Abstract
Vasoconstrictors activate phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2), leading to calcium mobilization, protein kinase C activation, and contraction. Our aim was to investigate whether PLC-δ1, a PLC isoform implicated in α1-adrenoreceptor signaling and the pathogenesis of hypertension, is involved in noradrenaline (NA) or endothelin (ET-1)-induced PIP2 hydrolysis and contraction. Rat mesenteric small arteries were studied. Contractility was measured by pressure myography, phospholipids or inositol phosphates were measured by radiolabeling with 33Pi or myo-[3H]inositol, and caveolae/rafts were prepared by discontinuous sucrose density centrifugation. PLC-δ1 was localized by immunoblot analysis and neutralized by delivery of PLC-δ1 antibody. The PLC inhibitor U73122, but not the negative control U-73342, markedly inhibited NA and ET-1 contraction but had no effect on potassium or phorbol ester contraction, implicating PLC activity in receptor-mediated smooth muscle contraction. PLC-δ1 was present in caveolae/rafts, and NA, but not ET-1, stimulated a rapid twofold increase in PLC-δ1 levels in these domains. PLC-δ1 is calcium dependent, and removal of extracellular calcium prevented its association with caveolae/rafts in response to NA, concomitantly reducing NA-induced [33P]PIP2 hydrolysis and [3H]inositol phosphate formation but with no effect on ET-1-induced [33P]PIP2 hydrolysis. Neutralization of PLC-δ1 by PLC-δ1 antibody prevented its caveolae/raft association and attenuated the sustained contractile response to NA compared with control antibodies. In contrast, ET-1-induced contraction was not affected by PLC-δ1 antibody. These results indicate the novel and selective role of caveolae/raft localized PLC-δ1 in NA-induced PIP2 hydrolysis and sustained contraction in intact vascular tissue.
Keywords: signal transduction, vascular smooth muscle, phosphoinositide, caveolae, lipid rafts
vascular tone is an important determinant of peripheral vascular resistance and blood pressure, and increased responsiveness to the vasoconstrictors noradrenaline (NA) and endothelin (ET)-1 contributes to pathological states, such as vasospasm and hypertension (9, 15). In vascular tissues, NA and ET-1 activate G protein-coupled receptors (GPCR) linked to the phosphoinositide (PI) signaling system. Activation of this pathway results in phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis, producing the second messengers inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 induces calcium release from intracellular stores and triggers extracellular calcium influx through activation of store-operated calcium channels, leading to contraction, while DAG activates protein kinase C (PKC), implicated in both contractility and proliferation (43). Additionally, PIP2 hydrolysis and increased intracellular calcium induce actin cytoskeleton reorganization, further facilitating contraction (50). Thus vasoconstrictor hormone activation of the PI signaling system is central to regulation of small artery tone and peripheral vascular resistance, and aberrations in this signaling pathway may underlie alterations in the peripheral vasculature in cardiovascular disease.
Hydrolysis of PIP2 is mediated by PI-specific phospholipase C (PLC), a family of 11 isoforms (reviewed in Ref. 40), of which PLC-β1, -β2, -δ1, and -γ1 have been identified in vascular smooth muscle (VSM) (28). Of these, previous research has implicated PLC-β2 in α-adrenergic responses (28). Cotransfection studies have shown that α1-adrenoreceptors (AR) can couple via Gαq family members to PLC-β (46), and, in rat tail artery, α1-AR stimulation induced the translocation of PLC-β2 from the cytosol to the plasma membrane (28). However, GPCR agonists can also activate PLC-γ (31) and PLC-δ1 (26, 35), and so the PLC isoforms involved in vasoconstrictor-stimulated PIP2 hydrolysis in VSM are still unclear. Studies have shown that α1-AR agonists activate PLC-δ1 (35) in a calcium-dependent manner (40) and that its activity is increased in hypertension (23, 27). Taken together, this suggests that PLC-δ1 may be important for GPCR responses in VSM.
It is now accepted that subcellular localization and targeting of signaling molecules are important for regulating signal transduction in biological systems. In rat mesenteric small arteries (RMSA), hydrolysis of PIP2 in response to both NA and ET-1 is restricted to caveolae/lipid rafts (12) plasma membrane microdomains implicated in VSM signal transduction and contractility (7). Recently, a PLC-δ1 binding protein, p122/RhoGAP, has been detected in caveolae (49), suggesting that PLC-δ1 may also localize to caveolae/rafts and could potentially be involved in PIP2 hydrolysis within these domains. Given the importance of the PI pathway in VSM contraction and the lack of data on the microdomain localization of PLC isoforms in vascular tissues, we investigated whether PLC-δ1 is involved in NA- and ET-1-induced PIP2 hydrolysis in caveolae/lipid rafts and its functional role in small-artery contraction. As there is evidence that α1-AR regulates blood pressure (44), and increased PLC-δ1 activity has been implicated in hypertension (27), these studies are of particular relevance in the context of this disease.
MATERIALS AND METHODS
The investigation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals, published by the US National Institutes of Health (publication no. 85–23, revised 1996), The University of Manchester Animal Experimentation Guidelines, and the U.K. Animals (Scientific Procedures) Act 1986. Experiments were performed with the approval of the Review Board of the University of Manchester and the Home Office.
Animals and incubation conditions.
Adult female Sprague-Dawley rats (8–10 wk of age, body weight 180–220 g) were used for all experiments. A minimum of three and maximum of five animals were used for each treatment, as detailed in Figs. 1–8 legends. The mesentery was excised and placed in ice-cold physiological salt solution. Mesenteric small arteries (internal diameter <400 μm) were cleaned of adherent fat and connective tissue and dissected from the mesenteric bed. Unless stated otherwise, arteries were equilibrated in 1 ml of tissue culture media M199 (Invitrogen) for 1 h at 37°C, before stimulation with NA (15 μM) or ET-1 (100 nM) for various time points.
Fig. 1.
