Abstract
GABAB receptor function is upregulated in the paraventricular nucleus (PVN) of the hypothalamus in spontaneously hypertensive rats (SHR), but it is unclear whether this upregulation occurs pre- or postsynaptically. We therefore determined pre- and postsynaptic GABAB receptor function in retrogradely labeled spinally projecting PVN neurons using whole cell patch-clamp recording in brain slices in SHR and Wistar-Kyoto (WKY) rats. Bath application of the GABAB receptor agonist baclofen significantly decreased the spontaneous firing activity of labeled PVN neurons in both SHR and WKY rats. However, the magnitude of reduction in the firing rate was significantly greater in SHR than in WKY rats. Furthermore, baclofen produced larger membrane hyperpolarization and outward currents in labeled PVN neurons in SHR than in WKY rats. The baclofen-induced current was abolished by either including G protein inhibitor GDPβS in the pipette solution or bath application of the GABAB receptor antagonist in both SHR and WKY rats. Blocking N-methyl-d-aspartic acid receptors had no significant effect on baclofen-elicited outward currents in SHR. In addition, baclofen caused significantly greater inhibition of glutamatergic excitatory postsynaptic currents (EPSCs) in labeled PVN neurons in brain slices from SHR than WKY rats. By contrast, baclofen produced significantly less inhibition of GABAergic inhibitory postsynaptic currents (IPSCs) in labeled PVN neurons in SHR than in WKY rats. Although microinjection of the GABAB antagonist into the PVN increases sympathetic vasomotor tone in SHR, the GABAB antagonist did not affect EPSCs and IPSCs of the PVN neurons in vitro. These findings suggest that postsynaptic GABAB receptor function is upregulated in PVN presympathetic neurons in SHR. Whereas presynaptic GABAB receptor control of glutamatergic synaptic inputs is enhanced, presynaptic GABAB receptor control of GABAergic inputs in the PVN is attenuated in SHR. Changes in both pre- and postsynaptic GABAB receptors in the PVN may contribute to the control of sympathetic outflow in hypertension.
Keywords: autonomic nervous system, synaptic transmission, hypertension, γ-aminobutyric acid, baclofen
hypertension is often associated with elevated sympathetic vasomotor tone (1, 7, 20, 50). The paraventricular nucleus (PVN) of the hypothalamus plays an important role in the control of sympathetic outflow through projections to the intermediolateral cell column of the spinal cord and rostral ventrolateral medulla (15, 19, 43, 46, 49). Previous studies have suggested that the PVN is critically involved in the development and maintenance of hypertension. For example, lesions of the PVN attenuate the development of hypertension and lower the blood pressure in spontaneously hypertensive rats (SHR) (12). However, the cellular mechanisms regulating the augmented sympathetic outflow in hypertension are not fully known.
γ-Aminobutyric acid (GABA) is a ubiquitous inhibitory neurotransmitter in the central nervous system, including the PVN. The inhibitory actions of GABA in the PVN are mediated primarily through ionotropic GABAA receptors, which are ligand-gated chloride channels, and metabotropic GABAB receptors, which are G protein-coupled receptors. GABAB receptors are distributed in both presynaptic and postsynaptic sites in the hypothalamus (33, 37, 38). Previous studies have shown that the GABAB receptor function is upregulated in the hypothalamus in hypertension. For example, microinjection of the GABAB receptor agonist baclofen into the ventromedial hypothalamus produces a larger decrease in blood pressure, heart rate, and sympathetic nerve activity in SHR than in Wistar-Kyoto (WKY) rats (51). Also, microinjection of baclofen into the PVN produces a greater inhibitory effect on sympathetic outflow in SHR than in normotensive WKY rats (29). Furthermore, the GABAB receptor antagonist CGP-55845 significantly increases the firing activity of PVN presympathetic neurons in SHR but not in WKY rats, suggesting that the GABAB receptor is tonically involved in the regulation of the excitability of PVN output neurons in SHR (28). Although these studies provide evidence that GABAB receptor function is enhanced in the hypothalamus in SHR, it remains unclear whether upregulation of GABAB receptors occurs pre- or postsynaptically in the PVN in SHR.
