Abstract
The objective of this study was to test the hypothesis that the mechanism mediating left ventricular (LV) dysfunction in the aging rat heart involves, in part, changes in cardiac cytoskeletal components. Our results show that there were no significant differences in heart rate, LV pressure, or LV diameter between conscious, instrumented young [5.9 ± 0.3 mo (n = 9)] and old rats [30.6 ± 0.1 mo (n = 10)]. However, the first derivative of LV pressure (LV dP/dt) was reduced (8,309 ± 790 vs. 11,106 ± 555 mmHg/s, P < 0.05) and isovolumic relaxation time (τ) was increased (8.7 ± 0.7 vs. 6.3 ± 0.6 ms, P < 0.05) in old vs. young rats, respectively. The differences in baseline LV function in young and old rats, which were modest, were accentuated after β-adrenergic receptor stimulation with dobutamine (20 μg/kg), which increased LV dP/dt by 170 ± 9% in young rats, significantly more (P < 0.05) than observed in old rats (115 ± 5%). Volume loading in anesthetized rats demonstrated significantly impaired LV compliance in old rats, as measured by the LV end-diastolic pressure and dimension relationship. In old rat hearts, there was a significant (P < 0.05) increase in the percentage of LV collagen (2.4 ± 0.2 vs. 1.3 ± 0.2%), α-tubulin (92%), and β-tubulin (2.3-fold), whereas intact desmin decreased by 51%. Thus the cardiomyopathy of aging in old, conscious rats may be due not only to increases in collagen but also to alterations in cytoskeletal proteins.
Keywords: aging cardiomyopathy, left ventricular systolic function, left ventricular diastolic function, tubulin
the effects of age on cardiac function have been explored extensively in anesthetized rats (16, 24, 33, 37, 44). Almost all studies demonstrate the development of cardiomyopathy with age, characterized by depressed left ventricular (LV) systolic and diastolic function, LV hypertrophy (LVH), and increased collagen in the heart (21, 23). The first goal of this study was to determine the extent to which LV function was depressed in conscious, chronically instrumented rats. A second goal was to examine the extent to which LV diastolic compliance was altered in the aging rat heart. LV dysfunction with age has largely been attributed to altered excitation-contraction mechanisms, a shift in myosin heavy chain (MHC) protein from a faster to a slower isoform, and an increase in collagen in the heart (22). Our recent finding that stiffness increases with age at the level of the single myocyte (27) led us to hypothesize that material property changes in aging hearts may be due to more than just changes in collagen. Therefore, the third goal of this work was to measure cellular protein changes in the LV of young and old Fischer 344 × Brown Norway F1 hybrid (F344×BN) rats, focusing on intracellular proteins, e.g., tubulin and desmin. Although cytoskeletal and membrane proteins have been examined in other states of LV dysfunction (7, 8, 13, 20), their effects in aging remain to be elucidated.
MATERIALS AND METHODS
Animals.
F344×BN rats were obtained from the National Institute on Aging (NIA). Physiology studies were conducted on young (5.9 ± 0.3 mo old; n = 9) and old rats [30.6 ± 0.1 mo old (aged, not senescent) n = 10] (26, 28). A parallel group of young (5.5 ± 0.2 mo old; n = 7) and old Fischer 344 × Brown Norway (F344×BN) rats (30.3 ± 0.3 mo old; n = 7) were used only for tissue harvest, and samples were prepared for immunoblotting (Western blot analyses), pathology (collagen measures), and immunohistochemistry. All procedures and protocols were approved by the Institutional Animal Care and Use Committee of the University of Medicine and Dentistry of New Jersey-New Jersey Medical School and followed the National Institutes of Health guidelines for the care and use of laboratory animals.
Implantation of instrumentation.
