Abstract
There is increasing evidence that TGF-β family member cytokine bone morphogenetic protein (BMP)-4 plays different pathophysiological roles in the pulmonary and systemic circulation. Upregulation of BMP-4 has been linked to atherosclerosis and hypertension in the systemic circulation, whereas disruption of BMP-4 signaling is associated with the development of pulmonary hypertension. To test the hypothesis that BMP-4 elicits differential effects in the pulmonary and systemic circulation, we compared the prooxidant and proinflammatory effects of BMP-4 in cultured human coronary arterial endothelial cells (CAECs) and pulmonary arterial endothelial cells (PAECs). We found that BMP-4 (from 0.3 to 10 ng/ml) in CAECs increased O2•− and H2O2 generation, induced NF-κB activation, upregulated ICAM-1, and induced monocyte adhesiveness to ECs. In contrast, BMP-4 failed to induce oxidative stress or endothelial activation in PAECs. Also, BMP-4 treatment impaired acetylcholine-induced relaxation and increased O2•− production in cultured rat carotid arteries, whereas cultured rat pulmonary arteries were protected from these adverse effects of BMP-4. Thus, we propose that BMP-4 exerts prooxidant, prohypertensive, and proinflammatory effects only in the systemic circulation, whereas pulmonary arteries are protected from these adverse effects of BMP-4. The vascular bed-specific endothelial effects of BMP-4 are likely to contribute to its differential pathophysiological role in the systemic and pulmonary circulation.
Keywords: systemic, oxidative stress, endothelial dysfunction, pulmonary
transforming growth factor (TGF) superfamily member cytokines bone morphogenetic protein (BMP)-4 and BMP-2 (which is related to BMP-4 by its amino acid sequence, acts on the same receptors, but is transcribed from an entirely different gene) were originally described as factors regulating the formation of cartilage and bone (22). Subsequent studies revealed that they have a host of other functions related to embryonic development, patterning, and cell differentiation as well. First, genetic analysis of patients with primary pulmonary arterial hypertension (PAH) indicated that a vascular BMP/BMP receptor (BMPR) system plays an important role in vascular physiology (9, 23). Studies in the last decade have shown that multiple loss-of-function mutations affecting BMP-2/4 signaling [both in BMPR2 and activin receptor-like kinase 1] (4–9) lead to vascular remodeling promoting the development of idiopathic PAH (18, 33). While it is now clear that mutations in BMPRs can predispose to PAH, it is unclear that if in idiopathic PAH the mutation is germline (i.e., passed along to all somatic cells from germinative cells) and why only pulmonary arteries (but not vessels from the systemic circulation) are affected by the disease. These observations raised the possibility that the BMP system plays different roles in the pulmonary and systemic circulation.
Recently, expression of BMPs and BMPRs has been demonstrated in arteries of the systemic circulation as well(1–4, 7, 10, 11, 15, 19, 21, 26, 31, 32). Previous studies by these and other laboratories provided strong evidence that BMPs act as inflammatory cytokines in systemic arteries promoting endothelial activation (3, 7, 19, 25, 26). Accordingly, recent studies confirmed a striking upregulation of BMPs in atherosclerotic lesions (10, 26, 31, 34) and in pathophysiological conditions known to promote atherosclerosis (7, 24, 27). We have shown that, in vitro, both BMP-4 (31) and BMP-2 (3) elicit endothelial dysfunction in arteries from the systemic circulation. Accordingly, when injected in vivo, BMP-4 elicited significant systemic hypertension (19). Our recent studies demonstrated that both BMP-4 and BMP-2 induce oxidative stress in endothelial cells (ECs) of systemic arteries by activating NAD(P)H oxidase, and this effect is responsible for BMP-induced endothelial dysfunction (3, 16, 19, 25). BMP-2/4-induced oxidative stress has also been linked to NF-κB activation, ICAM induction, and increases in monocyte adhesiveness of coronary arterial ECs (CAECs) (3, 16, 25, 26).
Taken together, BMPs exert predominantly proinflammatory and prooxidant effects in systemic arteries. Yet, it has not been reported whether pulmonary arteries are protected from the proinflammatory and prooxidant effects of BMP-4. Thus, in the present study, we compared BMP-4 -induced cellular ROS generation, NF-κB activation, ICAM induction, and monocyte adhesiveness in cultured pulmonary arterial ECs (PAECs) and CAECs. We also determined the effect of BMP-4 treatment on endothelial vasorelaxation and cellular ROS production in cultured rat pulmonary, coronary, and carotid arteries.
METHODS
Animals.
All animal use protocols were approved by the Institutional Animal Care and Use Committee of New York Medical College (Valhalla, NY). Male Wistar rats (n = 10, Taconic Biotechnology) were killed by an injection of pentobarbital sodium (50 mg/kg ip), and intrapulmonary branches (third order) of the pulmonary artery, septal coronary artery (third order), and carotid arteries were harvested using microsurgery instruments, as previously reported (3, 27).
Experiments on EC cultures.
Primary human CAECs (Cell Applications, San Diego, CA) and human PAECs (Cell Applications) were cultured as previoulsy described (3, 6, 7, 30). After passage 4, cells were treated with recombinant BMP-4 (0.3–10 ng/ml, R&D Systems) for 24 h (3, 8).
Vessel culture and functional experiments.
Isolated segments of carotid arteries and pulmonary arteries were maintained in vessel culture under sterile conditions in F-12 medium (GIBCO-BRL) containing antibiotics (100 UI/l penicillin and 100 mg/l streptomycin) and supplemented with 5% FCS (GIBCO-BRL/Invitrogen) as previously described (3). Arteries were treated with BMP-4 (10 ng/ml) for 24 h. Endothelial function was assessed as previously described (3). In brief, cultured arteries were cut into ring segments of 2 mm in length and mounted on 40-μm stainless steel wires in myograph chambers (Danish Myo Technology, Atlanta, GA) containing Krebs buffer solution (118 mM NaCl, 4.7 mM KCl, 1.5 mM CaCl2, 25 mM NaHCO3, 1.1 mM MgSO4, 1.2 mM KH2PO4, and 5.6 mM glucose, at 37°C, gassed with 95% air and 5% CO2) for measurements of isometric tension. After an equilibration period of 1 h, during which optimal passive tension was applied to the rings (as determined from the vascular length-tension relationship), vessels were contracted by phenylephrine (PE; 10−6 mol/l) or the thromboxane A2 analog U-46619 (10−6 mol/l, as coronary arteries do not contract in response to PE), and relaxations to acetylcholine (from 10−9 to 10−4 mol/l) were obtained.