The GABAB receptor is a heterodimeric complex composed of a single GABAB1 subunit for membrane ligand binding and a single GABAB2 subunit for intracellular signaling through G proteins (4, 6, 22, 23). GABAB receptors are G protein-coupled receptors, which can hyperpolarize the neurons by activating inwardly rectifying K+ channels (32, 39, 45). On the other hand, activation of presynaptic GABAB receptors inhibits both GABAergic and glutamatergic inputs to spinally projecting PVN neurons (11). To study whether upregulation of GABAB receptors in the PVN of SHR occurs pre- or postsynaptically, we determined the possible functional changes in pre- and postsynaptic GABAB receptors in spinally projecting PVN neurons in SHR by using in vivo retrograde labeling and in vitro whole cell recordings in brain slices.
MATERIALS AND METHODS
Animals.
Male WKY rats and SHR (11–13 wk old; Harlan, Indianapolis, IN) were used in this study. The surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of The University of Texas M. D. Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals. The blood pressure was measured in each rat by using a noninvasive tail-cuff system (model 29-SSP; IITC Life Science, Woodland Hills, CA). We measured blood pressure every day for at least 1 wk before the electrophysiological experiments.
Retrograde labeling of spinally projecting PVN neurons.
Rats were anesthetized by intraperitoneal injection of a mixture of ketamine (70 mg/kg) and xylazine (6 mg/kg), and the spinal cord at the T1 to T4 level was exposed through dorsal laminectomy. A glass pipette (Drummond Scientific, Broomall, PA) filled with rhodamine-labeled fluorescent microsphere suspension (FluoSpheres, 0.04 μm; Molecular Probes, Eugene, OR) was positioned at 500 μm from the midline and 500 μm below the surface of the spinal cord with a micromanipulator. FluoSpheres were pressure-ejected (50 nl, Nanojector II; Drummond Scientific) bilaterally into the intermediolateral cell column region of the spinal cord in three or four separate injections. The microinjection was monitored under a surgical microscope. After injection, the rats were returned to their cages to recover for 3–5 days to permit FluoSpheres to be transported to the PVN. After surgery, rats were treated prophylactically with an antibiotic (enrofloxacin; 5 mg/kg subcutaneously daily for 3 days) and an analgesic (buprenorphine; 0.2–0.5 mg/kg subcutaneously every 12 h for 2 days).
Slice preparation.
Hypothalamic slices containing the PVN were prepared from the FluoSphere-injected rats. Briefly, under 2% isoflurane anesthesia, the rat was quickly decapitated, and the brain was immediately removed and placed in ice-cold 95% O2-5% CO2-saturated artificial cerebral spinal fluid (aCSF). A tissue block containing the hypothalamus was glued onto the stage of a vibrating microtome (Technical Product International, St. Louis, MO). Coronal hypothalamic slices containing the PVN were cut 300 μm thick, as described previously (11, 28). The slices were then transferred to a storage chamber to incubate in the aCSF continuously gassed with 95% O2-5% CO2 at 34°C for at least 1 h before electrophysiological recordings were performed. The aCSF solution contained (in mM) 124.0 NaCl, 3.0 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.4 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3.
Electrophysiological recordings.
