Abstract
Nitric oxide (NO) has been shown to be the endothelium-derived relaxing factor (EDRF), and its impairment contributes to a variety of cardiovascular disorders. Recently, it has been recognized that nitrite can be an important source of NO; however, questions remain regarding the activity and mechanisms of nitrite bioactivation in vessels and its physiological importance. Therefore, we investigated the effects of nitrite on in vivo hemodynamics in rats and in vitro vasorelaxation in isolated rat aorta under aerobic conditions. Studies were performed to determine the mechanisms by which nitrite is converted to NO. In anesthetized rats, nitrite dose dependently decreased both systolic and diastolic blood pressure with a threshold dose of 10 μM. Similarly, nitrite (10 μM-2 mM) caused vasorelaxation of aortic rings, and NO was shown to be the intermediate factor responsible for this activity. With the use of electrochemical as well as electron paramagnetic resonance (EPR) spectroscopy techniques NO generation was measured from isolated aortic vessels following nitrite treatment. Reduction of nitrite to NO was blocked by heating the vessel, suggesting that an enzymatic process is involved. Organ chamber experiments demonstrated that aortic relaxation induced by nitrite could be blocked by both hemoglobin and soluble guanylyl cyclase (sGC) inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one (ODQ). In addition, both electrochemical and EPR spin-trapping measurements showed that ODQ inhibits nitrite-mediated NO production. These findings thus suggest that nitrite can be a precursor of EDRF and that sGC or other heme proteins inhibited by ODQ catalyze the reduction of nitrite to NO.
Keywords: soluble guanylate cyclase, aerobic nitrite reduction, vasorelaxation, blood pressure, heme proteins
nitric oxide (NO) is an important and highly versatile signaling molecule with a wide variety of functions in the cardiovascular system including regulation of systemic blood pressure, blood flow, and regional vascular tone (38). It was first discovered by Furchgott and Zawadzki (14) to be an endothelium-derived relaxing factor (EDRF), which was subsequently identified as NO by Palmer et al. (44) and Ignarro et al. (21). It is now well recognized that in biological tissues NO is generated by specific NO synthases (NOSs) that metabolize arginine to citrulline with the formation of NO (2, 15). The biological activity of NO is acutely terminated by oxidation to nitrite and nitrate.
Vasodilator therapies with organic nitrates, such as glycerol trinitrite and isosorbide dinitrite, have been an integral part of treatment for more than 130 years to bring relief to patients suffering from coronary disease (7). The beneficial vasodilator effects of organic nitrates mainly stem from their enzymatic metabolism to NO and subsequent activation of the vascular NO/cyclic GMP (cGMP) pathway (13). Despite potent vasodilatory effects, the use of organic nitrites has been limited because of well-defined side effects and development of tolerance (45).
Nitrite (NO2−) is an endogenous metabolite of NO oxidation under normal physiological conditions (28). Nitrite has long been believed to be relatively inert at the micromolar levels normally found in vivo, and, until recently, nitrite was thought to be inactive as a vasodilator on the basis of a report by Lauer et al. (30) in humans. In 1995, we first reported that nitrite can be a source of NO in ischemic tissues where it is reduced back to NO (65). Recent works by a number of laboratories (3, 5, 48, 55, 61) have shown that nitrite can be recycled back to bioactive NO in both the circulation and tissues and has the potential to be a supplemental source of NO and provide therapeutic benefit. Despite initial controversy, potent vasodilating effects of nitrite have now been reported in mice, rats, dogs, sheep, primates, and humans (5, 6, 35, 37).
Several mechanisms for the reduction of nitrite to NO have been identified by us and others including 1) nonenzymatic disproportionation at low pH (65), 2) enzyme-independent reduction (50, 64), 3) enzymatic conversion by xanthine oxidoreductase (XOR) (17, 31, 32), 4) reduction by P450 (33), and 5) reduction by deoxyhemoglobin (deoxyHb) (27, 40). Recently, hemoglobin (Hb)-mediated, hypoxia-induced vasodilation and NO production have been proposed; however, this remains highly controversial in view of the potent NO scavenging by Hb (25). Most recently, it has been reported that physiological concentrations of nitrite can cause modest arterial dilation in both normoxia and hypoxia and that aortic vasorelaxation does not require Hb (36). Thus further investigation is needed to characterize the process and mechanisms of NO generation from nitrite in vessels.
Therefore, we have functionally and analytically studied the process of nitrite-induced vasodilation in vascular tissue and provide definitive evidence that nitrite can be a precursor of EDRF and that soluble guanylate cyclase (sGC) or other heme-enzymes blocked by sGC inhibitors catalyze the reduction of nitrite to NO.
MATERIALS AND METHODS
This study was reviewed by the Institutional Laboratory Animal Care and Use Committee at The Ohio State University, carried out according to the approved guidelines, and conforms with the Guidelines for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Animal Preparations and In Vivo BP Measurements
Male Sprague-Dawley rats weighing 300–500 g were purchased from Harlan (Indianapolis, IN). Rats were anesthetized with a combination of low-dose pentobarbital sodium (30 mg/kg ip) and isoflurane inhalation. After adequate anesthesia and necessary shaving, rats were placed on a heated surgical plate to maintain body temperature at 37°C. Intubation was then performed with a rat tracheal catheter (Introcan Safety 14 G-2; B. Braun Medical, Bethlehem, PA), and rats were ventilated with room air with a rodent ventilator (model no. 683; Harvard Apparatus, Holliston, MA). The average breathing rate was 100 strokes/min, and the average tidal volume was 1.0 cm3. Under aseptic conditions, a small incision was made at right carotid area, the right carotid artery was dissected and freed from surrounding tissues, and a catheter was then inserted into the right carotid artery for blood pressure (BP) measurement. The left jugular vein was similarly dissected and cannulated for intravenous administration of nitrite. Both arterial and venous cannula were flushed with heparinized saline (10 U/ml) to prevent blood clotting. BP was continuously monitored via a MLT0699 BP transducer connected to the PowerLab/400 multichannel data acquisition system with PowerLab Chart software (ADInstruments, Castle Hill, Australia). The BP signal was digitally processed to yield systolic and diastolic pressures as well as heart rate. After surgery, at least 30 min was allowed for stabilization of BP. Following each nitrite administration, 5–10 min was allowed for the BP to stabilize. Nitrite was administered as a concentrated 200 mM stock dissolved in PBS, pH 7.4, without calcium and magnesium, and it was infused slowly over 1–3 min to achieve the final concentration calculated according to the body weight and circulating blood volume of each animal as outlined (41). The dose-dependent effects of in vivo nitrite were determined by measuring the decrease in BP from baseline, and this was expressed as the percentage decrease with respect to the maximal BP decrease from baseline by 2 mM nitrite.
Aortic Preparations and Functional Measurements
All in vitro experiments were performed on thoracic aortic rings except for electron paramagnetic resonance (EPR) experiments that used whole thoracic and abdominal aorta. After anesthesia with pentobarbital sodium (60 mg/kg), aorta were excised from heparinized rats, placed in ice-cold buffer, cleaned of loosely adhering fat and connective tissue, and cut into rings 10 mm in length for electrochemical analyzer experiments or 5 mm in length for functional organ bath experiments.
