Abstract
We examined the modulation of intrinsic (i.e., spontaneous) detrusor contractions by the urothelium and the lamina propria through optical mapping approaches. Normal adult and spinal cord-transected (SCT) rat bladders were stained with Ca2+- and voltage-sensitive dyes, and optical activity generated from intrinsic contractions was mapped from the mucosal surface of whole bladder sheets. Both normal adult and SCT rat bladders displayed intrinsic contractions, where normal bladders showed low-amplitude, high-frequency contractions with disorganized patterns of activity. In contrast, in the SCT animals there were high-amplitude, low-frequency contractions that displayed an organized spread of membrane potential and intracellular Ca2+. The difference in contractile activity was mirrored in the Ca2+ and membrane potential maps of bladder sheets. Normal bladders showed multiple initiation sites across the mucosal surface, whereas SCT bladders showed only one or two fixed initiation sites localized to the dome. The magnitude of intrinsic contractions could be enhanced by stretch or low-dose arecaidine (50 nM), a muscarinic-specific agonist. Partial removal of the mucosa decreased the amplitude of the intrinsic contractions and decreased the response to stretch or arecaidine. Optical mapping of mucosa-denuded sheets, where enhanced spontaneous activity was abolished, or application of 1 μM nifedipine to remove smooth muscle signals, but not the mucosal signals, shows that intrinsic activity in pathological bladders is driven by the mucosal layer. In summary, we suggest an urotheliogenic origin for intrinsic activity, where structures within the mucosal layer organize and thereby enhance intrinsic detrusor contractions.
Keywords: intrinsic or spontaneous contractions, spinal cord transection, bladder overactivity, Ca2+- and voltage-sensitive dyes
contractile activity of the bladder is a result of coordinated activation of the embedded efferent nerves to activate muscarinic and purinergic receptors on the detrusor smooth muscle, inducing a micturition response. However, intrinsic bladder contractile activities (observed in vitro and in vivo), which are not large enough to cause micturition (thus also referred to as nonvoiding contractions), have been described from various species (30, 44). These contractions seem to occur in normal healthy bladders during the filling phase when the efferent nerves are effectively silent, and thus intrinsic activity was initially hypothesized to be myogenic in origin, possibly as a mechanism to keep the bladder wall taut against the growing bolus of urine as the bladder fills. This is supported by observations that detrusor smooth muscle has spontaneous Ca2+ transients or Ca2+ sparks (6, 22), which are thought to contribute to the excitability of the cell. Intrinsic contractions have been demonstrated to be more prominent in neonatal rats than adults (33), and these predominant contractions reemerge in pathology (e.g., detrusor overactivity), increasing their amplitude in response to bladder filling.
There may also be influence from the central nervous system (CNS) over intrinsic activity as the addition of a ganglionic blocker increases the amplitude of intrinsic bladder contractions (30), leading to the theory that they may play a role in pathological conditions where CNS inhibition over the lower urinary tract has become compromised. This has also been demonstrated with in vitro brain stem-spinal cord-bladder preparations in neonatal rats, where when the connection from the spinal cord to the bladder is severed there is an increase in the amplitude of intrinsic contractions of the bladder (45, 46). This indicates there is an inhibitory control from the spinal cord over intrinsic bladder activity, but does not appear to be a driving force. This may contribute to the increase in intrinsic contractions in bladder pathologies such as outlet obstruction or spinal cord damage, where there are significant changes to the tissue architecture and decreased or lost innervation of the bladder (13).
These observations indicate an intrinsic mechanism in the bladder that controls spontaneous activity of detrusor smooth muscle. Potential structures within the bladder wall that could regulate activity include the urothelial layer, lamina propria myofibroblasts (LPM), and interstitial cell (IC)-like cells found throughout the bladder wall. Urothelial cells have neuronal-like properties and release factors such as ATP (15), nitric oxide (NO·) (2, 5), prostaglandins (11, 40), and ACh (21, 32) in response to stretch or pharmacological stimulation. Distinct networks of IC-like cells or myofibroblasts found throughout the bladder of various species (18, 29) including humans (43) and have been implicated in driving intrinsic activity in the bladder. This is done potentially by responding to signaling factors released from the urothelial layer of the bladder during filling to regulate contractile activity. IC-like cells and LPM in the bladder have been shown to have spontaneous action potentials (23, 48); in the case of LPM, the activity appears to be mediated by an inward Cl− current, leading to Ca2+ release from intracellular stores (54). It is hypothesized that these cells may communicate to the detrusor to modulate smooth muscle intrinsic activity. For example, LPM have been shown to respond to ATP (54) and NO (19) (but not ACh) (54) and, from their relative position to the urothelium, may respond to factors released from urothelial cells during bladder filling. The change in LPM activity may lead to changes in smooth muscle intrinsic activity. The mechanism by which LPM communicate to the smooth muscle has yet to be determined; however, it may involve signal transduction through the network of detrusor IC-like cells or afferent nerves that have been shown to be in close proximity to LPM (53). In the bladders of neonatal and spinal cord-transected (SCT) rats, connexin expression has been shown to significantly increase in the urothelium and lamina propria, compared with normal adult animals (26). Enhanced connectivity within these layers may contribute to the high-amplitude, rhythmic, intrinsic contractions seen in these animals.
