Abstract
Endocardial mapping has suggested that Purkinje fibers may play a role in the maintenance of long-duration ventricular fibrillation (LDVF). To determine the influence of Purkinje fibers on LDVF, we chemically ablated the Purkinje system with Lugol solution and recorded endocardial and transmural activation during LDVF. Dog hearts were isolated and perfused, and the ventricular endocardium was exposed and treated with Lugol solution (n = 6) or normal Tyrode solution as a control (n = 6). The left anterior papillary muscle endocardium was mapped with a 504-electrode (21 × 24) plaque with electrodes spaced 1 mm apart. Transmural activation was recorded with a six-electrode plunge needle on each side of the plaque. Ventricular fibrillation (VF) was induced, and perfusion was halted. LDVF spontaneously terminated sooner in Lugol-ablated hearts than in control hearts (4.9 ± 1.5 vs. 9.2 ± 3.2 min, P = 0.01). After termination of VF, both the control and Lugol hearts were typically excitable, but only short episodes of VF could be reinduced. Endocardial activation rates were similar during the first 2 min of LDVF for Lugol-ablated and control hearts but were significantly slower in Lugol hearts by 3 min. In control hearts, the endocardium activated more rapidly than the epicardium after 4 min of LDVF with wave fronts propagating most often from the endocardium to epicardium. No difference in transmural activation rate or wave front direction was observed in Lugol hearts. Ablation of the subendocardium hastens VF spontaneous termination and alters VF activation sequences, suggesting that Purkinje fibers are important in the maintenance of LDVF.
Keywords: electrophysiology, mapping, Lugol ablation, activation rate gradient
despite improvements in defibrillator technology and availability, sudden cardiac death due to ventricular fibrillation (VF) persists as one of the leading causes of death in the developed world (43, 48). Purkinje fibers have been implicated as a source for the initiation of VF. Studies have shown that the Purkinje fiber system may play a role in some cases of idiopathic VF (20, 30). The Purkinje fiber system has also been implicated in the onset of arrhythmias during postinfarct ischemia and reperfusion (4, 5, 46). Modeling studies have demonstrated that the Purkinje-myocardial junctions may be responsible for focal activations that lead to sustained tachyarrhythmias (8).
Purkinje fibers are more fatigue resistant and continue to function during ischemia longer than working myocardial tissue (6, 18, 19). A recent study demonstrated that during the first 10 min of VF in isolated dog hearts wave fronts propagate both from the working myocardium into the Purkinje system and from the Purkinje system into the working myocardium, suggesting that the Purkinje fiber system may play an integral role in the maintenance of long-duration VF (LDVF) (38).
If the Purkinje system plays an integral role in maintaining LDVF activation patterns, then ablation of the Purkinje system should result in slowing of the VF activation rate and premature termination of LDVF. In this study, we tested this hypothesis using an isolated, perfused dog heart model in which we recorded endocardial and transmural activation during LDVF in hearts with the endocardium treated with normal Tyrode solution (control) or with Lugol solution to ablate the endocardium containing the Purkinje fibers.
METHODS
All of the animals were managed in accordance with the guidelines established by the American Heart Association on research animal use (1), and the protocol was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Animal preparation.
Twelve mongrel dogs (22.5 ± 2.5 kg, mean ± SD) from Marshall Bioresources, North Rose, NY were fasted overnight and anesthetized with sodium thiopental (25 mg/kg iv), intubated, and mechanically ventilated with 2–3% isoflurane in 100% oxygen. Lead II surface ECG, core body temperature, arterial blood gases, arterial blood pressure, and serum electrolytes were monitored and maintained within normal levels until the hearts were excised and externally perfused.
Heart isolation and preparation.
The heart was exposed via a medial sternotomy approach. The heart was excised and the left ventricular anterior papillary muscle and adjacent endocardium was exposed with an incision through the right ventricle (RV) and the septum, as described previously (38). The heart was perfused through a catheter in the left main coronary artery with a continuous flow (150 ml/min) of modified Tyrode solution (in mmol/l: 123 NaCl, 4.5 KCl, 1.8 CaCl2, 0.98 MgCl, 20 NaHCO3, 1.01 NaH2PO4, and 11 dextrose, plus 0.04 g/l bovine albumin). The heart was submerged in a warmed (37°C) and oxygenated Tyrode solution bath.
Pretreatment mapping.