Functional measurements of vascular tone were performed as described previously with slight modification (4, 63). Briefly, aortic rings were mounted horizontally in organ chambers filled with 20 ml of buffer (37°C, pH 7.4) of the following composition (all in mM): 118 NaCl, 4.7 KCl, 1.19 CaCl2, 1.2 MgSO4, 1.1 KH2PO4, 25 NaHCO3, 11 glucose, and 4.6 HEPES. Several rings were cut from the same aorta and studied in parallel. In some rings, the endothelium was removed by placing a piece of stainless steel wire in the lumen and rubbing the aortic ring gently over a wet blotting paper. Each ring was connected to an isometric force transducer and suspended in an organ chamber bubbled with 95% O2-5% CO2 gas. Aortic rings were gradually stretched to the optimal point of their length-tension curve as determined by contraction to phenylephrine (10 μM). The aortic segments were allowed to equilibrate for 75 min, with the solution in the chambers changed every 15 min, and then their contractility was tested by application of phenylephrine (10 μM) for 20 min. Following stable phenylephrine contraction, the vasorelaxant effects of cumulative addition of nitrite (0.001–2 mM) were determined by measuring the tension and expressing this as the percentage relaxation with respect to the maximal phenylephrine contraction. To assess the role of NO in the process of nitrite-induced vasodilation under normoxic conditions, ferrous human Hb A was used. To assess the involvement of NO-cGMP pathway in nitrite-induced vasodilation under normoxic conditions, the effects of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one (ODQ) (16) was investigated before and after administration of nitrite. ODQ was dissolved in 100% DMSO and added to the organ bath to give a final concentration of 10 μM. In the pretreatment group, ODQ was first added at the peak of phenylephrine contraction, and, 10 min after ODQ treatment, nitrite was then added and the relaxation measured. In the posttreatment group, after nitrite-induced vasorelaxation in phenylephrine-contracted aortic rings, ODQ was then added.
Effects of various known inhibitors/agents were tested on nitrite-induced vasorelaxation in aerobic conditions. The inhibitors/agents were the following: 1) xanthine oxidase inhibitor, oxypurinol (43); 2) mitochondrial electron transport inhibitors, rotenone and myxothiazol (29); 3) cytochrome P-450 inhibitor, 17-octadecynoic acid (17-ODYA) (19); and 4) NOS inhibitor, NG-nitro-l-arginine methyl ester (l-NAME) (11). Stock solutions for oxypurinol, rotenone, and 17-ODYA were made with DMSO. Myxothiazol was dissolved in methanol, and l-NAME was dissolved in deionized water. The final concentrations of DMSO and methanol were less than 0.2% in the organ baths. Inhibitors were used according to their reported selective concentration for each pathway. Each inhibitor was applied at the stable phenylephrine contraction and incubated for 10–15 min, and then nitrite (500 μM) was added. Vehicle (DMSO and methanol) effects for these inhibitors were also measured on nitrite-induced relaxation.
Electrochemical Experiments
These experiments were performed as reported previously (63) with modified organ chambers so the solution in the chambers could be rapidly stirred to efficiently dissolve air into solution with no gas bubbling. The aortic rings were opened longitudinally and fixed to a custom-made plastic block that was attached to the bottom of a water-jacketed chamber that was then filled with buffer that was continuously stirred (63). Nitrite was added to the chamber containing the aortic preparation, and electrochemical measurement of the NO concentration derived from the administration of the nitrite was performed using an ISO-NOP Clark-type NO electrode (WPI, Sarasota, FL). Similar experiments were performed for denuded aortic preparations.
EPR Experiments
EPR measurements were performed using Bruker ER 300 or EMX spectrometers operating at X-band as described previously with slight modification (56, 59). NO formation was measured using the Fe2+-diethyldithiocarbamate (DETC)2 complex, Fe2+-DETC, which forms a stable NO-Fe+2-DETC complex that exhibits a characteristic triplet EPR spectrum. Aorta was incubated in PBS with 0.5% BSA, nitrite (500 μM), ammonium-iron (II) sulfate (1 mM), and DETC (2 mM), pH 7.4 at 37°C, and then frozen in liquid nitrogen. Measurements were performed in an EPR finger Dewar at 77 K using a TM110 microwave cavity with 100 kHz modulation frequency, 4.0 G modulation amplitude, 20 mW microwave power. To assess the effects of carbon monoxide (CO) on nitrite-induced NO, aortic preparations in PBS were bubbled with 99.5% CO gas for 3 min, and NO production from nitrite (1 mM) was determined with Fe2+-DETC.
Optical Spectroscopy
UV and visible spectra for heme proteins, oxymyoglobin (oxyMb) and cytoglobin (Cygb), were detected in the absence and presence of ODQ (30 μM) with a Cary 50 Bio UV-Visible spectrophotometer (Varian, Palo Alto, CA).
Mb preparation.
Myoglobin (Mb) was prepared as reported (52). Briefly, to obtain reduced Mb, equine Mb from skeletal muscle was first dissolved in PBS buffer (pH 7.4) to a 2.0 mM concentration and then reduced with 5 mM dithionate under anaerobic conditions for 45 min. This mixture was quickly separated on a G-25 Sephadex column (Sigma, St. Louis, MO) and then dialyzed three times in PBS buffer for 9 h through Slide-A-Lyser Dialysis Cassette 3.5K (Pierce, Rockford, IL). PBS containing pure oxyMb (6 μM) was placed in a cuvette, and the baseline UV/visible spectrum was recorded at room temperature. For ODQ effect, pure oxyMb (6 μM) was mixed with ODQ (30 μM), and the spectra were recorded.
Hb preparation.
Hb was prepared in a manner similar to that reported above for Mb.
Expression and purification of recombinant Cygb.
The expression plasmids (human CYGB cDNA in pET3a) were obtained from Dr. Thorsten Burmester (Mainz, Germany) and transformed into Escherichia coli and Cygb expressed and purified as reported (10). Cygb fractions were concentrated by Amicon filtration (PM10) and passed though a Sephacryl S 200 column and then pooled and stored at −80°C. PBS containing Cygb (5 μM) was placed in a cuvette and the baseline UV/visible spectrum recorded at room temperature. For ODQ effect, Cygb (5 μM) was mixed with ODQ (30 μM), and the spectra were recorded.
Chemicals and Reagents
Ammonium iron(II) sulfate hexahydrate, BSA, DMSO, DETC, sodium nitrite (nitrite), ODQ, human HbA (ferrous stabilized), skeletal Mb (equine), l-NAME, rotenone, oxypurinol, and myxothiazol were purchased from Sigma. 17-ODYA was purchased from Biomol (Plymouth Meeting, PA). 15N-sodium nitrite was purchased from Cambridge Isotope Laboratories (Andover, MA), and PBS without calcium or magnesium was obtained from GIBCO/Invitrogen (Carlsbad, CA). All other chemicals were of the highest grade available.