Phospholipase C (PLC) activity is required for noradrenaline (NA) and endothelin (ET)-1 contraction. The contractile response of rat mesenteric small arteries (RMSA) to NA (15 μM; A), ET-1 (100 nM; B), high-potassium physiological salt solution (KPSS; 50 mM; C), or phorbol 12,13-dibutyrate (PdBu; 1 μM; D) was obtained in the presence of PLC inhibitor U73122 (3 μM) or its negative control U73342 (3 μM), as described in materials and methods. Values are means ± SE (n = 3). *P < 0.05.
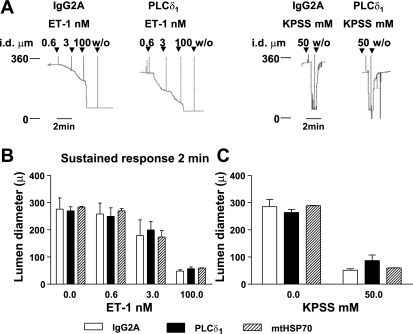
Fig. 8.
PLC-δ1 antibody does not affect contraction to ET-1 or KPSS. Lumen diameter was recorded from arteries mounted in a pressure myograph following delivery of 3-μg anti-IgG2A or anti-PLC-δ1 or anti-mtHSP70, as described in materials and methods. A: arteries were stimulated with ET-1 or KPSS, as indicated. The tracings represent 4 separate experiments. B: lumen diameter at 2 min after addition of ET-1 was recorded. Values are means ± SE; n = 4 PLC-δ1 or IgG2A, n = 3 mtHSP70. C: lumen diameter at 1 min after addition of KPSS was recorded. Values are means ± SE; n = 6 PLC-δ1 n = 5 IgG2A, n = 3 mtHSP70.
Isolation of caveolae/raft microdomains.
Caveolae/raft-enriched microdomains were purified from RMSA, as described previously (12). Briefly, arteries were homogenized in 0.5 M Na2CO3 (pH 11), the protein concentration was adjusted to 1 mg/ml, and 450 μl of homogenate were separated by discontinuous (40%:35%:5%) sucrose density gradient centrifugation. Thirteen fractions were collected, and fractions 2–5 at the 5–35% interface were pooled as caveolae/rafts. Using this method, we have shown that these fractions are enriched in caveolae and lipid raft markers: caveolin-1, cholesterol, and ganglioside GM1 (12).
Immunoblotting.
Proteins in the caveolae/raft fractions were precipitated with 5% trichloroacetic acid and processed for SDS-PAGE and Western blot analysis with monoclonal anti-PLC-δ1 diluted 1:200 (Clone S-11–2, Upstate), or polyclonal anti-PLC-δ1 diluted 1:500 (H-140, Santa Cruz Biotechnology), or anti-caveolin-1 diluted 1:10,000 (Clone 2297, Transduction Laboratories). Signals were developed by horseradish peroxidase-conjugated secondary antibody and chemiluminescence (Pierce). Signal intensity was quantified by densitometry (BioRad GS800 Densitometer), and images showing saturation were not used in analysis. Following caveolae/raft preparation, fresh dilutions of PLC-δ1 antibody detected a doublet; the upper band corresponding to the expected molecular mass of PLC-δ1 was used for densitometry.
Cell culture and transfection of plasmids for specificity of PLC-δ1 antibodies.
Mouse embryonic fibroblast cells (MEF) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Mouse PLC-δ1, human PLC-δ3, and rat PLC-δ4 were subcloned into FLAG-tagged expression vector pcDNA3 (21) and transiently expressed in MEF cells using Lipofectamine Plus (Invitrogen). At 24 h after transfection, cells were lysed in RIPA buffer and processed for immunoblotting. VSM cells (VSMC) were isolated from Sprague-Dawley rat thoracic aorta by enzymatic digestion (17) and maintained in Dulbecco's modified Eagle's medium/15% fetal bovine serum. Total cell lysates for immunoblotting were prepared by lysing cells in RIPA buffer.
Measurement of endogenously labeled phospholipids.
RMSA phospholipids were labeled with 33Pi, tissue was processed for caveolae/raft separation, lipids were extracted, and [33P]PIP2 was separated from other [33P]phospholipids, as previously described (37). Radiolabeled lipids were quantified by electronic autoradiography (InstantImager, Perkin Elmer) and identified by use of cochromatographed standards.
Removal of extracellular calcium.
To determine the effect of calcium removal, tissues were incubated in either calcium-free HEPES buffer containing 1 mM EGTA for 10 min (36), or in nominally calcium-free HEPES buffer for 30 min, before stimulation with agonist (NA, 15 μM; ET-1, 100 nM) or vehicle (distilled H2O).
[3H]inositol phosphate accumulation.
Total [3H]inositol phosphate ([3H]InsPx) accumulation was determined by incubating arteries for 2 h with 10 μCi myo-[3H]inositol, as described previously (38). Following radiolabeling, vessels were transferred to 0.5 ml M199 or calcium-free HEPES buffer containing 10 mM LiCl for 10 min before stimulation with 15 μM NA or vehicle for 1 min. Reactions were terminated by homogenization of arteries in 0.5-ml ice-cold 10% trichloroacetic acid. Samples were neutralized, and the [3H]InsPx fraction was recovered by Dowex anion exchange chromatography column and quantified using liquid scintillation counting (6).
Antibody delivery.
