Abstract
N-oleoyldopamine (OLDA), a bioactive lipid originally found in the mammalian brain, is an endovanilloid that selectively activates the transient receptor potential vanilloid type 1 (TRPV1) channel. This study tests the hypothesis that OLDA protects the heart against ischemia and reperfusion (I/R) injury via activation of the TRPV1 in wild-type (WT) but not in gene-targeted TRPV1-null mutant (TRPV1−/−) mice. Hearts of WT or TRPV1−/− mice were Langendorffly perfused with OLDA (2 × 10−9 M) in the presence or absence of CGRP8–37 (1 × 10−6 M), a selective calcitonin gene-related peptide (CGRP) receptor antagonist; RP-67580 (1 × 10−6 M), a selective neurokinin-1 receptor antagonist; chelerythrine (5 × 10−6 M), a selective protein kinase C (PKC) antagonist; or tetrabutylammonium (TBA, 5 × 10−4 M), a nonselective K+ channel antagonist, followed by 35 min of global ischemia and 40 min of reperfusion (I/R). Left ventricular end-diastolic pressure (LVEDP), left ventricular developed pressure (LVDP), coronary flow (CF), and left ventricular peak positive dP/dt (+dP/dt) were evaluated after I/R. OLDA improved recovery of cardiac function after I/R in WT but not TRPV1−/− hearts by increasing LVDP, CF, and +dP/dt and by decreasing LVEDP. CGRP8–37, RP-67580, chelerythrine, or TBA abolished the protective effect of OLDA in WT hearts. Radioimmunoassay showed that the release of substance P (SP) and CGRP after OLDA treatment was higher in WT than in TRPV1−/− hearts, which was blocked by chelerythrine or TBA. Thus OLDA exerts a cardiac protective effect during I/R injury in WT hearts via CGRP and SP release, which is abolished by PKC or K+ channel antagonists. The protective effect of OLDA is void in TRPV1−/− hearts, supporting the notion that TRPV1 mediates OLDA-induced protection against cardiac I/R injury.
Keywords: N-oleoyldopamine, transient receptor potential vanilloid 1, ischemia-reperfusion, substance P, calcitonin gene-related peptide, protein kinase C antagonist, potassium ion channel antagonist, gene knockout
the transient receptor potential vanilloid 1 (TRPV1) channel is a nonselective cation channel mainly expressed in primary sensory neurons and sensory C- and A- fibers (17), although TRPV1 mRNA and proteins have recently been found in vascular smooth muscle and endothelial cells (15, 35, 52). TRPV1 can be activated by physical and chemical stimuli, including noxious heat, protons, vanilloid compounds (6, 10), or endogenous arachidonic acid derivatives (21, 44), which include at least three kinds of lipids: unsaturated N-acyldopamines [e.g., N-arachidonoyldopamine (NADA), N-oleoyldopamine (OLDA)] (7, 20); lipoxygenase products [e.g., 12-(S)- and 15-(S)-hydroperoxyeicosatetraenoic, leukotriene B4] (21, 39); and the N-acylethanolamines (e.g., anandamide, N-oleoylethanolamine, N-linoleoylethanolamine) (29, 38, 58). These compounds may activate TRPV1 and other receptors with different affinities and are increasingly recognized as an important class of signaling molecules affecting pain, inflammation, and tissue injury (47).
For example, anandamide may activate TRPV1 as well as cannabinoid receptors, CB1 and CB2, but its potency for TRPV1 is much less than capsaicin (3, 58). Anandamide may protect against ischemia-reperfusion (I/R) injury through activation of CB1 or CB2 in the heart, brain, and liver (1, 42, 45) and may cause release of nitric oxide through TRPV1 activation in arterial mesenteric bed (35). However, the role of TRPV1 in anandamide-induced protective effects is controversial and unclear given the facts that higher concentrations of anandamide are required to stimulate TRPV1 (44) and that the efficacy of anandamide as a TRPV1 agonist is influenced by CB1 receptor activation (39). Although 12(S)- and 15-(S)-hydroperoxyeicosatetraenoic acid (HpETE) has also been shown to protect against myocardial I/R injury through TRPV1 activation (39), 12(S)-HpETE possesses lower potency when compared with capsaicin (21). Although NADA possesses nanomolar potency for TRPV1 and causes substance P (SP) and calcitonin gene-related peptide (CGRP) release (20), it also has the similar potency for CB1 receptors (3). In contrast to these endogenous lipids that have either low or indistinct affinity or potency, or are rapidly taken up or degraded after binding to TRPV1, OLDA is the most potent and selective endogenous agonist of TRPV1 that has very low affinity for cannabinoid receptors. For example, OLDA has been shown to be 50 times more potent on TRPV1 than on CB1 receptors and at least 30 times more potent than capsaicin (7, 40). Moreover, OLDA is a stable compound that stays for hours in biomembranes, which may result from a slower rate of metabolism that provides for a longer period of receptor activation (55). Regardless, the role of OLDA in protecting the heart against I/R injury is unknown.