Measurement of ROS.
In BMP-4-treated CAECs and PAECs, the production of O2•− was measured using the dihydroethidine (DHE) staining method (3, 5) with modifications. In brief, cells were grown in 96-well plates (treated with or without BMP-4 for 24 h). After the culture period, cells were incubated with DHE (3 × 10−6 mol/l at 37°C), and the time course of the build up of ethidium bromide (EB, the fluorescent reaction product of DHE and O2•−) fluorescence was recorded for 30 min using a Tecan Infinite M200 plate reader (excitation: 520 nm and emission: 620 nm). The slope factor, representing cellular O2•− production, was calculated and normalized to Hoechst 33258 fluorescence (excitation: 352 nm and emission: 461 nm), representing the DNA content/cell mass (Hoechst 33258 loading was performed after DHE loading to avoid any interference with the EB signal). The production of O2•− in segments of the same carotid and pulmonary arteries that were used for functional experiments was assessed using a modified DHE staining method, as previously described (3, 5).
The cell-permeant oxidative fluorescent indicator dye 5(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (C-H2DCFDA; Invitrogen) was used to assess H2O2 production in PAECs and CAECs (in serum-free conditions to avoid noise due to noncellular esterase activity), as we have previoulsy reported (5). C-H2DCFDA is a 2′7′-dichlorofluorescein derivative that has longer retention within cells. In brief, cells grown in 96-well plates were treated with C-H2DCFDA (10−5 mol/l at 37°C), and the time course of the build up of green fluorescence (excitation: 485 nm and emission: 530 nm) was recorded for 30 min. The slope factor, representing cellular H2O2 production, was calculated and normalized to Hoechst 33258 fluorescence (excitation: 352 nm and emission: 461 nm), representing the DNA content/cell mass.
Quantitative real-time PCR.
Total RNA from tissues and cell pellets was isolated with a Mini RNA Isolation Kit (Zymo Research, Orange, CA) and was reverse transcribed using Superscript II RT (Invitrogen) as previously described (3, 4, 7). Real-time RT-PCR techniques were used to analyze mRNA expression using the Strategen MX3000, as previously reported (3, 4, 7). Samples were run in triplicate. The efficiency of the PCR was determined using dilution series of a standard vascular sample. Quantification was performed using the ΔΔCT method (where CT is threshold cycle). The housekeeping gene hypoxanthine phosphoribosyltransferase was used for internal normalization. The oligonucleotides used for quantitative real-time RT-PCR are shown in Table 1. Fidelity of the PCR was determined by melting temperature analysis and visualization of the product on a 2% agarose gel.
Table 1.
Oligonucleotides for real-time RT-PCR
| mRNA Targets | Species | Description (Alternative Name) |
Primers |
|
|---|---|---|---|---|
| Sense | Antisense | |||
| BMP-4 | Rat | Bone morphogenetic protein-4 | 5′-GAATAAGAACTGCCGTCG-3′ | 5′-CCTTGTCGTACTCGTCC-3′ |
| BMP-2 | Rat | Bone morphogenetic protein-2 | 5′-TCAAGCCAAACACAAACAG-3′ | 5′-CGCTAAGCTCAGTGGG-3′ |
| BMPR1a | Rat | Bone morphogenetic protein receptor, type IA (ALK3) | 5′-GCTTCAGACATAGGACTTCAG-3′ | 5′-TGGACAGGATAGTGAGTAACC-3′ |
| BMPR1b | Rat | Bone morphogenetic protein receptor, type IB | 5′-AGAAGCATCCTTAGCCCAAG-3′ | 5′-GGTTCCTCTGTGTCTGTCC-3′ |
| BMPR2 | Rat | Bone morphogenetic protein receptor, type II | 5′-CCAGTCCTGATGAACATGAACC-3′ | 5′-TTCCCTCCTGTCTACCAAACG-3′ |
| ACVR2a | Rat | Activin A receptor, type IIA | 5′-GCTTGTGAATGTGTAGTGTGC-3′ | 5′-GCAACCGTGGAACTGAGG-3′ |
| ACVR2b | Rat | Activin A receptor, type IIB | 5′-ATCAGGAGGTCGGTCAACAG-3′ | 5′-TGGGCTTAGATGCTGGACTC-3′ |
| ACVR1 | Rat | Activin A receptor, type I (ALK2) | 5′-AAGACAGCGGCAAGTTGAATC-3′ | 5′-AAGGGACGCAGCAACAGAC-3′ |
| ACVRL1 | Rat | Activin A receptor type II-like 1 (ALK1) | 5′-CCGAAGCCTGAGTTGATGGAC-3′ | 5′-GCCCGAGCCCTACTTTGC-3′ |
| CHRD | Rat | Chordin | 5′-TACACATTGCCACTTACAC-3′ | 5′-CCTCTGAGCCATAGAATCC-3′ |
| NOG | Rat | Noggin | 5′-GCCAGCACTATCTACACATCC-3′ | 5′-CAGCAGCGTCTCGTTCAG-3′ |
| SMAD-1 | Rat | Mothers against decapentaplegic homolog 1 (MADH1) | 5′-CTGTCTCATCCGCTCCAC-3′ | 5′-GTCTGTATTGTAAACGCCATAAG-3′ |
| SMAD-4 | Rat | 5′-CACTGGTTGTTATGTATGAAGGAG-3′ | 5′-ACAGGTGACTTAACTTTGAGAAC-3′ | |
| SMAD-5 | Rat | 5′-CAGCACATATCCCAACTC-3′ | 5′-GGCATGATCTGAGGAATC-3′ | |
| gp91phox | Rat | Cytochrome b-245, β-polypeptide (NOX2) | 5′-GGATGAATCTCAGGCCAA-3′ | 5′-TTAGCCAAGGCTTCGG-3′ |
| p47phox | Rat | Neutrophil cytosolic factor 1 (NCF-1), NADPH oxidase organizer 2 (NOXO2) | 5′-AGCACCAAGAGGAAACT-3′ | 5′-CCTAGCAATACCCGTGGA-3′ |
| p22phox | Rat | Cytochrome b-245, α-polypeptide (CYBA) | 5′-TGCCAGTGTGATCTACC-3′ | 5′-AGCTATTAACCATGTTTATTACAGT-3′ |
| NOX1 | Rat | NADPH oxidase homolog 1 | 5′-TGAATCTTGCTGGTTGACACTTGC-3′ | 5′-GAGGGACAGGTGGGAGGGAAG-3′ |
| NOX4 | Rat | Renox | 5′-TGCCTCCATCAAGCCAAG-3′ | 5′-TTCCAGTCATCCAGTAGAGTG-3′ |
| HPRT | Rat | Hypoxanthine phosphoribosyltransferase 1 (housekeeping gene) | 5′-AAGACAGCGGCAAGTTGAATC-3′ | 5′-AAGGGACGCAGCAACAGAC-3′ |
| ICAM-1 | Human | Intercellular adhesion molecule 1 | 5′-CTCTCGCTCTGTCACC-3′ | 5′-GGAAGTCTGGGCAATGT-3′ |
| Id1 | Human | DNA-binding protein inhibitor | 5′-CGCATCTTGTGTCGCTGAAG-3′ | 5′-GGGAGACCCACAGAGCAC-3′ |
| HPRT | Human | Hypoxanthine phosphoribosyltransferase 1 (housekeeping gene) | 5′-AGATGGTCAAGGTCGCAAG-3′ | 5′-TTCATTATAGTCAAGGGCATATCC-3′ |
Western blot analysis.