Data Analysis
All results are expressed as the means ± SE. Data were analyzed either by two-tailed Student's t-test for paired data from the same experiment and unpaired data from different experiments. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Concentration-dependent effects of nitrite on in vivo hemodynamics of anesthetized rats.
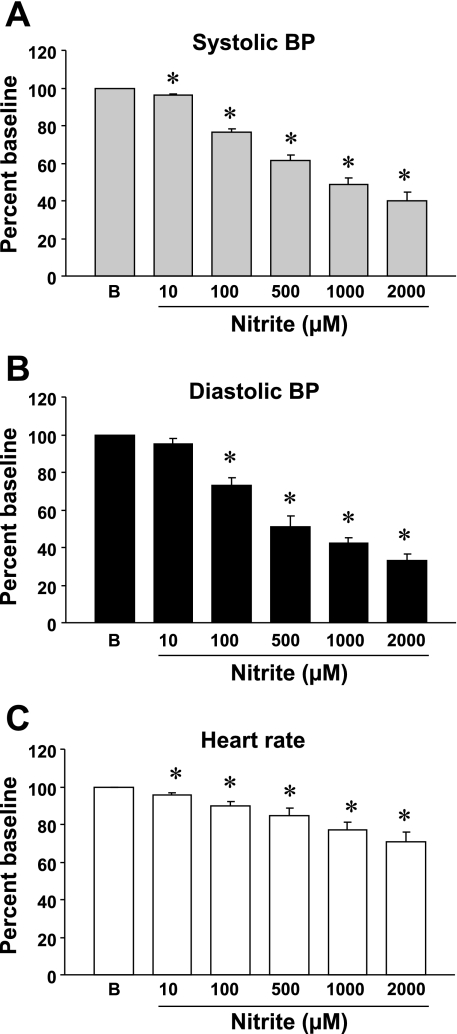
Nitrite, administered noncumulatively, significantly reduced BP and heart rate in anesthetized rats (Fig. 1, A, B, and C) at normal body temperature (37°C). Baseline systolic BP, diastolic BP, and heart rate in anesthetized rats were 134 ± 6 mmHg, 116 ± 6 mmHg, and 322 ± 8 bpm, respectively, n = 4. Nitrite at 1 μM had no clear effect on the BP, but, at 10 μM and higher concentrations, it significantly decreased BP in a concentration-dependent manner (Fig. 1). Systolic BPs at 10 μM, 100 μM, 500 μM, 1 mM, and 2 mM nitrite were 128 ± 6, 102 ± 4, 83 ± 6, 65 ± 5, and 53 ± 6 mmHg, respectively, and diastolic BPs were 111 ± 7, 86 ± 7, 59 ± 6, 49 ± 8, and 39 ± 4 mmHg, respectively. Corresponding heart rates were 308 ± 10, 290 ± 12, 272 ± 16, 248 ± 13, and 227 ± 19 beats/min, respectively. The percentage decrease in both systolic and diastolic BP by nitrite, calculated as the percentage decrease with respect to the maximal BP decrease from the baseline by 2 mM nitrite, was comparable for each concentration (Fig. 1, A and B).
Fig. 1.
Concentration-dependent effects of nitrite on the blood pressure (BP) and heart rate of anesthetized rats with normal body temperature (37°C). A: systolic BP. B: diastolic BP. C: Heart rate. Ordinate BP is expressed as percentage baseline (B) that was assigned as 100%; abscissa, concentration of nitrite in μM. Each symbol with a vertical bar represents the mean ± SE of 4 experiments with different animals. *P < 0.05 vs. baseline.
Concentration-dependent effects of nitrite on isolated rat aorta.
Nitrite applied cumulatively relaxed aortic rings precontracted with phenylephrine in aerobic conditions at pH 7.4, 37°C (Fig. 2A). In the neutral buffer environment, the threshold concentration of nitrite to induce vasorelaxation was 10 μM, and the aortic segment relaxed almost to basal tone at 2 mM concentration (Fig. 2B). We also observed that the precontracted endothelium-denuded aortic rings relaxed in an identical manner (data not shown) as seen for the endothelium-intact aortic rings. The extent of relaxation caused by 10 μM, 100 μM, 1 mM, and 2 mM nitrite was 5 ± 2, 38 ± 3, 88 ± 5, and 97 ± 3% of phenylephrine contraction, respectively, n = 5 (Fig. 2B).
Fig. 2.
Cumulative concentration-dependent effects of nitrite on isolated rat aorta precontracted with phenylephrine (10 μM) under aerobic conditions (pH 7.4, 37°C). A: representative tracing for the effects of nitrite on the precontracted aortic ring over time. B: average data for the vasorelaxant effects of nitrite; ordinate, relaxant effects expressed as the percentage relaxation from the maximal phenylephrine contraction; abscissa, concentration of nitrite in mM. Each point represents the mean ± SE of 5 experiments.
Role of NO and effect of oxyhemoglobin on nitrite-induced vasorelaxation.
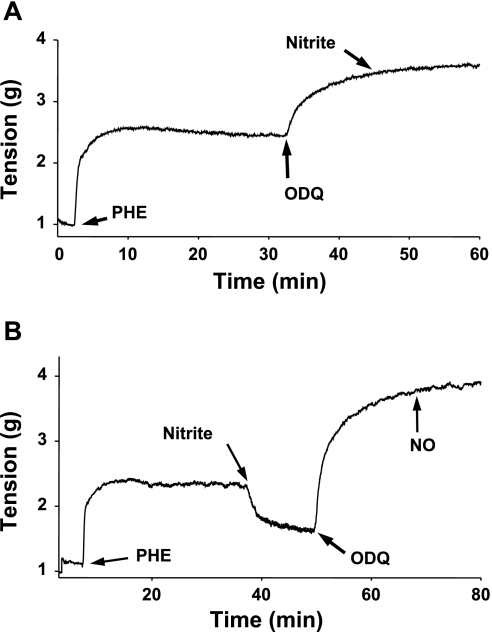
Oxyhemoglobin (oxyHb) is a highly effective NO scavenger, and its vasoconstrictant effect was examined under aerobic conditions at pH 7.4 (37°C). When phenylephrine-contracted aortic rings were prerelaxed with nitrite (100 μM) application of 20 μM oxyHb immediately abolished the nitrite-induced relaxation (Fig. 3A). Of note, although oxyHb rapidly scavenges NO, it also much more slowly oxidizes nitrite over a period of minutes. Since the constriction effect of oxyHb was almost instant this suggests that it is due to NO scavenging rather than nitrite consumption. To further confirm this, experiments were also performed with higher nitrite concentrations so that oxyHb could not possibly scavenge the added nitrite. With 500 μM nitrite, 20 μM oxyHb rapidly reversed the observed relaxation, but as expected, after 10–20 min, this effect was reversed as the oxyHb was oxidized (Fig. 3B).
Fig. 3.