Chariot protein transfection reagent (ActiveMotif) was used to deliver antibodies to intact RMSA. This transfection reagent is able to deliver antibodies into cells while preserving their ability to localize to the proper cellular compartment and to recognize antigens within the cell (32). Recently, Chariot has been used successfully to deliver proteins to lung and vascular tissue (4, 25, 30). Chariot/antibody complexes were prepared and used according to the manufacturer's instructions. Briefly, dissected arteries were incubated in 0.5 ml Leibovitz medium (L15, Invitrogen) for 30 min at 37°C. For each transfection, 6 μl Chariot in 100 μl 40% dimethyl sulfoxide were mixed with 3 μg antibody in 100 μl phosphate-buffered saline and incubated at room temperature for 30 min to allow the complex to form. The arteries were transferred to a fresh microfuge tube, overlaid with 200 μl Chariot/antibody complex, and mixed gently. L15 medium (400 μl) was added, and the tissues were incubated for 1 h at 37°C before addition of 750 μl L15 and a further 2 h incubation at 37°C. Following incubation, arteries were washed in phosphate-buffered saline and mounted in a pressure arteriograph for measurement of contractility or transferred to 0.5 ml M199 for 15 min at 37°C before stimulation with NA (15 μM, 2 min) or vehicle (distilled H2O) and processed for caveolae/raft separation. Antibodies delivered were as follows: anti-PLC-δ1 clone S-11–2 (mouse monoclonal IgG2A, Upstate) and, as controls, anti-mouse IgG2A (Dako) or anti-mitochondrial heat shock protein 70 (mtHSP70) clone JG1 (mouse monoclonal IgG3, Affinity BioReagents).
Measurement of contractile responses.
Contractile responses of small arteries were measured using pressure myography (Living Systems), as described previously (12). After equilibration at 20 mmHg and 37°C in physiological salt solution (pH 7.4, gassed with 5% CO2 in air), intraluminal pressure was raised to 70 mmHg, and the vessel left to stabilize for 15 min before addition of 50 mM KPSS (high-potassium physiological salt solution, molar substitution with NaCl) or cumulative concentrations of NA (0.6, 3, and 15 μM) or ET-1 (0.6, 3, and 100 nM) at 2-min intervals. A dual-chamber organ bath was used, allowing study of paired arterial segments in each experiment: a control artery that had received anti-mouse-IgG2A or anti-mtHSP70, and a test artery that had received anti-PLC-δ1. The concentrations of agonist used were chosen to cause minimal, submaximal, and maximal contraction. The total protocol took ∼90 min, thus minimizing the time for degradation of antibodies.
To study the effect of PLC inhibition on contractility, nontransfected arteries were incubated in 3 μM U73122 or 3 μM U73342 for 30 min before addition of 15 μM NA, 100 nM ET-1, 1 μM 12, 13-dibutyrate (PdBu), or 50 mM KPSS. To minimize nonspecificity, the concentration of U73122 was titrated from 0.5 to 10 μM against 15 μM NA and 50 mM KPSS (not shown). At 10 μM U73122, a concentration frequently used to inhibit PI-PLC (2) contraction to 50 mM KPSS was completely abolished, suggesting nonspecific effects on contractility; 5 μM also reduced contraction to 50 mM KPSS, whereas preincubation with 3 μM for 30 min had no effect on the response to 50 mM KPSS but did inhibit 15 μM NA. Accordingly, we used U73122 and the negative control U73342 at 3 μM in the present study. Individual segments were used for each treatment.
For all experiments, endothelial function was tested by the addition of 10 μM acetylcholine to arteries precontracted with 15 μM NA or 50 mM KPSS; any tissues that failed to dilate were discounted from the study.
Materials.
ET-1 was purchased from Calbiochem, TLC Plates (Merck 5721) and “HiPerSolv” grade solvents were from VWR International (Leics, UK). [33P]PO4 (specific activity 370 MBq/ml) was from Amersham International (Amersham, Bucks, UK), and myo-[3H]inositol (specific activity 370–925 GBq/mmol) from NEN. NA and all other chemicals were supplied by Sigma Chemicals.
Statistical analysis.
Comparisons between two groups were analyzed by Student's t-test, whereas comparisons between multiple groups were analyzed by repeated-measures two-way ANOVA comparison. P < 0.05 was considered statistically significant, with n = number of experiments as indicated.
RESULTS
Inhibition of PLC activity reduces NA- and ET-1-induced contraction.
To investigate the role of PLC in vasoconstrictor hormone-induced contraction, we first measured RMSA contractility in response to NA and ET-1 in the presence of the PLC inhibitor U73122 (3 μM) or its negative control U73342 (3 μM) (8). To avoid specificity issues, inhibitor concentrations were titrated from 0.5 to 10 μM to give the minimum effective concentration (3 μM; not shown), while agonist concentrations were chosen as those that induce PIP2 hydrolysis and maximum contraction in RMSA (12). As can be seen, PLC inhibition substantially blocked contraction to NA (15 μM) or ET-1 (100 nM) with both initial (30 s) and sustained components of the response markedly reduced in the presence of U73122 compared with U73342 (Fig. 1, A and B). In contrast, PLC inhibition did not affect non-GPCR-mediated contraction induced by depolarization with 50 mM KPSS (Fig. 1C) or direct PKC activation with phorbol ester (phorbol 12,13-dibutyrate; 1 μM) (Fig. 1D), indicating that the effect of U73122 on NA and ET-1-induced contraction is specific.
PLC-δ1 in NA and ET-1-induced PIP2 hydrolysis.
Having shown that PLC activity is involved in NA and ET-1-induced RMSA contraction, we investigated whether PLC-δ1 was involved in PIP2 hydrolysis. Recently, we found that PIP2 hydrolysis in response to NA and ET-1 occurs solely in caveolae/rafts in RMSA (12). Accordingly, we investigated whether PLC-δ1 was present in, or associated with, these domains following stimulation. Immunoblot analysis of fractions from sucrose density centrifugation showed the presence of PLC-δ1 in the buoyant caveolin-1-enriched fractions corresponding to caveolae rafts (12) (Fig. 2). In untreated tissue, 6.4 ± 1.1% of total PLC-δ1 were localized to caveolae/rafts. NA stimulated a rapid (20 s) and transient increase in PLC-δ1 levels of caveolae/rafts (2.05 ± 0.3-fold peak; Fig. 3) but had no significant effect on its levels in either of the noncaveolae/raft fractions (not shown). Of the four PLC subtypes, PLC-δ isoforms are the most sensitive to calcium (1). Consistent with this, removal and chelation of extracellular calcium prevented the NA-stimulated translocation of PLC-δ1 to caveolae/rafts (Fig. 3). This was not due to EGTA, as a similar effect was observed in nominally calcium-free buffer (Fig. 3).