Western blot analysis was performed as previously described (3, 7) using primary antibodies directed against BMP-4 (Abcam) and gp91phox (Upstate Biotechnology). Anti-β-actin (Abcam) was used for normalization purposes.
Transient transfection and luciferase assays.
The effect of BMP-4 on NF-κB activity in PAECs and CAECs was tested by a reporter gene assay, as previously described (3). We used a NF-κB reporter composed of a NF-κB response element upstream of firefly luciferase (NF-κB-Luc, Stratagene) and a Renilla luciferase plasmid under the control of the cytomegalovirus promoter (as an internal control). All transfections were performed with Novafector (Venn Nova, Pompano Beach, Fl) following the manufacturer's protocols. The pDsRed-Monomer vector (Clontech, Mountain View, CA), which expresses red fluorescent DsRed-monomer fluorescent protein (6), was used to assess the efficiency of the endothelial transfection. Using flow cytometry (Guava EasyCyte Mini, Guava Technologies, Hayward, CA), we determined that the transfection efficiency was ∼65% by this method. Firefly and Renilla luciferase activities were assessed after 42 h using the Dual-Luciferase Reporter Assay Kit (Promega) and a luminometer. Pyrrolidine dithiocarbamate (10−5 mol/l), an inhibitor of NF-κB activation, was used as a control.
Monocyte adhesion assays.
We measured the adhesion of fluorescently labeled human monocytic (THP-1) cells to confluent monolayers of CAECs and PAECs using a microplate-based assay as previously described (3). In brief, cells were grown to confluence in 96-well plates and treated with BMP-4 (0.3–10 ng/ml, incubation time: 24 h, at 37°C). TNF-α was used as a positive control. THP-1 cells were labeled with the fluorescent dye BCECF (5 μmol/l final concentration, Molecular Probes, Eugene, OR) in serum-free RPMI medium for 45 min at 37°C. Cells were then washed twice with prewarmed (37°C) RPMI medium. PMA (10−6 mol/l)-pretreated fluorescently labeled THP-1 cells (5 × 105 cells/well) were added the microplate wells containing confluent cells (medium removed, incubation time: 45 min, at 37°C). Nonadherent THP-1 cells were removed by careful washing (three times with prewarmed RPMI medium). PBS (200 μl) was added to each well, and fluorescence was then measured using an Tecan fluorescent plate reader (excitation: 485 nm and emission: 528 nm). Controls included the measurement of total fluorescence of labeled cells before adhesion, the measurement of autofluorescence of unlabeled cells, and measurement of monocyte adhesion to EC-free microplate wells.
Data analysis.
Data were normalized to respective control mean values. Data are expressed as means ± SE. Statistical analyses of data were performed by Student's t-test or by two-way ANOVA followed by the Tukey post hoc test, as appropriate. P < 0.05 was considered statistically significant.
RESULTS
Expression of BMP-2/4, BMPRs, and other components of the BMP/SMAD signaling pathway in systemic and pulmonary arteries.
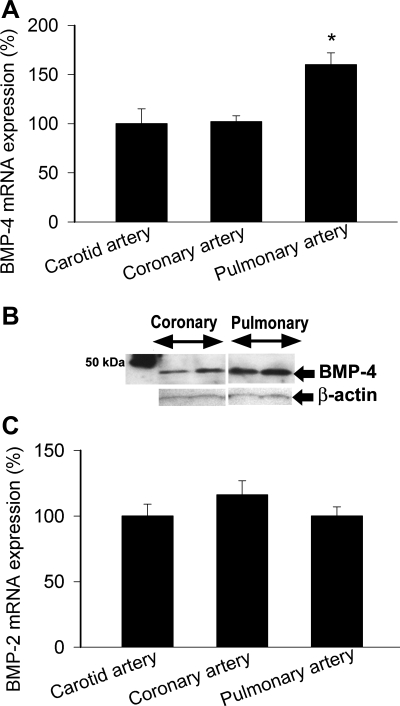
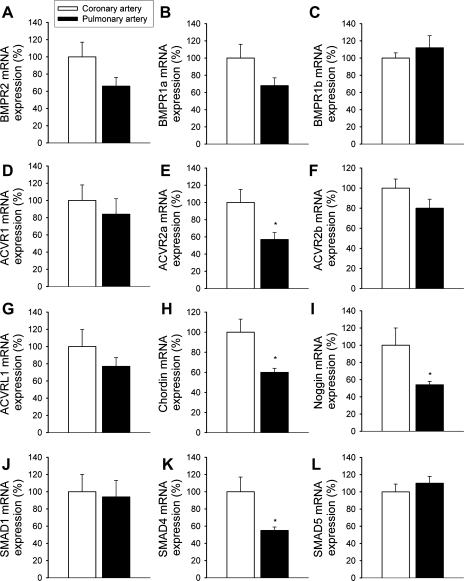
The expression of BMP-4 mRNA was more pronounced in intrapulmonary branches of the pulmonary artery than in coronary arteries and carotid arteries (Fig. 1A). BMP-4 protein was also more abundantly expressed in pulmonary arteries compared with coronary arteries (Fig. 1B). The expression of BMP-2 did not differ significantly between vascular beds (Fig. 1C). Analysis of the expression profile of BMPRs [BMPR1a, BMPR1b, BMPR2, activin receptor (ACVR)2a, ACVR2b, ACVR1, and activin A receptor, type II-like kinase 1 (ACVRL1); Fig. 2, A–G], BMP antagonists (chordin and noggin; Fig. 2, H and I), and SMAD signaling molecules (Fig. 2, J–L) revealed that key components of the BMP/SMAD signaling pathway are expressed in both coronary arteries and pulmonary arteries. Among them, the expression of chordin, noggin, ACVR2a, and SMAD4 was significantly different in coronary and pulmonary arteries.