During myocardial ischemia, TRPV1-positive sensory nerves integrate and respond to multiple ischemic metabolites and cause chest pain (31). The responses of TRPV1-positive sensory nerves include transmitting signals to the central nervous system as well as releasing sensory neurotransmitters such as SP and CGRP, which coexpress in TRPV1-positive sensory neurons (17, 18) and may play an important role in protecting the heart from ischemic injury and damage. Indeed, we have previously shown that TRPV1 gene knockout impairs preconditioning and postischemic protection against myocardial injury in mice (49, 56). The present study was designed to use the most potent endogenous TRPV1 agonist known to date, namely OLDA, as a model compound to define whether OLDA may protect the heart from I/R injury by increasing SP and CGRP release following TRPV1 activation. With the use of wild-type (WT) and TRPV−/− mice, this study examines whether OLDA protects the heart from I/R injury by activating TRPV1, leading to SP and CGRP release in WT mice, and whether the protective effect of OLDA is absent in TRPV−/− mice. We also used the K+ channel antagonist and the protein kinase C (PKC) antagonist to examine whether blockade of these pathways may modulate OLDA-induced cardiac protective effects.
MATERIALS AND METHODS
Langendorff heart preparation and measurements of cardiac function.
Male TRPV1 gene knockout (TRPV1−/−) strain B6.129S4-TRPV1tm1Jul and matching control WT strain C57BL/6J mice (25–30 g) were used (Jackson Laboratory, Bar Harbor, ME). Mice were heparinized (500 U/kg ip) and anesthetized with pentobarbital sodium (50 mg/kg ip). Hearts from TRPV1−/− and WT mice were cannulated and retrogradely perfused at 37°C and 80 mmHg with Krebs-Henseleit buffer (in mmol/l: 118 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 25 NaHCO3, 0.5 Na-EDTA, and 11 glucose, saturated with 95% O2-5% CO2, pH 7.4) through the aorta in a noncirculating Langendorff apparatus as described previously (49). A water-filled balloon was inserted in the left ventricle and adjusted to a left ventricular end-diastolic pressure (LVEDP) of 5–8 mmHg. The distal end of the catheter was connected to a Digi-Med Heart Performance Analyzer via a pressure transducer. Coronary flow (CF) was continuously measured using an ultrasonic flow probe placed in the aortic perfusion line. Hearts were paced at 400 beats/min except during sustained global ischemia to avoid inducing excessive ventricular tachyarrhythmia during reperfusion, and pacing was reinitiated 3 min after reperfusion. Left ventricular developed pressure (LVDP, peak systolic pressure − LVEDP) and left ventricular (LV) peak positive dP/dt (+dP/dt) during isovolumic contractions were used as indexes of LV systolic function; LVEDP was used as an index of LV diastolic function. The experiments were approved by the Michigan State University Animal Care and Use Committee.
Experimental protocols.
All hearts were allowed to stabilize for 25 min and then perfused at 1% of the CF rate with 1) normal controls (nonischemic) as vehicle; 2) OLDA (2 × 10−9 M; Cayman Chemical); 3) OLDA plus CGRP8–37 (10−6 M; Sigma), a selective CGRP receptor antagonist; 4) OLDA plus RP-67580 (10−6 M; Tocris Bioscience), a selective neurokinin 1 (NK1) receptor antagonist; 5) OLDA plus chelerythrine (5 × 10−6 M; Calbiochem), a general PKC antagonist; or 6) OLDA plus tetrabutylammonium (TBA, 5 × 10−4 M; Sigma), a nonselective K+ channel antagonist. Antagonists were added to the perfusate 5 min before adding OLDA and continued for an additional 5 min with OLDA perfusion. Hearts were subsequently subjected to 35 min of no-flow normothermic global ischemia followed by 40-min of reperfusion. Additional antagonists without OLDA groups were performed in which WT hearts were perfused with the same concentrations of antagonists as above before subjected to I/R.
Lactate dehydrogenase release.
In addition to the measurement of cardiac function, cardiac injury was assessed by measuring lactate dehydrogenase (LDH) release. Perfusion effluent was collected during the first 5–15 min of I/R and stored at 80°C until analyzed. Total LDH levels were determined with the use of a Cytotoxicity Detection Kit (LDH) (Roche Applied Science). The data were expressed as absorbance units released per milliliter per minute per gram of heart wet tissues.
Evaluation of myocardial infarct size.
The risk area and infarct size were measured after 40 min of postischemia reperfusion. Hearts were perfused for 10 min at a flow rate of 2 ml/min with a 1% 2,3,5-triphenyltetrazolium chloride (TTC) dissolved in Krebs buffer. Hearts were then removed and sliced perpendicularly along the long axis from apex to base in 2-mm sections. Sections were incubated for another 10 min at 37°C in 1% TTC. Both sides of each slice were photographed and delineated, and photos were quantified with ImageJ version 1.37v (National Institutes of Health). Because hearts were subjected to global ischemia, the total cross-sectional areas were defined as the total risk areas. The infarcted area to total risk area ratio (%infarct size) of the two sides of each slice was calculated and multiplied by the weight of the slice.
Measurement of SP and CGRP.
WT and TRPV1−/− hearts were cut into pieces and put into tubes containing Krebs-Henseleit buffer with 1 μM phosphoramidon and 1 μM captopril and saturated with 95% O2-5% CO2 at 37°C continuously for 60 min (the stabilization period). In OLDA-treated groups, OLDA (2 × 10−9 M) was added and incubated for 60 min in WT and TRPV−/− hearts. To examine the effects of the PKC antagonist and the K+ channel antagonist on OLDA-induced SP and CGRP release, chelerythrine (5 × 10−6 M) and TBA (5 × 10−4 M) were added 5 min before adding OLDA. The samples were purified and analyzed by radioimmunoassay as recommended by the supplier. Commercially available rat CGRP and SP radioimmunoassay kits (Peninsula Laboratories) were used to determine SP and CGRP release that was normalized by the wet heart weight.
Immunofluorescence assay of TRPV1 receptors.