If, indeed, enhanced intrinsic bladder contractions leads to detrusor overactivity, further understanding of the mechanisms involved may elucidate potential therapeutic targets. The mainstay pharmaceutical treatment used for overactive bladder symptoms are antimuscarinics, which were initially thought to act on detrusor smooth muscle. However, as antimuscarinics work during the filling phase of the bladder when parasympathetic efferents are effectively silent, hence the muscle seems an unlikely target, particularly for urgency symptoms. In this study, we examined the role of mucosal muscarinic receptors in driving intrinsic contractions in normal and SCT rat bladders and whether cells in the bladder mucosal layer can enhance intrinsic smooth muscle activity.
MATERIALS AND METHODS
Animals.
Control female adult Sprague-Dawley rats (4 mo old, Harlan) and SCT female rats (T8–T9 spinal level) were used in this study, as has been described earlier (26). For the SCT surgery, animals were anesthetized with 2% isoflurane/98% O2, and a laminectomy was performed. The dura and spinal cord were completely transected, and an absorbable hemostatic sponge (Vetspon, Novartis Animal Health) was used to pack between the cut ends of the cord. The muscle and skin were sutured, and the animal was allowed to recover with prophylactic antibiotics (ampicillin, 100 mg/kg twice daily, intramuscularly for 2 wk). In the first 1–2 wk after the operation, the animals required their bladders to be emptied at least twice a day by gentle abdominal compressions, until they developed their own spinal cord-mediated micturition reflexes (10). SCT animals were used for experiments 2 wk postsurgery. All animals used were anesthetized with 2% isoflurane/98% O2, bladders were excised, and the animals were euthanized by a thoracotomy while still under anesthesia. The tissue was immediately placed into oxygenated physiological saline (Tyrode's solution; see below). All procedures conformed to institutional guidelines and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Tissue preparation.
Bladders were cannulated with a 22-gauge needle per urethra to load the tissue with fluorochromes intravesically. Bladders were first instilled with 1–2 ml of the voltage-sensitive dye di-4-ANEPPS (10 μM, Molecular Probes) and incubated at 37°C for 30 min in Ca2+-free Tyrode's solution (see below). The dye was removed, and the bladder lumen was washed through with Tyrode's. Next, the Ca2+-sensitive dye rhod-2-AM (10 μM, Molecular Probes) was instilled into the bladder and incubated under the same conditions as given for the voltage dye.
After staining, the bladders were cut from outlet to dome along the dorsal aspect to form a sheet. In partially mucosa-denuded preparations, the mucosal layer was dissected under a microscope by cutting in through the lamina propria, with care taken not to damage the smooth muscle. The outlet was pinned to the fixed platform (with the mucosal surface facing up) in the recording chamber machined from thermally conductive, electrically insulating epoxy resin. The dome was tied with 5-0 suture to a bar made of ultralightweight stainless steel tubing, which was then connected to a tension transducer. Contractile activity was measured through a tension transducer attached to an oil hydraulic micromanipulator (Narishige) computer controlled through a stepper motor to allow precise increments of stretch.
The recording chambers were placed on a Peltier block (University of Pittsburgh Machine and Electronics shops) to maintain the temperature at 37 ± 0.5°C and superfused with Tyrode's at a rate of 1 ml/min. Bladder sheets were stretched to 1 g of resting tension, and all preparations were allowed to equilibrate for at least 30 min.
Optical recording system.
Intracellular Ca2+ and membrane potential, Vm, were recorded from the entire bladder mucosal surface using a custom-built dual-photodiode array system as described previously (26, 27). Briefly, fluorescence images of the bladder mucosal surface were focused on two 16 × 16-element photodiode arrays (C675-103, Hamamatsu, Bridgewater, NJ) with each diode, having a sensing area of 0.95 × 0.95 mm and an interpixel spacing of 1.1 mm. The photodiode array output was amplified (LBC-2 Argo Transdata, Clinton, CT), digitized with 12-bit resolution (DAP 3400/a, Microstar Laboratories, Bellevue, WA), and stored in the computer's memory. A typical file was sampled at 2 kHz for 30–60 s, consisting of 256 Ca2+, 256 Vm, and 16 instrumentation channels; the maximum sampling rate is 4,000 frames/s. The alignment and focusing of the preparation with respect to the photodiodes were accomplished by viewing the tissue with a CCD camera and a superimposed 16 × 16 graticule (Graticules, Tonbridge, UK) that is in exact registry with both photodiode arrays.