Representative tracings for the effects of hemoglobin (Hb) on nitrite-induced vasorelaxation in aerobic conditions (pH 7.4, 37°C). Aortic rings were first contracted with phenylephrine (PHE) (10 μM), and Hb (20 μM) was then added after nitrite. A: representative tracing for the effect of Hb on nitrite (100 μM) prerelaxed aortic ring. B: representative tracing for the effect of Hb on nitrite (500 μM) prerelaxed aortic ring. Ordinate, aortic tension expressed in grams (g); abscissa, time in min. Arrows show the time at which different agents were added. Each experiment was repeated at least 5 times, and findings were reproducible as shown.
Role of sGC and effect of ODQ on nitrite-induced vasorelaxation.
The influence of ODQ, a sGC inhibitor, on nitrite-induced vasorelaxation was examined in two series of experiments under aerobic conditions at pH 7.4 (37°C) as described in materials and methods. In the first series, ODQ (10 μM) was seen to produce marked augmentation of phenylephrine-induced contraction, and with subsequent addition of nitrite there was no vasorelaxant effect (Fig. 4A). In the second series, following phenylephrine-induced contraction, nitrite was added, and relaxation was seen. However, ODQ not only reversed the nitrite-induced relaxation but also it further augmented the contraction above the tension seen with phenylephrine alone (Fig. 4B). Also, with subsequent addition of authentic NO (3 μM), from NO gas, no relaxation was seen.
Fig. 4.
Effects of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one (ODQ) on nitrite-induced vasorelaxation under aerobic conditions (pH 7.4, 37°C). Aortic rings were first contracted with phenylephrine (10 μM), and ODQ (10 μM) or nitric oxide (NO) (3 μM) was then added either before or after nitrite (500 μM). A: representative tracing for the effect of ODQ pretreatment on the nitrite-induced vasorelaxation. B: representative tracing for the effect of ODQ on nitrite prerelaxed aortic ring and followed by the effect of NO. Ordinate, aortic tension expressed in grams (g); abscissa, time in min. Arrows show the time at which different agents were added. Each protocol was repeated at least 5 times, and findings were reproducible as shown.
Effects of different inhibitors on nitrite-induced vasorelaxation.
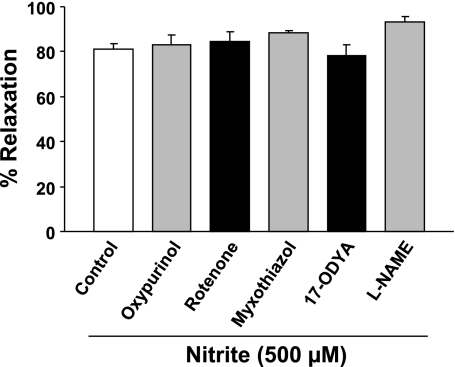
Involvement of several potential mechanisms for nitrite-mediated vasorelaxation was examined under aerobic conditions at pH 7.4 (37°C) using selective inhibitors/agents. The DMSO and methanol vehicles had only minor relaxant effects on the phenylephrine-contracted aortic rings, 5.4 ± 0.5 and 3.9 ± 0.6%, respectively, n = 4, and nitrite-induced (500 μM) aortic relaxation was not affected by DMSO or methanol. Importantly, oxypurinol (50 μM), rotenone (10 μM), myxothiazol (10 μM), 17-ODYA (50 μM), and l-NAME (100 μM) did not inhibit or decrease the nitrite-induced vasorelaxation (Fig. 5). This indicates that this aortic relaxation under aerobic conditions is not due to XOR, mitochondrial electron transport chain, cytochrome P450, or NOS.
Fig. 5.
Effects of different inhibitors on nitrite-induced vasorelaxation of aorta under aerobic conditions. At stable phenylephrine (10 μM) contraction, the aortic ring was first treated with an inhibitor for 10–15 min, and nitrite-induced vasorelaxation was then determined. The bars shown from left to right are nitrite (500 μM) alone (control), oxypurinol (50 μM) plus nitrite, rotenone (10 μM) plus nitrite, myxothiazol (10 μM) plus nitrite, 17-octadecynoic acid (17-ODYA) (50 μM) plus nitrite; and NG-nitro-l-arginine methyl ester (l-NAME) (100 μM) plus nitrite. Average data for the vasorelaxant effects of nitrite were expressed as the percentage relaxation from the phenylephrine contraction. Each symbol with a vertical bar represents the mean ± SE of ≥4 experiments.
Electrochemical measurements of nitrite-induced NO.
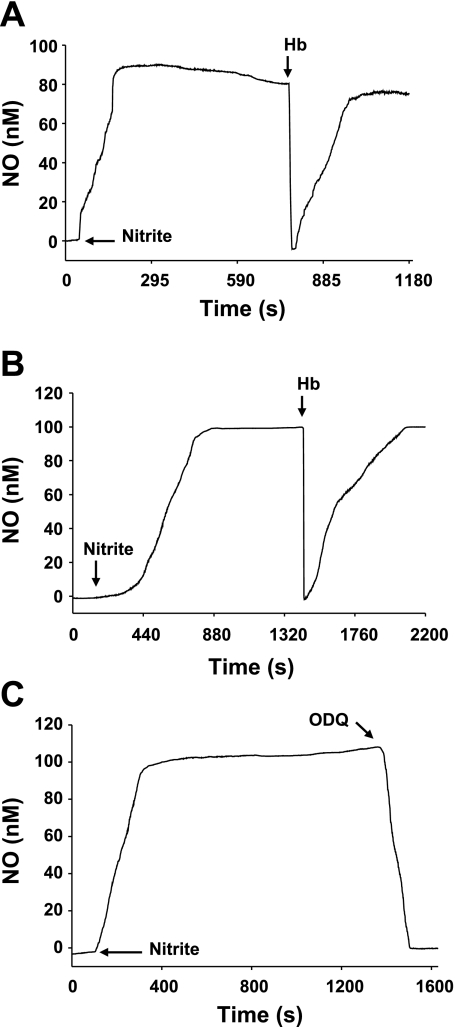
To detect nitrite-induced NO generation from isolated aortic segments under aerobic conditions (pH 7.4, 37C), a modified organ bath and an electrochemical analyzer were used. First, nitrite-induced NO was determined in the aortic preparations, and the influence of oxyHb was then examined. Nitrite (500 μM) induced a marked increase in NO concentration (∼90 nM) from both endothelium-intact (Fig. 6A) and endothelium-denuded (Fig. 6B) aortic preparations, and administration of oxyHb (50 μM) totally but transiently abolished this NO. To examine whether sGC or similar heme proteins blocked by ODQ are involved in nitrite-induced NO generation under aerobic conditions (pH 7.4, 37°C), the effect of ODQ (10 μM) was determined. ODQ completely inhibited the nitrite-induced NO generation (Fig. 6C).
Fig. 6.
