90: 456–461, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han H, Fuortes M, Nathan C. Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation. J Exp Med 197: 63–75, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem 271: 695–701, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Hunter T Treatment for chronic myelogenous leukemia: the long road to imatinib. J Clin Invest 117: 2036–2043, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med 334: 13–18, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Kohno T, Matsuda E, Sasaki H, Sasaki T. Protein-tyrosine kinase CAKbeta/PYK2 is activated by binding Ca2+/calmodulin to FERM F2 alpha2 helix and thus forming its dimer. Biochem J 410: 513–523, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature 376: 737–745, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Dy RC, Cance WG, Graves LM, Earp HS. Interactions between two cytoskeleton-associated tyrosine kinases: calcium-dependent tyrosine kinase and focal adhesion tyrosine kinase. J Biol Chem 274: 8917–8924, 1999. [DOI] [PubMed] [Google Scholar]
- 24.McMullen M, Keller R, Sussman M, Pumiglia K. Vascular endothelial growth factor-mediated activation of p38 is dependent upon Src and RAFTK/Pyk2. Oncogene 23: 1275–1282, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Meneely GR, Ball CO. Experimental epidemiology of chronic sodium chloride toxicity and the protective effect of potassium chloride. Am J Med 25: 713–725, 1958. [DOI] [PubMed] [Google Scholar]
- 26.Meneely GR, Tucker RG, Darby WJ, Auerbach SH. Chronic sodium chloride toxicity: hypertension, renal failure and vascular lesions. Ann Intern Med 39: 991–998, 1953. [DOI] [PubMed] [Google Scholar]
- 27.Mozes MM, Bottinger EP, Jacot TA, Kopp JB. Renal expression of fibrotic matrix proteins and of transforming growth factor-beta (TGF-beta) isoforms in TGF-beta transgenic mice. J Am Soc Nephrol 10: 271–280, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med 4: 1449–1452, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Pandey P, Avraham S, Kumar S, Nakazawa A, Place A, Ghanem L, Rana A, Kumar V, Majumder PK, Avraham H, Davis RJ, Kharbanda S. Activation of p38 mitogen-activated protein kinase by PYK2/related adhesion focal tyrosine kinase-dependent mechanism. J Biol Chem 274: 10140–10144, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. TGF-beta signaling in vascular fibrosis. Cardiovasc Res 74: 196–206, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Sanders PW, Gibbs CL, Akhi KM, MacMillan-Crow LA, Zinn KR, Chen YF, Young CJ, Thompson JA. Increased dietary salt accelerates chronic allograft nephropathy in rats. Kidney Int 59: 1149–1157, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Schiffrin EL Vascular stiffening and arterial compliance. Implications for systolic blood pressure. Am J Hypertens 17: 39S–48S, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Tahara S, Fukuda K, Kodama H, Kato T, Miyoshi S, Ogawa S. Potassium channel blocker activates extracellular signal-regulated kinases through Pyk2 and epidermal growth factor receptor in rat cardiomyocytes. J Am Coll Cardiol 38: 1554–1563, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Tai LK, Okuda M, Abe J, Yan C, Berk BC. Fluid shear stress activates proline-rich tyrosine kinase via reactive oxygen species-dependent pathway. Arterioscler Thromb Vasc Biol 22: 1790–1796, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Weir MR, Dworkin LD. Antihypertensive drugs, dietary salt, and renal protection: how low should you go and with which therapy? Am J Kidney Dis 32: 1–22, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Wynn TA Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117: 524–529, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ying WZ, Sanders PW. Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-β1. Am J Physiol Renal Physiol 275: F18–F24, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-β1 in rat aortic endothelium. Am J Physiol Heart Circ Physiol 277: H1293–H1298, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Ying WZ, Sanders PW. Dietary salt intake activates MAP kinases in the rat kidney. FASEB J 16: 1683–1684, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-β in rats. Am J Physiol Renal Physiol 274: F635–F641, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Ying WZ, Sanders PW. Increased dietary salt activates rat aortic endothelium. Hypertension 39: 239–244, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Ying WZ, Sanders PW. The interrelationship between TGF-β1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol 285: F902–F908, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Yu H, Li X, Marchetto GS, Dy R, Hunter D, Calvo B, Dawson TL, Wilm M, Anderegg RJ, Graves LM, Earp HS. Activation of a novel calcium-dependent protein-tyrosine kinase. Correlation with c-Jun N-terminal kinase but not mitogen-activated protein kinase activation. J Biol Chem 271: 29993–29998, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Yu HCM, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 98: 2621–2628, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell 124: 929–942, 2006. [DOI] [PubMed] [Google Scholar]