The LV tissue blocks were cut to a thickness of 10 μm. The tissues sections were incubated with the primary antibody (goat anti-TRPV1, dilution 1:2,500; Santa Cruz Biotechnology) and then with horseradish peroxidase-conjugated donkey anti-goat IgG secondary antibody (dilution 1:200; Jackson ImmunoResearch). The sections were subsequently incubated with fluorescein isothiocyanate conjugated to tyramide (TSA kit; PerkinElmer Life Sciences) following the protocol recommended by the manufacturer.
Statistical analysis.
All values are expressed as means ± SE. Differences among groups measured at the end of the I/R experiments, the release of SP, CGRP, and LDH, and the infarct size were performed by one-way ANOVA followed by the Tukey-Kramer multiple-comparison test. Results were considered statistically significant at P < 0.05.
RESULTS
OLDA protection against I/R injury was impaired in TRPV1−/− hearts.
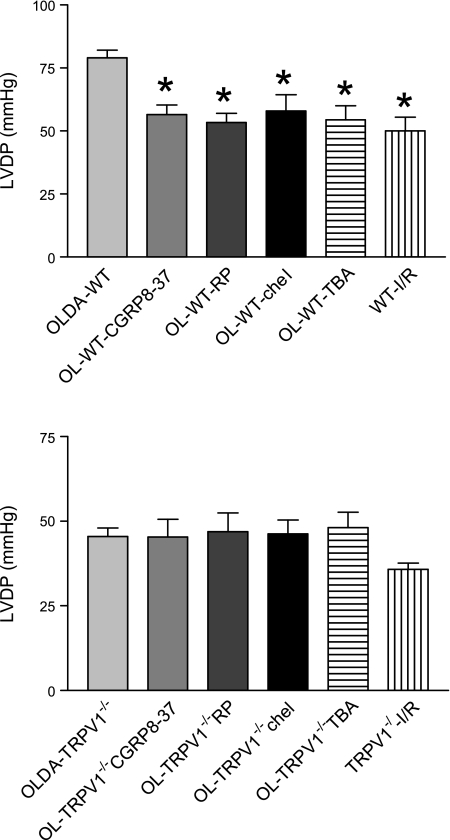
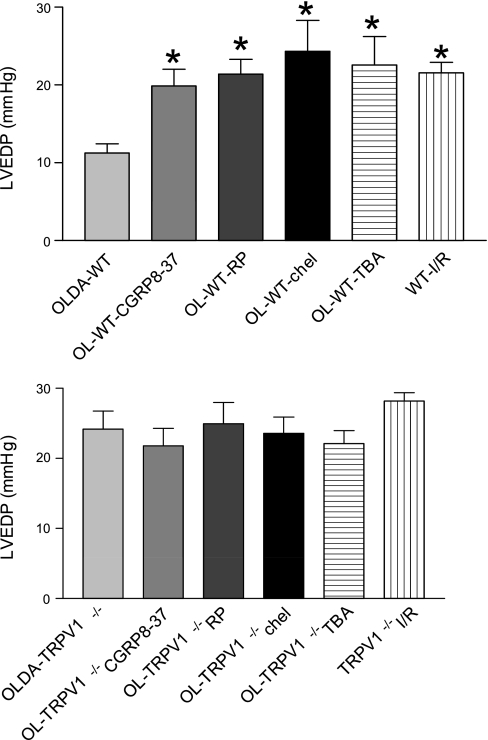
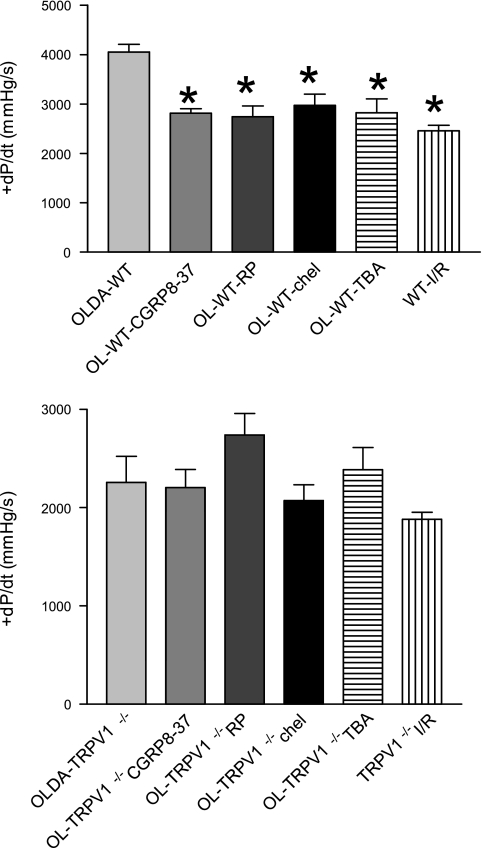
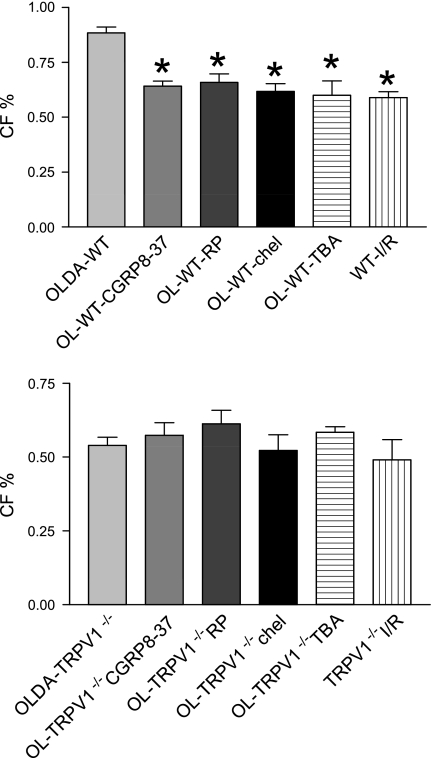
There were no statistically significant differences in hemodynamics between groups at the baseline (data not shown). After I/R, OLDA pretreatment inhibited the increase in LVEDP and improved recovery by increasing LVDP, CF, and +dP/dt in WT but not in TRVR1−/− hearts (Figs. 1–4). Thus OLDA protected WT hearts against ischemic injury, whereas it had no protective effect on TRPV1−/− hearts.
