Abstract
Hydrogen sulfide (H2S) is an endogenously produced gaseous signaling molecule with diverse physiological activity. The potential protective effects of H2S have not been evaluated in the liver. The purpose of the current study was to investigate if H2S could afford hepatoprotection in a murine model of hepatic ischemia-reperfusion (I/R) injury. Hepatic injury was achieved by subjecting mice to 60 min of ischemia followed by 5 h of reperfusion. H2S donor (IK1001) or vehicle were administered 5 min before reperfusion. H2S attenuated the elevation in serum alanine aminotransferase (ALT) by 68.6% and aspartate aminotransferase (AST) by 70.8% compared with vehicle group. H2S-mediated cytoprotection was associated with an improved balance between reduced glutathione (GSH) vs. oxidized glutathione (GSSG), an attenuated formation of lipid hydroperoxides, and an increased expression of thioredoxin-1 (Trx-1). Furthermore, H2S inhibited the progression of apoptosis after I/R injury by increasing the protein expression of heat shock protein (HSP-90) and Bcl-2. These results indicate that H2S protects the murine liver against I/R injury through an upregulation of intracellular antioxidant and antiapoptotic signaling pathways.
Keywords: apoptosis, liver, glutathione, thioredoxin, heat shock protein 90
hepatic ischemia-reperfusion (I/R) injury occurs in diverse clinical settings, including liver transplantation, lobectomy (pringle maneuver), veno-occlusive disease including Budd-Chiari syndrome, congestive heart failure, shock states, and resuscitation (10, 14, 18, 20, 38). Liver injury induced by I/R represents a continuum of processes (26) that may produce profound hepatocellular injury and ultimately result in morbidity and mortality (25). The number of liver transplantations performed each year has been on the rise for last 20 years, but there exists a large imbalance between the number of available donors and patients waiting for transplantation. The noted increase in demand and shortage of supply has forced the consideration of cadaveric or steatotic grafts (19), which have a higher susceptibility to I/R injury and a much higher risk of primary nonfunction and mortality (34). Therefore, minimizing the adverse effects of hepatic I/R injury could increase the number of patients that may undergo a successful transplantation (11). However, at present there is no therapeutic treatment plan or strategy available to prevent hepatic I/R injury (26).
Experimental evidence suggests that liver I/R injury is biphasic: early I/R injury occurs with the initiation of an inflammatory cascade involving numerous reactive oxygen species, reactive nitrogen species (14, 38), chemokines, and cytokines (10, 28, 38), followed by neutrophil-mediated hepatic injury occurring at 6–24 h of reperfusion (18, 20). Additionally, a consequence of I/R injury is the disruption of intracellular energy metabolism and enzyme function, resulting in a depletion of ATP and an accumulation of intracellular sodium and edema (11), suggesting that the mitochondria play a role in the pathology of I/R injury (31). The role of necrosis was considered vital for I/R injury in the past, but it is now known that apoptosis also plays a significant role in cellular damage after I/R injury (12, 20).
Hydrogen sulfide (H2S) has long been considered as a toxic environmental pollutant emerging from sewers, marshes, and volcanic eruptions. Recently, H2S has been recognized alongside nitric oxide and carbon monoxide as an endogenously produced gaseous signaling molecule (29, 36, 39). In mammalian cells, H2S is produced by two heme containing enzymes, cystathionine β-synthase and cystathionine γ-lyase, the activity of which depends on pyridoxal 5′-phosphate (36, 39). Cystathionine β-synthase is primarily responsible for production of H2S in the central nervous system (8), while cystathionine γ-lyase is primarily expressed in peripheral tissues, including vascular and nonvascular smooth muscle (30, 36, 37, 48). Since the recent discovery that H2S is a powerful physiological signaling molecule, experimental studies (30) have begun to characterize its biological profile. H2S promotes vascular smooth muscle relaxation and induces vasodilation of isolated blood vessels (3, 27, 48). H2S has also been shown to inhibit leukocyte-endothelial cell interactions in vivo (45) indicating an anti-inflammatory action. It has also become evident that H2S is a potent antioxidant (24, 40, 44) and under more chronic conditions upregulates antioxidant defenses (23, 24). It has also been demonstrated that H2S effectively inhibits apoptosis of a number of cell types (6, 32, 35), and this effect has been shown to promote cytoprotection.