Whole cell voltage-clamp recordings were performed in labeled PVN neurons in brain slices. A slice was placed in a recording chamber (Warner Instruments, Hamden, CT) and held to the bottom of the chamber by nylon mesh attached to a U-shaped stainless steel weight. The recording chamber was continuously perfused (3 ml/min) with aCSF at 34°C maintained by an in-line solution heater and a temperature controller (model TC-324; Warner Instruments). It took ∼1.5 min to completely exchange the solution inside the recording chamber. The labeled PVN neurons were briefly identified by using an upright microscope (BX51WI; Olympus, Tokyo, Japan) with a combination of epifluorescence illumination and differential interference contrast optics. The recording electrode was pulled from borosilicate capillaries (1.2-mm outer diameter, 0.68-mm inner diameter; World Precision Instruments, Sarasota, FL) by using a micropipette puller (P-97; Sutter Instruments, Novato, CA). The resistance of the pipette was 3–7 MΩ when it was filled with internal solution containing (in mM) 110.0 Cs2SO4, 2.0 MgCl2, 0.1 CaCl2, 1.1 EGTA, 10.0 HEPES, 2.0 MgATP, and 0.3 Na2GTP (pH was adjusted to 7.25 with 1 M CsOH, 280–300 mosmol/kgH2O). After a gigaohm seal was formed, brief negative pressure was used to obtain the whole cell configuration. Signals were processed using an Axopatch 200B amplifier (Molecular Devices, Foster City, CA), filtered at 1–2 kHz, digitized at 20 kHz using Digidata 1320A (Molecular Devices), and saved to the hard drive of a computer.
The spontaneous firing activity of labeled PVN neurons was recorded using the whole cell current-clamp technique. Recording of the firing activity of labeled PVN neurons began about 5 min after the whole cell access was established and the firing activity reached a steady state. Spontaneous firing activity was recorded from the labeled neurons with resting membrane potentials of −50 mV or lower and with action potential overshoot >10 mV.
To assess the postsynaptic GABAB receptor function, we recorded the GABAB currents induced by direct puff application of baclofen to labeled PVN neurons. To record the postsynaptic current mediated by GABAB receptors, we used a pipette solution containing (in mM) 130.0 potassium gluconate, 10.0 NaCl, 1.6 MgCl2, 0.1 EGTA, 10 HEPES, 2 MgATP, and 0.3 Na2GTP. The pH was adjusted to 7.25 with 1 M KOH (280–300 mosmol/kgH2O). Puff application of baclofen was done using a Pressure System IIe (Toohey, Fairfield, NJ). The puff pipette (∼5-μm tip diameter) was placed ∼150 μm away from the recorded cell (30). Positive pressure (∼4 lb./in.2 and 400-ms duration) was applied to eject baclofen solution onto the recorded cell to elicit a current at a holding potential of −60 mV in the presence of 1 μM tetrodotoxin.
To assess the presynaptic action of baclofen, we recorded the spontaneous and miniature excitatory postsynaptic currents (sEPSCs and mEPSCs, respectively) at a holding potential of −70 mV in the presence of 10 μM bicuculline. The spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded at a holding potential of 0 mV in the presence of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μM). The mIPSCs were recorded in the presence of 20 μM CNQX and 1 μM tetrodotoxin. To eliminate the possible postsynaptic action mediated by GABAB receptors, a general G protein inhibitor, guanosine 5′-O-(2-thiodiphosphate) (GDPβS; 1 mM), was added into the pipette solution in some experiments (36). A sodium channel blocker, lidocaine N-ethylbromide (QX-314; 10 mM), was included in the pipette solution to suppress action potential generation.
All the drugs were freshly prepared in aCSF before the recording and delivered by syringe pumps at final concentrations. Baclofen (11, 24) and CGP-55845 (5) were used as GABAB receptor agonist and antagonist, respectively. MK-801 (54) and 2-amino-5-phosphonopentanoic acid (AP5) (30) were used to block N-methyl-d-aspartic acid (NMDA) receptors. Bicuculline methiodide and CNQX disodium were used to block GABAA and non-NMDA receptors, respectively. The drugs were purchased from Sigma (St. Louis, MO) except for tetrodotoxin and QX-314, which were purchased from Alomone Labs (Jerusalem, Israel).
Data analysis.
Data are means ± SE. The firing activity and membrane potentials were analyzed over a period of 3–5 min before, during, and after drug application. The junction potential was corrected off-line based on the composition of the internal and external solution used for the recordings. The amplitude of baclofen-induced currents was analyzed using Clampfit 8.2 (Molecular Devices). The GABAB current was presented as current density normalized by cell capacitance. The firing rate, amplitude, and frequency of sEPSCs and sIPSCs were analyzed off-line using a peak detection program (MiniAnalysis; Synaptosoft, Leonia, NJ). Events were detected by setting a threshold above the noise level. The effect of drugs on the firing rate, GABAB current, amplitude, and the frequency of sEPSCs and sIPSCs was analyzed using ANOVA with Dunn's post hoc test. Two-way ANOVA was used to compare the difference of the baclofen-induced inhibitory effect on the frequency and amplitude of sEPSCs and sIPSCs between SHR and WKY rats. P < 0.05 was considered to be statistically significant.