A 504-electrode plaque (24 columns × 21 rows with 1-mm spacing) was placed over the endocardial insertion of the anterior-lateral papillary muscle. Unipolar electrograms were recorded with reference to a grounding electrode attached to the aortic root. Electrograms were band passed between 0.5 and 500 Hz, recorded at 14 bit resolution, and sampled at 2 KHz. If the heart was in VF following the isolation and perfusion procedure, it was defibrillated with a 30- to 50-J biphasic shock delivered with pediatric paddles from a Lifepak 12 defibrillator (Medtronic Physio-Control, Redmond, WA). The diastolic pacing threshold was determined with bipolar pacing with hook electrodes high on the septum on the left ventricle (LV) endocardial surface adjacent to the left bundle branch that descended toward the anterior-lateral papillary muscle, ∼2–3 cm from the plaque. Intrinsic and paced beats were recorded with the plaque.
Lugol ablation procedure.
The plaque was temporarily removed, and the heart was taken out of the perfusion bath. The perfusion pump was turned off, and the entire LV and RV endocardium was sprayed with a small spray bottle containing either 1) Lugol solution (5 g I2 and 10 g KI dissolved in 100 ml deionized H2O; n = 6) or 2) normal Tyrode solution (n = 6). The solution was allowed to remain on the endocardium for 20 s, after which the perfusion pump was turned on and the endocardium was rinsed with 500 ml of Tyrode solution. The heart was then returned to the perfusion bath and was allowed to stabilize for 5 min.
Posttreatment mapping.
The plaque was returned to the same location over the anterior-lateral papillary muscle and four plunge needles were inserted, each just outside the midpoint of one of the four edges of the plaque. Each needle contained six electrodes 2 mm apart, with the most endocardial electrode 1 mm from the endocardium. Plunge needle recordings were made with the same mapping system and with the same parameters as the plaque recordings. Paced beats were again recorded. VF was then induced with a 9-V battery held briefly against the RV. Perfusion was terminated, and VF was recorded continuously until it spontaneously terminated.
Post-VF termination pacing and reinduction of VF.
Within 30–60 s of the spontaneous termination of VF, the heart was paced at 10 times diastolic threshold at a cycle length of 500 ms. In four control hearts and four Lugol hearts, a 9-V battery was then placed in contact with the right ventricle for 1–2 s to determine whether VF was reinducible.
Quantification of activation rate and VF progression.
Activations were identified by a computer algorithm for the plaque and plunge needle recordings during the first 5 s of each minute for the first 10 min of VF or, if VF stopped spontaneously in less than 10 min, while VF persisted. Activations were chosen as the maximum negative derivatives that exceeded a minimum of −0.20 V/s within a 50-ms search window (15, 38). Activation picks were manually overread by visual inspection so that only activations within the working myocardium were detected, and average activation rates were determined. Channels with activity that did not meet the slope criteria were excluded from the analysis. Purkinje activations were identified manually as rapid, short-duration activations, typically 1–2 ms in duration, with a minimum downslope exceeding −0.20 V/s, following the criteria that we have used previously (38), which were adapted from the criteria used by others for identifying Purkinje activation in canines (4, 5, 7, 25, 31, 32, 38, 42).
The direction of propagation along each plunge needle was quantified by linking working myocardial activations identified on each electrode to working myocardial activations on adjacent electrodes. An activation that occurred within 20 ms of an activation of a neighboring electrode (2 mm away) was categorized as propagating from one electrode to the other, and the direction of propagation (endocardial to epicardial or endocardial to epicardial) was determined (2).
Statistical analysis.
Differences in activation rate and propagation direction between control and Lugol hearts were determined by ANOVA repeated-measures tests (SAS, SAS Institute, Cary, NC). If the repeated-measures test showed that the data from the Lugol hearts was different from the control hearts, unpaired equal variance t-tests were conducted to determine at what VF duration a significant difference between the treatment and control group was observed. Paired t-tests were conducted when appropriate, such as when activation rates from the same animals were compared for the same time periods from different locations (such as comparing endocardial and epicardial activation rates in the same treatment groups). Significant differences were noted when P ≤ 0.05. Data are reported as means ± SD.
RESULTS
Plaque recordings.
Purkinje fiber and working myocardial activations were recorded in all animals during intrinsic and paced beats before hearts were treated with Lugol or control solution. After Lugol ablation, Purkinje fiber activity was no longer recorded, while Purkinje activation continued in the Tyrode-treated hearts (Fig. 1). During unperfused LDVF, Purkinje activations in the control hearts were commonly recorded with bidirectional propagation between the Purkinje system and working myocardium, as previously reported (38). Purkinje and myocardial activations were identified throughout VF in control hearts, whereas only myocardial activations were identified in Lugol-ablated hearts (Fig. 2).