Effects of Hb and ODQ on electrochemical measurements of nitrite-induced NO from aortic rings in aerobic conditions (pH 7.4, 37°C). Nitrite (500 μM) was first added to the organ bath to generate NO from the aortic preparation, and Hb (50 μM) or ODQ (10 μM) was then added at the steady-state level of NO. A: representative tracing for the effect of Hb on nitrite-induced NO in endothelium-intact ring. B: representative tracing for the effect of Hb on nitrite-induce NO in an endothelium-denuded ring. C: representative tracing for the effect of ODQ on nitrite-induced NO in endothelium-intact ring. Ordinate, NO concentration in nM; abscissa, time in s. Arrows show the time at which different drugs were added. Findings were reproducible with each experiment, n ≥ 4.
EPR measurements of nitrite-induced NO.
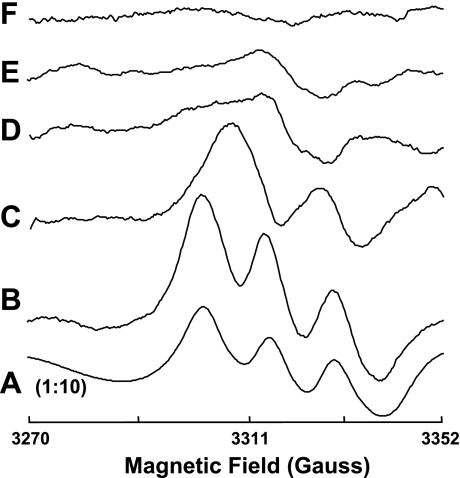
To further measure nitrite-induced NO from aortic tissue under aerobic conditions, EPR spin-trapping measurements were performed (Fig. 7). The typical triplet spectrum of NO-Fe2+-DETC was seen when NO (15 μM) was added to aortic tissue (Fig. 7A). A similar triplet EPR spectrum of 14NO-Fe2+-DETC was produced by addition of natural abundance 14N nitrite (500 μM) to aortic tissue (Fig. 7B). When aortic tissue was exposed to 15N nitrite (500 μM), the prominent doublet signal of 15NO-Fe2+-DETC was detected definitively demonstrating that the NO was nitrite derived (Fig. 7C).
Fig. 7.
Representative electron paramagnetic resonance (EPR) spectra for NO-Fe2+-DETC complex formed from isolated rat aorta in the presence of added nitrite or NO at 77 K. EPR measurements were performed using a Bruker ER 300 spectrometer operating at X-band, 9.77 GHz. Aorta were incubated in PBS with 0.5% BSA, nitrite (500 μM), ammonium-iron(II) sulfate(1 mM), and diethyldithiocarbamate (DETC) (2 mM), pH 7.4 at 37°C with the following: A: NO (15 μM); B: 14N nitrite (500 μM); C: 15N nitrite (500 μM); D: high temperature (100°C, 8 min) and 14N nitrite (500 μM); and E: ODQ (20 μM) and 14N nitrite (500 μM). F: background effects for 14N nitrite (500 μM) without aorta. Each spectrum is representative of at least 5 independent experiments. Please note that the A is presented at 1:10 magnitude of all others.
To examine whether the nitrite-mediated NO generation occurs through an enzymatic process and whether sGC or similar heme proteins were involved, the effects of high temperature (100°C, 8 min) and ODQ (20 μM) were determined. Figure 7D shows that the 14NO-Fe2+-DETC signal from aortic tissue was completely abolished by preheating. Importantly, pretreatment of the aortic tissue with ODQ abolished the 14NO-Fe2+-DETC signal (Fig. 7E). No 14NO-Fe2+-DETC signal was seen in the absence of aortic tissue (Fig. 7F).
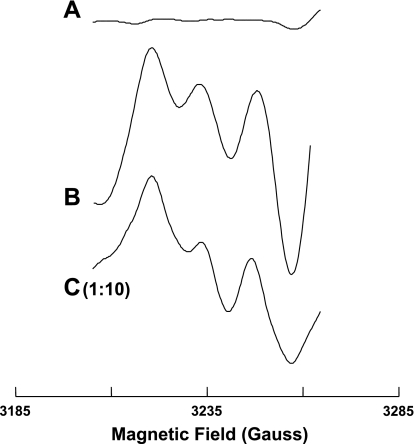
We also examined the effects of CO on nitrite-mediated NO generation. Figure 8C shows the typical triplet of NO-Fe2+-DETC complex seen when NO (5 μM) was added to aortic tissue preexposed to CO, and Fig. 8B shows the EPR spectra of 14NO-Fe2+-DETC produced by 14N nitrite from aortic tissue. Pretreatment of aorta with CO abolished this nitrite-derived 14NO-Fe2+-DETC signal (Fig. 8A). Thus CO blocks nitrite-mediated NO generation from aorta.
Fig. 8.
Representative EPR spectra for NO-Fe2+-DETC complex formed from isolated rat aorta with added nitrite or NO at 77 K. EPR spin-trapping measurements were performed using a Bruker EMX spectrometer operating at X-band, 9.53 GHz, and aorta were prepared as described in Fig. 7. Treatment of aorta with CO totally abolished 14NO-Fe2+-DETC signal for nitrite (A). B: EPR spectra for 14NO-Fe2+-DETC complex produced by 14N nitrite (1 mM) from aortic tissue; C: typical triplet of NO-Fe2+-DETC complex from NO (5 μM) with aortic tissue treated with CO. Each spectrum is representative of at least 5 independent experiments. Please note that the C is presented at 1:10 magnitude of all others.
Specificity of ODQ: possible involvement of other heme proteins.
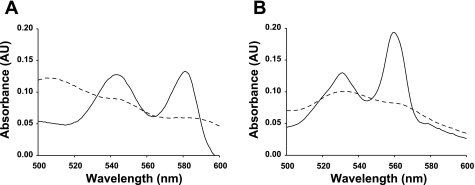
To test whether ODQ is specific for the heme of sGC, UV visible spectra for oxyMb and Cygb were detected in room air-equilibrated buffer in the absence and presence of ODQ. Figure 9A shows the visible spectrum of pure oxyMb (6 μM) with two distinct peaks at 543 and 581 nm wavelength. After the addition of ODQ (30 μM), these peaks were quenched. Figure 9B shows the similar visible spectrum of pure reduced Cygb (5 μM) with two distinct peaks at 531 and 560 nm wavelength. In the presence of ODQ (30 μM), these peaks were also quenched. Thus ODQ not only inhibits sGC but also interacts with other heme proteins such as Mb and Cygb.
Fig. 9.
Representative absorbance spectrum of oxymyoglobin (oxyMb) and reduced cytoglobin (Cygb). OxyMb (6 μM) and reduced Cygb (5 μM) in the absence (solid line) and presence of ODQ (dashed line) and UV/visible spectra are shown between 500 and 600 nm wavelength (pH 7.4, room temperature). A: effect of ODQ (30 μM) on oxyMb peaks between 540 to 580 nm; B: effect of ODQ (30 μM) on Cygb peaks between 530 to 580 nm. Ordinate, absorbance in arbitrary units (AU); abscissa, wavelength in nm. The spectra are representative of 4 independent experiments with fresh oxyMb and Cygb preparations.