The bladder was illuminated by collimated light from a 100-W tungsten-halogen lamp, directed through a band-pass filter (520 ± 20 nm) to a 575-nm long-wave-pass dichroic mirror (Omega Optical, Brattleboro, VT) and 85 mm, f1:1.4 lens (Nikon). Fluorescence was collected by the same lens and passed through the 575-nm long-wave-pass dichroic mirror to block excitation light. The signals were then split with a 635-nm long-wave-pass dichroic mirror: the rhod-2 signal (595-nm range) was sent to the Ca2+ camera, and the di-4-ANNEPS signal (>700 nm) was transmitted to the voltage camera.
Solutions.
Preparations were bathed in Tyrode's solution containing (in mM) 118 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgCl2·6H2O, 1.8 CaCl2, 14 glucose, gassed with 95% O2-5%CO2, pH 7.4. Ca2+-free HEPES-Tyrode's solution contained (in mM) 132 NaCl, 4.0 KCl, 0.4 NaH2PO4, 6.1 glucose, 5.0 Na pyruvate, and 10.0 HEPES, pH 7.35. Arecaidine, atropine, hexamethonium, and nifedipine were added to the physiological solution as aliquots from aqueous stock solutions. All chemicals were from Sigma.
Statistical analysis.
Quantitative data are shown as mean ± SD. Differences between normal data sets were tested with Student's t-test, and the null hypothesis was rejected when P < 0.05.
RESULTS
Intrinsic contractile activity in normal and SCT rat bladders and response to stretch.
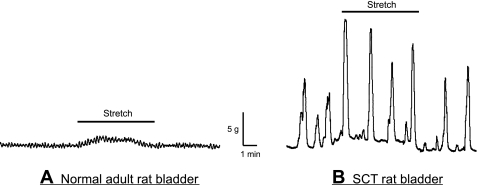
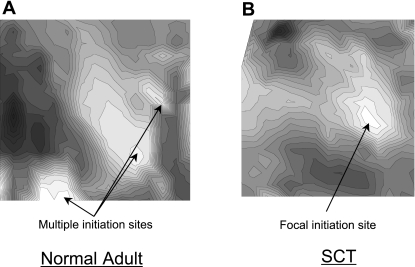
The two experimental groups, normal adult and SCT rats, demonstrated significant differences in their intrinsic bladder activity. The SCT group demonstrated that in bladder sheet preparations there were high-amplitude contractions (1.8 ± 0.6 g/mm2, n = 7) with a regular periodicity (2.4 ± 0.9 contractions/min, n = 7). Normal adult bladders showed much smaller contractions (0.4 ± 0.2 g/mm2, n = 6) with a higher frequency (4.5 ± 0.7 contractions/min, n = 6) compared with SCT animals. Figure 1 shows examples of tension traces from SCT and normal rat bladders. The addition of stretch to 10% of resting length caused an increase in the frequency and amplitude of spontaneous contractions in the SCT preparations but not in normal bladders (amplitude: 60 ± 21% of control and frequency: 25 ± 10% of control, n = 6). A similar degree of stretch did not cause an enhancement of normal adult activity. Optical maps taken from the mucosal surface of SCT and normal rat bladder sheets demonstrated that there were differences in the patterns of activity. Normal rat bladder sheets showed multiple initiation sites across the surface with no fixed pattern. On the other hand, SCT rat bladder sheets showed a focal initiation site in the dome region (Fig. 2), with most preparations displaying no more than one or two initiation sites. The different patterns of optical activity appeared to correlate with the difference in the contractile activity between the normal and pathological bladders. The one or two initiation sites seen in the SCT bladder sheets resulted in larger amplitude more coordinated intrinsic smooth muscle contractions with a slower rate, as the number of initiations sites determines the frequency. The larger number of initiation sites in the normal adult bladder resulted in smaller uncoordinated intrinsic contractions with a higher frequency.
Fig. 1.
Response of intrinsic contractions in spinal cord-transected (SCT) and normal adult rat bladder sheets to 10% stretch of their resting length. Stretch did not significantly alter the intrinsic contractions in the normal adult rat bladder (A); however, intrinsic contractions in SCT rat bladder sheets increased in amplitude in response to a similar degree of stretch (B).
Fig. 2.
Ca2+ isochronal maps imaged from the mucosal surface of a normal adult and SCT rat bladder sheet. Normal adult bladders displayed multiple initiation sites across the mucosal surface with no specific pattern (A) while SCT rat bladders displayed 1 or 2 stable focal initiation site(s) in the dome region (B).
Effect of low-dose muscarinic receptor agonists on intrinsic contractions.