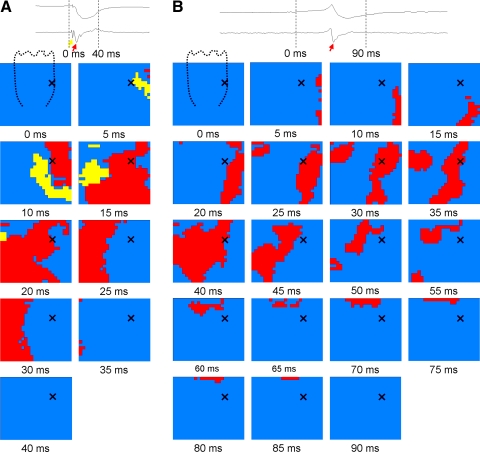
Fig. 1.
Purkinje activations recorded during a paced beat before (A) but not after (B) Lugol ablation. A: the voltage electrogram (top trace) and the temporal derivative (bottom trace) at the top of the panel were recorded from the location indicated by an X on the plaque below. The arrows in the electrograms denote Purkinje (yellow arrow) and working myocardial (red arrows) activations. The plaque below represents the 504 electrodes. The dashed line in the frame of 0 ms represents the approximate location of the anterior papillary muscle. The timing of the frame (in ms) is shown below each panel. Electrodes with derivatives >−0.2 V/s at the time frame shown are inactive (blue). Purkinje activations (yellow) and activations of the working myocardium (red) are shown when the current frame electrogram signal has a derivative of ≤−0.2 V/s. Before Lugol ablation, activation from a paced beat first appears in the Purkinje system (yellow) and then in the working myocardium (red). The wave front activates the entire plaque in less than 40 ms. B: a paced beat in the same heart following Lugol ablation. The activation proceeds from the area to the right of the papillary muscle to the area to the left of the papillary muscle, with the papillary muscle activating last. The wave front takes nearly 90 ms to activate the entire mapped region.
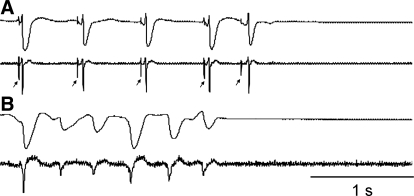
Fig. 2.
Electrical activations were recorded until long-duration ventricular fibrillation (LDVF) abruptly terminated. A: an electrogram (top) and its temporal derivative (bottom) from a plaque in a control heart demonstrate the termination of LDVF. Purkinje activations were recorded in control hearts until the termination of LDVF. B: an electrogram (top) and its temporal derivative (bottom) in a Lugol-ablated heart show spontaneous termination of LDVF. No Purkinje activations were recorded in Lugol-treated hearts.
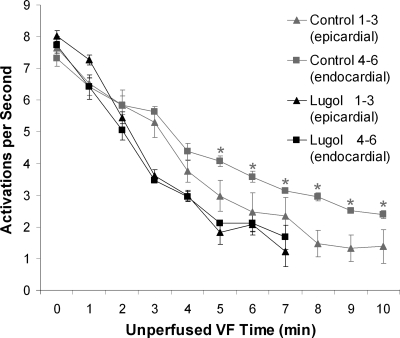
LDVF spontaneously terminated (Fig. 2) earlier in the Lugol-ablated hearts than in the control hearts (4.9 ± 1.5 vs. 9.2 ± 3.2 min, respectively, P < 0.05). In both control and Lugol-ablated hearts, the VF activation rate slowed to approximately two activations per second and then abruptly ceased (Fig. 3A). The mean cycle length of the plaque electrodes for the last five cycles before spontaneous termination of VF was not significantly different (ANOVA repeated-measures test) for control and Lugol-treated hearts (Table 1).
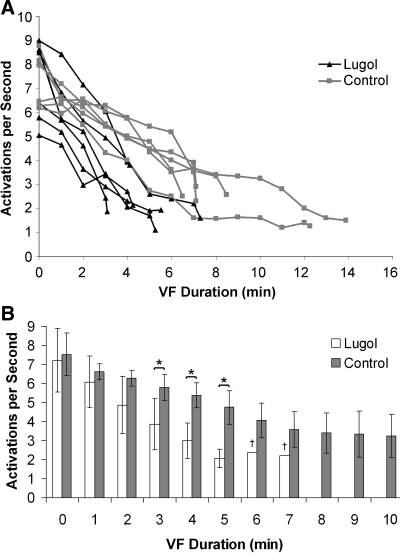
Fig. 3.
Activation rates of plaque electrodes and ventricular fibrillation (VF) termination times. A: mean activation rates recorded by the plaque electrodes and VF termination times as determined by the plaque and plunge needle recordings are shown for each Lugol-ablated (red) and control (blue) heart. B: average activation rates (mean with bars showing standard deviation) for all Lugol-ablated (red) together and all control (blue) hearts in which VF persisted are shown. VF durations with P < 0.05 by an unpaired t-test are marked with an asterisk. Statistics were not performed for minutes 6 and 7 (marked with †) because VF persisted in only 1 Lugol-ablated heart at this time. VF did not last more than 7 min in any Lugol-ablated heart.