The animals were anesthetized with ketamine and acepromazine (65 mg/kg ip and 2 mg/kg ip, respectively). A left intercostal thoracotomy was performed. A miniature solid-state pressure gauge (P1.5; Konigsberg Instruments; Pasadena, CA) was inserted into the LV for the measurements of LV pressure and the first derivative of LV pressure (LV dP/dt). A Micro-Renathane tubing (Braintree Scientific) venous catheter (7-in. length) was placed for drug injection, whereas a Rena Pulse tubing (Braintree Scientific) catheter (8-in. length) was placed in the ascending aorta for aortic pressure measurements. LV pressure or aortic pressure was measured using a 1.4-F micromanometer (Millar Instruments) under anesthesia as described above. The right carotid artery was exposed via a midline ventral incision and dissected free from neighboring structures. A pair of ultrasonic crystals were placed on the opposing LV surfaces to measure LV diameter and fractional shortening. Animals were allowed to recover for 1–2 wk before the initiation of experiments.
Hemodynamics.
Rats were placed in a restraining box designed for conscious studies. The fluid-filled catheter was connected to a pressure transducer (DTX Plus; Becton Dickinson) and calibrated with a manometer. The implanted instrumentation was connected to an amplifier with an electronic filter setup. Data were displayed and recorded on a computer through a data acquisition system (Notocord) with a 1,000-Hz sampling rate. The aortic pressure measured with a fluid-filled catheter was used to cross-calibrate the offset for the Konigsberg, where the LV systolic pressure is matched with the systolic arterial pressure as previously described (9). Baseline physiological measurements were then recorded and parameters calculated with Notocord software, including dP/dt, heart rate, LV end-diastolic pressure (EDP), end-systolic pressure (ESP), the Weiss isovolumic relaxation time constant (τ), end-diastolic diameter (EDD), and end-systolic diameter (ESD). LV fractional shortening was calculated as [(EDD − ESD)/EDD] × 100. Responses to dobutamine (5, 10, 15, and 20 μg/kg iv) were also examined in conscious young and old rats as previously described (9).
Additional young and old F344xBN rats were used for assessment of LV compliance, i.e., the LV pressure-dimension relationship. The basic surgical procedure and anesthesia were as described above. While LV pressures and dimensions were recorded continuously, acute volume loading was performed using intravenous infusion of saline to increase LVEDP by ∼15 mmHg.
Western blot analysis.
Extracts for cytoskeletal and membrane proteins were prepared from frozen LV tissue from young and old F344×BN rats in a 2% SDS buffer containing 10 mM Tris, pH 7.5, 100 mM NaCl, 1 mM EDTA, and Sigma protease inhibitor (3 μl/ml), as previously described (30). Proteins were analyzed by Western blotting as previously described (46). In brief, equal amounts of each sample were loaded onto gels for SDS-PAGE using the Bio-Rad Minigel system. A 10% gel was used for β1-integrin (20 μg/well), α-tubulin (10 μg/well), β-tubulin (20 μg/well), and desmin (10 μg/well); an 8% gel was used for α-actinin (10 μg/well) and vinculin (10 μg/well); and a 6% gel was used for talin (10 μg/well). Gels were transferred to nitrocellulose membranes with a wet transfer cell. The blots were probed with primary antibodies and incubated overnight at 4°C. The following antibodies were used: β1-integrin, 610468 (Becton Dickinson); α-tubulin, T6199 (Sigma); β-tubulin, T7816 (Sigma); desmin, D8281(Sigma); α-actinin, A5044 (Sigma); vinculin, V9133 (Sigma); and talin, T3287 (Sigma). The primary antibody was applied at a concentration of 1:1,000 for all proteins. Secondary antibodies coupled to horseradish peroxidase were used. The immunopositive bands were visualized by using Western Lightning chemiluminescence reagent (PerkinElmer). All Western blot exposures were in the linear range of detection, and the intensities of the resulting bands were quantified using Quantity One software on a GS-800 densitometer (Bio-Rad). Data were normalized by reprobing the plots for β-actin at 1:50,000 concentration and rocking at room temperature for 1 h.
Measurement of advanced glycation end products.