Fig. 2.
PLC-δ1 is present in caveolae/rafts. RMSA were processed for caveolae/raft isolation, and fraction content was analyzed by immunoblot for PLC-δ1 (upper portion of membrane) and caveolin-1 (lower portion of membrane), as described in materials and methods. The small panel on the right is a longer exposure of the membrane showing PLC-δ1 in fractions 2 and 3. mes, RMSA total tissue homogenate before fractionation.
Fig. 3.
NA stimulates PLC-δ1 translocation to caveolae/rafts in an extracellular calcium-dependent manner. RMSA were stimulated with NA (15 μM) for various time points up to 5 min in the presence or absence of extracellular calcium. Caveolae/rafts (fractions 2–5), noncaveolae/rafts-1 (fractions 6–9), and noncaveolae/rafts-2 (fractions 10–13) were isolated, and PLC-δ1 content was analyzed by Western blot, as described in materials and methods. A: immunoblots showing the effect of NA on PLC-δ1 distribution (top panel 1.26 mM calcium; bottom panel, calcium-free buffer with 1 mM EGTA). B: immunoblots showing the effect of 2-min NA on PLC-δ1 distribution (top panel 1.26 mM calcium; bottom panel, nominally calcium-free buffer). C: densitometric data of the PLC-δ1 signal in caveolae/rafts for 0, 20-s, 2-min, and 5-min stimulation with 15 μM NA in 1.26 mM calcium (NA + calcium) or 0 mM calcium + 1 mM EGTA (NA − calcium). D: densitometric data of the PLC-δ1 signal in caveolae/rafts following 15 μM NA for 2 min in 1.26 mM calcium or nominally calcium-free buffer. Values are means ± SE of %total PLC-δ1. Where a doublet was detected, the upper band that corresponded to the expected molecular mass was analyzed; any image showing saturation was not used in analysis. *P < 0.05 for NA compared with basal, and NA compared with calcium-free + NA; n = 5. Cav/raft, caveolae/raft; Non Cav/raft-1, noncaveolae/raft-1; Non Cav/raft-2, noncaveolae/raft-2.
If PIP2 hydrolysis required PLC-δ1, extracellular calcium removal, by preventing PLC-δ1 relocalization to caveolae/rafts, would also be expected to reduce hydrolysis in response to NA. Indeed, at 20 s of NA stimulation (time point of PLC-δ1 association with caveolae/rafts), [33P]PIP2 hydrolysis was reduced in the absence of extracellular calcium (Fig. 4A). This was not due to an effect on [33P]PIP2 production, as turnover of [33P]PIP, the precursor of PIP2, was unaffected (not shown). Furthermore, removal and chelation of extracellular calcium also prevented NA-induced [3H]InsPx accumulation (Fig. 4B), confirming that PIP2 hydrolysis was reduced.
Fig. 4.
Extracellular calcium removal reduces NA-stimulated [33P]phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis and [3H]inositol phosphate (InsPx) accumulation. A: RMSA were labeled with 33Pi and stimulated with NA (15 μM) for 20 s in the presence or absence of extracellular calcium. Caveolae/rafts were isolated (fractions 2–5), and lipids extracted. [33P]PIP2 content was analyzed as described in materials and methods. Values are means ± SE of %decrease in [33P]PIP2 from basal in caveolae/rafts; n = 5. *P < 0.05. B: [3H]inositol labeled RMSA were stimulated with vehicle (distilled H2O, control) or 15 μM NA for 1 min in the presence or absence of extracellular calcium. [3H]InsPx were extracted and quantified as detailed in materials and methods. Values are means ± SE; n = 5. *P < 0.05 for NA compared with basal.
In contrast, ET-1 had no effect on PLC-δ1 levels in either caveolae/raft or noncaveolae/raft fractions at any of the time points studied (Fig. 5, A and B). In addition, removal and chelation of extracellular calcium had no significant effect on ET-1-stimulated [33P]PIP2 hydrolysis in caveolae/rafts at 20 s (Fig. 5C), suggesting that the calcium-dependent PLC-δ1 is not involved in the response to this agonist.
Fig. 5.
ET-1 does not induce PLC-δ1 association with caveolae/rafts, and ET-1-induced [33P]PIP2 hydrolysis is independent of extracellular calcium. RMSA were stimulated with ET-1 (100 nM) for various time points up to 5 min; caveolae/rafts (fractions 2–5), noncaveolae/rafts-1 (fractions 6–9), and noncaveolae/rafts-2 (fractions 10–13) were isolated; and PLC-δ1 content analyzed as described in materials and methods. A: immunoblot showing the effect of ET-1 on PLC-δ1 distribution. B: densitometric data of the PLC-δ1 signal in caveolae/rafts expressed as means ± SE of %total PLCδ1 (n = 5). Any image showing saturation was not used in analysis; where a doublet was detected, the upper band that corresponded to the expected molecular mass was analyzed. C: RMSA were labeled with 33Pi and stimulated with ET-1 (100 nM) for 20 s in the presence or absence of extracellular calcium. Caveolae/rafts were isolated (fractions 2–5), and lipids extracted. [33P]PIP2 content was analyzed as described in materials and methods. Values are means ± SE of %decrease [33P]PIP2 from basal in caveolae/rafts (n = 5).
Role of PLC-δ1 in NA and ET-1-induced contraction.