Table 1.
Mean cycle length of last 5 cycles before VF termination
| 5th | 4th | 3rd | 2nd | Last Cycle | |
|---|---|---|---|---|---|
| Lugol | 429±230 | 472±277 | 535±271 | 468±207 | 471±164 |
| Control | 481±215 | 467±214 | 500±188 | 531±162 | 539±203 |
Values are means ± SD, in ms. VF, ventricular fibrillation.
An ANOVA repeated-measures test showed that the treatment group (control vs. Lugol) and the duration of VF were significant predictors of the mean activation rate recorded from the plaque. Although mean activation rates were not statistically different during 0–2 min of VF, the mean activation rates for 3–5 min of VF were slower for the Lugol-ablated hearts than for the control hearts (Fig. 3B).
Plunge needle recordings.
A repeated-measures analysis of the plunge needle recordings showed that activation rate was significantly related to VF duration and to treatment group (Lugol vs. control). The activation rate decreased as VF duration increased for both groups. In the control animals, after 4 min of VF, an activation rate gradient developed in which the endocardium continued to activate at a rapid rate while the epicardial tissue activation rate decreased (Fig. 4).
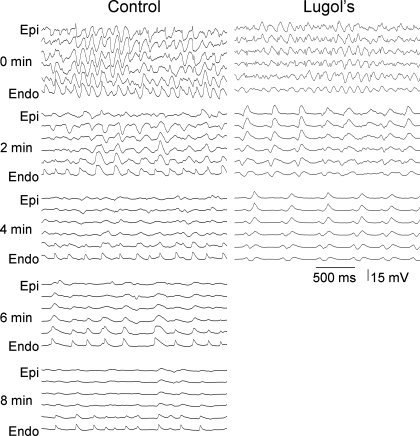
Fig. 4.
Recordings from the 6 electrodes of a plunge needle in a control and a Lugol-ablated heart every 2 min during VF. As VF progresses, an activation rate gradient develops in the control animals in which the endocardium (Endo) activates more rapidly than the epicardium (Epi). In the Lugol-ablated heart, the endocardium does not activate more rapidly than the epicardium. VF terminated at 8.25 min in the control heart and at 4.5 min in the Lugol-treated heart shown in this figure.
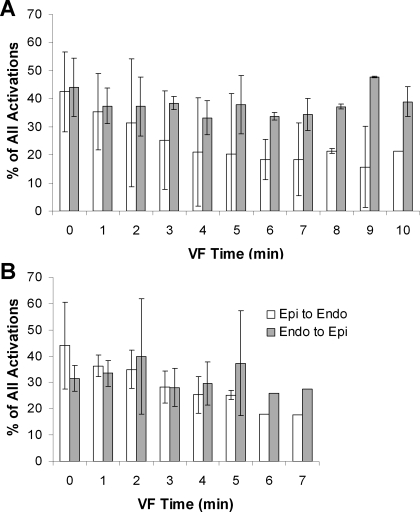
An activation rate gradient did not develop in Lugol-ablated hearts (Fig. 5). An ANOVA repeated-measures test determined that the mean activation rate of the three most endocardial electrodes was different from the mean activation rate of the three most epicardial electrodes in the control hearts but not in the Lugol-ablated hearts. Paired t-tests showed a significant difference developed in the fifth minute of VF in the control hearts, which persisted until VF spontaneously terminated. Although a repeated-measures test showed that wave fronts traveled from the endocardium toward the epicardium significantly more often than from the epicardium toward the endocardium in control hearts, particularly after the second minute of VF, activation did not propagate significantly more often in one direction than in the other in the Lugol-ablated hearts (Fig. 6).
Fig. 5.
Mean activation rate of the 3 most endocardial and the 3 most epicardial plunge needle electrodes during VF. An activation rate gradient developed after 4 min of VF in the control hearts with the endocardial electrodes activating significantly faster than the epicardial electrodes but did not develop in the Lugol-ablated hearts. Mean activation rate with the standard deviation is shown. Statistically different values are denoted with asterisks.
Fig. 6.
Mean direction of the VF wave fronts along the plunge needles. Wavefronts in control hearts propagated significantly more often from the endocardium toward the epicardium than in the opposite direction (A), whereas there was no preferential direction of propagation in Lugol-ablated hearts (B).
Post-VF termination pacing and VF reinduction.
After spontaneous termination of VF, consistent capture was observed in five of the control hearts and intermittent capture was observed in one control heart. In the Lugol hearts, five exhibited consistent capture and one was unexcitable with stimulation at 10 times diastolic threshold. After spontaneous termination of VF and pacing, short-lived VF was reinduced in control and Lugol hearts (5 ± 4 and 6 ± 11 s in duration, respectively).