Advanced glycation end products (AGEs) were determined as previously described (35). Briefly, defatted LV tissues (n = 5/group) were minced and washed with cold saline, resuspended in 0.5 M acetic acid, and digested for 24 h at 4°C in the presence of 1 mg/ml pepsin (Sigma). Samples were centrifuged at 15,000 g for 45 min. The resulting supernatant was considered the pepsin-soluble fraction, whereas the pellet was washed with cold saline, resuspended in 0.2 M Tris·HCl, pH 7.4, and incubated for 24 h at 37°C with 300 U/ml collagenase type VII (Sigma) containing 100 mM CaCl2. After centrifugation at 15,000 g for 45 min, the supernatant was regarded as the collagenase-soluble fraction. Both the pepsin-soluble fraction and the collagenase-soluble fraction samples were subjected to fluorescence emission spectra at 370-nm excitation and 440-nm emission wavelengths (Hitachi F-3010). The quantity of glycation-induced fluorescence was expressed as the relative fluorescence per milligram of dry weight of the tissue.
Histological analysis.
Histological analyses of the heart sections for collagen were conducted as described previously (15). LV specimens were fixed with 10% neutral buffered formalin, embedded in paraffin, and sectioned at 6-μm thickness. Interstitial fibrosis was evaluated by picric acid Sirius red staining. The positively stained (red) fibrotic area was measured and expressed as a percentage of total area.
Statistics.
All data are means ± SE. Statistical significance was determined by calculating a probability value (P) with a Student's t-test, where values of P < 0.05 were considered significant. The regression for the LVEDP-diameter relationship was calculated using linear regression.
RESULTS
Hemodynamic measurements in conscious, chronically instrumented rats.
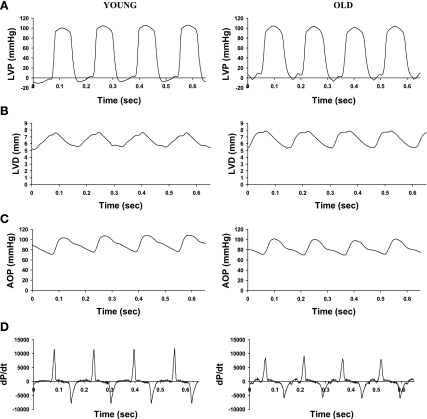
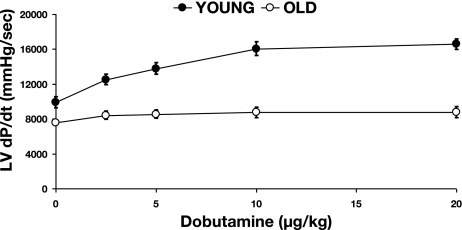
An example of phasic waveforms of LV function in a young and old conscious rat is shown in Fig. 1, and baseline hemodynamic values are shown in Table 1. There were no significant differences in LV systolic pressure, LVEDP, mean arterial pressure, and heart rate between young and old conscious rats. However, there was a significant (P < 0.05) decrease in LV +dP/dt (11,106 ± 555 vs. 8,309 ± 790 mmHg/s) and LV −dP/dt (−7,133 ± 328 vs. −5,647 ± 492 mmHg/s). The LV isovolumic relaxation time constant τ was significantly (P < 0.05) increased in old (8.9 ± 0.7 ms) compared with young rats (6.3 ± 0.6 ms). There was no difference in LVEDD between young and old rats. However, LV fractional shortening was significantly (P < 0.05) reduced in old (18.8 ± 2.1%) compared with young rats (26.4 ± 1.6%). The differences in baseline LV function were accentuated after β-adrenergic receptor (β-AR) stimulation with dobutamine, which increased LV dP/dt by 170 ± 9% in young rats, significantly (P < 0.05) more than that observed in old rats (115 ± 5%) with a 20 μg/kg dose (Fig. 2).
Fig. 1.
Sample waveforms of left ventricular (LV) pressure (LVP; A), LV diameter (LVD; B), aortic pressure (AOP; C), and the first derivative of LVP (dP/dt; D) acquired from young and old conscious rats.
Table 1.