Our data suggest that PLC-δ1 is involved in NA-induced PIP2 hydrolysis and so might be expected to regulate the contractile response to this agonist. However, as there are no PLC isoform-selective inhibitors, we were unable to use a pharmacological approach to investigate this. Accordingly, using Chariot protein transfection reagent, we delivered PLC-δ1 antibodies to intact RMSA to neutralize endogenous PLC-δ1. To check the specificity of the PLC-δ1 antibodies, FLAG-tagged mouse PLC-δ1, human PLC-δ3, and rat PLC-δ4 were expressed in MEF cells (21). Expression of the PLC-δ isoforms was demonstrated by anti-FLAG immunoblot (Fig. 6A); neither monoclonal PLC-δ1 raised against full-length human PLC-δ1, nor polyclonal PLC-δ1 raised against amino acids 1–140 at the NH2-terminus of human PLC-δ1 showed detectable cross-reactivity with PLC-δ3 or PLC-δ4 (Fig. 6A), confirming isoform specificity of these antibodies. In addition, the monoclonal PLC-δ1 antibody recognized a single major band at the expected molecular mass of ∼85 kDa on an immunoblot of RMSA and VSMC homogenate (Fig. 6B). The polyclonal PLC-δ1 antibody detected two major bands on an immunoblot of RMSA of ∼85 and 70–75 kDa (Fig. 6B). However, only an 85-kDa band was detected in VSMC lysates, suggesting that the 70- to 75-kDa band was of nonsmooth muscle cell origin. Accordingly, the polyclonal anti-PLC-δ1 was used for immunoblot only, and the monoclonal anti-PLC-δ1 antibody was used to neutralize endogenous PLC-δ1.
Fig. 6.
Specificity of PLC-δ1 antibodies. A: lysates from mouse embryonic fibroblast (MEF) cells transfected with vector alone, FLAG-PLC-δ1, FLAG-PLC-δ3, or FLAG-PLC-δ4 were analyzed by immunoblot (IB) with anti-FLAG, anti-PLC-δ1 monoclonal antibody (MAb), or anti-PLC-δ1 polyclonal antibody (PAb), as described in materials and methods. B: lysates from RMSA and vascular smooth muscle cells (VSMC), 20 μg protein loaded, were analyzed by IB with anti-PLC-δ1 MAb or anti-PLCδ1 PAb, or anti-mitochondrial heat shock protein 70 (mtHSP70) antibody, as described in materials and methods.
Delivery of 3-μg monoclonal anti-PLC-δ1, but not mouse anti-IgG2A, inhibited NA-induced association of PLC-δ1 with caveolae/rafts (Fig. 7). Furthermore, while delivery of 3-μg anti-PLC-δ1 had no effect on the initial rapid contraction to NA (Fig. 7), tension during the sustained response was not maintained, compared with a paired artery that had received 3 μg of anti-mouse IgG2A (Fig. 7). In contrast, the contractile response to ET-1 or KPSS was similar between anti-PLC-δ1 and anti-mouse IgG2A-treated arteries (Figs. 7 and 8). As an additional control, we delivered anti-mtHSP70 (mouse monoclonal), which recognizes rat mtHSP70 (Fig. 6) and would be expected to bind in tissues but not affect contraction. As can be seen, delivery of anti-mtHSP70 had no effect on the contractile response to NA, ET-1, or KPSS (Figs. 7 and 8), indicating that the observed effect of anti-PLC-δ1 was due to its interaction with PLC-δ1 and not a nonspecific antibody response.
Fig. 7.
PLC-δ1 antibody attenuates the sustained phase of NA-induced contraction. A: representative immunoblots of PLC-δ1 in caveolae/rafts following delivery of 3 μg anti-IgG2A or anti-PLC-δ1 and stimulation with NA (15 μM), with corresponding caveolin-1 to indicate caveolae enrichment. Figures indicate values obtained by densitometric analysis of corresponding bands in arbitrary units normalized to control. B: densitometric data of the PLC-δ1 signal in caveolae/rafts expressed as means ± SE of %total PLC-δ1 (n = 3). Any image showing saturation was not used in analysis; where a doublet was detected, the upper band that corresponded to the expected molecular mass was analyzed. *P < 0.05, NA compared with control. C–E: lumen diameter was recorded from arteries mounted in a pressure myograph following delivery of 3 μg anti-IgG2A or anti-PLC-δ1 or anti-mtHSP70, as described in materials and methods. C: arteries were stimulated with NA as indicated. The tracings represent 4 separate experiments. D: the maximum decrease in lumen diameter occurring before 30 s. E: lumen diameter at 2 min after addition of NA was recorded. Values are means ± SE; n = 4 PLC-δ1 or IgG2A, n = 3 mtHSP70. *P < 0.05 compared with anti-IgG2A. w/o, Washout.
DISCUSSION
The PI signaling system is central to the regulation of vascular contractility and peripheral vascular resistance. The hydrolysis of PIP2 by PLC is a key reaction involved in initiation and regulation of VSM contraction. However, it is still unclear which PLC isoforms mediate this reaction in response to vasoconstrictors. Previous expression studies in nonmuscle cells have shown that α1-ARs can couple to PLC-δ1 (10, 16, 20), although whether this occurs in tissues and is of any physiological significance has not been addressed. Here, we present the first evidence that PLC-δ1 is involved in NA-induced contraction in VSM tissue.