DISCUSSION
The primary findings of this study are the following: 1) A significant transmural activation rate gradient did not develop in Lugol-ablated hearts, whereas it did develop after 4 min of VF in Tyrode-treated hearts. 2) Ablation of the Purkinje system caused VF activation rate to slow significantly after 2 min of VF. 3) Ablation of the Purkinje system led to spontaneous termination of VF significantly earlier than in Tyrode-treated hearts. All of these findings suggest that the Purkinje system is important for the maintenance of LDVF.
Previous studies have shown that during early LDVF in a dog model, endocardial and epicardial activation rates are not significantly different (2, 11, 29, 44). As VF progresses beyond the first few minutes, an activation rate gradient develops in which the epicardium slows more rapidly than the endocardium (2, 11, 29, 44). This activation rate gradient could be due to at least two factors: 1) Purkinje fibers, which are limited to the subendocardium in dogs (3, 17, 36, 37), are more resistant to ischemia caused by VF (6, 16, 18, 19, 26), thus maintaining a rapid activation rate near the endocardium, or 2) the endocardial tissue does not become ischemic as rapidly as the epicardial tissue because it is exposed to a large reservoir of oxygenated blood in the ventricular cavities.
A study of LDVF in dogs by Cha et al. (11) compared endocardial and epicardial activation rates in three groups: control animals with the ventricles filled with blood (n = 3), the RV filled with air (n = 2), and RV endocardium ablated with Lugol solution (n = 2). Hook electrodes were used to study the activation rate of the epicardium and endocardium during prolonged VF. Cha and coworkers demonstrated that the control and air-treated animals developed the transmural gradient of activation rate, but the Lugol-ablated animals did not. Although the results were in a limited number of animals, this result supported the hypothesis that the subendocardial Purkinje system is responsible for the activation rate gradient.
In our study, wave fronts generally propagated in a direction from the endocardium toward the epicardium in the plunge needle recordings in the control animals as reported previously (2, 11, 29). This finding is consistent with activation propagating from the Purkinje system to the working myocardium and then passing transmurally toward the epicardium. In the Lugol-ablated hearts there was no longer a preferential transmural direction of propagation; wave fronts did not propagate toward the epicardium any more often than the propagated toward the endocardium (Fig. 6). Also, the transmural activation rate slowed more rapidly than in the control hearts during VF so that after 2 min of VF the activation rate was significantly slower in the Lugol-treated hearts than in the control hearts (Fig. 3). In addition, Lugol ablation of the Purkinje system significantly shortened the duration of sustained VF. All of these findings suggest that, although reentrant activity in the working myocardium may be the dominant driver of activation during the first 2 min of VF, Purkinje fiber activation plays an important role in wave front activation after 2 min of LDVF.
In a canine model similar to the model in the present study, Tabereaux et al. (38) demonstrated that during LDVF activation wave fronts detected on the endocardium may 1) conduct from the Purkinje system into the myocardium, 2) conduct from the myocardium into the Purkinje system, 3) arise focally from the Purkinje system, or 4) arise focally or as breakthrough from the working myocardium (38). In the present study, Purkinje activations were entirely eliminated within the mapped regions after Lugol ablation. Therefore, the changes in VF termination time and VF activation rate observed may be attributed to the contribution of the Purkinje fiber system to LDVF maintenance.
Several studies have described different phases of activity as VF progresses over several minutes (22, 23, 45). Early VF is characterized by rapid, chaotic activation, followed by a brief period with a greater degree of organization with larger, more repetitive wave fronts. Panoramic optical mapping of continuously perfused, nonischemic, fibrillating swine hearts demonstrated that most wave fronts were continuous and persistent on the heart throughout the mapped episodes (35). Nonepicardial sources of activation (i.e., sources that appear de novo on the epicardium) were not necessary for VF maintenance. Although this may be true for short-duration or perfused VF, as global ischemia progresses the incidence of epicardial reentry decreases (22). After several minutes of unperfused VF, there is a period of increased conduction block, smaller wave fronts, and slower activation rates (22, 23, 45). This period is accompanied by an increased incidence of epicardial focal or breakthrough activity. These changes in VF characteristics may indicate that whereas early VF is driven primarily by reentrant activity in the working myocardium, VF persisting more than a few minutes may be driven by a different mechanism.