Baseline hemodynamic values in young and old conscious F344×BN rats
|
Young |
Old
|
|||
|---|---|---|---|---|
| Value | n | Value | n | |
| LVSP, mmHg | 106±2 | 9 | 103±5 | 10 |
| LVEDP, mmHg | 5.2±1.3 | 9 | 7.0±1.3 | 10 |
| +dP/dt, mmHg/s | 11,106±555 | 9 | 8,309±790* | 10 |
| −dP/dt, mmHg/s | −7,133±328 | 9 | −5,647±491* | 10 |
| Weiss τ, ms | 6.3±0.6 | 9 | 8.7±0.7* | 10 |
| LV EDD, mm | 7.57±0.50 | 7 | 7.59±0.20 | 9 |
| Fractional shortening, % | 26.4±1.6 | 7 | 18.8±2.1* | 9 |
| Heart rate, beats/min | 383±9 | 9 | 376±15 | 10 |
Values are means ± SE measured in young and old Fischer 344 × Brown Norway (F344×BN) rats. LVSP, left ventricular systolic pressure; LVEDP, LV end-diastolic pressure; ±dP/dt, first derivatives of LV pressure; Weiss τ, Weiss isovolumic relaxation time constant; LV EDD, LV end-diastolic diameter.
P < 0.05 vs. young rats.
Fig. 2.
Responses of LV dP/dt to increasing doses of dobutamine (5, 10, 15, and 20 μg/kg) in conscious rats are shown, which indicate that inotropic responses to sympathetic stimulation are significantly depressed in old (n = 7) compared with young rats (n = 7).
LVEDP-diameter relationship with volume loading in anesthetized rats.
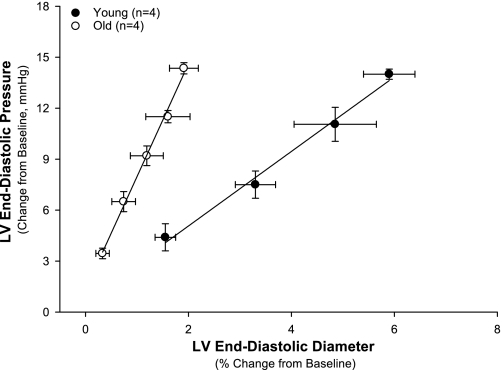
LV compliance was evaluated in anesthetized rats after implantation of a Millar micromanometer to measure LV pressure and of ultrasonic dimension crystals to measure LV diameter (Fig. 3). The relationship between LVEDP and diameter with volume loading was shifted significantly (P < 0.05) to the left in the old rats, demonstrating that the LV was stiffer. Regression analysis of linear data demonstrated a difference (P < 0.05) in the slopes.
Fig. 3.
LV compliance was evaluated in anesthetized old and young rats after implantation of a Millar micromanometer to measure LVP and of ultrasonic crystals on opposing LV surfaces to measure LVD. The relationship between LV end-diastolic pressure and diameter with volume loading was shifted significantly (P < 0.05) to the left in the old rats, demonstrating that the LV was stiffer. Slopes of regression lines are 7.57 ± 0.25 for old rats and 2.34 ± 0.05 for young rats.
Cellular matrix proteins.
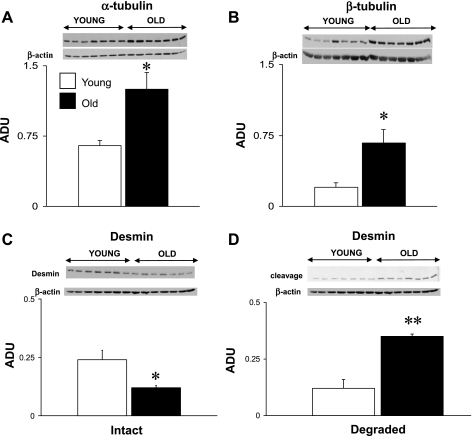
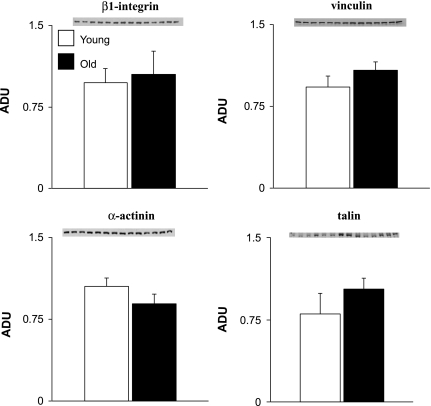
There were significant (P < 0.05) increases in α-tubulin (92%) and β-tubulin (2.3-fold) and a decrease in intact desmin (51%) in old rats (Fig. 4). The desmin degradation products increased twofold in the myocardium of old rats, which might explain the reduction of the intact desmin protein (Fig. 4C). There were no significant changes in β1-integrin, vinculin, α-actinin, and talin (Fig. 5).