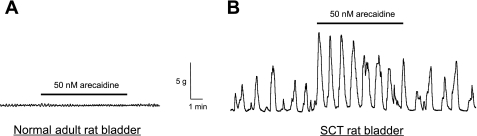
The effects of stretch in SCT rat bladders could be mimicked by low-dose arecaidine (50 nM), a muscarinic-specific agonist (Fig. 3A). Spontaneous intrinsic contractions of the bladder have been shown to be responsive to low-dose muscarinic stimulation that becomes more sensitive in models of bladder outlet obstruction (12). Therefore, we examined the effect of low-dose muscarinic receptor agonists on spontaneous contractions in normal adult and SCT rat bladder sheets. In normal adult bladders (n = 6), addition of 50 nM arecaidine did not cause an increase in amplitude or frequency of spontaneous contractions but could cause a small increase in baseline tension (26 ± 7.9%). In SCT bladders (n = 6), there was an increase in the amplitude (92 ± 45% from control) that did not significantly alter the frequency (2.5 ± 1.0 contractions/min) of the intrinsic activity. The response to arecaidine was also accompanied by an increase in baseline tension (33 ± 16%). The increase in amplitude was significantly greater in SCT bladders compared with controls (unpaired Student's t-test, P < 0.05). The presence of 10 μM hexamethonium, a general nicotinic receptor antagonist, had no significant effect on the arecaidine response in both control and SCT bladders. In addition, atropine could inhibit the stretch-mediated enhancement (n = 3, not shown). This indicates that the enhancement is mediated through muscarinic, rather than nicotinic receptors.
Fig. 3.
Effect of low-dose (50 nM) arecaidine on intrinsic contractions in normal and SCT rat bladder sheets. A: addition of 50 nM arecaidine did not significantly change the intrinsic contractions in normal adult rat bladders. B: SCT rat bladders showed an enhanced sensitivity to muscarinic stimulation, with a significant increase in the amplitude of contractile activity.
Role of urothelium/lamina propria in stretch and arecaidine-enhanced spontaneous contractions.
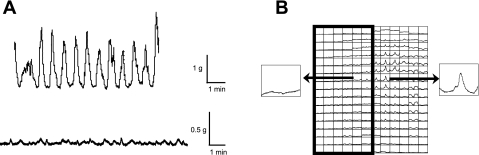
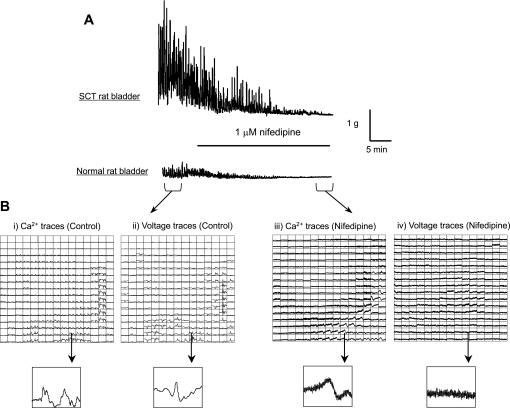
To demonstrate the involvement of urothelial/suburothelial muscarinic receptors in driving intrinsic contractile activity, bladder sheets were partially denuded of the mucosal layer. This allowed optical imaging of the bladder with or without urothelium in the same preparation to distinguish between muscle and urothelial muscarinic receptor activation. In SCT bladders (n = 3), dissection of one-half the mucosal surface from the bladder sheet resulted in a significant reduction of amplitude, but not the frequency of intrinsic contractions, 0.3 ± 0.2 g/mm2 and 2.0 ± 1.0 contractions/min, respectively. Figure 4A shows the comparison of spontaneous contractile activity between an intact and partially denuded SCT rat bladder sheet. Addition of 50 nM arecaidine to mucosa-denuded SCT preparations only resulted in the increase in baseline tension similar to mucosa-intact preparations (n = 3, data not shown). Optical mapping showed that focal large-amplitude intrinsic activity was initiated only on the portion of the preparation with an intact mucosa (Fig. 4B). To further demonstrate the role of the mucosa in generating spontaneous activity, 1 μM nifedipine was added to the perifusion solution to stop smooth muscle contractions by inhibiting L-type Ca2+ channels. After addition of nifedipine, intrinsic muscle contractions and tension generation ceased and voltage optical activity was abolished; however, Ca2+ transients remained (Fig. 5). This implies that there is spontaneous activity that does not originate from the smooth muscle that influences intrinsic contractile activity.
Fig. 4.
A: tension traces from a mucosa-intact SCT rat bladder sheet (top) and partially mucosa-denuded SCT rat sheet (bottom). Dissection of the mucosa from half of the surface area of the SCT rat bladder sheet resulted in a significant decrease in the contractile activity. B: Ca2+ transient trace map from a partially denuded normal adult rat bladder sheet. The denuded region is denoted by the boxed region. Intrinsic Ca2+ transients originate from the mucosa-intact region and did not appear to propagate into the denuded area.