Previous studies (1, 6, 35, 47) have provided insights into the protective actions of H2S in the setting of hypoxia-reoxygenation and I/R injury. However, to date the potential cytoprotective effects of H2S have not been evaluated in hepatic I/R injury. The aim of present study was to investigate the effects of an exogenous administration of H2S on the severity of I/R injury in an in vivo murine model of hepatic I/R injury.
MATERIALS AND METHODS
H2S donor.
Sodium sulfide (Na2S, IK1001) was produced by Ikaria (Seattle, WA) by using H2S gas (Matheson, Newark, CA) as a starting material. Na2S was formulated to pH neutrality and iso-osmolarity. Na2S (stock solution at 0.55 mg/ml and 7.1 mM) was diluted in normal (0.9%) saline to the desired concentration in a rapid fashion, immediately before administration For acute hepatic I/R experiments, normal saline (100 μl) or H2S donor (0.3, 1, and 2 mg/kg) in a final volume of 100 μl was injected intravenously into the inferior vena cava using a 32-gauge needle at 5 min before reperfusion.
Animals.
Mice (C57BL6/J) were used for the study. All mice were male, 8–10 wk of age, and were purchased from Jackson Laboratories. All experimental procedures were approved by the Institute for Animal Care and Use Committee at Albert Einstein College of Medicine and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Hepatic I/R protocol.
The hepatic I/R protocol has been described previously (5). In the present study, mice were subjected to 60 min of hepatic ischemia and either 1, 5, or 24 h of reperfusion. Mice were anesthetized with xylazine (8 mg/kg) and ketamine (100 mg/kg), constituted in normal saline and administered intravenously. Core body temperature of the mice was monitored continuously during the entire period of surgery using a rectal probe, and a heat lamp was used to maintain the core body temperature at 37 ± 0.4°C. A midline laparotomy incision was performed to expose the liver. A microaneurysm clamp was applied to the hepatic artery and portal vein resulting in ischemia of the left lateral and median lobes of the liver. The procedure leads to segmental (70%) hepatic ischemia so as to prevent mesenteric venous congestion by allowing portal decompression through the caudate and right lobe of the liver. The liver was then repositioned back to its original position and kept moist throughout the period of ischemia (60 min) using gauze soaked with normal saline. Mice were injected with heparin (100 U/Kg) to prevent clotting before reperfusion. Sham surgeries were identical except liver ischemia was not performed using microaneurysm clamp. Study drug (Na2S; IK1001) or vehicle was injected intravenously 5 min before reperfusion. After the ischemia period was completed, the clamp was removed and the liver was reperfused for different periods (1, 5, or 24 h) according to the protocol for the various experimental endpoints as described below. Liver tissue collected for Western blot analysis and glutathione assay was snap frozen in liquid nitrogen and stored at −80°C till further processing of the tissue.
Determination of liver transaminases.
At 5 h of reperfusion, mice were anesthetized again as described in Hepatic I/R protocol. Blood was collected from the inferior vena cava with a 20-gauge needle and placed in a microtainer serum separator tube. The blood sample was kept on ice for 15 min and then centrifuged at 14,000 g for 12 min. The serum samples were then analyzed for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) using a commercially available kit (Thermo Electron, Melbourne, Australia) by spectrophotometric method. AST and ALT are released into blood circulation after hepatocellular injury and can be used to estimate extent of injury.
Lipid hydroperoxide assay.
Quantification of lipid peroxidation was done to assess the extent of hepatic tissue oxidative injury. Lipid peroxidation results in the formation of highly unstable and reactive hydroperoxides of both saturated and unsaturated lipids. Hepatic tissue was collected at 1 and 5 h reperfusion. Lipid hydroperoxides were measured using a commercially available kit (Cayman Chemicals) according to the manufacturer's recommendations. The assay is based on the principle that hydroperoxides are highly unstable and react with ferrous ions readily to produce ferric ions. The resulting ferric ions are detected using thiocynate as the chromogen. Hepatic lipid hydroperoxide (LPO) is reported in micromolars.
Glutathione assay.
Hepatic tissue was collected for glutathione assay at 1, 5, or 24 h of reperfusion. Tissue levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured using a commercially available kit (Cayman Chemicals). The GSH-to-GSSG ratio was calculated for each sample according to manufacturer's recommendations.