Fig. 1.
A: expression of bone morphogenetic protein (BMP)-4 mRNA in rat coronary arteries, carotid arteries, and pulmonary arteries. Analysis of mRNA expression was performed by quantitative real-time RT-PCR. Hypoxanthine phosphoribosyltransferase (HPRT) was used for normalization. Data are means ± SE; n = 6 for each group. *P < 0.05. B: original Western blot showing greater BMP-4 protein expression in rat pulmonary arteries than in coronary arteries. Identical results were found in 3 independent experiments. C: expression of BMP-2 mRNA in rat coronary arteries, carotid arteries, and pulmonary arteries. Data are means ± SE; n = 6 for each group.
Fig. 2.
mRNA expression of BMP receptors (BMPRs; A–G), BMP antagonists (H and I), and SMAD signaling molecules (J–L) in rat coronary and pulmonary arteries. A: BMPR1a; B: BMPR1b; C: BMPR2; D: activin receptor (ACVR)2a; E: ACVR2b; F: ACVR1; G: activin A receptor, type II-like kinase (ACVRL)1; H: chordin; I: noggin; J: SMAD1; K: SMAD4; L: SMAD5. Analysis of mRNA expression was performed by quantitative real-time RT-PCR. HPRT was used for normalization. Data are means ± SE; n = 5–6 for each group. *P < 0.05.
Effect of BMP-4 on endothelial vasodilator function and ROS production in systemic and pulmonary arteries.
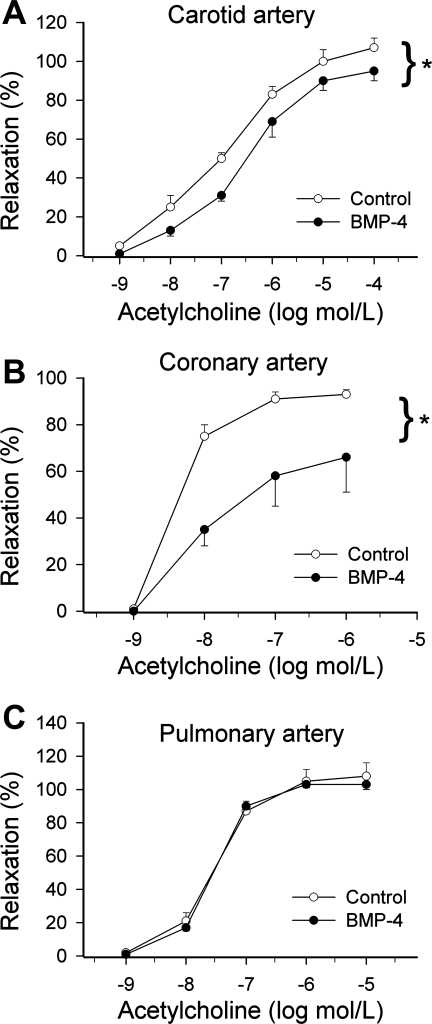
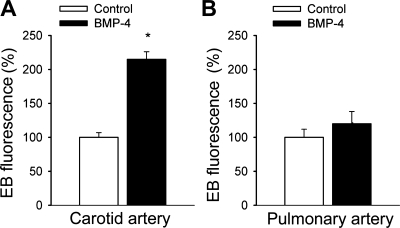
BMP-4 treatment resulted in a significant impairment of acetylcholine-induced relaxation of cultured rat carotid and coronary arteries (Fig. 3, A and B). In contrast, BMP-4 elicited no alterations of vascular relaxations to acetylcholine in cultured segments of the pulmonary artery (Fig. 3C). In BMP-4-treated carotid arteries, there was a significant increase in EB fluorescence, indicating that BMP-4 promotes endothelial O2•− generation in this vascular bed (Fig. 4A). In contrast, BMP-4 treatment did not result in significant increases in EB staining in pulmonary arteries (Fig. 4B).
Fig. 3.
Relaxations to acetylcholine in ring preparations of rat carotid arteries (A), coronary arteries (B), and pulmonary arteries (C) maintained in vessel culture (for 24 h) in the absence and presence of recombinant BMP-4 (10 ng/ml for 24 h). Data are means ± SE; n = 3–6 for each group. *P < 0.05. vs. untreated vessels.
Fig. 4.
Results from dihydroethidine (DHE) staining experiments. Time courses of the build up of ethidium bromide (EB) fluorescence in carotid artery segments (A) and pulmonary artery segments (B) were obtained. Arteries were maintained in vessel culture (for 24 h) in the absence and presence of recombinant BMP-4 (10 ng/ml for 24 h). Bar graphs are summary data for slope factors normalized to tissue mass, representing tissue O2•− production. Data are means ± SE; n = 5 in each group. *P < 0.05.
Effect of BMP-4 on ROS production in CAECs and PAECs.