Fig. 4.
Comparison of expression levels of α-tubulin (A), β-tubulin (B), intact desmin (C), and degraded (cleaved) desmin (D) in young and old LV rat samples. The Western blots were normalized with β-actin. There were significant (*P < 0.05) increases in α- and β-tubulin and a decrease in intact desmin in the old rat hearts. The degraded desmin products were significantly increased in the old compared with young rats (**P < 0.01). ADU, arbitrary densitometric units.
Fig. 5.
Comparison of expression levels of β1-integrin, vinculin, α-actinin, and talin in young and old LV rat samples. There were no significant differences in the expression of these proteins.
Extracellular matrix proteins.
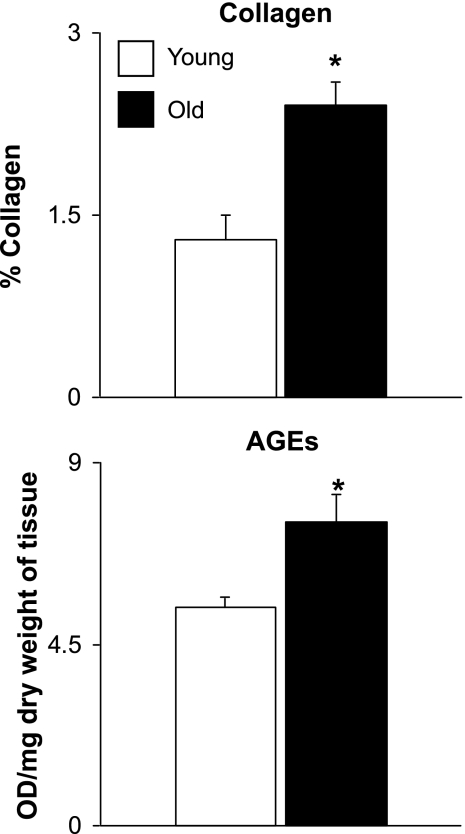
There was a significant (P < 0.05) 34% increase in the ratio of LV to tibial length in old rats (213 ± 6.3 mg/cm) compared with young rats (160 ± 4.7 mg/cm). There was an 81.9% increase (P < 0.05) in collagen deposition in the LV (Fig. 6) in old rats as shown by immunohistostaining. AGEs in the old rat LV were significantly (P < 0.05) increased compared with those in young rat LV (Fig. 6).
Fig. 6.
Comparison of collagen in young and old LV rat samples. The collagen concentration (top) and advanced glycation end products (AGEs; bottom) were significantly increased in old rat LV (*P < 0.05). OD, optical density.
DISCUSSION
We examined the effects of aging on LV hemodynamics in conscious, chronically instrumented rats and found significant differences in systolic and diastolic LV function in the old rats, directionally consistent with most prior studies in aging rats (5, 37). There were significantly greater differences in response to β-AR stimulation with dobutamine in old compared with young rats (Fig. 2). In part, this could be attributed to the additional LV dysfunction induced by β-AR desensitization, which is known to occur with age (16, 22, 33, 37, 39, 44). Chronic sympathetic stimulation may support LV function in old rats, but reserve LV function is lost due to β-AR desensitization (22, 24, 39).