DISCUSSION
The results of the present study demonstrate that, under aerobic conditions, exogenous nitrite caused a dose-dependent decrease in the BP of anesthetized rats in parallel with a concentration-dependent vasorelaxation of rat aorta in vitro. Associated key findings are that 1) nitrite produced NO from isolated rat aorta under aerobic conditions; 2) both vasorelaxation and NO generation were inhibited by oxyHb; 3) both vasorelaxation and NO generation were abolished by a sGC inhibitor ODQ; and 4) EPR spectra of nitrite-derived NO from aorta were abolished by heat, ODQ, and CO. Thus our in vivo and in vitro functional data in concert with electrochemical and spectroscopic findings demonstrate that the dose-dependent vasodilatory effects of exogenous inorganic nitrite are due to NO formation with this process mediated by sGC and/or other heme enzymes under aerobic conditions.
Vasodilatory action of nitrite under aerobic conditions.
Despite initial controversy (30), recent studies have repeatedly shown that under normoxic conditions, exogenous nitrite can cause potent vasodilation in both animals and humans (5, 6, 35, 36). In vivo BP lowering and in vitro vasorelaxing effects of nitrite in the present study were within the same concentration range (Figs. 1 and 2), and the magnitude of nitrite-induced changes were comparable both in vivo and in vitro, thus reaffirming that the vasodilating effects of nitrite are present in aerobic conditions. We observed that the threshold concentration of nitrite was at ∼10 μM for both BP and vasorelaxant effects, which is within the range of normal physiological tissue levels. Our findings are also consistent with published reports where similar concentrations of nitrite decreased BP (3, 60) and/or elicited aortic vasorelaxation in aerobic conditions (6, 37, 57). In a recent study now ongoing in our laboratory, we have performed in vivo thermodilution measurements of the dose-dependent effect of nitrite on cardiac output (Cardiomax-III; Columbus Instruments, Columbus, OH) and observed that the nitrite-induced decreases in systolic, diastolic, and mean arterial pressure (MAP) occur without alterations in cardiac output. Since MAP depends on both cardiac output and systemic vascular resistance (SVR), the decreases in blood pressure seen in our experiments are due to vasodilation with decrease in SVR.
Typical nitrite levels in plasma are in the range of 0.3 to 1.0 μM, and the concentration in aortic tissue is 10–20 μM (5, 49). Since the threshold concentration of nitrite to elicit vasorelaxant effects was also 10 μM, this suggests that basal nitrite levels have only slight effects on vascular tone but that increases associated with pharmacological administration or disease can elicit prominent vasodilatation.
Nitrite-induced vasorelaxation: role of NO and sGC.
With its well-recognized NO-scavenging effect (25, 51), the inhibition of relaxation by oxyHb serves as a classical measure of the role of NO in vasorelaxation. In the present study, we observed that nitrite caused dose-dependent vasodilation, and this was inhibited by oxyHb demonstrating a critical role of NO in this process. Furthermore, this relaxation was totally blocked by the sGC blocker ODQ indicating a key role of NO-mediated activation of sGC. Although Hb has also been proposed to play key roles as a nitrite reductase in hypoxia and as a donor of NO at the site of hypoxia through S-nitrosohemoglobin (24, 27, 51, 55), from our studies under aerobic conditions, it is clear that Hb does not facilitate nitrite-mediated vascular relaxation but blocks this process.
Nitrite-mediated NO formation was directly measured in aerobic aorta by both electrochemical (Fig. 6) and EPR spin-trapping techniques (Figs. 7 and 8). Importantly, both nitrite-induced vasodilation (Fig. 3) and nitrite-induced NO generation (Fig. 6A) were acutely inhibited by oxyHb. Thus a nitrite-NO-vasodilation pathway exists in aerobic vessels, and cell-free Hb is a potent scavenger, not a source of this nitrite-induced NO. Dalsgaard et al. (6) reported that oxyHb inhibits nitrite-induced vasodilation, whereas deoxyHb preserves it. Recently, Isbell et al. (22) also demonstrated that nitrite-dependent vasodilation is inhibited at high oxygen fractional saturation, whereas vasodilation is promoted when Hb deoxygenates. These results are consistent with deoxyHb both scavenging and generating NO but only scavenging by oxyHb.
Since NO is paramagnetic and reacts to form high-affinity complexes with a variety of metal chelates and metalloproteins (20), the distinctive EPR spectra of these NO complexes can provide real-time measurements of NO generation. Our EPR studies of aortic vessels demonstrated prominent nitrite-derived NO generation with the typical triplet EPR spectra of natural abundance 14NO (Fig. 7B). Isotope tracer studies with 15N nitrite demonstrated that the NO was nitrite derived since the characteristic doublet 15NO signal was observed. Nitrite-induced NO was absent when the aortic tissue was preheated (Fig. 7D), suggesting that it is derived from an enzymatic process. This is consistent with a previous report where both NOS-derived and nitrite-derived NO generation showed a similar thermal sensitivity (1).
Mechanisms of nitrite-induced NO formation.
Recently, several potential mechanisms have been proposed for nitrite-mediated vasodilation during hypoxia (6, 8, 27, 28). Enzymatic pathways including xanthine oxidase, mitochondrial electron transport complexes, cytochrome P450 reductase, and endothelial NOS have been shown to reduce nitrite to NO (17, 27, 31–33, 35, 40, 48, 50, 58, 64, 65). In general, these pathways are greatly increased under anaerobic or hypoxic conditions, and questions have remained regarding their role in the nitrite-mediated NO generation in aerobic vessels. However, in the present study, we observed that under aerobic conditions, selective inhibitors of each of the above pathways such as oxypurinol, rotenone, myxothiazol, 17-ODYA, and l-NAME did not block nitrite-induced aortic vasorelaxation. Dejam et al. (8) reported that allopurinol did not inhibit but enhanced nitrite-induced vasodilation in humans. Dalsgaard et al. (6) also demonstrated no effects of allopurinol and myxothiazol on nitrite-induced aortic vasorelaxation during hypoxia. Recently, we have reported that xanthine oxidase and aldehyde oxidase do play a critical role in nitrite-induced NO generation in the heart and liver under anaerobic conditions (34). However, we showed that NO generation from nitrite in these tissues was dependent on reducing substrate concentrations and inhibited by increasing oxygen tension. From these prior studies and our present results, it is clear that the mechanisms of nitrite reduction in anaerobic tissues are quite different from that in normoxic vessels. Thus our present findings indicate that a novel pathway is involved in nitrite-induced aortic vasorelaxation and NO production in aerobic vessels.
CO binds with high affinity to the ferrous iron of heme proteins and blocks the heme and any heme-mediated electron transfer (26). In the presence of CO, there was no detectable EPR spectrum for nitrite-induced NO from the aortic tissue (Fig. 8A). These results thus indicate that heme proteins in aortic tissue are involved in nitrite-induced NO formation.
The heme-containing sGC is a major intracellular effector enzyme of NO (9) and mediates many biological actions such as vascular smooth muscle relaxation by increasing cGMP. Binding of NO or NO donors to the ferrous heme iron of sGC induces a change in heme geometry and activates the enzyme catalyzing the formation of cGMP from GTP (9). ODQ has been shown to be a potent inhibitor of sGC without production of superoxide (16). The mechanism of action of ODQ is believed to be specifically related to oxidation of the heme moiety (53), and this greatly decreases the sensitivity of the prosthetic heme group of sGC for NO and thus prevents cGMP formation.