Fig. 1.
The changes of left ventricular end-diastolic pressure (LVEDP) at the end of ischemia-reperfusion (I/R). Wild-type (WT) and transient receptor potential vanilloid 1-null mutant (TRPV1−/−) hearts were retrogradely perfused in a Langendorff apparatus and subjected to N-oleoyldopamine (OLDA, 2 × 10−9 M, at 1% of coronary flow rate) for 10 min in the presence or absence of the antagonists calcitonin gene-related protein (CGRP)-8–37, RP-67580, chelerythrine, or tetrabutylammonium (TBA), and then I/R. Antagonists were added to the perfusate 5 min before OLDA and continued for 5 min after OLDA. Hearts were paced at 400 beats/min during the initial equilibration period. Pacing was terminated during ischemia and reinitiated at 3 min into the reperfusion period. In the ischemia control groups, WT and TRPV1−/− hearts were equilibrated for 40 min, followed by I/R (WT-I/R and TRPV1−/− I/R). Values are means ± SE; n = 6–11. *P < 0.05 vs. OLDA-WT.
Fig. 2.
The changes of LVEDP at the end of I/R. WT and TRPV1−/− hearts were treated as described in Fig. 1. Values are means ± SE; n = 6–11 mice. *P < 0.05 vs. OLDA-WT.
Fig. 3.
The changes of left ventricular (LV) peak positive dP/dt (+dP/dt) at the end of I/R. WT and TRPV1−/− hearts were treated as described in Fig. 1. Values are means ± SE; n = 6–11. *P < 0.05 vs. OLDA-WT.
Fig. 4.
The changes of coronary flow (CF, %baseline) at the end of I/R. WT and TRPV1−/− hearts were treated as described in Fig. 1. Values are means ± SE; n = 6–11. *P < 0.05 vs. OLDA-WT.
Blockade of the CGRP receptor impaired OLDA protection.
To determine whether endogenous CGRP plays a role in OLDA-induced cardiac protection, the selective CGRP receptor antagonist CGRP8–37 (10−6 M) was given. CGRP8–37 blocked OLDA-induced cardioprotective effects in WT mice by increasing LVEDP and decreasing LVDP, CF, and +dP/dt in WT but not TRVR1−/− hearts (Figs. 1–4). CGRP8–37 (10−6 M) had no effect on cardiac function in WT hearts without I/R (data not shown).
Blockade of the SP receptor impaired OLDA protection.
The effect of endogenous SP on OLDA-induced cardiac protection was assessed by pretreatment of the hearts with the NK1 receptor antagonist RP-67580 (10−6 M). The protective effects of OLDA were suppressed in the presence of RP-67580 by increasing LVEDP and decreasing LVDP, CF, and +dP/dt in WT but not TRVR1−/− hearts (Figs. 1–4). RP-67580 (10−6 M) had no effect on cardiac function in WT hearts without I/R (data not shown).
Blockade of PKC impaired OLDA protection.
The effect of PKC activation on OLDA-induced cardiac protection was assessed by pretreatment with the PKC inhibitor chelerythrine (5 × 10−6 M). The protective effects of OLDA were suppressed in the presence of chelerythrine by increasing LVEDP and decreasing LVDP, +dP/dt, and CF in WT but not TRVR1−/− hearts (Figs. 1–4). Chelerythrine (5 × 10−6 M) had no effect on cardiac function in WT hearts without I/R (data not shown).
Blockade of K+ channel impaired OLDA protection.
The effect of a nonselective K+ channel antagonist TBA on OLDA-induced cardiac protection was assessed. The protective effects of OLDA were suppressed in the presence of TBA (5 × 10−4 M) by increasing LVEDP and decreasing LVDP, +dP/dt, and CF in WT hearts but not TRVR1−/− hearts (Figs. 1–4). TBA (5 × 10−4 M) had no effect on cardiac function in WT hearts without ischemia (data not shown).
Measurements of LDH and infarct area.
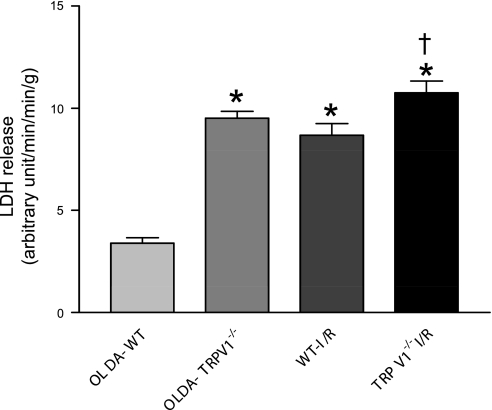
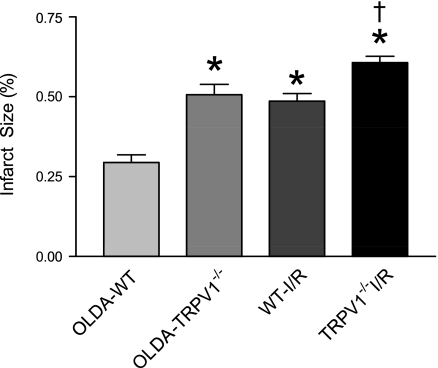
LDH levels and the infarct area after I/R were significantly lower in WT hearts treated with OLDA than WT hearts treated with vehicle or TRPV1−/− hearts with or without OLDA treatment (Figs. 5 and 6), indicating that OLDA protects hearts in WT, but it's protection is impaired in TRPV1−/−.