The Purkinje system activates more rapidly than the working myocardium in LDVF because Purkinje fibers are more resistant to the global ischemia caused by the lack of perfusion during VF than are working myocardial cells (6, 16, 18, 19, 26). This may be due to the increased metabolic load required for the contractions of the myocardial cells and to the increased glycogen stored in Purkinje fibers (21, 40). In addition, in dogs as well as in humans, the Purkinje fibers are near the endocardium so that they are less ischemic than the working myocardium because of the diffusion of oxygen from the near by LV cavity. Although the refractory period for Purkinje fibers in sinus rhythm is longer than for myocardial cells, Purkinje fibers accommodate so that their refractory period shortens more than myocardial cells when activated rapidly (34). The rapid activation rate observed in VF may cause the refractory period of the Purkinje fibers to become shorter than the refractory period of the working myocardium, thus enabling the Purkinje fibers to activate at a faster rate than the working myocardium.
The VF activation rate in both control and Lugol-ablated hearts slowed to one to three activations per second and then spontaneously terminated. The Lugol-ablated hearts slowed to this activation rate faster than the control hearts. In most cases in both the control and Lugol groups, the working myocardium was excitable after termination of LDVF and VF could be reinitiated. However, this reinitiated VF spontaneously terminated within a few seconds. There may be a minimum required activation rate needed to sustain the intramural reentry thought to be important in maintaining VF (12, 41, 47). Recent studies in pigs have indicated that the percentage of wave fronts that appear de novo in the working myocardium increases as the duration of VF increases, and that the Purkinje system is a likely source of these activation fronts (28). The Purkinje system, therefore, may play a crucial role in sustaining VF past 3–5 min by maintaining the activation rate above the minimum activation rate required for intramural reentry that maintains VF.
Limitations.
The process of isolating, perfusing with Tyrode solution, and opening the heart to allow direct mapping of the endocardium may have altered VF activation patterns (33). However, similar activation patterns such as the development of the activation gradient in control but not Lugol hearts (11, 44) and increased endocardial origination of activation wave fronts as LDVF progresses (2) are consistent with previous intact canine heart studies. Control studies were performed in which the protocol for the control hearts and Lugol-ablated hearts was the same except that Tyrode solution was substituted for the Lugol solution to ablate the Purkinje system and the subendocardium. This allowed us to compare the results with the only difference being the Lugol ablation procedure. Although there are a number of experiments that study LDVF in canine hearts (2, 11, 22, 38, 44), the time to spontaneous termination of VF has not previously been reported for either intact nor isolated hearts, making direct comparisons to the presented work difficult.
The Lugol ablation procedure not only eliminates the Purkinje fiber system but also causes a subendocardial layer of necrosis 200–500 μm thick (13, 14), but the bulk of the myocardium remains intact in this model. This technique has been used by numerous investigators to study the effect of ablating the Purkinje system (9, 10, 13, 14, 24, 27, 32, 39).
The combination of the Lugol ablation of the subendocardium and the decreased amplitude of activation wave fronts as LDVF progressed prevented quantitative analysis of VF wave front characteristics. The trabeculated surface of the endocardium also made quantitative analysis of cohesive wave fronts problematic. The plaque was placed over the base of the anterior papillary muscle because this region is relatively flat and is where early myocardial activation occurs via Purkinje-myocardial junctions during sinus rhythm. Mapping in broader or other areas would provide more complete information about the role of the Purkinje system in VF but also presents additional technical difficulties.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-28429, HL-66256, HL-64184, HL-85370, and T32 HL-07457-16.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.AHA Special Report. Position of the American Heart Association on research animal use. Circulation 71: 849A–850A, 1985. [PubMed] [Google Scholar]