In the present study, ODQ produced marked contraction in isolated aortic rings precontracted with phenylephrine (Fig. 4), indicating the involvement of constitutively active sGC in arterial smooth muscle. It is important to note that nitrite-induced vasorelaxation was absent when ODQ was applied either before (Fig. 4A) or after (Fig. 4B) nitrite, suggesting the possibility of a direct interaction between sGC and nitrite in addition to the classical NO-cGMP pathway. Since ODQ totally abolished nitrite-mediated vasodilation, we questioned whether it would also block nitrite-mediated NO generation. Interestingly, ODQ completely blocked this NO formation and abolished the EPR spectra of nitrite-derived NO (Fig. 7E). We further noted that nitrite-induced vasorelaxation and NO formation was endothelium independent (Fig. 6B). Therefore, blockade of nitrite-induced vasorelaxation and NO generation with ODQ treatment suggests that nitrite and/or its secondary NO may be directly interacting with aortic smooth muscle sGC.
Nitrite-induced-NO pathway: are other heme proteins involved?
Although ODQ has been widely utilized and considered as a specific sGC inhibitor, it has also been reported that ODQ can interfere with other heme proteins (12). ODQ has been spectroscopically shown to interact with and oxidize the ferrous heme of sGC (52); however, it may also target the heme group of other proteins such as Hb (39).
It has been recently hypothesized that the heme globin family (hemoglobin, myoglobin, cytoglobin, and neuroglobin) can function as nitrite reductases (27, 35, 43, 48). Mb is an important intracellular oxygen-binding protein and a potent NO scavenger (62). Although Mb has been reported to be present in human aorta, it has not been detected (46) and is believed not to be present in rat aorta (23). Interestingly, in rat cardiac homogenates, deoxyMb has been shown to be a nitrite reductase that generates NO and regulates mitochondrial respiration (54). Moreover, a nitrite reductase function of deoxyMb has been reported in hypoxic wild-type but not in Mb knockout heart (47). Recently, a small novel heme protein, Cygb, with similar characteristics to Mb has been identified in a broad range of tissues (18). We observed that the optical spectra of ferrous Mb (Fig. 9A) and Cygb (Fig. 9B) were altered by ODQ with apparent oxidation of the heme to the ferric state as also occurs for sGC (42), demonstrating that ODQ not only targets the heme of sGC but also that of Mb, Cygb, and perhaps other similar heme proteins (43).
In conclusion, under aerobic conditions, nitrite evokes a dose-dependent vasorelaxation of precontracted rat aorta and also causes a dose-dependent decrease in blood pressure in anesthetized rats. Both functional and analytical studies demonstrate that the vasorelaxant effect of nitrite is mediated by NO formation mediated through sGC or other heme proteins inhibitable by ODQ. Taken together, our findings provide strong evidence that a ferrous heme protein in aortic smooth muscle is involved in nitrite-induced NO production under normal aerobic conditions. Future research with the use of improved molecular probes and antibodies will be required to address the precise role of sGC, Cygb, or the other heme proteins in the process of nitrite-mediated NO production and vasodilation in normoxic vessels.
GRANTS
This work was supported by NIH grants HL63744, HL65608, and HL38324.
Acknowledgments
The authors thank Drs. Haitao Li, Yeong-Renn Chen, and Larry Druhan for helpful advice and Dr. Fuchun Yang for technical help in cardiac output measurements.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agvald P, Adding LC, Gustafsson LE. Influence of oxygen, temperature and carbon dioxide on nitric oxide formation from nitrite as measured in expired gas from in situ perfused rabbit lungs. Vascul Pharmacol 43: 441–448, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Andrews KL, Triggle CR, Ellis A. NO and the vasculature: where does it come from and what does it do? Heart Fail Rev 7: 423–445, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bryan NS, Fernandez BO, Bauer SM, Garcia-Saura MF, Milsom AB, Rassaf T, Maloney RE, Bharti A, Rodriguez J, Feelisch M. Nitrite is a signaling molecule and regulator of gene expression in mammalian tissues. Nat Chem Biol 1: 290–297, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem 282: 879–887, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon 3rd RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Dalsgaard T, Simonsen U, Fago A. Nitrite-dependent vasodilation is facilitated by hypoxia and is independent of known NO-generating nitrite reductase activities. Am J Physiol Heart Circ Physiol 292: H3072–H3078, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Darius H Role of nitrates for the therapy of coronary artery disease patients in the years beyond 2000. J Cardiovasc Pharmacol 34, Suppl 2: S15–S20, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon 3rd RO, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation 116: 1821–1831, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Denninger JW, Marletta MA. Guanylate cyclase and the·NO/cGMP signaling pathway. Biochim Biophys Acta 1411: 334–350, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Dewilde S, Kiger L, Burmester T, Hankeln T, Baudin-Creuza V, Aerts T, Marden MC, Caubergs R, Moens L. Biochemical characterization and ligand binding properties of neuroglobin, a novel member of the globin family. J Biol Chem 276: 38949–38955, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Dudzinski DM, Igarashi J, Greif D, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol 46: 235–276, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Feelisch M, Kotsonis P, Siebe J, Clement B, Schmidt HH. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation. Mol Pharmacol 56: 243–253, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Fung HL Biochemical mechanism of nitroglycerin action and tolerance: is this old mystery solved? Annu Rev Pharmacol Toxicol 44: 67–85, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980. [DOI] [PubMed] [Google Scholar]
- 15.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J 3: 2007–2018, 1989. [PubMed] [Google Scholar]
- 16.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol 48: 184–188, 1995. [PubMed] [Google Scholar]
- 17.Godber BL, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J Biol Chem 275: 7757–7763, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Hankeln T, Ebner B, Fuchs C, Gerlach F, Haberkamp M, Laufs TL, Roesner A, Schmidt M, Weich B, Wystub S, Saaler-Reinhardt S, Reuss S, Bolognesi M, De Sanctis D, Marden MC, Kiger L, Moens L, Dewilde S, Nevo E, Avivi A, Weber RE, Fago A, Burmester T. Neuroglobin and cytoglobin in search of their role in the vertebrate globin family. J Inorg Biochem 99: 110–119, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Harder DR, Campbell WB, Roman RJ. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res 32: 79–92, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Ignarro LJ, Ballot B, Wood KS. Regulation of soluble guanylate cyclase activity by porphyrins and metalloporphyrins. J Biol Chem 259: 6201–6207, 1984. [PubMed] [Google Scholar]
- 21.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA 84: 9265–9269, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isbell TS, Gladwin MT, Patel RP. Hemoglobin oxygen fractional saturation regulates nitrite-dependent vasodilation of aortic ring bioassays. Am J Physiol Heart Circ Physiol 293: H2565–H2572, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Ishibashi T, Hamaguchi M, Kato K, Kawada T, Ohta H, Sasage H, Imai S. Relationship between myoglobin contents and increases in cyclic GMP produced by glyceryl trinitrate and nitric oxide in rabbit aorta, right atrium and papillary muscle. Naunyn Schmiedebergs Arch Pharmacol 347: 553–561, 1993. [DOI] [PubMed] [Google Scholar]