Studies in VSM have identified and implicated PLCs-β, -γ, and -δ in PIP2 hydrolysis stimulated by GPCR agonists (26, 28, 31). Although PLC-β2 was reported as the major isoform involved in NA responses in rat caudal artery (28), the PLC isoforms expressed in RMSA and involved in GPCR signaling have yet to be fully identified. Caveolae/rafts and/or caveolins are implicated in smooth muscle contraction and the pathophysiology of vascular disease (7, 29), and disruption of these domains reduces ET-1- and NA-induced contraction in vascular tissues (14, 22, 41). Previously, we have shown that NA and ET-1 induce PIP2 hydrolysis solely within caveolae/rafts in RMSA (12). Here, we have identified PLC-δ1 within caveolae/rafts and found that stimulation with NA, but not ET-1, induced a rapid and transient translocation of PLC-δ1 to caveolae/rafts of RMSA. Furthermore, we observed that removal of extracellular calcium prevented NA-stimulated PLC-δ1 translocation to caveolae/rafts, with concomitant reduction of PIP2 hydrolysis and [3H]InsPx production, suggesting that calcium is upstream of PLC-δ1 activation. Consistent with this, previous studies have suggested that PLC-δ1 acts to amplify the calcium signal initiated by agonist activation of PLCs-β, -γ, or -ɛ. Unlike other PLC isoforms, PLC-δ1 is not active at basal calcium levels, but its activity increases markedly following a rise in calcium (39). Furthermore, keratinocytes from PLC-δ1 null mice do not show a sustained rise in calcium following PLC-γ activation, but reintroduction of PLC-δ1 restores the response (34), and, in smooth muscle cells, expression of dominant-negative PLC-δ1 inhibits the sustained but not the initial phase of PI hydrolysis in response to Gi/o agonists (33). Moreover, as we observed a decrease but not abolition of PIP2 hydrolysis and InsPx production in the absence of extracellular calcium, our data support a role for PLC-δ1 as an amplifier of PLC signaling. Importantly, extracellular calcium removal had no significant effect on ET-1-stimulated PIP2 hydrolysis, consistent with the lack of effect of ET-1 on PLC-δ1 distribution and indicating the agonist-specific activation of PLC isoforms in RMSA. Additionally, as ET-1 caused a comparable decrease in [33P]PIP2 levels as NA, despite not activating PLC-δ1, this further suggests that GPCR agonists differentially regulate PLCs in VSM.
Both NA and ET-1 increase intracellular calcium in RMSA (41), but PLC-δ1 did not appear to be required for ET-1 responses, suggesting that a rise in intracellular calcium cannot be the only activator of PLC-δ1. Indeed, increased intracellular calcium alone does not cause maximal activation of PLC-δ1 (5, 26), and many agonists that increase intracellular calcium do not activate this isoform (40). Therefore, GPCR agonists must use additional mechanisms to regulate PLC-δ1 activity; for example, interactions of PLC-δ1 with atypical G protein Gh, Rho, Rho-GAP, Ral/calmodulin, and PLC-β (16, 18, 19, 33, 42) have all been observed to modulate PLC-δ1 activation in response to various agonists. Therefore, although increased intracellular calcium is required for PLC-δ1 activation, different agonists must also use other signaling molecules to modulate the response, most probably dependent on the coupling of specific G proteins to agonist receptors, in addition to the proteins expressed within the cells. The additional mechanisms of PLC-δ1 regulation are currently the subject of further study.
Subcellular localization and targeting of signaling molecules are now accepted to be important for regulation of signal transduction in biological systems. In this study, NA but not ET-1 caused PLC-δ1 to associate with caveolae/rafts, suggesting that targeting to specific membrane sites may also function in regulation of its activity. However, although removal of extracellular calcium prevented PLC-δ1 association with caveolae/rafts, it is not clear from our experiments whether it is the rise in calcium that “directs” PLC-δ1 to these domains. Although interaction of PLC-δ1 with membranes is dependent on its pleckstrin homology domain that binds inositol 4,5-bisphosphate (the PIP2 head group) (48), the enzyme also possesses a C2 domain. These domains are implicated in lipid binding and calcium-dependent targeting of proteins to membranes (11), and, indeed, the PLC-δ1 C2 domain binds phosphatidylserine and associates with membranes both in vitro and in vivo in a calcium-dependent manner (3). However, the interactions of these domains with calcium, PIP2, and IP3, and their relative contributions to the regulation of PLC-δ1 are currently unclear.
Agonist-induced contraction of tonic smooth muscle has two components. An initial rapid response is triggered by IP3-mediated mobilization of calcium with subsequent activation of myosin light chain (MLC) kinase and inhibition of MLC phosphatase. This is followed by a sustained phase involving calcium sensitization of MLC phosphorylation through PLC/DAG/PKC/CPI-17 and RhoA/Rho kinase pathways (13, 43), suggesting that PLC activity is important for both components of contraction. Consistent with this, PLC inhibition reduced both initial and sustained contractile responses to both NA and ET-1, with no effect observed on contraction induced by membrane depolarization or direct activation of PKC by PdBu. To minimize potential nonspecific effects of the PLC inhibitor U73122 and its negative control, inhibitor concentrations were titrated down to a minimum effective dose (3 μM; data not shown). As this dose was still able to inhibit NA- and ET-1-mediated contraction, but was below the level required for reported PLC-unrelated effects of U73122, such as blockade of α1-ARs (2), this is consistent with a role for PLC in the contractile responses to these agonists.
To address the functional role of PLC-δ1 in smooth muscle contraction, we delivered PLC-δ1 antibodies, previously shown to effectively block PLC-δ1 activity (33), to intact RMSA. Whereas this resulted in an inhibition of PLC-δ1 association with caveolae/rafts and attenuation of NA-induced contraction, in contrast, there was no significant effect on ET-1-induced contraction, consistent with the inability of ET-1 to alter PLC-δ1 distribution and the selective role of PLC-δ1 in NA-induced PIP2 hydrolysis, as discussed above. Importantly, PLC-δ1 antibodies attenuated only the sustained contraction to NA, further implying that PLC-δ1 is activated secondary to PLC-β and consistent with a role for this enzyme as an amplifier of PIP2 hydrolysis. This was found to be a specific effect, as neither anti-IgG2A nor mtHSP70 antibodies had an effect on contraction. Moreover, this is in agreement with recent studies in smooth muscle cells, where sustained PIP2 hydrolysis in response to somatostatin was dependent on PLC-δ1 (33), and in bradykinin-stimulated PC12 cells, where PLC-δ1 was activated subsequent to PLC-β activation (26). To study the role of PLC-δ1 in a physiological context, we used intact vascular tissue with a functional endothelium. Therefore, it is possible that the responses we observed were modulated by the endothelium. However, as both α1-AR and ETA receptors are found predominantly on smooth muscle cells, this suggests the effects on contractility would be primarily of smooth muscle origin. Taken together, this is the first direct evidence that PLC-δ1 has a functional role in maintenance of contraction and further links activation of PI signaling in caveolae/rafts to the contractile response.