Although baseline LV stiffness, as assessed by LVEDP and diameter, did not change with aging, LV stiffness increased with volume loading (Fig. 3). The relationship between LVEDP and dimension we observed in old compared with young rats resembles closely the data published by Asif et al. (3) in anesthetized dogs. A prior echocardiography study by Walker et al. (43) found diastolic dysfunction in aging rats (3–6 mo old), and Rozenberg et al. (36) and Cappelli et al. (6) found diastolic dysfunction in aging rat papillary muscle. Whether LV stiffness increases with aging in response to stress (e.g., increased LV end-diastolic volume) has been controversial. For example, Kass (17) showed in elderly patients that LV stiffness increases with volume load, as assessed using the LV diastolic pressure-volume component of the LV pressure-volume loop; the original data were included in an article by Frenneaux and Williams (10). Similar data on the diastolic portion of the pressure-volume load were found by Pacher et al. (33) in aging rat hearts with increased preload. Brenner et al. (5) did not find increased LV stiffness with aging in normal rat hearts with a simple increase in LV volume but, in contrast, found increased LV diastolic stiffness with ischemia-induced increases in LV volume.
One explanation for the increased LV stiffness is an increase in LV collagen. We found that LV collagen was increased in the old rats, consistent with what has been described by others previously (1, 12). In addition, the conjugation of AGEs was significantly higher in the LV of old rats than in young rats (Fig. 6), which is also consistent with previous studies (19, 25). Further evidence supporting the AGE hypothesis is also provided by the observation that the AGE cross-link breaker can reverse age-related increases in myocardial stiffness (2, 3).
The major finding of the current investigation demonstrates that cellular proteins change with aging, which furthers the novel hypothesis that a component of cardiac dysfunction, which occurs with aging, results from changes occurring at the myocyte level (27). Western blot analysis showed a significant 92% increase in α-tubulin (Fig. 4A) and a 2.3-fold increase in β-tubulin (Fig. 4B). The generally accepted role of the microtubular network is to serve as an intracellular transport system for proteins and lipids (14); however, its role in cell mechanics and the development of aging cardiomyopathy has not been identified previously.
Although the microtubules (or rather, tubulin) have been implicated in β-AR signaling, the role of tubulin in the regulation of β-AR pathway in adult cardiac muscle is still a subject of debate. Rasenick's group (45) has shown that free tubulin can transfer GTP to a subunit of G proteins. Transfer is initially to Gαi and then to Gαs; thus, depending on the experimental conditions, tubulin either inhibits or activates adenylyl cyclase. Malan et al. (29) also recently proposed an effect of proliferated microtubulin on the Gi signaling pathway through the muscarinic receptor. It also was reported that microtubulin, disrupted by colchicine, attenuates the response to β-AR stimulation (11, 29), and the proliferation of tubulin has been implicated in the depression of contractility in cardiac hypertrophy (42).
We also observed a decrease in the intermediate filament desmin (Fig. 4C). Desmin maintains myocyte cytoskeletal architecture through connections to the Z disk, contractile apparatus, subsarcolemmal cytoskeleton and the nuclei, and cellular organelles (34). Desmin's function has been demonstrated in several studies using desmin knockout mice, which develop hypertrophy and eventually suffer from cardiomyopathy accompanied by myocyte death and extracellular matrix changes (32, 34, 40). The effect of desmin on cardiac global function has been studied in Langendorff preparations, where the LV weight was found to increase along with higher diastolic pressure and lower developed pressures (34). At the cellular level it has been suggested that desmin may be involved in supporting sarcomere alignment and force transmission (34), where the contractile apparatus generates less active force without desmin (4). Studies also have indicated that desmin influences the position, movement, and activity of mitochondria in cardiac muscle (18, 31), which in turn could explain their abnormal function and appearance in transgenic mice (32, 34, 40).
Interestingly, many of the changes in proteins observed in the current study also have been observed in LVH and heart failure (13). For example, there are several cardiomyopathies attributed to alterations in desmin: animals with desmin knocked out develop severe cardiomyopathy (32, 34, 40). In addition, alterations in tubulin have been proposed as the cause of LV dysfunction in LVH (38, 41, 42). Therefore, it appears that the cardiomyopathy of the aging rat heart shares some of the same features that occur in the cardiomyopathy of cardiac hypertrophy and failure, most importantly, the alterations in cytoskeletal proteins.