Fig. 5.
Effect of 1 μM nifedipine on intrinsic activity of the rat bladder. A: tension traces from SCT and normal rat bladder sheets. Addition of 1 μM nifedipine to the perifusion solution abolished the intrinsic contractions in both preparations. B: Ca2+ and voltage trace maps from before (i and ii) and after (iii and iv) addition of nifedipine. Before the addition of nifedipine, Ca2+ transients and voltage signals are clearly observed. After nifedipine is added, voltage activity is completely abolished. However, Ca2+ transients are still observed from the mucosal surface, indicating intrinsic activity may originate from cells in the mucosa.
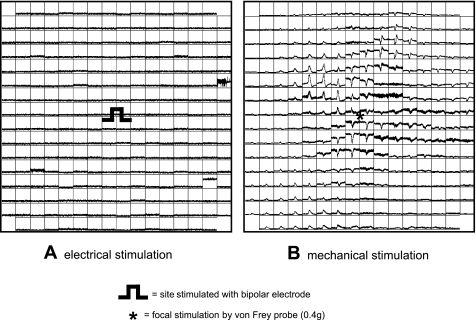
The urothelium lacks voltage-gated Ca2+ channels and is therefore not electrically excitable, but we and others have shown it to be mechanically sensitive (Fig. 6). The urothelium has been shown to express a variety of mechanosensitive channels including epithelial Na+ channels (ENaC) and TRPV1, which can affect vesicular ATP release (4, 14). As exocytosis in urothelial cells is a Ca2+-dependent mechanism (52), increased Ca2+ transient activity in the urothelium may correspond to increased ATP release. This could lead to increased activation of LPM, that in turn increase the activity of the smooth muscle. The data here show that there is Ca2+ activity in the mucosa that may enhance smooth muscle contractions.
Fig. 6.
Ca2+ trace map from an isolated mucosa sheet of a normal adult rat and the response to electrical (A) and mechanical stimulation (B). The mucosal sheet did not respond to electrical stimulation but showed propagation of Ca2+ transients across its surface in response to focal mechanical stimulation.
DISCUSSION
In this study, we examined the characteristics of intrinsic bladder contractile activity in normal adult and SCT rats. There were significant differences in the pattern of intrinsic activity between normal adult and SCT rat bladders. Normal rat bladders showed low-amplitude contractions with no apparent pattern to the activity. This was demonstrated by the optical recordings where there were multiple voltage- and Ca2+-initiation sites spread randomly across the mucosal surface. In contrast, bladders from SCT rats showed high-amplitude contractions with a regular periodicity. Accordingly, the pattern of Ca2+ and voltage activity was more organized and therefore of greater magnitude and lower frequency, with activity originating from only one or rarely two fixed initiation sites in the dome. This is similar to the activity we have described in neonatal bladders (39, 49), which also display a focal initiation site (27). For this study, we utilized whole bladder sheets to examine the activity from the entire mucosal surface of the bladder. However, to form the sheet we had to cut along the dorsal aspect and this may cause damage to any preexisting initiation sites.
It could be hypothesized that the organized activity seen in SCT and neonatal rat bladders has a similar mechanism. Indeed, neonatal and SCT rat bladders have increased expression of connexin (Cx) 26 and 43 compared with adult bladders in the urothelium and lamina propria, respectively (26). A similar increase has also been described in a rat model of bladder outlet obstruction (20). In addition, increased Cx43 expression has been demonstrated in the lamina propria of human bladders with idiopathic overactivity compared with normal bladders (47). It is reasonable to propose that after spinal cord resection there is loss of neuronal control over the bladder, resulting in the recapitulation of neonatal-like spontaneous activity. The neonatal activity is likely a result of the underdeveloped innervation of the bladder and the intrinsic spontaneous bladder contractions in both SCT animals and neonates may serve as a mechanism to cope with the lack/loss of voluntary voiding function and the distension that results within the bladder.