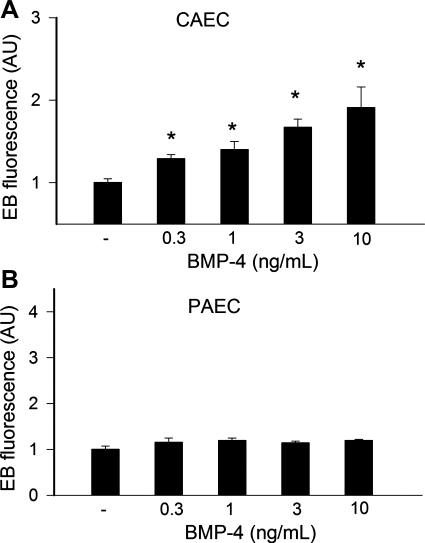
To test the effect of BMP-4 directly on endothelial ROS generation, cultured CAECs and PAECs were treated with BMP-4. Previously, we have shown that the acute administration of BMPs can rapidly activate NAD(P)H oxidase in CAECs, likely via activation of PKC (3). In addition, chronic treatment with BMP4 also upregulated NAD(P)H oxidase expression in systemic ECs, resulting in sustained increases in ROS production (25, 26). Because our working hypothesis was that BMP4 acts as a proatherogenic cytokine in the systemic circulation, in the present study we chose a 24-h timepoint to test the chronic effects of BMP-4 on endothelial ROS production. Plate reader-based hydroethidine staining assays showed that, compared with untreated controls, chronic treatment with BMP-4 significantly increased EB staining in CAECs (Fig. 5A), whereas it did not have any significant effect on O2•− production in PAECs (Fig. 5B). The NADPH oxidase inhibitors apocynin (3 × 10−4 mol/l) and diphenyleneiodonium (DPI; 10−6 mol/l) prevented BMP-4-induced increases in EB fluorescence in CAECs (10 ng/ml BMP-4 + apocynin: 94 ± 7% and 10 ng/ml BMP-4 + DPI: 97 ± 18%). Apocynin and DPI had no significant effect on EB staining in BMP-4-treated PAECs.
Fig. 5.
A: compared with untreated controls, BMP-4 elicited significant increases in O2•− production in coronary arterial endothelial cells (CAECs), as indicated by the intensive EB fluorescent signal (plate reader-based DHE staining assay). B: in pulmonary arterial endothelial cells (PAECs), BMP-4 treatment (for 24 h) did not affect O2•− generation. AU, arbitrary units. Data are means ± SE; n = 8 for each group. *P < 0.05. vs. untreated cells.
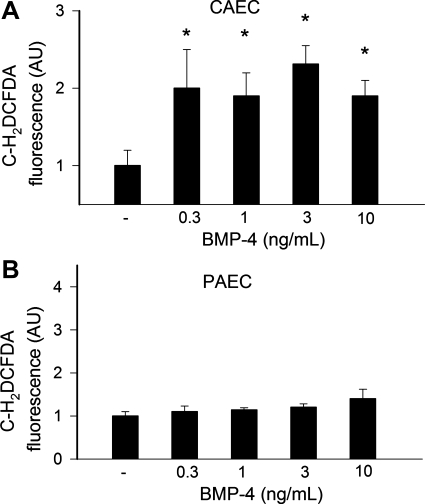
Plate reader-based C-H2DCFDA assays yielded identical results, showing that BMP-4 treatment significantly increased cellular C-H2DCFDA fluorescence in CAECs (Fig. 6A), whereas it did not have any significant effect on H2O2 production in PAECs (Fig. 6B). Apocynin (3 × 10−4 mol/l) and DPI (10−6 mol/l) prevented BMP-4 induced increases in C-H2DCFDA fluorescence in CAECs (10 ng/ml BMP-4 + apocynin: 107 ± 18% and 10 ng/ml BMP-4 + DPI: 89 ± 22%). Apocynin and DPI had no significant effect on C-H2DCFDA fluorescence in BMP-4-treated PAECs.
Fig. 6.
A: compared with untreated controls, BMP-4 elicited significant increases in H2O2 production in CAECs, as indicated by the intensive 5(and 6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (C-H2DCFDA) fluorescent signal (plate reader-based C-H2DCFDA assay). B: in PAECs, BMP-4 treatment (for 24 h) did not affect H2O2 generation. Data are means ± SE; n = 8 for each group. *P < 0.05. vs. untreated cells.
Comparison of NAD(P)H oxidase expression in coronary and pulmonary arteries.
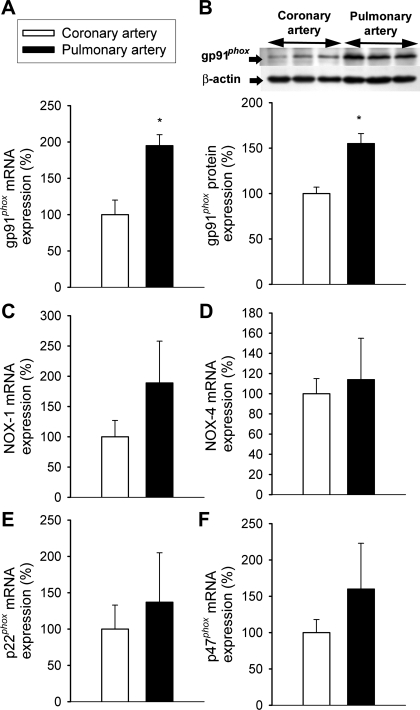
To test the hypothesis that differences in the expression of NAD(P)H oxidases may contribute to the differential effects of BMP-4 on endothelial NAD(P)H oxidase-derived ROS production in the systemic and pulmonary vasculature, we compared the expression of NAD(P)H oxidase in rat coronary and pulmonary arteries. We found that the expression of gp91phox (both at the mRNA and protein level) was more prominent in pulmonary arteries than in coronary vessels (Fig. 7, A and B). mRNA expression of NAD(P)H oxidase 1, NAD(P)H oxidase 4, p47phox, and p22phox did not differ significantly between pulmonary arteries and coronary arteries (Fig. 7, C–F).
Fig. 7.
A: mRNA expression of gp91phox in rat coronary and pulmonary arteries. Analysis of mRNA expression was performed by quantitative real-time RT-PCR. HPRT was used for normalization. Data are means ± SE; n = 5 for each group. *P < 0.05. B: original Western blot showing the expression of gp91phox protein in rat coronary and pulmonary arteries. β-Actin content was used for normalization. Bar graphs are summary densitometric data (data are expressed as percentages of control mean values ± SE). Data from 2 independent experiments were pooled; n = 6 in each group. *P < 0.05. C–F: mRNA expression of NAD(P)H oxidase 1 (NOX1; C), NAD(P)H oxidase 4 (NOX4; D), p47phox (E), and p22phox (F) did not differ significantly between pulmonary arteries and coronary arteries (n = 5 in each group).
Effect of BMP-4 on NF-κB activation in CAECs and PAECs.