Western blot analysis.
Western blot analysis was performed to evaluate the protein expression of cleaved caspase-3, Bcl-2, thioredoxin-1 (Trx-1), and 90-kDa heat shock protein (HSP-90) as described previously (16). Briefly, livers (ischemic lobes only) of sham, vehicle plus I/R and H2S plus I/R from nondiabetic mice were excised after 5 and 24 h after hepatic I/R. Samples were homogenized in 1 ml of ice-cold RIPA lysis buffer. Homogenates were then centrifuged at 1,300 g to remove any cellular debris. The pellet was discarded, and the supernatant was again centrifuged at 16,000 g for 30 min at 4°C. The resultant supernatant (cytosolic fraction) was collected. The resulting pellet was resuspended in 300 μl of RIPA lysis buffer. Protein assay was done with the use of Bio-RAD DC protein assay as per recommendations from manufacturer. An equal amount of protein (25 μg) was loaded into each well and separated on 7–12% polyacryilamide gel (7% for HSP-90, 12% for cleaved caspase 3, Bcl-2, and Trx-1). Protein was transferred to immunoblot polyvinylidene difluoride membrane and then blocked in 5% milk in Tris-buffered saline Tween-20 at room temperature. Membranes were then incubated with primary antibodies (mouse anti-Trx-1, 1:2,000; mouse anti-HSP-90, 1:5,000; mouse anti-cleaved caspase 3, 1:500, and mouse anti-Bcl-2, 1:1,500) overnight at 4°C. Membranes were then washed and incubated with horseradish peroxidase-linked anti-rabbit IgG (1:10,000) at room temperature. Membranes were then washed, followed by incubation with ECL reagents (Amersham), and then exposed to film. Membranes were then stripped and incubated with either mouse anti-α-tubulin (1:10,000) or anti-cytochrome c oxidase (COX-IV; 1:20,000) overnight at 4°C. Membranes were then washed and incubated with horseradish peroxidase-linked anti-rabbit or anti-goat secondary (1:10,000), followed by incubation with ECL (Amersham) reagents, and then exposed to film. Anti-α-tubulin antibodies were purchased from Santa Cruz Biotechnology, and all other antibodies were purchased from Cell Signaling Technology. Densitometric analysis was performed using Image J software from the National Institutes of Health.
Statistical analysis.
Data were analyzed by one-way ANOVA with post-Turkey multiple comparison test and two-way ANOVA with post hoc Bonferroni analysis wherever appropriate using Prism software (San Diego, CA). Data are reported as means ± SE. P values <0.05 were considered to be statistically significant.
RESULTS
H2S attenuated hepatic I/R injury.
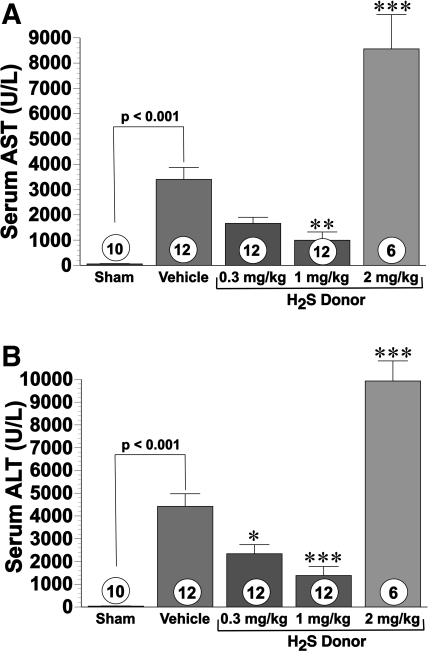
Intravenous administration of H2S donor at doses of 0.3 and 1.0 mg/kg before reperfusion limited serum elevations of the liver transaminases AST and ALT in a dose-dependent manner after 60 min of ischemia and 5 h of reperfusion (Fig. 1, A and B) . Specifically, the 0.3 mg/kg dose reduced serum AST by 51.6% (P < 0.05) and serum ALT by 47% (P < 0.05). The 1.0 mg/kg dose significantly reduced serum AST by 70.8% (P < 0.01) and serum ALT by 68.6% (P < 0.001). In sharp contrast to lower doses, 2.0 mg/kg of the H2S donor increased serum AST by 151% and ALT by 124%, compared with the vehicle. Additionally, doses higher than 2 mg/kg were associated with significantly higher mortality (data not shown). Thus 1 mg/kg was considered the most effective dose for hepatocellular protection and was investigated in further experiments. Both vehicle- and H2S-donor-treated mice had significantly higher liver transaminases as a consequence of I/R injury, compared with sham group. Sham values for AST were 58.4 ± 12.8 U/l, and ALT values in sham-operated controls were 38.3 ± 9.4 U/l.