In this study, the intrinsic spontaneous contractions in SCT rat bladders could be enhanced by either stretch or low-dose arecaidine, and the enhancement was inhibited by atropine but not hexamethonium. This is similar to observations seen in other species, where muscarinic receptor activation (17) enhanced spontaneous contractions of in vitro whole bladder preparations. Again, neonatal rat bladders also show higher sensitivity to muscarinic receptor agonists compared with adult animals (27, 38), further indicating neonatal and SCT rat bladder spontaneous activity may have a similar underlying mechanism. Sensitivity of these contractions to muscarinic receptor agonists has also demonstrated to be increased in a partial bladder outlet-obstructed rat model (12). In this study, removal of the urothelium reduced the intrinsic activity, and the response to low-dose arecaidine was also diminished. Therefore, this suggests that intrinsic activity is modulated by muscarinic receptors, most likely on cells in the urothelial/suburothelial region. Immunohistochemical studies have demonstrated extensive expression of muscarinic and nicotinic receptors throughout the urothelium and lamina propria with apparent species variations in the subtype distribution (7, 8, 56). In this study, there did not appear to be a significant contribution from nicotinic receptors on altering intrinsic contractions or optical activity. However, activation of urothelial nicotinic receptors has been shown to alter bladder-voiding reflexes (1). Therefore, there may be a role for urothelial nicotinic receptors in altering bladder sensory function; however, a precise mechanism has yet to be defined. Also, there is evidence that expression of M2 and M3 receptors are altered on the urothelium and IC-like cells (37) in different bladder pathologies. This upregulation may also account for the altered sensitivity of pathological bladders to muscarinic receptor agonists. It could be postulated that antimuscarinic drugs used routinely to treat overactive bladder symptoms may be acting on multiple sites within in the bladder.
In this study, it was found that removal of the mucosa caused a decrease in the spontaneous activity in SCT rat bladder sheets. This does appear to contradict studies that suggest there is an inhibitory effect of the urothelium on detrusor contractile activity (31, 34). It could be hypothesized that under pathological conditions there is a change in the activity of the urothelium from inhibition to stimulation of spontaneous contractions. The urothelium releases many factors in response to stretch, including ACh, ATP, NO·, substance P, etc. The targets for these released compounds are thought to include urothelial cells, LPM, and suburothelial afferent nerves. Therefore, the urothelium may be acting as a mechanical and chemical sensor to respond to changes in bladder distension or changes in urine composition. Changes in urothelial function have been attributed to various bladder pathologies. An example is the alteration in urothelial ATP release described in umbrella cells from bladders with feline interstitial cystitis, which is thought to be a contributing factor to afferent sensitization (3, 41).
One of the potential targets for factors released from the urothelium are the sensory afferent terminals that densely innervate the suburothelial region of the bladder. Sensitization of sensory nerves, specifically c-fibers, is thought to lead to urgency symptoms and detrusor overactivity that can be part of the symptoms of bladder overactivity (9, 55). It has been suggested that P2X2/3 receptors found on bladder sensory nerve terminals (51) may be activated by enhanced ATP release from the urothelium in pathologies such as spinal cord injury (42) to cause afferent sensitization.
There is extensive tissue remodeling in SCT due to bladder-sphincter dyssynergia and the accompanying outlet obstruction. There are also increased connective tissue deposition and smooth muscle hypertrophy. It is therefore possible that the increased contractile activity is due to the increase in muscle mass along with compliance changes. There is also evidence that there is increased spontaneous Ca2+ release in smooth muscle cells due to alterations in BK and SK channel expression (24, 50) as deletion of either channel leads to bladder overactivity (25, 35). These are likely to contribute to the increased force and excitability of the muscle seen in pathology, and it is hypothesized that these myogenic changes drive the increase in spontaneous contractions. However, this theory does not account for the decrease in the frequency of spontaneous contractions along with their inhibition by gap junction blockers (26, 36), which indicates a possibility that pacemaker-like cells mediate this activity, as there is little gap connectivity between detrusor smooth muscle cells (26, 47).
Another possible target for urothelial factors are the LPM, which have been shown to have functional ATP receptors that enhance the spontaneous firing of these cells (54). It may be possible that increased ATP release from the urothelium under pathological conditions enhances the activity of LPM and promotes pacemaker-like activity. There is also an accompanying increase in gap junction connectivity in these cells in pathology (26) that could account for the more organized spontaneous activity seen in these bladders. However, the mechanism by which the LPM communicate with the smooth muscle cells is still not clear. It has been suggested that LPM could potentially communicate with the detrusor through adhesion molecules. In human bladders, LPM have been shown to express cadherin-11 (28), an adhesion molecule also expressed on the surface of detrusor smooth muscle cells. Formation of adhesion junctions may allow the LPM to transmit signals directly to the detrusor layer or through other networks of IC-like cells.
Bladder overactivity may have neurogenic and/or myogenic origins; however, each does not completely explain the mechanism(s) of enhanced intrinsic bladder activity. There is increasing evidence for the peripheral modulation of intrinsic bladder activity. The autonomous theory for intrinsic bladder activity suggests that intramural nerves or networks of interstitial-like cells may be involved in enhancing intrinsic contractions of the smooth muscle (16). We propose a theory of urotheliogenic origin for modulation of intrinsic activity, where factors released from the urothelium act on suburothelial structures including LPM and afferent nerve terminals.