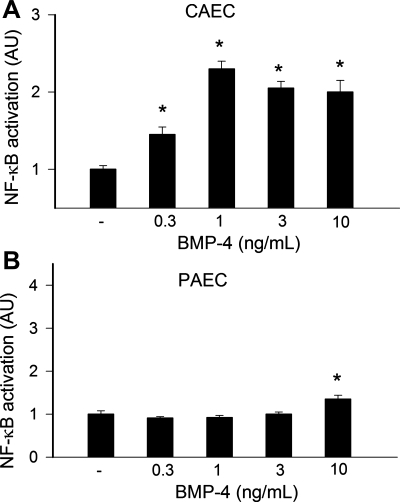
To determine the effect of BMP-4 on NF-κB activation, we transiently transfected CAECs and PAECs with a NF-κB-driven reporter gene construct and then treated cells with BMP-4. A significant increase in luciferase activity over the vector control was noted upon stimulation with low concentrations of BMP-4 in CAECs (Fig. 8A). Interestingly, BMP-4 did not enhance the transcriptional activity of NF-κB in PAECs in the concentration range of 0.3–3 ng/ml. Small but significant NF-κB activation was observed only at the highest BMP-4 concentration administered (10 ng/ml; Fig. 8B).
Fig. 8.
Reporter gene assay showing the effects of BMP-4 on NF-κB reporter activity in CAECs (A) and PAECs (B). Cells were transiently cotransfected with NF-κB-driven firefly luciferase and cytomegalovirus-driven Renilla luciferase constructs followed by BMP-4 stimulation. After 24 h, cells were lysed and subjected to luciferase activity assays. After normalization, the relative luciferase activity was obtained from 7 independent transfections. Data are means ± SE. *P < 0.05. vs. untreated cells.
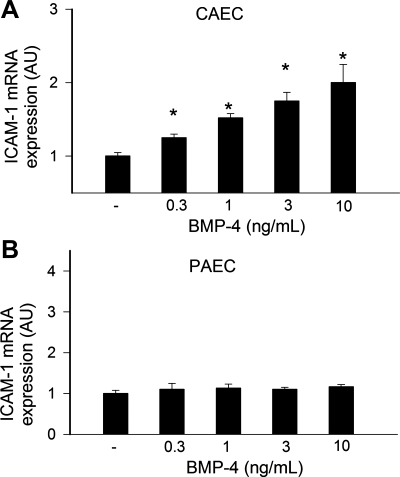
Effect of BMP-4 on ICAM-1expression in CAECs and PAECs.
We also found that BMP-4 significantly upregulated the expression of the known NF-κB target ICAM-1 in CAECs (Fig. 9A). In contrast, BMP-4 had no significant effect on ICAM-1 expression in PAECs (Fig. 9B). As positive control, we assessed the expression of Id1. The Id1 gene encodes a negative regulator of basic helix-loop-helix transcription factors, which has been shown to be a direct target of BMP proteins in multiple cell types. We found that BMP-4 upregulated Id1 in both CAECs (control: 100 ± 19%, 1 ng/ml BMP-4: 210 ± 21%, 3 ng/ml BMP-4: 264 ± 40%, and 10 ng/ml BMP-4: 230 ± 25%, P < 0.05) and PAECs (control: 100 ± 15%, 1 ng/ml BMP-4: 182 ± 23%, 3 ng/ml BMP-4: 185 ± 27%, and 10 ng/ml BMP-4: 186 ± 19%, P < 0.05).
Fig. 9.
Effect of BMP-4 on ICAM-1 mRNA expression in CAECs (A) and PAECs (B). Analysis of mRNA expression in cells treated with BMP4 for 24 h was performed by quantitative real-time RT-PCR. HPRT was used for normalization. Data are means ± SE; n = 6 for each group. *P < 0.05. vs. untreated controls.
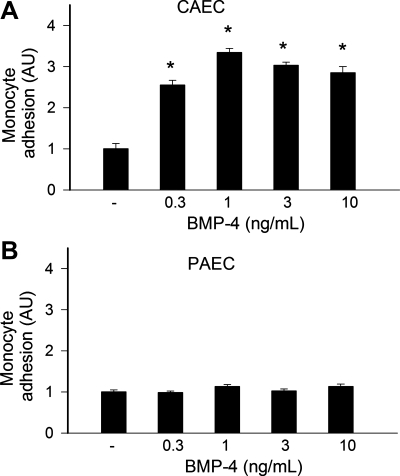
Effect of BMP-4 on monocyte adhesiveness to CAECs and PAECs.
BMP-4 treatment significantly increased monocyte adherence to cultured CAECs in a concentration-dependent manner (Fig. 10A). In contrast, BMP-4 failed to induce increases in monocyte adhesiveness in PAECs (Fig. 10B).
Fig. 10.
Results of the monocyte adhesion assay (see methods). A: treatment of CAECs with increasing concentrations of BMP-4 for 24 h significantly increased the adhesion of fluorescently labeled PMA-stimulated THP-1 monocytes. B: monocyte adhesiveness to PAECs was unaffected by BMP-4 treatment. Data are means ± SE; n = 6 for each group. *P < 0.05. vs. untreated controls.
DISCUSSION
There were three major findings in this study. First, we have shown that BMP-4 is more abundant in pulmonary arteries than in vessels from the systemic circulation (Fig. 1, A and B). The expression of BMP-2 did not differ between these vascular beds (Fig. 1C). BMP-2 is related to BMP-4 by its amino acid sequence, and they act on the same receptors. However, BMP-2 is transcribed from an entirely different gene, and our previous studies have shown that the promoter regions of the two genes and their transcriptional regulation show little similarity (3, 4). Analysis of the expression profile of BMPRs (BMPR1a, BMPR1b, BMPR2, ACVR2a, ACVR2b, ACVR1, and ACVRL1; Fig. 2, A–G), BMP antagonists (chordin and noggin; Fig. 2, H and I), and SMAD signaling molecules (Fig. 2, J–L) revealed that key components of the BMP/SMAD signaling pathway are transcribed in both coronary arteries and pulmonary arteries.