Fig. 1.
Serum transaminases were measured in mice after 60 min of hepatic ischemia and 5 h of reperfusion. A: aspartate aminotransferase (AST) levels (U/l); B: alanine aminotransferase (ALT levels; U/l). Hydrogen sulfide (H2S) donor therapy (0.3 and 1.0 mg/kg) significantly reduced hepatic release of AST and ALT. *P < 0.05 vs. vehicle; **P < 0.01 vs. vehicle; ***P < 0.001 vs. vehicle; numbers inside bars represent number of animals investigated in each group.
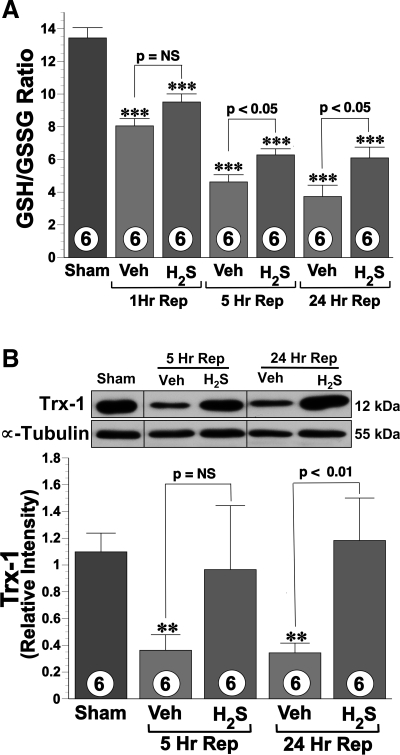
H2S increased hepatic tissue GSH-to-GSSG ratio.
The GSH-to-GSSG ratio (Fig. 2A) was calculated for sham and vehicle- and H2S-treated mice (n = 6 in each group). There was a significant decrease (P < 0.001) in the GSH-to-GSSG ratio in both the vehicle and H2S groups as consequence of I/R injury compared with sham. However, H2S attenuated the I/R-induced reduction significantly (P < 0.05) when compared with the vehicle-treated mice at 5 and 24 h reperfusion. Additionally, similar trend was observed at 1 h of reperfusion. Specifically, the ratios in the H2S-treated mice were 18.1, 35.8, and 63.3% higher than the vehicle-treated mice at 1, 5, and 24 h of reperfusion, respectively.
Fig. 2.
A: hepatic tissue reduced glutathione (GSH)-to-oxidized glutathione (GSSG) ratio at 1, 5, and 24 h after 60 min of ischemia. Data are presented for sham-operated controls, mice receiving vehicle (Veh), and mice treated with the H2S donor (1.0 mg/kg). H2S therapy significantly preserved the GSH-to-GSSG ratio compared with the vehicle group. B: representative immunoblot and densitometric analysis (bottom) of hepatic thioredoxin-1 (Trx-1) protein expression. Trx-1 protein levels remained significantly elevated in animals receiving H2S at 24 h after reperfusion (Rep). **P < 0.01 vs. sham; ***P < 0.001 vs. sham; n = 6 in each group.
Trx-1 expression was preserved by H2S after I/R injury.
Western blot analysis for protein expression of Trx-1 (Fig. 2B) revealed that Trx-1 levels decreased significantly (P < 0.05) in the vehicle group at 5 and at 24 h of reperfusion compared with the sham group. Conversely, Trx-1 levels in the H2S-treated group (1.0 mg/kg) remained at levels similar to those of the sham group at both 5 and 24 h of reperfusion. The mean expression of Trx-1 was higher at both 5 and 24 h of reperfusion in H2S group compared with vehicle-treated mice, but a statistical significance was only observed for at the 24 h time point (P < 0.01).