In summary, we have demonstrated the increased magnitude and decreased frequency of intrinsic contractions in bladders from SCT rats that are mediated by a fixed focal initiation site in the dome and appear to originate from within the mucosa. This is in contrast to normal adult rats, where activity is multifocal and has no fixed pattern and originates from the smooth muscle cells. This organized pattern of activity may be due to the formation of a syncytium of LPM via gap junctions as has been previously described (26). There is also increased sensitivity of intrinsic contractions to muscarinic receptor agonists, and the enhanced intrinsic activity seen in SCT rat bladders were dependent on the presence of the mucosal layer. This suggests that muscarinic receptors in the mucosal region are involved in detrusor overactivity and may be the site of action for antimuscarinic drugs. These studies also suggest that the LPM may be another potential therapeutic target in which to modulate signal transduction from the urothelium to the detrusor in pathological bladders.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-064280 and grants from Pfizer, Ltd. to A. Kanai and Y. Ikeda.
Acknowledgments
We thank Travis Wheeler (Manager), Josh Byler, and Greg Szekeres of the Machine and Electronic Shops, of the Departments of Cell Biology and Physiology and Pharmacology and Chemical Biology, University of Pittsburgh, for the fabrication of equipment used in these studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol 290: F103–F110, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol Renal Physiol 275: F226–F229, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol 285: F423–F429, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci 5: 856–860, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22: 8063–8070, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brading AF Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. J Physiol 570: 13–22, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braverman AS, Lebed B, Linder M, Ruggieri MR. M2 mediated contractions of human bladder from organ donors is associated with an increase in urothelial muscarinic receptors. Neurourol Urodyn 26: 63–70, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bschleipfer T, Schukowski K, Weidner W, Grando SA, Schwantes U, Kummer W, Lips KS. Expression and distribution of cholinergic receptors in the human urothelium. Life Sci 80: 2303–2307, 2007. [DOI] [PubMed] [Google Scholar]
- 9.de Groat WC A neurologic basis for the overactive bladder. Urology 50: 36–52, 1997. [DOI] [PubMed] [Google Scholar]
- 10.de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst 30, Suppl: S71–S77, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Downie JW, Karmazyn M. Mechanical trauma to bladder epithelium liberates prostanoids which modulate neurotransmission in rabbit detrusor muscle. J Pharmacol Exp Ther 230: 445–449, 1984. [PubMed] [Google Scholar]
- 12.Drake M, Gillespie J, Hedlund P, Harvey I, Lagou M, Andersson KE. Muscarinic stimulation of the rat isolated whole bladder: pathophysiological models of detrusor overactivity. Auton Autacoid Pharmacol 26: 261–266, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Drake MJ, Hedlund P, Mills IW, McCoy R, McMurray G, Gardner BP, Andersson KE, Brading AF. Structural and functional denervation of human detrusor after spinal cord injury. Lab Invest 80: 1491–1499, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Du S, Araki I, Mikami Y, Zakoji H, Beppu M, Yoshiyama M, Takeda M. Amiloride-sensitive ion channels in urinary bladder epithelium involved in mechanosensory transduction by modulating stretch-evoked adenosine triphosphate release. Urology 69: 590–595, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes—a possible sensory mechanism? J Physiol 505: 503–511, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillespie JI The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int 93: 478–483, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie JI, Harvey IJ, Drake MJ. Agonist- and nerve-induced phasic activity in the isolated whole bladder of the guinea pig: evidence for two types of bladder activity. Exp Physiol 88: 343–357, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie JI, Markerink-van Ittersum M, de Vente J. cGMP-generating cells in the bladder wall: identification of distinct networks of interstitial cells. BJU Int 94: 1114–1124, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Gillespie JI, Markerink-van Ittersum M, De Vente J. Endogenous nitric oxide/cGMP signalling in the guinea pig bladder: evidence for distinct populations of sub-urothelial interstitial cells. Cell Tissue Res 325: 325–332, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Haefliger JA, Tissieres P, Tawadros T, Formenton A, Beny JL, Nicod P, Frey P, Meda P. Connexins 43 and 26 are differentially increased after rat bladder outlet obstruction. Exp Cell Res 274: 216–225, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Hanna-Mitchell AT, Beckel JM, Barbadora S, Kanai AJ, de Groat WC, Birder LA. Non-neuronal acetylcholine and urinary bladder urothelium. Life Sci 80: 2298–2302, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashitani H, Brading AF. Ionic basis for the regulation of spontaneous excitation in detrusor smooth muscle cells of the guinea-pig urinary bladder. Br J Pharmacol 140: 159–169, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol 559: 567–581, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol 551: 893–903, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda Y, Fry CH, Hayashi F, Stolz DB, Griffiths D, Kanai AJ. The role of gap