Many lines of evidence have suggested that BMPs function as prooxidant, prohypertensive, and proatherogenic mediators in systemic arteries (3, 16, 19, 25). The second interesting finding of the present study was that while BMP-4 elicited significant endothelial dysfunction in systemic arteries (3, 31) (Fig. 3, A–B), pulmonary arteries were completely protected from the adverse functional effects of BMP-4 (Fig. 3C). As noted at the outset, our previous studies have provided ample evidence that BMPs can upregulate and activate NAD(P)H oxidase in systemic ECs (3, 19, 25, 26, 31). First, BMP4 treatment (both in vitro and in vivo) induces endothelial dysfunction and O2•− generation in systemic arteries, which can be reversed by the NAD(P)H oxidase inhibitor apocynin (3, 19, 31). Although recent studies have indicated that apocynin at high concentrations may also exert direct antioxidant effects (14), there is also strong molecular biology evidence to support the role of NAD(P)H oxidases in BMP-induced oxidative stress. Second, we previously have shown that the administration of BMPs activated ERK1/2 phosphorylation in CAECs within 5 min (3), which could be attenuated by inhibition of NAD(P)H oxidase (3). These data strongly support the view that BMPs can elicit rapid increases in ROS generation in systemic ECs, likely via the activation of PKC (3). Third, BMP4 also upregulates NAD(P)H oxidase expression in systemic ECs (19, 25, 26). In systemic arteries and CAECs, BMP-induced oxidative stress (and endothelial dysfunction) has been shown to be sustained and present both in vitro after a 24-h BMP treatment (3, 31) and in animals chronically infused with BMP-4 (19). Because our working hypothesis was that BMP-4 acts as a proatherogenic cytokine in the systemic circulation, in the present study we chose a 24-h time point to test its chronic effects on endothelial ROS production. Fourth, BMP4-mediated ROS generation and activation of systemic ECs can be inhibited by knockdown of NAD(P)H oxidase 1 (by short interfering RNA). Moreover, BMP-4 induced activation of NAD(P)H oxidases, endothelial dysfunction, and hypertension in wild-type mice were prevented in p47phox-null mice (25). These data are in line with the evidence that BMP-4 fails to induce monocyte adhesion to ECs from p47phox−/− mice, but its ability to cause endothelial activation was restored when cells were transfected with p47phox plasmid (19, 25, 26). Taken together, these results strongly suggest that NAD(P)H oxidases are implicated in the endothelial oxidative stress induced by BMP-4 in the systemic circulation. Importantly, our present study confirmed that BMP-4 elicits oxidative stress in cultured carotid arteries (Fig. 4A) and CAECs (Figs. 5A and 6A). In contrast, we found that in cultured pulmonary arteries (Fig. 4B) and PAECs (Figs. 5B and 6B) BMP-4 did not increase O2•− and H2O2 generation.
From a pathophysiological standpoint, the increase in endothelial oxidative stress that attends the upregulation of BMPs in the walls of systemic arteries would be expected to contribute to increased vasoconstriction and induction of hypertension (19) and the progression of atherosclerotic plaque development (16). Pulmonary arteries, in which BMP-4 does not induce ROS production, do not exhibit BMP-4 induced endothelial dysfunction and are known to be resistant to atherogenesis. It is likely that the differential effect of BMP-4 on NADPH oxidase activity is responsible for the phenomenon that high circulating levels of BMP-4 selectively induce systemic (but not pulmonary) hypertension (19). Interestingly, BMP-4 has also been reported to induce oxidative stress in human umbilical vein ECs (25), suggesting that in this regard ECs from the arterial and venous side of the systemic circulation are more similar than CAECs and PAECs.
One could hypothesize that the differential effects of BMP-4 on endothelial NAD(P)H oxidase-derived ROS production may be a consequence of a lower expression of NAD(P)H oxidases in the pulmonary vasculature. However, this hypothesis could be refuted because we found that in pulmonary arteries both the regulatory and oxidase subunits of NAD(P)H oxidase are abundantly expressed (Fig. 7). Interestingly, the expression of gp91phox, the oxidase subunit of the major endothelial NAD(P)H oxidase, was even higher in pulmonary arteries than in coronary vessels (Fig. 7, A and B), extending recent findings in bovine coronary arteries and pulmonary vessels (12). A more likely explanation is that BMPR activation may induce different intracellular signaling events in PAECs and CAECs. Previous findings have shown that in CAECs, BMPs activate NAD(P)H oxidase likely via a pathway that involves PKC activation [as suggested by the inhibitory effect of chelerythrine on this process (3)]. Indeed, our previous studies have shown that endothelial NADPH oxidase activity in systemic arteries is regulated by PKC in a calcium-dependent manner (28, 29). Furthermore, there is evidence for the association of the BMPR complex with PKC (13). Thus, future studies should compare the effect of BMP-4 on PKC in both cell types.
It is also possible that different subsets of BMPRs are expressed in systemic and pulmonary ECs, which would predominantly activate different signaling pathways. In support of this point of view, our study revealed that the expression pattern of BMPRs seemed to differ in systemic arteries and pulmonary vessels (Fig. 2). Further studies are evidently needed to determine which receptor is responsible for NADPH oxidase activation in CAECs and whether the differences between BMP-induced ROS generation can be explained by the differential expression of BMPRs and/or their downstream targets. While it is unlikely that SMAD-dependent expression of Id1 is critical for acute BMP-induced ROS production in CAECs, it has yet to be determined whether SMAD-1 or Id1 are needed for the upregulation of NAD(P)H oxidase in ECs by chronic BMP-4 treatment. Recent studies have shown that BMP antagonists (e.g., follistatin, noggin, and matrix gla protein-1) are expressed in systemic arterial ECs, which seem to regulate BMP activity in the vascular wall (1). BMP antagonists are also expressed in pulmonary arteries. Yet, it is unlikely that BMP antagonists are responsible for the blunted BMP-4 responses in these vessels, because their expression is not enriched in pulmonary arteries (Fig. 2, H and I).
Recent studies have demonstrated a striking upregulation of BMPs in atherosclerosis-prone vascular regions and atherosclerotic lesions (10, 20, 25, 26, 34), and hypotheses have been put forward that endothelium-derived BMPs contribute to vascular calcification (for reviews, see Refs. 15 and 17). In vitro, BMPs have been shown to exert proinflammatory effects in systemic arteries eliciting endothelial activation and thereby enhancing monocyte adhesiveness (1, 3, 7, 25, 26). There is evidence that in ECs of systemic arteries, BMPs activate NF-κB and the expression of adhesion molecules, at least in part, via NADPH oxidase-dependent pathways (3, 26). In addition to clarifying the differential effects of BMP-4 on ROS generation with respect to endothelial dysfunction, the third important finding of this study suggested that BMP-4 also exerts proinflammatory effects selectively in systemic arteries. In this context, we have demonstrated that while BMP-4 induces NF-κB activation (Fig. 8A) and ICAM-1 expression in CAECs (Fig. 9A), these proinflammatory effects are substantially blunted in PAECs (Figs. 8B and 9B). Accordingly, BMP-4 significantly increased monocyte adhesiveness to CAECs (Fig. 10A), whereas monocyte adhesion to PAECs were not induced by BMP-4 (Fig. 10B). We attribute the lack of BMP-4-induced endothelial activation in PAECs to the superior resistance of these cells to the prooxidant effects of BMP-4.