GRANTS
This work was supported in part by National Institutes of Health Grants AG014121, HL033107, HL059139, HL069752, AG027211, AG023137, HL069020, and AG023567.
Acknowledgments
We appreciate the helpful editorial assistance provided by Lauren Danridge.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res 67: 871–885, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Aronson D Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens 21: 3–12, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci USA 97: 2809–2813, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balogh J, Merisckay M, Li Z, Paulin D, Arner A. Hearts from mice lacking desmin have a myopathy with impaired active force generation and unaltered wall compliance. Cardiovasc Res 53: 439–450, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation 104: 221–226, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Cappelli V, Forni R, Poggesi C, Reggiani C, Ricciardi L. Age-dependent variations of diastolic stiffness and collagen content in rat ventricular myocardium. Arch Int Physiol Biochim 92: 93–106, 1984. [DOI] [PubMed] [Google Scholar]
- 7.Collins JF, Pawloski-Dahm C, Davis MG, Ball N, Dorn GW, 2nd, Walsh RA. The role of the cytoskeleton in left ventricular pressure overload hypertrophy and failure. J Mol Cell Cardiol 28: 1435–1443, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Cooper G 4th. Cardiocyte cytoskeleton in hypertrophied myocardium. Heart Fail Rev 5: 187–201, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Cui Y, Shen YT, Kalthof B, Iwase M, Sato N, Uechi M, Vatner SF, Vatner DE. Identification and functional role of beta-adrenergic receptor subtypes in primate and rodent: in vivo versus isolated myocytes. J Mol Cell Cardiol 28: 1307–1317, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Frenneaux M, Williams L. Ventricular-arterial and ventricular-ventricular interactions and their relevance to diastolic filling. Prog Cardiovasc Dis 49: 252–262, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Gomez AM, Kerfant BG, Vassort G. Microtubule disruption modulates Ca2+ signaling in rat cardiac myocytes. Circ Res 86: 30–36, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Hacker TA, McKiernan SH, Douglas PS, Wanagat J, Aiken JM. Age-related changes in cardiac structure and function in Fischer 344 × Brown Norway hybrid rats. Am J Physiol Heart Circ Physiol 290: H304–H311, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Hein S, Kostin S, Heling A, Maeno Y, Schaper J. The role of the cytoskeleton in heart failure. Cardiovasc Res 45: 273–278, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Hirokawa N Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279: 519–526, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, Wight DC, Wagner TE, Ishikawa Y, Homcy CJ, Vatner SF. Adverse effects of chronic endogenous sympathetic drive induced by cardiac Gsα overexpression. Circ Res 78: 517–524, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol Heart Circ Physiol 271: H1215–H1228, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Kass DA Age-related changes in ventricular-arterial coupling: pathophysiologic implications. Heart Fail Rev 7: 51–62, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Kay L, Li Z, Mericskay M, Olivares J, Tranqui L, Fontaine E, Tiivel T, Sikk P, Kaambre T, Samuel JL, Rappaport L, Usson Y, Leverve X, Paulin D, Saks VA. Study of regulation of mitochondrial respiration in vivo. An analysis of influence of ADP diffusion and possible role of cytoskeleton. Biochim Biophys Acta 1322: 41–59, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Kohn RR, Cerami A, Monnier VM. Collagen aging in vitro by nonenzymatic glycosylation and browning. Diabetes 33: 57–59, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Kostin S, Heling A, Hein S, Scholz D, Klövekorn WF, Schaper J. The protein composition of the normal and diseased cardiac myocyte. Heart Fail Rev 2: 245–260, 1998. [Google Scholar]