Vascular tone is an important determinant of vascular resistance and blood pressure, and increased responsiveness to sympathetic nervous activity contributes to pathological states, such as vasospasm and hypertension. Vasoconstrictor hormone activation of the PI signaling system is central to regulation of small-artery tone, and increased activity of this pathway in hypertension has been reported recently (27). Of particular relevance to our study, there is evidence that α1D-ARs regulate blood pressure (44), are upregulated in the aorta of spontaneously hypertensive rats (SHR) (47), and are implicated in the development of hypertension (45). Evidence also suggests that PLC-δ1 is involved in hypertension, with studies indicating increased PLC-δ1 activity in human hypertension (27) and in aorta from SHR (23). Additionally, a PLC-δ1 polymorphism that results in a missense mutation in the catalytic domain was found to cosegregate with low blood pressure in SHR (24). Our data showing that PLC-δ1 is involved in sustained NA-induced contraction of small arteries suggests that aberrations in this signaling pathway may underlie alterations in the peripheral vasculature in cardiovascular disease. Accordingly, further understanding of the mechanisms involved in α1-AR activation of PLC-δ1 may offer potential targets for intervention.
GRANTS
The study was funded by the British Heart Foundation.
Acknowledgments
We are grateful to Kiyoko Fukami, Life Sciences, Tokyo University of Pharmacy and Life Science, Tokyo, Japan, for the generous gift of PLC-δ isoform cDNAs and to Dr. Andrew Gilmore, University of Manchester, UK, for help with their expression.
Present address of C. J. Clarke: Department of Biochemistry and Molecular Biology, Medical University of South Carolina, Charleston, SC 29425.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allen V, Swigart P, Cheung R, Cockcroft S, Katan M. Regulation of inositol lipid-specific phospholipase cdelta by changes in Ca2+ ion concentrations. Biochem J 327: 545–552, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altmann C, Steenpass V, Czyborra P, Hein P, Michel MC. Comparison of signaling mechanisms involved in rat mesenteric microvessel contraction by noradrenaline and sphingosylphosphorylcholine. Br J Pharmacol 138: 261–271, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ananthanarayanan B, Das S, Rhee SG, Murray D, Cho W. Membrane targeting of C2 domains of phospholipase C-delta isoforms. J Biol Chem 277: 3568–3575, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol 28: 555–562, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Banno Y, Okano Y, Nozawa Y. Thrombin-mediated phosphoinositide hydrolysis in Chinese hamster ovary cells overexpressing phospholipase C-delta 1. J Biol Chem 269: 15846–15852, 1994. [PubMed] [Google Scholar]
- 6.Batty IH, Carter AN, Challiss RAJ, Hawthorne JN. Receptor-linked phosphoinositide metabolism. In: Neurochemistry, edited by Turner AJ and Bachelard HS. Oxford, UK: Oxford University Press, 1997, p. 229–268.
- 7.Bergdahl A, Sward K. Caveolae-associated signalling in smooth muscle. Can J Physiol Pharmacol 82: 289–299, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther 255: 756–768, 1990. [PubMed] [Google Scholar]
- 9.Brenner R, Perez GJ, Bonev AD, Eckman DR, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the β1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Lin F, Iismaa S, Lee KN, Birckbichler PJ, Graham RM. Alpha1-adrenergic receptor signaling via Gh is subtype specific and independent of its transglutaminase activity. J Biol Chem 271: 32385–32391, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Cho W, Stahelin RV. Membrane binding and subcellular targeting of C2 domains. Biochim Biophys Acta 1761: 838–849, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Clarke CJ, Ohanian V, Ohanian J. Norepinephrine and endothelin activate diacylglycerol kinases in caveolae/rafts of rat mesenteric arteries: agonist-specific role of PI3-kinase. Am J Physiol Heart Circ Physiol 292: H2248–H2256, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos GJ, Semba S, Kitazawa K, Eto M, Kitazawa T. Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle. Circ Res 100: 121–129, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dreja K, Voldstedlund M, Vinten J, Tranum-Jensen J, Hellstrand P, Sward K. Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler Thromb Vasc Biol 22: 1267–1272, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Feldstein C, Romero C. Role of endothelins in hypertension. Am J Ther 14: 147–153, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Feng JF, Rhee SG, Im MJ. Evidence that phospholipase delta1 is the effector in the Gh (transglutaminase II)-mediated signaling. J Biol Chem 271: 16451–16454, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Griendling KK, Rittenhouse SE, Brock TA, Eckstein LS, Gimbrone LM, Alexander RW. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II stimulated vascular smooth muscle cells. J Biol Chem 261: 5901–5906, 1986. [PubMed] [Google Scholar]
- 18.Guo Y, Rebecchi M, Scarlata S. Phospholipase Cbeta2 binds to and inhibits phospholipase Cdelta1. J Biol Chem 280: 1438–1447, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Homma Y, Emori Y. A dual functional signal mediator showing RhoGAP and phospholipase C-delta stimulating activities. EMBO J 14: 286–291, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Im MJ, Russell MA, Feng JF. Transglutaminase II: a new class of GTP-binding protein with new biological functions. Cell Signal 9: 477–482, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Irino Y, Cho H, Nakamura Y, Nakahara M, Furutani M, Suh PG, Takenawa T, Fukami K. Phospholipase C delta-type consists of three isozymes: bovine PLCdelta2 is a homologue of human/mouse PLCdelta4. Biochem Biophys Res Commun 320: 537–543, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Je HD, Gallant C, Leavis PC, Morgan KG. Caveolin-1 regulates contractility in differentiated vascular smooth muscle. Am J Physiol Heart Circ Physiol 286: H91–H98, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Kato H, Fukami K, Shibasaki F, Homma Y, Takenawa T. Enhancement of phospholipase C δ1 activity in the aortas of spontaneously hypertensive rats. J Biol Chem 267: 6483–6487, 1992. [PubMed] [Google Scholar]