junctions in spontaneous activity of the rat bladder. Am J Physiol Renal Physiol 293: F1018–F1025, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, de Groat W, Fry C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol 292: F1065–F1072, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuijpers KA, Heesakkers JP, Jansen CF, Schalken JA. Cadherin-11 is expressed in detrusor smooth muscle cells and myofibroblasts of normal human bladder. Eur Urol 52: 1213–1221, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Lagou M, De Vente J, Kirkwood TB, Hedlund P, Andersson KE, Gillespie JI, Drake MJ. Location of interstitial cells and neurotransmitters in the mouse bladder. BJU Int 97: 1332–1337, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Levin RM, Ruggieri MR, Velagapudi S, Gordon D, Altman B, Wein AJ. Relevance of spontaneous activity to urinary bladder function: an in vitro and in vivo study. J Urol 136: 517–521, 1986. [DOI] [PubMed] [Google Scholar]
- 31.Levin RM, Wein AJ, Krasnopolsky L, Atta MA, Ghoniem GM. Effect of mucosal removal on the response of the feline bladder to pharmacological stimulation. J Urol 153: 1291–1294, 1995. [PubMed] [Google Scholar]
- 32.Lips KS, Wunsch J, Zarghooni S, Bschleipfer T, Schukowski K, Weidner W, Wessler I, Schwantes U, Koepsell H, Kummer W. Acetylcholine and molecular components of its synthesis and release machinery in the urothelium. Eur Urol 51: 1042–1053, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Maggi CA, Santicioli P, Meli A. Postnatal development of myogenic contractile activity and excitatory innervation of rat urinary bladder. Am J Physiol Regul Integr Comp Physiol 247: R972–R978, 1984. [DOI] [PubMed] [Google Scholar]
- 34.Meng E, Young JS, Brading AF. Spontaneous activity of mouse detrusor smooth muscle and the effects of the urothelium. Neurourol Urodyn 27: 79–87, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem 279: 36746–36752, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Miyazato M, Sugaya K, Nishijima S, Oda M, Ogawa Y. A gap junction blocker inhibits isolated whole bladder activity in normal rats and rats with partial bladder outlet obstruction. Biomed Res 27: 203–209, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Mukerji G, Yiangou Y, Grogono J, Underwood J, Agarwal SK, Khullar V, Anand P. Localization of M2 and M3 muscarinic receptors in human bladder disorders and their clinical correlations. J Urol 176: 367–373, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Ng YK, de Groat WC, Wu HY. Muscarinic regulation of neonatal rat bladder spontaneous contractions. Am J Physiol Regul Integr Comp Physiol 291: R1049–R1059, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oh SJ, Lee KH, Kim SJ, Kim KW, Kim KM, Choi H. Active properties of the urinary bladder: in vitro comparative studies between adult and neonatal rats. BJU Int 85: 1126–1133, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Pinna C, Zanardo R, Puglisi L. Prostaglandin-release impairment in the bladder epithelium of streptozotocin-induced diabetic rats. Eur J Pharmacol 388: 267–273, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Roppolo JR, Tai C, Booth AM, Buffington CA, de Groat WC, Birder LA. Bladder Adelta afferent nerve activity in normal cats and cats with feline interstitial cystitis. J Urol 173: 1011–1015, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Salas NA, Somogyi GT, Gangitano DA, Boone TB, Smith CP. Receptor activated bladder and spinal ATP release in neurally intact and chronic spinal cord injured rats. Neurochem Int 50: 345–350, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shafik A, El-Sibai O, Shafik AA, Shafik I. Identification of interstitial cells of Cajal in human urinary bladder: concept of vesical pacemaker. Urology 64: 809–813, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Sibley GN A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J Physiol 354: 431–443, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugaya K, de Groat WC. Inhibitory control of the urinary bladder in the neonatal rat in vitro spinal cord-bladder preparation. Brain Res Dev Brain Res 138: 87–95, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Sugaya K, De Groat WC. Micturition reflexes in the in vitro neonatal rat brain stem-spinal cord-bladder preparation. Am J Physiol Regul Integr Comp Physiol 266: R658–R667, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int 90: 118–129, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol 171: 938–943, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Szell EA, Somogyi GT, de Groat WC, Szigeti GP. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. Am J Physiol Regul Integr Comp Physiol 285: R809–R816, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol 289: F604–F610, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21: 5670–5677, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang E, Truschel S, Apodaca G. Analysis of hydrostatic pressure-induced changes in umbrella cell surface area. Methods 30: 207–217, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int 91: 89–93, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol 559: 231–243, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimura N Bladder afferent pathway and spinal cord injury: possible mechanisms inducing hyperreflexia of the urinary bladder. Prog Neurobiol 57: 583–606, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Zarghooni S, Wunsch J, Bodenbenner M, Bruggmann D, Grando SA, Schwantes U, Wess J, Kummer W, Lips KS. Expression of muscarinic and nicotinic acetylcholine receptors in the mouse urothelium. Life Sci 80: 2308–2313, 2007. [DOI] [PubMed] [Google Scholar]