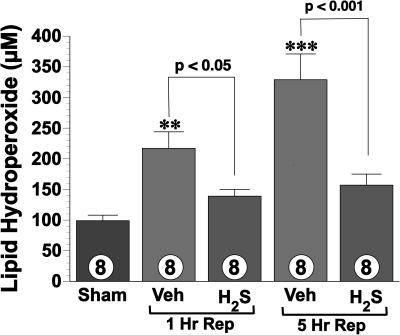
H2S attenuated oxidative stress.
Lipid peroxidation was used as measure of hepatic oxidative stress after I/R injury. LPO levels (Fig. 3) were measured for sham and vehicle- and H2S-treated mice (n = 8 in each group) after I/R injury. The vehicle group exhibited significantly higher levels of lipid hydroperoxides at 1 h postreperfusion and 5 h postreperfusion compared with sham-operated controls, as a consequence of I/R injury. Conversely, H2S-treated mice displayed LPO levels similar to sham at both 1 and 5 h. H2S-treated mice had significant reductions in lipid peroxidation by 36% at 1 h (P < 0.05) and 52% at 5 h (P < 0.001) reperfusion compared with vehicle.
Fig. 3.
Lipid hydroperoxide levels (μM) in hepatic tissue lyaste after 1 and 5 h of reperfusion after 60 min of ischemia. Treatment with H2S (1.0 mg/kg) significantly attenuated hepatic formation of lipid hydroperoxides. **P < 0.01 vs. sham; ***P < 0.001 vs. sham; numbers inside bars represent number of animals investigated in each group.
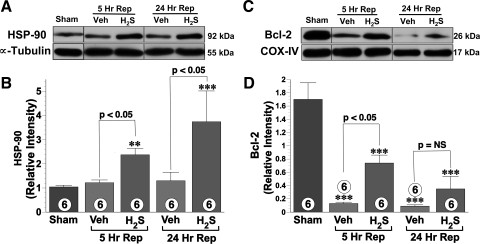
HSP-90 mediated protective effects of H2S.
After I/R injury, the hepatic HSP-90 protein levels in the vehicle-treated mice remained at levels similar to those observed in the sham mice (Fig. 4, A and B). Conversely, the administration of H2S significantly increased the expression of HSP-90 compared with the levels observed in the sham (P < 0.01) and vehicle-treated mice (P < 0.05) at both 5 and 24 h of reperfusion.
Fig. 4.
A and B: 90-kDa heat shock protein (HSP-90) protein expression in hepatic tissue, representative immunoblots, and densitometric analysis are shown. H2S therapy (1.0 mg/kg) significantly increased HSP-90 protein expression at both 5 and 24 h of repefusion compared with vehicle group. C and D: representative immunoblot and densitometric analysis of hepatic Bcl-2 protein expression in the mitochondrial fraction at 5 and 24 h of reperfusion. Bcl-2 protein expression decreased significantly in both vehicle and H2S groups. However, Bcl-2 levels were relatively preserved in animals treated with H2S donor therapy. COX-IV, cytochrome c oxidase. **P < 0.01 vs. sham; ***P < 0.001 vs. sham; n = 6 for each group.
H2S increased mitochondrial Bcl-2 levels and inhibited apoptosis.
To gain insights into apoptotic signaling, we evaluated the protein expression of the antiapoptotoic protein Bcl-2 at 5 and 24 h after ischemia (Figs. 4, C and D). I/R injury led to a significant reduction in Bcl-2 expression in both the vehicle- and H2S (P < 0.001)-treated groups compared with the sham group. However, Bcl-2 protein levels were significantly (P < 0.05) higher in H2S-treated mice compared with vehicle-treated mice at 5 h reperfusion. However, at 24 h of reperfusion, Bcl-2 was not increased in the H2S-treated group.
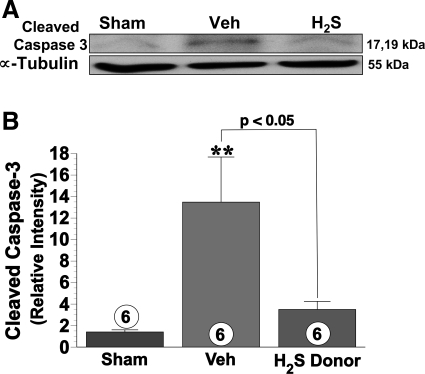
Western blot analysis of the hepatic tissue lysate (Fig. 5, A and B) obtained after 60 min of ischemia and 24 h of reperfusion revealed a significant (P < 0.01) increase in the expression of cleaved caspase-3 in the vehicle group compared with the sham group. In contrast, the expression of cleaved caspase-3 in the H2S-treated group only increased slightly.