- 24.Katsuya T, Higaki J, Miki T, Kohara K, Yagisawa H, Tanase H, Mikami H, Serikawa T, Nojima H, Ogihara T. Hypotensive effect associated with a phospholipase C-delta 1 gene mutation in the spontaneously hypertensive rat. Biochem Biophys Res Commun 187: 1359–1366, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Keller M, Lidington D, Vogel L, Peter BF, Sohn HY, Pagano PJ, Pitson S, Spiegel S, Pohl U, Bolz SS. Sphingosine kinase functionally links elevated transmural pressure and increased reactive oxygen species formation in resistance arteries. FASEB J 20: 702–704, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Kim YH, Park TJ, Lee YH, Baek KJ, Suh PG, Ryu SH, Kim KT. Phospholipase C-delta1 is activated by capacitative calcium entry that follows phospholipase C-beta activation upon bradykinin stimulation. J Biol Chem 274: 26127–26134, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Kosugi T, Osanai T, Kamada T, Nakano T, Okumura K. Phospholipase C activity is enhanced in skin fibroblasts obtained from patients with essential hypertension. J Hypertens 21: 583–590, 2003. [DOI] [PubMed] [Google Scholar]
- 28.LaBelle EF, Wilson K, Polyak E. Subcellular localization of phospholipase C isoforms in vascular smooth muscle. Biochim Biophys Acta 1583: 273–278, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Li XA, Everson WV, Smart EJ. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc Med 15: 92–96, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Maron MB, Folkesson HG, Stader SM, Walro JM. PKA delivery to the distal lung air spaces increases alveolar liquid clearance after isoproterenol-induced alveolar epithelial PKA desensitization. Am J Physiol Lung Cell Mol Physiol 289: L349–L354, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Marrero MB, Paxton WG, Duff JL, Berk BC, Bernstein KE. Angiotensin II stimulates tyrosine phosphorylation of phospholipase C-γ1 in vascular smooth muscle cells. J Biol Chem 269: 10935–10939, 1994. [PubMed] [Google Scholar]
- 32.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol 19: 1173–1176, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Murthy KS, Zhou H, Huang J, Pentyala SN. Activation of PLC-delta1 by Gi/o-coupled receptor agonists. Am J Physiol Cell Physiol 287: C1679–C1687, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura Y, Fukami K, Yu H, Takenaka K, Kataoka Y, Shirakata Y, Nishikawa S, Hashimoto K, Yoshida N, Takenawa T. Phospholipase Cdelta1 is required for skin stem cell lineage commitment. EMBO J 22: 2981–2991, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, Im MJ, Graham RM. Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science 264: 1593–1596, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Ohanian J, Ohanian V, Shaw L, Bruce C, Heagerty AM. Involvement of tyrosine phosphorylation in endothelin-1-induced calcium-sensitization in rat small mesenteric arteries. Br J Pharmacol 120: 653–661, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohanian J, Ollerenshaw J, Collins P, Heagerty AM. Agonist-induced production of 1,2-diacylglycerol and phosphatidic acid in intact resistance arteries–evidence that accumulation of diacylglycerol is not a prerequisite for contraction. J Biol Chem 265: 8921–8928, 1990. [PubMed] [Google Scholar]
- 38.Ollerenshaw J, Heagerty AM, Swales JD. Noradrenaline stimulation of the phosphoinositide system: evidence for a novel hydrophobic inositol-containing compound in resistance arterioles. Br J Pharmacol 94: 363–370, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev 80: 1291–1335, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Rhee SG Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281–312, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw L, Sweeney MA, O'Neill SC, Jones CJ, Austin C, Taggart MJ. Caveolae and sarcoplasmic reticular coupling in smooth muscle cells of pressurised arteries: the relevance for Ca(2+) oscillations and tone. Cardiovasc Res 69: 825–835, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Sidhu RS, Clough RR, Bhullar RP. Regulation of phospholipase C-delta1 through direct interactions with the small GTPase Ral and calmodulin. J Biol Chem 280: 21933–21941, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83: 1325–1358, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. The alpha1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest 109: 765–775, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villalobos-Molina R, Lopez-Guerrero JJ, Ibarra M. Functional evidence of alpha1D-adrenoceptors in the vasculature of young and adult spontaneously hypertensive rats. Br J Pharmacol 126: 1534–1536, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu D, Katz A, Lee CH, Simon MI. Activation of phospholipase C by alpha 1-adrenergic receptors is mediated by the alpha subunits of Gq family. J Biol Chem 267: 25798–25802, 1992. [PubMed] [Google Scholar]
- 47.Xu KM, Tang F, Han C. Alterations of mRNA levels of alpha 1-adrenoceptor subtypes with maturation and ageing in different rat blood vessels. Clin Exp Pharmacol Physiol 24: 415–417, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Yagisawa H, Sakuma K, Paterson HF, Cheung R, Allen V, Hirata H, Watanabe Y, Hirata M, Williams RL, Katan M. Replacements of single basic amino acids in the pleckstrin homology domain of phospholipase C-delta1 alter the ligand binding, phospholipase activity, and interaction with the plasma membrane. J Biol Chem 273: 417–424, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Yamaga M, Sekimata M, Fujii M, Kawai K, Kamata H, Hirata H, Homma Y, Yagisawa H. A PLCdelta1-binding protein, p122/RhoGAP, is localized in caveolin-enriched membrane domains and regulates caveolin internalization. Genes Cells 9: 25–37, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol 65: 761–789, 2003. [DOI] [PubMed] [Google Scholar]