- 2.Allison JS, Qin H, Dosdall DJ, Huang J, Newton JC, Allred JD, Smith WM, Ideker RE. The transmural activation sequence in porcine and canine left ventricle is markedly different during long-duration ventricular fibrillation. J Cardiovasc Electrophysiol 18: 1306–1312, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Antzelevitch C, Sicouri S, Litovsky SH, Lukas A, Krishnan SC, Di Diego JM, Gintant GA, Liu DW. Heterogeneity within the ventricular wall: electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ Res 69: 1427–1449, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Arnar DO, Bullinga JR, Martins JB. Role of the Purkinje system in spontaneous ventricular tachycardia during acute ischemia in a canine model. Circulation 96: 2421–2429, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Arnar DO, Martins JB. Purkinje involvement in arrhythmias after coronary artery reperfusion. Am J Physiol Heart Circ Physiol 282: H1189–H1196, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Bagdonas AA, Stuckey JH, Piera J, Amer NS, Hoffman BF. Effects of ischemia and hypoxia on the specialized conducting system of the canine heart. Am Heart J 61: 206–218, 1961. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Haim SA, Cable DG, Rath TE, Carmen L, Martins JB. Impulse propagation in the Purkinje system and myocardium of intact dogs. Am J Physiol Heart Circ Physiol 265: H1588–H1595, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Berenfeld O, Jalife J. Purkinje-muscle reentry as a mechanism of polymorphic ventricular arrhythmias in a 3-dimensional model of the ventricles. Circ Res 82: 1063–1077, 1998. [DOI] [PubMed] [Google Scholar]
- 9.Cates AW, Smith WM, Ideker RE, Pollard AE. Purkinje and ventricular contributions to endocardial activation sequence in perfused rabbit right ventricle. Am J Physiol Heart Circ Physiol 281: H490–H505, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Cha YM, Birgersdotter-Green U, Wolf PL, Peters BB, Chen PS. The mechanism of termination of reentrant activity in ventricular fibrillation. Circ Res 74: 495–506, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Cha YM, Uchida T, Wolf PL, Peters BB, Fishbein MC, Karagueuzian HS, Chen PS. Effects of chemical subendocardial ablation on activation rate gradient during ventricular fibrillation. Am J Physiol Heart Circ Physiol 269: H1998–H2009, 1995. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Mandapati R, Berenfeld O, Skanes AC, Jalife J. High-frequency periodic sources underlie ventricular fibrillation in the isolated rabbit heart. Circ Res 86: 86–93, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Chen PS, Wolf PL, Cha YM, Peters BB, Topham SL. Effects of subendocardial ablation on anodal supernormal excitation and ventricular vulnerability in open-chest dogs. Circulation 87: 216–229, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Damiano RJ, Smith PK, Tripp HF Jr, Asano T, Small KW, Lowe JE, Ideker RE, Cox JL. The effect of chemical ablation of the endocardium on ventricular fibrillation threshold. Circulation 74: 645–652, 1986. [DOI] [PubMed] [Google Scholar]
- 15.Evans FG, Rogers JM, Smith WM, Ideker RE. Automatic detection of conduction block based on time-frequency analysis of unipolar electrograms. IEEE Trans Biomed Eng 46: 1090–1097, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Fixler DE, Wheeler M, Huffines D. Extent of myocardial flow from luminal collateral circulation. J Appl Physiol 37: 282–285, 1974. [DOI] [PubMed] [Google Scholar]
- 17.Forsgren S, Eriksson A, Kjorell U, Thornell LE. The conduction system in the human heart at midgestation—immunohistochemical demonstration of the intermediate filament protein skeleton. Histochemistry 75: 43–52, 1982. [DOI] [PubMed] [Google Scholar]
- 18.Friedman PL, Stewart JR, Fenoglio JJ, Wit AL. Survival of subendocardial Purkinje fibers after extensive myocardial infarction in dogs. Circ Res 33: 597–611, 1973. [DOI] [PubMed] [Google Scholar]
- 19.Gilmour RF, Zipes DP. Different electrophysiological responses of canine endocardium and epicardium to combined hyperkalemia, hypoxia, and acidosis. Circ Res 46: 814–825, 1980. [DOI] [PubMed] [Google Scholar]
- 20.Haissaguerre M, Shah DC, Jais P, Shoda M, Kautzner J, Arentz T, Kalushe D, Kadish A, Griffith M, Gaita F, Yamane T, Garrigue S, Hocini M, Clementy J. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet 359: 677–678, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Henry CG, Lowry OH. Enzymes and metabolites of glycogen metabolism in canine cardiac Purkinje fibers. Am J Physiol Heart Circ Physiol 248: H599–H605, 1985. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Rogers JM, Killingsworth CR, Singh KP, Smith WM, Ideker RE. Evolution of activation patterns during long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol 286: H1193–H1200, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Huizar JF, Warren MD, Shvedko AG, Kalifa J, Moreno J, Mironov S, Jalife J, Zaitsev AV. Three distinct phases of VF during global ischemia in the isolated blood-perfused pig heart. Am J Physiol Heart Circ Physiol 293: H1617–H1628, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Janse MJ Vulnerability to ventricular fibrillation. Chaos 8: 149–156, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Joyner RW, Picone J, Veenstra R, Rawling D. Propagation through electrically coupled cells. Effects of regional changes in membrane properties. Circ Res 53: 526–534, 1983. [DOI] [PubMed] [Google Scholar]
- 26.Lazzara R, el-Sherif N, Scherlag BJ. Electrophysiological properties of canine Purkinje cells in one-day-old myocardial infarction. Circ Res 33: 722–734, 1973. [DOI] [PubMed] [Google Scholar]