RESULTS
The mean systolic arterial blood pressure of 11- to 13-wk-old SHR (220 ± 12 mmHg, n = 29 rats) was significantly higher than that of age-matched WKY rats (132 ± 11 mmHg, n = 32 rats). Whole cell voltage-clamp and current-clamp recordings were performed on a total of 150 FluoSphere-labeled PVN cells (83 from SHR and 67 from WKY rats). The resting membrane potentials, input resistance, cell capacitance, and access resistance of labeled PVN neurons were not significantly different between WKY rats and SHR (Table 1).
Table 1.
Electrophysiological properties of labeled PVN neurons in WKY and SHR
| No. of Cells | Membrane Potential, mV | Input Resistance, MΩ | Cell Capacitance, pF | Access Resistance, MΩ | |
|---|---|---|---|---|---|
| WKY | 42 | −59.1±4.5 | 492.6±25.6 | 29.7±1.5 | 17.3±1.5 |
| SHR | 60 | −57.9±3.6 | 516.1±18.9 | 31.9±1.2 | 16.2±1.2 |
Values are means ± SE measured in paraventricular neurons (PVN) of Wistar-Kyoto (WKY) and spontaneously hypertensive rats (SHR).
Effect of baclofen on spontaneous firing activity of labeled PVN neurons in WKY rats and SHR.
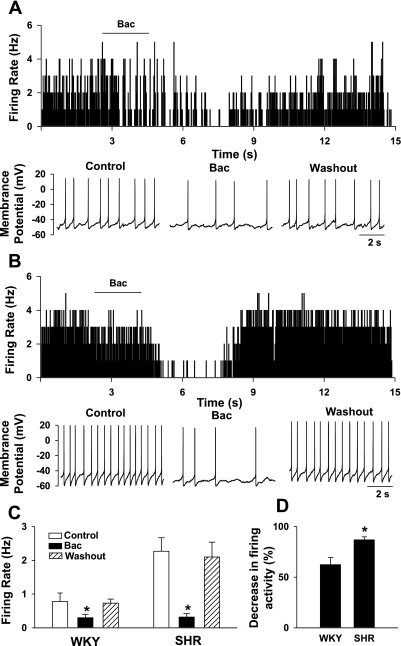
We first determined the effect of the GABAB receptor agonist baclofen on the spontaneous firing activity of labeled PVN neurons. The majority of the labeled PVN neurons displayed spontaneous discharges in WKY rats (12 of 15 cells, 75.0%) and SHR (11 of 14 cells, 78.6%). The baseline firing rate of PVN neurons was significantly higher in SHR (2.3 ± 0.5 Hz, n = 11 cells) than in WKY rats (0.8 ± 0.3 Hz, n = 12, P < 0.05). We tested the effect of baclofen on the firing activity only in labeled neurons with spontaneous activity. In WKY rats, bath application of 10 μM baclofen for 2 min significantly decreased the firing activity of labeled PVN neurons in 8 of 12 cells (Fig. 1). The membrane potential was hyperpolarized from −59.6 ± 1.5 to −64.3 ± 2.5 mV (P < 0.05) in these eight cells. The firing rate of the remaining four cells did not change significantly (1.6 ± 0.7 vs. 1.5 ± 0.8 Hz) in response to 10 μM baclofen. In SHR, bath application of 10 μM baclofen for 2 min significantly reduced the firing activity of labeled PVN neurons in 9 of 11 cells (Fig. 1). The membrane potential was hyperpolarized from −57.6 ± 1.3 to −63.8 ± 2.6 mV (P < 0.05) in these nine cells. Baclofen failed to change the firing activity in the remaining two cells (from 2.6 to 2.5 Hz in 1 cell and 1.6 to 1.5 Hz in another cell). However, the magnitude of the baclofen-induced decrease in the firing activity of PVN neurons was significantly larger in SHR than in WKY rats (Fig. 1D).