Fig. 5.
Representative hepatic tissue immunoblot (A) and densitometric analysis (B) show expression of cleaved caspase-3 at 24 h reperfusion in sham, vehicle, and H2S donor groups. Cleaved caspase-3 was significantly elevated in vehicle group compared with sham-operated controls. The H2S donor (1.0 mg/kg) attenuated the increase in hepatic cleaved caspase-3. **P < 0.01 vs. sham; numbers inside bars represent number of animals investigated in each group.
DISCUSSION
In the current study, administration of H2S before reperfusion significantly attenuated I/R injury in the liver. The conferred hepatoprotection was characterized by a significant attenuation of serum transaminases level and lipid peroxidation by an upregulation of antioxidant and antiapoptotic signaling.
I/R injury involves the activation of the inflammatory cascade characterized by leukocyte infiltration as well as generation of reactive oxygen and nitrogen species (21, 38). H2S possesses a number of signaling actions that are likely to attenuate the pathological aspects of I/R injury. Recently, Zanardo et. al. (45) observed that H2S inhibits aspirin-induced leukocyte adherence in mesenteric venules and subsequent diapedesis suggesting that H2S is a potent anti-inflammatory agent. H2S also directly scavenges of reactive oxygen and nitrogen species preventing tissue damage (36, 40). Furthermore, H2S reduces the protein carbonyl formation after burn and smoke injury, indicative of overall antioxidant effects (7). GSH is a potent intracellular antioxidant, and the GSH-to-GSSG ratio corresponds to the capacity of a cell to attenuate oxidative stress (9). Thioredoxin like glutathione is a regulator of cellular oxidation/reduction (redox) status and has been shown to protect against oxidative stress (41, 46) and to inhibit apoptosis (41). Moreover, mice over expressing the human thioredoxin gene (hTrx tg) are resistant to renal I/R injury (22). In our study, we observed that H2S preserved the GSH levels and Trx-1 expression after hepatic I/R injury, suggesting that H2S maintains the intracellular antioxidant capacity of hepatocytes. I/R injury involves oxidative damage not only to proteins and nucleic acids but also to lipids. Stress-induced lipid peroxidation has been associated with development of atherosclerosis, neurodegeneration, and even carcinogenesis (13). In the current study, H2S significantly attenuated lipid peroxidation suggesting its protective effects against oxidative stress.
Apoptosis plays a significant role in the pathophysiology of I/R injury (12, 20). Previously, Rose et. al. (32) demonstrated that H2S abolished β-phenyethyl isothiocyanate induced apoptotic cell death in the human adenocarcinoma cell line HCT116, suggesting its potential to be a potent inhibitor of apoptosis. We found that the protein expression of HSP-90 was elevated in H2S-treated mice. HSP-90 is known to attenuate the injury caused by stress by maintaining the conformation of proteins, refolding proteins damaged by stressful stimuli, and rendering cells more resistant to apoptosis. HSP-90, the most abundant heat shock protein present in eukaryotic cells, is constitutively expressed but is induced under conditions of stress like I/R injury (15). HSP-90 interacts with a variety of intracellular pathways to evade apoptosis and promote cell survival, including the phospatidylinositol 3-kinase-AKT pathway, IGF-1, and inositol hexakisphosphate kinase-2 (2, 42). Additionally HSP-90 is known to mediate the upregulation of Bcl-2 expression induced by VEGF further contributing to apoptosis inhibition (4). Bcl-2 is an antiapoptotic protein vital for the integrity of the mitochondrial membrane, as it prevents the release of proapoptotic protein such as cytochrome c (33). Rat liver transfected with human Bcl-2 gene (hBcl-2) has been shown to be resistant to I/R injury (43). In the present study, H2S therapy increased the protein expression of HSP-90 and Bcl-2 after I/R injury and also inhibited apoptosis, as revealed by decreased levels of cleaved caspse-3 in H2S-treated mice. H2S has previously been shown to protect the ischemic myocardium by preserving mitochondrial function (6). Therefore, it is possible that H2S mediates preservation of mitochondrial function after I/R injury by upregulating Bcl-2 expression.