- 24.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996. [DOI] [PubMed] [Google Scholar]
- 25.Joshi MS, Ferguson TB Jr, Han TH, Hyduke DR, Liao JC, Rassaf T, Bryan N, Feelisch M, Lancaster JR Jr. Nitric oxide is consumed, rather than conserved, by reaction with oxyhemoglobin under physiological conditions. Proc Natl Acad Sci USA 99: 10341–10346, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaczorowski DJ, Zuckerbraun BS. Carbon monoxide: medicinal chemistry and biological effects. Curr Med Chem 14: 2720–2725, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol 26: 697–705, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, Scheeren T, Gödecke A, Schrader J, Schulz R, Heusch G, Schaub GA, Bryan NS, Feelisch M, Kelm M. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med 35: 790–796, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Kozlov AV, Staniek K, Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett 454: 127–130, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Lauer T, Preik M, Rassaf T, Strauer BE, Deussen A, Feelisch M, Kelm M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc Natl Acad Sci USA 98: 12814–12819, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem 276: 24482–24489, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the effects of oxygen on xanthine oxidase-mediated nitric oxide formation. J Biol Chem 279: 16939–16946, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Liu X, Cui H, Chen YR, Cardounel AJ, Zweier JL. Characterization of the mechanism of cytochrome P450 reductase-cytochrome P450-mediated nitric oxide and nitrosothiol generation from organic nitrates. J Biol Chem 281: 12546–12554, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Cui H, Kundu TK, Alzawahra W, Zweier JL. Nitric oxide production from nitrite occurs primarily in tissues not in the blood: critical role of xanthine oxidase and aldehyde oxidase. J Biol Chem 283: 17855–17863, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 7: 156–167, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation 117: 670–677, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Modin A, Björne H, Herulf M, Alving K, Weitzberg E, Lundberg JO. Nitrite-derived nitric oxide: a possible mediator of 'acidic-metabolic' vasodilation. Acta Physiol Scand 171: 9–16, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 43: 109–142, 1991. [PubMed] [Google Scholar]
- 39.Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, Radomski MW, Moncada S. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci USA 93: 1480–1485, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagababu E, Ramasamy S, Abernethy DR, Rifkind JM. Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J Biol Chem 278: 46349–46356, 2003. [DOI] [PubMed] [Google Scholar]
- 41.National Institute of Health. Guidelines for Survival Bleeding of Mice and Rats (Online). http://oacu.od.nih.gov/ARAC/documents/Bleeding_120307_final.pdf [May 1, 2008].
- 42.Olesen SP, Drejer J, Axelsson O, Moldt P, Bang L, Nielsen-Kudsk JE, Busse R, Mülsch A. Characterization of NS 2028 as a specific inhibitor of soluble guanylyl cyclase. Br J Pharmacol 123: 299–309, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pacher P, Nivorozhkin A, Szabó C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58: 87–114, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987. [DOI] [PubMed] [Google Scholar]
- 45.Parker JD Nitrate tolerance, oxidative stress, and mitochondrial function: another worrisome chapter on the effects of organic nitrates. J Clin Invest 113: 352–354, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiu Y, Sutton L, Riggs AF. Identification of myoglobin in human smooth muscle. J Biol Chem 273: 23426–23432, 1998. [DOI] [PubMed] [Google Scholar]
- 47.Rassaf T, Flögel U, Drexhage C, Hendgen-Cotta U, Kelm M, Schrader J. Nitrite reductase function of deoxymyoglobin: oxygen sensor and regulator of cardiac energetics and function. Circ Res 100: 1749–1754, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Robinson JM, Lancaster JR Jr. Hemoglobin-mediated, hypoxia-induced vasodilation via nitric oxide: mechanism(s) and physiologic versus pathophysiologic relevance. Am J Respir Cell Mol Biol 32: 257–261, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez J, Maloney RE, Rassaf T, Bryan NS, Feelisch M. Chemical nature of nitric oxide storage forms in rat vascular tissue. Proc Natl Acad Sci USA 100: 336–341, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samouilov A, Kuppusamy P, Zweier JL. Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch Biochem Biophys 357: 1–7, 1998. [DOI] [PubMed] [Google Scholar]
- 51.Schechter AN, Gladwin MT. Hemoglobin and the paracrine and endocrine functions of nitric oxide. N Engl J Med 348: 1483–1485, 2003. [DOI] [PubMed] [Google Scholar]
- 52.Schenkman K, Marble DR, Burns DH, Feigl EO. Myoglobin oxygen dissociation by multiwavelength spectroscopy. J Appl Physiol 82: 86–92, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Schrammel A, Behrends S, Schmidt K, Koesling D, Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol Pharmacol 50: 1–5, 1996. [PubMed] [Google Scholar]
- 54.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circ Res 100: 654–661, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Single DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99–145, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Tiravanti E, Samouilov A, Zweier JL. Nitrosyl-heme complexes are formed in the ischemic heart: evidence of nitrite-derived nitric oxide formation, storage, and signaling in post-ischemic tissues. J Biol Chem 279: 11065–11073, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Van De Voorde J, Leusen I. Endothelium-dependent and independent relaxation of aortic rings from hypertensive rats. Am J Physiol Heart Circ Physiol 250: H711–H717, 1986. [DOI] [PubMed] [Google Scholar]
- 58.Vanin AF, Bevers LM, Slama-Schwok A, van Faassen EE. Nitric oxide synthase reduces nitrite to NO under anoxia. Cell Mol Life Sci 64: 96–103, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vanin AF, Liu X, Samouilov A, Stukan RA, Zweier JL. Redox properties of iron-dithiocarbamates and their nitrosyl derivatives: implications for their use as traps of nitric oxide in biological systems. Biochim Biophys Acta 1474: 365–377, 2000. [DOI] [PubMed] [Google Scholar]
- 60.Vleeming W, van de Kuil A, te Biesebeek JD, Meulenbelt J, Boink AB. Effect of nitrite on blood pressure in anaesthetized and free-moving rats. Food Chem Toxicol 35: 615–619, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Webb A, Bond R, McLean P, Uppal R, Benjamin N, Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc Natl Acad Sci USA 101: 13683–13688, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J Exp Biol 206: 2011–2020, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Yan Q, Liu Q, Zweier JL, Liu X. Potency of authentic nitric oxide in inducing aortic relaxation. Pharmacol Res 55: 329–334, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Zweier JL, Samouilov A, Kuppusamy P. Non-enzymatic nitric oxide synthesis in biological systems. Biochim Biophys Acta 1411: 250–262, 1999. [DOI] [PubMed] [Google Scholar]
- 65.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med 1: 804–809, 1995. [DOI] [PubMed] [Google Scholar]