Collectively, our experiments are the first to provide unambiguous evidence in support of the hypothesis that BMP-4 exerts prooxidant, prohypertensive, and proinflammatory effects only in the systemic circulation, whereas PAECs are resistant to these adverse effects of BMP-4. Future studies should determine whether alterations in BMPR signaling (e.g., due to mutations affecting BMPRs in idiopathic PAH) would unmask prooxidant and prohypertensive effects of BMP-4 in the pulmonary circulation as well. In addition, BMP-4 secreted within the vascular wall also affects the function and phenotype of vascular smooth muscle cells in an autocrine/paracrine manner (35). Thus, further studies are definitely needed to elucidate differential effects of BMP-4 on the phenotype and function of smooth muscle cells in systemic arteries and pulmonary vessels.
GRANTS
This work was supported by American Heart Association Grants 0430108N and 0435140N and National Heart, Lung, and Blood Institute Grants HL-077256 and HL-43023 (to Z. Ungari).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation 116: 1258–1266, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Cola C, Almeida M, Li D, Romeo F, Mehta JL. Regulatory role of endothelium in the expression of genes affecting arterial calcification. Biochem Biophys Res Commun 320: 424–427, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Csiszar A, Ahmad M, Smith KE, Labinskyy N, Gao Q, Kaley G, Edwards JG, Wolin MS, Ungvari Z. Bone morphogenetic protein-2 induces proinflammatory endothelial phenotype. Am J Pathol 168: 629–638, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csiszar A, Labinskyy N, Smith KE, Rivera A, Bakker ETP, Jo H, Gardner J, Orosz Z, Ungvari Z. Downregulation of BMP-4 expression in coronary arterial endothelial cells: role of shear stress and the cAMP/PKA pathway. Arterioscler Thromb Vasc Biol 27: 776–782, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Csiszar A, Labinskyy N, Zhao X, Hu F, Serpillon S, Huang Z, Ballabh P, Levy RJ, Hintze TH, Wolin MS, Austad SN, Podlutsky A, Ungvari Z. Vascular superoxide and hydrogen peroxide production and oxidative stress resistance in two closely related rodent species with disparate longevity. Aging Cell 6: 783–797, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Csiszar A, Smith K, Labinskyy N, Orosz Z, Rivera A, Ungvari Z. Resveratrol attenuates TNF-α-induced activation of coronary arterial endothelial cells: role of NF-κB inhibition. Am J Physiol Heart Circ Physiol 291: H1694–H1699, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation 111: 2364–2372, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genomics 17: 21–30, 2004. [DOI] [PubMed] [Google Scholar]
- 9.De Caestecker M, Meyrick B. Bone morphogenetic proteins, genetics and the pathophysiology of primary pulmonary hypertension. Respir Res 2: 193–197, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 21: 1998–2003, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Griethe W, Schmitt R, Jurgensen JS, Bachmann S, Eckardt KU, Schindler R. Bone morphogenic protein-4 expression in vascular lesions of calciphylaxis. J Nephrol 16: 728–732, 2003. [PubMed] [Google Scholar]
- 12.Gupte SA, Kaminski PM, Floyd B, Agarwal R, Ali N, Ahmad M, Edwards J, Wolin MS. Cytosolic NADPH may regulate differences in basal Nox oxidase-derived superoxide generation in bovine coronary and pulmonary arteries. Am J Physiol Heart Circ Physiol 288: H13–H21, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Hassel S, Eichner A, Yakymovych M, Hellman U, Knaus P, Souchelnytskyi S. Proteins associated with type II bone morphogenetic protein receptor (BMPR-II) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 4: 1346–1358, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Hruska KA, Mathew S, Saab G. Bone morphogenetic proteins in vascular calcification. Circ Res 97: 105–114, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Jo H, Song H, Mowbray A. Role of NADPH oxidases in disturbed flow- and BMP4- induced inflammation and atherosclerosis. Antioxid Redox Signal 8: 1609–1619, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res 99: 1044–1059, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest 101: 927–934, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, Jo H. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation 113: 2818–2825, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Mohler ER, 3rd Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation 103: 1522–1528, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Nimmagadda S, Geetha Loganathan P, Huang R, Scaal M, Schmidt C, Christ B. BMP4 and noggin control embryonic blood vessel formation by antagonistic regulation of VEGFR-2 (Quek1) expression. Dev Biol 280: 100–110, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Nomura S, Takano-Yamamoto T. Molecular events caused by mechanical stress in bone. Matrix Biol 19: 91–96, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Rabinovitch M The mouse through the looking glass: a new door into the pathophysiology of pulmonary hypertension. Circ Res 94: 1001–1004, 2004. [DOI] [PubMed] [Google Scholar]
- 24.San Martin A, Du P, Dikalova A, Lassegue B, Aleman M, Gongora MC, Brown K, Joseph G, Harrison DG, Taylor WR, Jo H, Griendling KK. Reactive oxygen species-selective regulation of aortic inflammatory gene expression in Type 2 diabetes. Am J Physiol Heart Circ Physiol 292: H2073–H2082, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 95: 773–779, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 278: 31128–31135, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol 23: 418–424., 2003. [DOI] [PubMed] [Google Scholar]
- 28.Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation 108: 1253–1258, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: Involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol 165: 219–226, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ungvari Z, Orosz Z, Rivera A, Labinskyy N, Xiangmin Z, Olson S, Podlutsky A, Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am J Physiol Heart Circ Physiol 292: H2417–H2424, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Ungvari ZI Endothelium-derived bone morphogenic protein antagonists may counteract the proatherogenic vascular effects of bone morphogenic protein 4. Circulation 116: 1221–1223, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, Sideras P, ten Dijke P. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation 106: 2263–2270, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Voelkel NF, Cool C, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest 114: 225S–230S, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Willette RN, Gu JL, Lysko PG, Anderson KM, Minehart H, Yue T. BMP-2 gene expression and effects on human vascular smooth muscle cells. J Vasc Res 36: 120–125, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA, Kawai N, Bloch KD. Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J Biol Chem 283: 3877–3888, 2008. [DOI] [PubMed] [Google Scholar]