Fig. 5.
The cardiac injury was assessed by the release of lactate dehydrogenase (LDH) during I/R. WT and TRPV1−/− hearts were retrogradely perfused in a Langendorff apparatus and subjected to OLDA followed by I/R (OLDA-WT and OLDA-TRPV1−/−), or subjected only to I/R as injury control (WT-I/R and TRPV1−/−I/R). Coronary outflow was collected during the first period of 10–20 min of I/R and sampled for the LDH content. Values are means ± SE; n = 5. P < 0.05 vs. OLDA-WT (*) and vs. WT-I/R (†).
Fig. 6.
Cardiac injury was assessed and expressed as the percent of infarct size. WT and TRPV1−/− hearts were retrogradely perfused in a Langendorff apparatus and treated with OLDA followed by I/R (OLDA-WT and OLDA-TRPV1−/−), or subjected only to I/R as injury control (WT-I/R and TRPV1−/−I/R). Risk area and infarct size were measured 30 min after I/R. Hearts were perfused for 10 min at a flow rate of 2 ml/min with a 1% 2,3,5-triphenyltetrazolium chloride (TTC) dissolved in Krebs buffer and then removed and incubated for another 10 min at 37°C in 1% TTC. Values are means ± SE; n = 5. P < 0.05 vs. OLDA-WT (*) and P < 0.05 vs. WT-I/R (†).
Release of SP and CGRP.
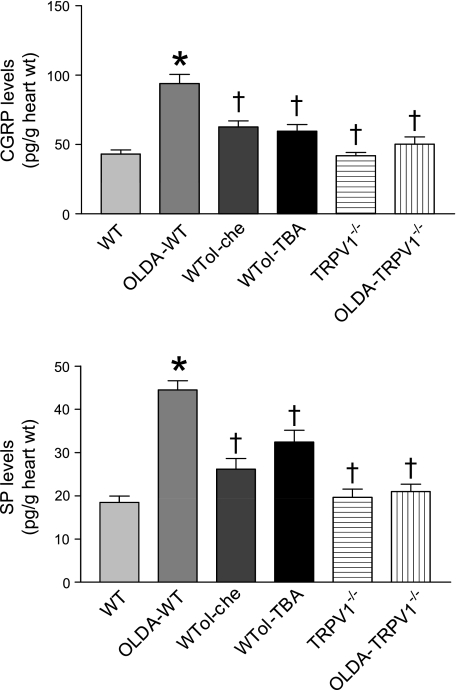
The release of SP and CGRP at baseline (normal control) was not different between WT and TRPV1−/− hearts. SP and CGRP release in WT but not TRPV1−/− hearts subjected to OLDA treatment increased remarkably compared with the baseline (Fig. 7). The PKC antagonist chelerythrine (5 × 10−6 M) and the nonselective K+ channel antagonist TBA (5 × 10−4 M) blocked OLDA-induced SP and CGRP release in WT hearts (Fig. 8).
Fig. 7.
Release of CGRP and substance P (SP) from isolated hearts subjected to OLDA (2 × 10−9 M) in the presence or absence of the protein kinase C (PKC) inhibitor chelerythrine (5 × 10−6 M) or the nonselective K+ channel antagonist TBA (5 × 10−4 M). WT and TRPV1−/− are the normal control groups. Values are means ± SE; n = 4. P < 0.05 vs. WT (*) and vs. OLDA-WT (†).
Fig. 8.
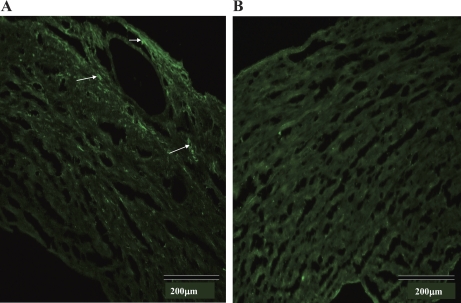
Immunofluorescence labeling of TRPV1 in WT (A) and TRPV1−/− (B) hearts. TRPV1 positive staining as indicated by the arrows is found in the myocardium and vessels in the left ventricle of the WT heart (A) but not the TRPV−/− heart (B). Negative staining was also found in WT control hearts in which the primary antibody was omitted from the immunofluorescence assay (data not shown).
TRPV1 expression in the heart.
TRPV1 immunofluorescence labeling was detected mainly in the myocardium and vascular tissues in the WT but not TRPV1−/− hearts, as indicated in Fig. 8. Negative staining was also found in WT control hearts in which the primary antibody was omitted from the immunofluorescence assay (data not shown).