- 21.Lakatta EG Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part III: cellular and molecular clues to heart and arterial aging. Circulation 107: 490–497, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Lakatta EG Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 73: 413–467, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part II: the aging heart in health: links to heart disease. Circulation 107: 346–354, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp Biochem Physiol A Mol Integr Physiol 132: 699–721, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan-Isik AF, LaCour KH, Yang X, Wilbert CJ, Sreejayan N, Ren J. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell 4: 57–64, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Froehlich J, Galis ZS, Lakatta EG. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension 33: 116–123, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Lieber SC, Aubry N, Pain J, Diaz G, Kim SJ, Vatner SF. Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol Heart Circ Physiol 287: H645–H651, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway × Fischer 344, and Fischer 344 × brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci 51: B54–B59, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malan D, Gallo MP, Bedendi I, Biasin C, Levi RC, Alloatti G. Microtubules mobility affects the modulation of L-type ICa by muscarinic and beta-adrenergic agonists in guinea-pig cardiac myocytes. J Mol Cell Cardiol 35: 195–206, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Mavroidis M, Capetanaki Y. Extensive induction of important mediators of fibrosis and dystrophic calcification in desmin-deficient cardiomyopathy. Am J Pathol 160: 943–952, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milner DJ, Mavroidis M, Weisleder N, Capetanaki Y. Desmin cytoskeleton linked to muscle mitochondrial distribution and respiratory function. J Cell Biol 150: 1283–1298, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milner DJ, Weitzer G, Tran D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J Cell Biol 134: 1255–1270, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacher P, Mabley JG, Liaudet L, Evgenov OV, Marton A, Hasko G, Kollai M, Szabo C. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am J Physiol Heart Circ Physiol 287: H2132–H2137, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paulin D, Li Z. Desmin: a major intermediate filament protein essential for the structural integrity and function of muscle. Exp Cell Res 301: 1–7, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Reddy GK AGE-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res 68: 132–142, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Rozenberg S, Tavernier B, Riou B, Swynghedauw B, Page CL, Boucher F, Leiris J, Besse S. Severe impairment of ventricular compliance accounts for advanced age-associated hemodynamic dysfunction in rats. Exp Gerontol 41: 289–295, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt U, del Monte F, Miyamoto MI, Matsui T, Gwathmey JK, Rosenzweig A, Hajjar RJ. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca2+-ATPase. Circulation 101: 790–796, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper G 4th. Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ Res 80: 281–289, 1997. [DOI] [PubMed] [Google Scholar]
- 39.Takagi G, Asai K, Vatner SF, Kudej RK, Rossi F, Peppas A, Takagi I, Resuello RR, Natividad F, Shen YT, Vatner DE. Gender differences on the effects of aging on cardiac and peripheral adrenergic stimulation in old conscious monkeys. Am J Physiol Heart Circ Physiol 285: H527–H534, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Thornell L, Carlsson L, Li Z, Mericskay M, Paulin D. Null mutation in the desmin gene gives rise to a cardiomyopathy. J Mol Cell Cardiol 29: 2107–2124, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Tsutsui H, Ishihara K, Cooper G 4th. Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science 260: 682–687, 1993. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsui H, Tagawa H, Kent RL, McCollam PL, Ishihara K, Nagatsu M, Cooper G 4th. Role of microtubules in contractile dysfunction of hypertrophied cardiocytes. Circulation 90: 533–555, 1994. [DOI] [PubMed] [Google Scholar]
- 43.Walker EM Jr, Nillas MS, Mangiarua EI, Cansino S, Morrison RG, Perdue RR, Triest WE, Wright GL, Studeny M, Wehner P, Rice KM, Blough ER. Age-associated changes in hearts of male Fischer 344/Brown Norway F1 rats. Ann Clin Lab Sci 36: 427–438, 2006. [PubMed] [Google Scholar]
- 44.Wanagat J, Wolff MR, Aiken JM. Age-associated changes in function, structure and mitochondrial genetic and enzymatic abnormalities in the Fischer 344 × Brown Norway F1 hybrid rat heart. J Mol Cell Cardiol 34: 17–28, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Wang N, Yan K, Rasenick MM. Tubulin binds specifically to the signal-transducing proteins, Gsα and Giα1. J Biol Chem 265: 1239–1242, 1990. [PubMed] [Google Scholar]
- 46.Yan L, Vatner DE, Kim SJ, Ge H, Masurekar M, Massover WH, Yang G, Matsui Y, Sadoshima J, Vatner SF. Autophagy in chronically ischemic myocardium. Proc Natl Acad Sci USA 102: 13807–13812, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]