Fig. 1.
Effect of baclofen on the firing activity of labeled paraventricular nucleus (PVN) neurons in Wistar-Kyoto (WKY) rats and spontaneously hypertensive rats (SHR). A and B, top: frequency histograms showing the effect of 10 μM baclofen on the firing activity of labeled PVN neurons in WKY rats (A) and SHR (B); bottom: representative tracings showing the spontaneous firing activity of the same neurons during control, application of baclofen, and washout. C: summary data showing the effects of baclofen (10 μM) on the firing activity of labeled PVN neurons in WKY rats and SHR. D: summary data showing the percent inhibition of the firing activity of labeled PVN neurons in WKY rats and SHR. Data are means ± SE. *P < 0.05 compared with control in C and with WKY rats in D. Bac, baclofen.
Baclofen-induced postsynaptic currents in labeled PVN neurons in WKY rats and SHR.
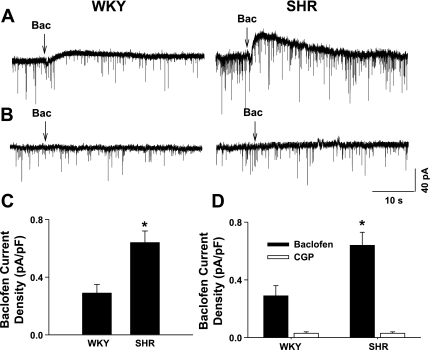
We next determined the postsynaptic current induced by puff application of baclofen to labeled PVN neurons at a holding potential of −60 mV in WKY rats and SHR. Since the capacitance of labeled neurons varied from 32 to 48 pF, we normalized the current by cell capacitance. Puff application of 10 μM baclofen to labeled PVN neurons did not evoke detectable outward currents in WKY rats (n = 4 cells) and SHR (n = 4 cells) (data not shown). When the baclofen concentration was increased to 100 μM, it evoked an outward current in 7 of 13 cells (53.9%) in WKY rats and 12 of 14 labeled PVN neurons (85%) in age-matched SHR (Fig. 2). The baclofen-sensitive current density (normalized by cell capacitance) was significantly higher in SHR than in WKY rats (Fig. 2C).
Fig. 2.
Baclofen-sensitive currents in labeled PVN neurons in WKY rats and SHR. A: original tracings showing currents elicited by puff application of 100 μM baclofen to labeled PVN neurons in WKY rat and SHR. Arrows indicate puff application of baclofen. B: raw tracings showing undetectable current by puff application of 100 μM baclofen when 1 mM GDPβS was included in the recording pipette solution in WKY rats and SHR. C: summary data showing the baclofen-sensitive current elicited by puff application of 100 μM baclofen in labeled PVN neurons in WKY rats and SHR. D: summary data showing baclofen-sensitive currents in the absence and presence of 2 μM CGP-55845 (CGP) in labeled PVN neurons in WKY rats and SHR. Data are means ± SE. *P < 0.05 compared with the corresponding value in WKY rats.
To examine whether baclofen-induced outward current was mediated through G proteins, we included 1 mM GDPβS, a competitive inhibitor of G proteins, in the internal pipette solution. When 1 mM GDPβS was included in the pipette solution, puff application of 100 μM baclofen failed to produce any outward current in all labeled PVN neurons in both WKY rats (n = 8 cells) or SHR (n = 6 cells) (Fig. 2B). We also determined whether the outward current elicited by baclofen in labeled PVN neurons was mediated by GABAB receptors. In a separate group of labeled PVN neurons, neurons with baclofen-sensitive currents were initially identified by puff application of 100 μM baclofen. Bath application of 2 μM CGP-55845, a specific GABAB receptor antagonist (44), for 4–5 min completely blocked the baclofen-induced outward current in all neurons tested in both WKY rats (n = 5 cells) and SHR (n = 7 cells) (Fig. 2D).