DISCUSSION
TRPV1 expressed in cardiac sensory nerves may function as a molecular sensor to detect tissue ischemia and to modulate cardiac function (33). It has been shown that capsaicin-sensitive sensory nerves play a role in attenuating inflammatory responses and protecting the heart from injury (13, 23, 25). Studies using TRPV1−/− mice showed that the TRPV1 contributes to cardiac protection (39, 49, 56) and protects against the onset of sepsis after endotoxin (10). OLDA is a member of the family of fatty acid amides that includes anandamide and NADA and is increasingly recognized as an important class of lipid signaling molecules (47). OLDA has been found in striatal, dorsal spinal cord, and dorsal root ganglia (47) and identified as the most selective and potent endogenous TRPV1 activator so far (6). Regardless its potentially important role, its pathophysiological function is unknown. The present study showed that OLDA significantly improved recovery of the cardiac function after I/R injury as well as decreased LDH release and the infarct size in WT but not in TRPV1−/− hearts. Basal contractile function and baseline SP and CGRP levels were not different between WT and TRPV1−/− hearts. These results indicate that OLDA protects the heart against I/R injury via activation of the TRPV1.
As immunofluorescence assay showed, TRPV1 is expressed in the myocardium and vascular tissues in WT hearts. Previous studies in rats showed that TRPV1 protein expression cannot be detected by immunohistochemistry although TRPV1 gene expression is detectable (57). In contrast, by the use of a signal amplification technology, Tyramide Signal Amplification (TSA) Systems that increases in sensitivity significantly, others have showed that TRPV1-positive afferent nerves distribute on the epicardial surface of the ventricle and around coronary vessels in rats (54). Our data obtained from mice are consistent with that of the latter study. TRPV1 can be activated in the setting of myocardial ischemia and mediates the sensation of angina (19). Indeed, TRPV1 may integrate and respond to multiple ischemic metabolites, serving as a polymodal detector of pain-producing chemical and physical stimuli. However, TRPV1 does not merely act in sensory capacity. Its activation also causes the release of CGRP, SP, and other neurokinins from sensory nerve terminals (14, 28, 48). CGRP is one of the most potent vasodilators identified to date in many species. In addition to vasodilation, CGRP has been suggested to play a protective role after myocardial infarction and vascular damage (5).
SP is colocalized with other sensory neuropeptides, especially CGRP and neurokinin A, in sensory nerve terminals (27). SP has been shown to be released from cardiac afferent fibers during myocardial ischemia to protect the heart from I/R injury (19, 43). SP-induced cyclooxygenase-2 and prostaglandin E2 expression may contribute to SP-induced cardiac protection (24, 51). We have shown that exogenous CGRP and SP added to the perfusion solution before ischemia improved recovery of the heart after I/R injury and that beneficial effects of these neuropeptides were evident not only in WT but also in TRPV1−/− hearts (53). These results indicate that substitution of SP and CGRP before I/R is capable of inducing preconditioning-like protection. In the present study, OLDA increased SP and CGRP release in WT but not in TRPV1−/− hearts. Blockade of the NK1 receptor with RP-67580 and the CGRP receptor with CGRP8–37 impaired OLDA-induced protective effects in WT but not TRPV1−/− hearts. These data suggest that endogenously released CGRP and SP following TRPV1 activation by OLDA contribute to OLDA-induced cardiac protection.
The PKC signaling cascade has been implicated to play an important role in I/R injury, and PKC antagonists abrogated ischemic preconditioning protection in the heart (4, 53). To assess the role of PKC in mediating the protective effect of OLDA against I/R injury, chelerythrine, a selective PKC antagonist, was used. The primary sequence predicts that TRPV1 contains many putative phosphorylation sites, and PKC- and protein kinase A-mediated phosphorylation of TRPV1 is critical for its functions (2, 26, 31, 36, 46). It has been shown that PKC-mediated phosphorylation of TRPV1 may significantly increase TRPV1-mediated effects, e.g., PKC potentiates and sensitizes heat-, protons-, or agonist-induced TRPV1 currents and TRPV1-induced increases in SP and CGRP release (31, 37, 46). In the present study, OLDA-induced SP and CGRP release can be blocked by chelerythrine, indicating that PKC modulates TRPV1 function by modulating TRPV1-induced neuorpeptide release. Although SP and CGRP release induced by OLDA may also activate PKC to protect the heart from I/R injury (24, 50), our data indicate chelerythrine-induced blockade of OLDA effects is probably mediated by inhibiting SP and CGRP release rather than affecting SP and CGRP effects.
It is well known that K+ channel antagonists can block the infarct-limiting effects of ischemic preconditioning, and agonists of the channel mimic the protective effect (16). TBA, a nonspecific calcium-activated K+ channel antagonist, was used to examine whether the K+ channel blocker may inhibit OLDA-mediated cardioprotective effects. We found that TBA impaired OLDA-induced I/R protection and SP and CGRP release. Although TRPV1 contains four subunits and each contains six transmembrane domains with similar topological features and consistent permeation characteristics similarly as the voltage-gated K+ channel (22, 31), no evidence shows that the K+ channel blocker TBA may block TRPV1. Thus TBA may affect OLDA action indirectly via inhibiting OLDA-induced SP and CGRP release. Indeed, it has been shown that CGRP opens K+ channels in patches on smooth muscle cells and hyperpolarizes arterial smooth muscle and dilates arteries through ATP-sensitive potassium channel (30).
Studies using rat basophilic leukemia-1 cells showed that OLDA is a potent inhibitor of 5-lipoxygenase (5-LOX) (41). It has been shown that myocardial I/R promotes the release and metabolism of leukotrienes (LTs), which are metabolites of arachidonic acid formed from the 5-LOX pathway and exert potent vasoactive and proinflammatory effects (12). LTs also play roles in I/R of skin, brain, and kidney (8, 11, 34), and 5-LOX inhibitors have been suggested to be useful in the treatment of conditions associated with I/R of the kidney (34). However, the effects of 5-LOX inhibitors on mycardial I/R are rather controversial. Studies of the 5-LOX inhibitor LY-233569 showed that it failed to reduce the myocardial infarct size after I/R in the dog (18), indicating that 5-LOX may not mediate acute reperfusion injury of ischemic myocardium and that OLDA-mediated cardioprotective effects observed in the present study may be 5-LOX pathway independent.