- 27.Lee JJ, Kamjoo K, Hough D, Hwang C, Fan W, Fishbein MC, Bonometti C, Ikeda T, Karagueuzian HS, Chen PS. Reentrant wavefronts in Wiggers' stage II ventricular fibrillation. Circ Res 78: 660–675, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Jin Q, Huang J, Cheng KA, Ideker RE. Intramural foci during long duration fibrillation in the pig ventricle. Circ Res 102: 1256–1264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton JC, Smith WM, Ideker RE. Estimated global transmural distribution of activation rate and conduction block during porcine and canine ventricular fibrillation. Circ Res 94: 836–842, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Nogami A, Sugiyasu A, Kubota S, Kato K. Mapping and ablation of idiopathic ventricular fibrillation from the Purkinje system. Heart Rhythm 2: 646–649, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Overholt ED, Joyner RW, Veenstra RD, Rawling D, Wiedmann R. Unidirectional block between Purkinje and ventricular layers of papillary muscles. Am J Physiol Heart Circ Physiol 247: H584–H595, 1984. [DOI] [PubMed] [Google Scholar]
- 32.Pollard AE, Spitzer KW, Burgess MJ. Contributions of the specialized conduction system to the activation sequence in the canine pulmonary conus. Am J Physiol Heart Circ Physiol 273: H446–H463, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Qin H, Kay MW, Chattipakorn N, Redden DT, Ideker RE, Rogers JM. Effects of heart isolation, voltage-sensitive dye, and electromechanical uncoupling agents on ventricular fibrillation. Am J Physiol Heart Circ Physiol 284: H1818–H1826, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Robinson RB, Boyden PA, Hoffman BF, Hewett KW. Electrical restitution process in dispersed canine cardiac Purkinje and ventricular cells. Am J Physiol Heart Circ Physiol 253: H1018–H1025, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Rogers JM, Walcott GP, Gladden JD, Melnick SB, Kay MW. Panoramic optical mapping reveals continuous epicardial reentry during ventricular fibrillation in the isolated swine heart. Biophys J 92: 1090–1095, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnabel PA, Richter J, Schmiedl A, Bach F, Bartels U, Ramsauer B, Gebhard MM, Bretschneider HJ. Patterns of structural deterioration due to ischemia in Purkinje fibres and different layers of the working myocardium. Thorac Cardiovasc Surg 39: 174–182, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Spach MS, Huang S, Armstrong SI, Canent RV Jr. Demonstration of peripheral conduction system in human hearts. Circulation 28: 333–338, 1963. [DOI] [PubMed] [Google Scholar]
- 38.Tabereaux PB, Walcott GP, Rogers JM, Kim J, Dosdall DJ, Robertson PG, Killingsworth CR, Smith WM, Ideker RE. Activation patterns of Purkinje fibers during long-duration ventricular fibrillation in an isolated canine heart model. Circulation 116: 1113–1119, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Taccardi B, Punske BB, Macchi E, Macleod RS, Ershler PR. Epicardial and intramural excitation during ventricular pacing: effect of myocardial structure. Am J Physiol Heart Circ Physiol 294: H1753–H1766, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tribulova N, Novakova S, Macsaliova A, Sass S, Thomas S, Goetzfried S, Podzuweit T, Manoach M. Histochemical and ultrastructural characterisation of an arrhythmogenic substrate in ischemic pig heart. Acta Histochem 104: 393–397, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Valderrábano M, Lee MH, Ohara T, Lai AC, Fishbein MC, Lin SF, Karagueuzian HS, Chen PS. Dynamics of intramural and transmural reentry during ventricular fibrillation in isolated swine ventricles. Circ Res 88: 839–848, 2001. [DOI] [PubMed] [Google Scholar]
- 42.Veenstra RD, Joyner RW, Rawling DA. Purkinje and ventricular activation sequences of canine papillary muscle. Effects of quinidine and calcium on the Purkinje-ventricular conduction delay. Circ Res 54: 500–515, 1984. [DOI] [PubMed] [Google Scholar]
- 43.Winslow RD, Mehta D, Fuster V. Sudden cardiac death: mechanisms, therapies and challenges. Nat Clin Pract Cardiovasc Med 2: 352–360, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Worley SJ, Swain JL, Colavita PG, Smith WM, Ideker RE. Development of an endocardial-epicardial gradient of activation rate during electrically induced, sustained ventricular fibrillation in dogs. Am J Cardiol 55: 813–820, 1985. [DOI] [PubMed] [Google Scholar]
- 45.Wu TJ, Lin SF, Hsieh YC, Ting CT, Chen PS. Ventricular fibrillation during no-flow global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol 17: 1112–1120, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Xing D, Martins JB. Triggered activity due to delayed afterdepolarizations in sites of focal origin of ischemic ventricular tachycardia. Am J Physiol Heart Circ Physiol 287: H2078–H2084, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Zaitsev AV, Berenfeld O, Mironov SF, Jalife J, Pertsov AM. Distribution of excitation frequencies on the epicardial and endocardial surfaces of fibrillating ventricular wall of the sheep heart. Circ Res 86: 408–417, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Zipes DP, Wellens HJJ. Sudden cardiac death. Circulation 98: 2334–2351, 1998. [DOI] [PubMed] [Google Scholar]