H2S is a potent reversible inhibitor of cytochrome c oxidase and has been shown to inhibit mitochondrial respiration in a dose-dependent manner (6). It is possible that supratherapeutic concentrations of H2S depress mitochondrial respiration irreversibly, affecting ATP synthesis and contributing further to I/R injury. However, to assess a complete side effect profile of H2S is beyond the scope of current study. Pharmacological preconditioning with H2S has been shown to protect the heart from I/R injury, and endogenous H2S has been suggested to play a role in ischemic preconditioning (1, 17). Further studies are required to assess role of H2S preconditioning in hepatic I/R injury. In the current study, H2S has been shown to be protective in nondiabetic animals but its role in conditions with enhanced oxidative stress including diabetes and obesity remains unknown. Clearly, additional studies are warranted to further our understanding regarding the efficacy of H2S under these pathological conditions.
In summary, our findings demonstrate that H2S significantly attenuates hepatic I/R injury via preservation of intracellular redox balance and by inhibition of apoptosis during the evolution of I/R injury. These results suggest that H2S is a promising therapeutic agent to protect against hepatic I/R injury.
GRANTS
The study was supported by National Institutes of Health (NIH) Grant 2RO1-HL-60849-08 and American Diabetes Association Grant 7-04-RA-59 to D. J. Lefer and by NIH Grant F32-DK-077380-01 to J. W. Calvert.
DISCLOSURES
The study was partially supported by a research grant from Ikaria. D. J. Lefer is a consultant for Ikaria.
Acknowledgments
We thank P. Hill (Ikaria) for formulation of the injectable H2S donor IK1001. We also thank R. Sellers for expert assistance with these studies.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther 316: 670–678, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty A, Koldobskiy MA, Sixt KM, Juluri KR, Mustafa AK, Snowman AM, van Rossum DB, Patterson RL, Snyder SH. HSP90 regulates cell survival via inositol hexakisphosphate kinase-2. Proc Natl Acad Sci USA 105: 1134–1139, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol 287: H2316–H2323, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Dias S, Shmelkov SV, Lam G, Rafii S. VEGF(165) promotes survival of leukemic cells by Hsp90-mediated induction of Bcl-2 expression and apoptosis inhibition. Blood 99: 2532–2540, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. Genetic overexpression of eNOS attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 291: H2980–H2986, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci USA 104: 15560–15565, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esechie A, Kiss L, Olah G, Horvath EM, Hawkins HK, Szabo C, Traber DL. Protective effect of hydrogen sulfide in a murine model of combined burn and smoke inhalation-induced acute lung injury. Clin Sci (Lond), 2008. [DOI] [PubMed]
- 8.Eto K, Ogasawara M, Umemura K, Nagai Y, Kimura H. Hydrogen sulfide is produced in response to neuronal excitation. J Neurosci 22: 3386–3391, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Exner R, Wessner B, Manhart N, Roth E. Therapeutic potential of glutathione. Wien Klin Wochenschr 112: 610–616, 2000. [PubMed] [Google Scholar]
- 10.Fan C, Zwacka RM, Engelhardt JF. Therapeutic approaches for ischemia/reperfusion injury in the liver. J Mol Med 77: 577–592, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury–a fresh look. Exp Mol Pathol 74: 86–93, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Georgiev P, Dahm F, Graf R, Clavien PA. Blocking the path to death: anti-apoptotic molecules in ischemia/reperfusion injury of the liver. Curr Pharm Des 12: 2911–2921, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Girotti AW Translocation as a means of disseminating lipid hydroperoxide-induced oxidative damage and effector action. Free Radic Biol Med 44: 956–968, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glantzounis GK, Salacinski HJ, Yang W, Davidson BR, Seifalian AM. The contemporary role of antioxidant therapy in attenuating liver ischemia-reperfusion injury: a review. Liver Transpl 11: 1031–1047, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Gray CC, Amrani M, Yacoub MH. Heat stress proteins and myocardial protection: experimental model or potential clinical tool? Int J Biochem Cell Biol 31: 559–573, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Gundewar S, Calvert JW, Elrod JW, Lefer DJ. Cytoprotective effects of N,N,N-trimethylsphingosine during ischemia- reperfusion injury are lost in the setting of obesity and diabetes. Am J Physiol Heart Circ Physiol 293: H2462–H2471, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Hu Y, Chen X, Pan TT, Neo KL, Lee SW, Khin ES, Moore PK, Bian JS. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflügers Arch 455: 607–616, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke H Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia-reperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol 290: G1083–G1088, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Jaeschke H Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol 284: G15–G26, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology 125: 1246–1257, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kang KJ Mechanism of hepatic ischemia/reperfusion injury and protection against reperfusion injury. Transplant Proc 34: 2659–2661, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Kasuno K, Nakamura H, Ono T, Muso E, Yodoi J. Protective roles of thioredoxin, a redox-regulating protein, in renal ischemia/reperfusion injury. Kidney Int 64: 1273–1282, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8: 661–670, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165–1167, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Koti RS, Seifalian AM, Davidson BR. Protection of the liver by ischemic preconditioning: a review of mechanisms and clinical applications. Dig Surg 20: 383–396, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc 37: 1653–1656, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Leffler CW, Parfenova H, Jaggar JH, Wang R. Carbon monoxide and hydrogen sulfide: gaseous messengers in cerebrovascular circulation. J Appl Physiol 100: 1065–1076, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtman SN, Lemasters JJ. Role of cytokines and cytokine-producing cells in reperfusion injury to the liver. Semin Liver Dis 19: 171–187, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Lowicka E, Beltowski J. Hydrogen sulfide (H2S)–the third gas of interest for pharmacologists. Pharmacol Rep 59: 4–24, 2007. [PubMed] [Google Scholar]
- 30.Moore PK, Bhatia M, Moochhala S. Hydrogen sulfide: from the smell of the past to the mediator of the future? Trends Pharmacol Sci 24: 609–611, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Plin C, Tillement JP, Berdeaux A, Morin D. Resveratrol protects against cold ischemia-warm reoxygenation-induced damages to mitochondria and cells in rat liver. Eur J Pharmacol 528: 162–168, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Rose P, Moore PK, Ming SH, Nam OC, Armstrong JS, Whiteman M. Hydrogen sulfide protects colon cancer cells from chemopreventative agent beta-phenylethyl isothiocyanate induced apoptosis. World J Gastroenterol 11: 3990–3997, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scarabelli TM, Knight R, Stephanou A, Townsend P, Chen-Scarabelli C, Lawrence K, Gottlieb R, Latchman D, Narula J. Clinical implications of apoptosis in ischemic myocardium. Curr Probl Cardiol 31: 181–264, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis 21: 105–113, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Sellke FW. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg 33: 906–913, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szabo C Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulphide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmacol 137: 139–145, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teoh NC, Farrell GC. Hepatic ischemia reperfusion injury: pathogenic mechanisms and basis for hepatoprotection. J Gastroenterol Hepatol 18: 891–902, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Wang R Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite “scavenger”? J Neurochem 90: 765–768, 2004. [DOI] [PubMed] [Google Scholar]
- 41.World CJ, Yamawaki H, Berk BC. Thioredoxin in the cardiovascular system. J Mol Med 84: 997–1003, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Xu W, Neckers L. Targeting the molecular chaperone heat shock protein 90 provides a multifaceted effect on diverse cell signaling pathways of cancer cells. Clin Cancer Res 13: 1625–1629, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Yanada S, Saitoh Y, Kaneda Y, Miwa N. Cytoprotection by bcl-2 gene transfer against ischemic liver injuries together with repressed lipid peroxidation and increased ascorbic acid in livers and serum. J Cell Biochem 93: 857–870, 2004. [DOI] [PubMed] [Google Scholar]
- 44.Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, Nishikawa H, Kawabata A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology 241: 11–18, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G, Wallace JL. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. FASEB J 20: 2118–2120, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Wang M, Xie HY, Zhou L, Meng XQ, Shi J, Zheng S. Role of reactive oxygen species in mediating hepatic ischemia-reperfusion injury and its therapeutic applications in liver transplantation. Transplant Proc 39: 1332–1337, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Z, Huang H, Liu P, Tang C, Wang J. Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening KATP channels. Can J Physiol Pharmacol 85: 1248–1253, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]