In summary, the present study provides direct evidence that OLDA, an endovanilloid, exerts a cardiac protective effect during I/R injury via activating TRPV1, leading to CGRP and SP release in WT mice. The PKC antagonist and the nonspecific K+ channel antagonist block OLDA-induced SP and CGRP release and impair the protective effect of OLDA. The protective effect of OLDA is void in TRPV1−/− hearts. Our data may have important clinical implications, suggesting that impairment of OLDA production or TRPV1 expression/function may render a rather more vulnerable I/R injury.
GRANTS
This work was support in part by the National Institutes of Health Grants HL-57853, HL-73287, and DK-67620 and by a grant from Michigan Economic Development.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Batkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Hasko G, Huffman JW, Gao B, Kunos G, Pacher P. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. FASEB J 21: 1788–1800, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35: 721–731, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Bisogno T, Melck D, Bobrov M, Gretskaya NM, Bezuglov VV, De Petrocellis L, Di Marzo V. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J 351: 817–824, 2000. [PMC free article] [PubMed] [Google Scholar]
- 4.Bolli R Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol 292: H19–H27, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev 84: 903–934, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Chu CJ, Huang SM, De Petrocellis L, Bisogno T, Ewing SA, Miller JD, Zipkin RE, Daddario N, Appendino G, Di Marzo V, Walker JM. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J Biol Chem 278: 13633–13639, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Ciceri P, Rabuffetti M, Monopoli A, Nicosia S. Production of leukotrienes in a model of focal cerebral ischaemia in the rat. Br J Pharmacol 133: 1323–1329, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark N, Keeble J, Fernandes ES, Starr A, Liang L, Sugden D, de Winter P, Brain SD. The transient receptor potential vanilloid 1 (TRPV1) receptor protects against the onset of sepsis after endotoxin. FASEB J 21: 3747–3755, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405: 183–187, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Dolan R, Hartshorn K, Andry C, McAvoy D. Systemic neutrophil intrinsic 5-lipoxygenase activity and CD18 receptor expression linked to reperfusion injury. Laryngoscope 108: 1386–1389, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Engler RL, Dahlgren MD, Morris DD, Peterson MA, Schmid-Schonbein GW. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol Heart Circ Physiol 251: H314–H323, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Ferdinandy P, Csont T, Csonka C, Tèorèok M, Dux M, Nâemeth J, Horvâath LI, Dux L, Szilvâassy Z, Jancsâo G. Capsaicin-sensitive local sensory innervation is involved in pacing-induced preconditioning in rat hearts: role of nitric oxide and CGRP? Naunyn Schmiedebergs Arch Pharmacol 356: 356–363, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Franco-Cereceda A, Saria A, Lundberg JM. Differential release of calcitonin gene-related peptide and neuropeptide Y from the isolated heart by capsaicin, ischaemia, nicotine, bradykinin and ouabain. Acta Physiol Scand 135: 173–187, 1989. [DOI] [PubMed] [Google Scholar]
- 15.Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, Shohami E, Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res 132: 87–92, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol 285: H921–H930, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci 23: 183–191, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Hahn RA, MacDonald BR, Simpson PJ, Wang L, Towner RD, Ho PP, Goodwin M, Breau AP, Suarez T, Mihelich ED. Characterization of LY233569 on 5-lipoxygenase and reperfusion injury of ischemic myocardium. J Pharmacol Exp Ther 256: 94–102, 1991. [PubMed] [Google Scholar]
- 19.Hua F, Ricketts BA, Reifsteck A, Ardell JL, Williams CA. Myocardial ischemia induces the release of substance P from cardiac afferent neurons in rat thoracic spinal cord. Am J Physiol Heart Circ Physiol 286: H1654–H1664, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Geppetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA 99: 8400–8405, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci USA 97: 6155–6160, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jan LY, Jan YN. A superfamily of ion channels (Abstract). Nature 345: 672, 1990. [DOI] [PubMed] [Google Scholar]
- 23.Katona M, Boros K, Sâantha P, Ferdinandy P, Dux M, Jancsâo G. Selective sensory denervation by capsaicin aggravates adriamycin-induced cardiomyopathy in rats. Naunyn Schmiedeberg Arch Pharmacol 370: 436–443, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Koon HW, Zhao D, Zhan Y, Rhee SH, Moyer MP, Pothoulakis C. Substance P stimulates cyclooxygenase-2 and prostaglandin E2 expression through JAK-STAT activation in human colonic epithelial cells. J Immunol 176: 5050–5059, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YJ, Xiao ZS, Peng CF, Deng HW. Calcitonin gene-related peptide-induced preconditioning protects against ischemia-reperfusion injury in isolated rat hearts. Eur J Pharmacol 311: 163–167, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole-cell and single-channel studies. J Neurosci 18: 6081–6092, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundberg JM, Franco-Cereceda A, Hua X, Hèokfelt T, Fischer JA. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur J Pharmacol 108: 315–319, 1985. [DOI] [PubMed] [Google Scholar]
- 28.Manzini S, Perretti F, De Benedetti L, Pradelles P, Maggi CA, Geppetti P. A comparison of bradykinin- and capsaicin-induced myocardial and coronary effects in isolated perfused heart of guinea-pig: involvement of substance P and calcitonin gene-related peptide release. Br J Pharmacol 97: 303–312, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Movahed P, Jonsson BA, Birnir B, Wingstrand JA, Jorgensen TD, Ermund A, Sterner O, Zygmunt PM, Hogestatt ED. Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem 280: 38496–38504, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature 344: 770–773, 1990. [DOI] [PubMed] [Google Scholar]
- 31.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem 277: 13375–13378, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Oseguera AJ, Islas LD, Garcâia-Villegas R, Rosenbaum T. On the mechanism of TBA block of the TRPV1 channel. Biophys J 92: 3901–3914, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan HL, Chen SR. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation 110: 1826–1831, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Patel NS, Cuzzocrea S, Chatterjee PK, Di Paola R, Sautebin L, Britti D, Thiemermann C. Reduction of renal ischemia-reperfusion injury in 5-lipoxygenase knockout mice and by the 5-lipoxygenase inhibitor zileuton. Mol Pharmacol 66: 220–227, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Poblete IM, Orliac ML, Briones R, Adler-Graschinsky E, Huidobro-Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol 568: 539–551, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature 408: 985–990, 2000. [DOI] [PubMed] [Google Scholar]
- 37.Premkumar LS, Qi ZH, Van Buren J, Raisinghani M. Enhancement of potency and efficacy of NADA by PKC-mediated phosphorylation of vanilloid receptor. J Neurophysiol 91: 1442–1449, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Ross RA Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol 140: 790–801, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sexton A, McDonald M, Cayla C, Thiemermann C, Ahluwalia A. 12-Lipoxygenase-derived eicosanoids protect against myocardial ischemia/ reperfusion injury via activation of neuronal TRPV1. FASEB J 21: 2695–2703, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Szolcsanyi J, Sandor Z, Petho G, Varga A, Bolcskei K, Almasi R, Riedl Z, Hajos G, Czeh G. Direct evidence for activation and desensitization of the capsaicin receptor by N-oleoyldopamine on TRPV1-transfected cell, line in gene deleted mice and in the rat. Neurosci Lett 361: 155–158, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Tseng CF, Iwakami S, Mikajiri A, Shibuya M, Hanaoka F, Ebizuka Y, Padmawinata K, Sankawa U. Inhibition of in vitro prostaglandin and leukotriene biosyntheses by cinnamoyl-beta-phenethylamine and N-acyldopamine derivatives. Chem Pharm Bull (Tokyo) 40: 396–400, 1992. [DOI] [PubMed] [Google Scholar]
- 42.Underdown NJ, Hiley CR, Ford WR. Anandamide reduces infarct size in rat isolated hearts subjected to ischaemia-reperfusion by a novel cannabinoid mechanism. Br J Pharmacol 146: 809–816, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ustinova EE, Bergren D, Schultz HD. Neuropeptide depletion impairs postischemic recovery of the isolated rat heart: role of substance P. Cardiovasc Res 30: 55–63, 1995. [PubMed] [Google Scholar]
- 44.Van Der Stelt M, Di Marzo V. Endovanilloids Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur J Biochem 271: 1827–1834, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Veldhuis WB, van der Stelt M, Wadman MW, van Zadelhoff G, Maccarrone M, Fezza F, Veldink GA, Vliegenthart JF, Bär PR, Nicolay K, Di Marzo V. Neuroprotection by the endogenous cannabinoid anandamide and arvanil against in vivo excitotoxicity in the rat: role of vanilloid receptors and lipoxygenases. J Neurosci 23: 4127–4133, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol 534: 813–825, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walker JM, Krey JF, Chen JS, Vefring E, Jahnsen JA, Bradshaw H, Huang SM. Targeted lipidomics: fatty acid amides and pain modulation. Prostaglandins Other Lipid Mediat 77: 35–45, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Wang DH The vanilloid receptor and hypertension. Acta Pharmacol Sin 26: 286–294, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation 112: 3617–3623, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Wang W, Jia L, Wang T, Sun W, Wu S, Wang X. Endogenous calcitonin gene-related peptide protects human alveolar epithelial cells through protein kinase Cepsilon and heat shock protein. J Biol Chem 280: 20325–20330, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Xiao CY, Yuhki K, Hara A, Fujino T, Kuriyama S, Yamada T, Takayama K, Takahata O, Karibe H, Taniguchi T, Narumiya S, Ushikubi F. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation 109: 2462–2468, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol 290: L1267–L1276, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol Heart Circ Physiol 266: H1145–H1152, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Zahner MR, Li DP, Chen SR, Pan HL. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J Physiol 551: 515–523, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zajac D, Matysiak Z, Czarnocki Z, Pokorski M. Membrane association of N-oleoyl-dopamine in rat brain. J Physiol Pharmacol 57, Suppl 4: 403–408, 2006. [PubMed] [Google Scholar]
- 56.Zhong B, Wang DH. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol 293: H1791–H1798, 2007. [DOI] [PubMed] [Google Scholar]
- 57.Zvara A, Bencsik P, Fodor G, Csont T, Hackler L Jr, Dux M, Fürst S, Jancsó G, Puskás LG, Ferdinandy P. Capsaicin-sensitive sensory neurons regulate myocardial function and gene expression pattern of rat hearts: a DNA microarray study. FASEB J 20: 160–162, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Zygmunt PM, Petersson J, Andersson DA, Chuang H, S2rgêard M, Di Marzo V, Julius D, Hèogestèatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999. [DOI] [PubMed] [Google Scholar]