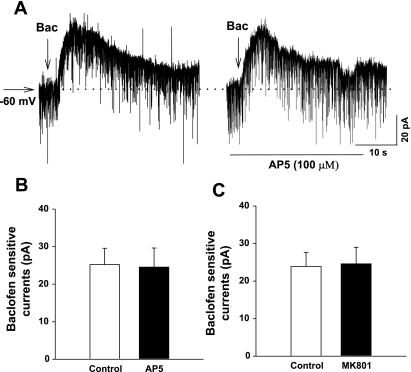
We then determined whether the increased baclofen-sensitive currents in labeled PVN neurons in SHR resulted from augmented glutamatergic inputs (3, 30, 35). Puff application of 100 μM baclofen elicited an outward current of 25.2 ± 4.36 pA in 13 of 15 labeled PVN neurons in SHR. The slice was then treated with NMDA receptor antagonists AP5 (100 μM, n = 6) or MK-801 (20 μM, n = 7) for 5 min. It has been shown that the NMDA receptor is completely blocked by 100 μM AP5 or 20 μM MK-801 (30, 54). Bath application of AP5 (100 μM) or MK-801 (20 μM) did not produce significant transmembrane current in these labeled PVN neurons in WKY rats and SHR. Puff application of 100 μM baclofen produced similar outward currents in these neurons in the presence of AP5 or MK-801 (Fig. 3).
Fig. 3.
Lack of effect of N-methyl-d-aspartic acid (NMDA) receptor antagonists on baclofen-elicited currents in labeled PVN neurons in SHR. A: raw tracings showing that puff application of 100 μM baclofen elicited outward currents in the absence and presence of 2-amino-5-phosphonopentanoic acid (AP5) in a labeled PVN neuron in SHR. B and C: summary data showing baclofen-elicited currents in labeled PVN neurons in SHR before and after blockade of NMDA receptors with AP5 (B) or MK-801 (C).
Effect of baclofen on sEPSCs in labeled PVN neurons in WKY rats and SHR.
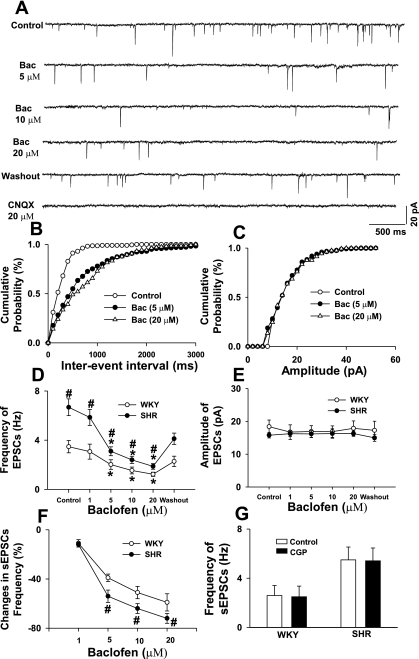
To assess the function of presynaptic GABAB receptors on glutamatergic afferent terminals in the PVN in WKY rats and SHR, we tested the effect of baclofen on glutamatergic sEPSCs. Inclusion of the G protein inhibitor GDPβS (1 mM) in the internal pipette solution had no significant effect on the baseline frequency (2.6 ± 0.6 vs. 2.7 ± 0.8 Hz in WKY rats and 5.5 ± 0.8 vs. 5.6 ± 0.9 Hz in SHR) and amplitude (16.3 ± 0.8 vs. 16.2 ± 1.2 pA in WKY rats and 16.5 ± 1.0 vs. 17.5 ± 0.9 pA in SHR) of sEPSCs in either WKY rats or SHR. In WKY rats, bath application of 1 to 20 μM baclofen dose-dependently reduced the frequency, but not the amplitude, of sEPSCs in all of the labeled PVN neurons (Fig. 4, A–E, n = 9 cells). The sEPSCs were completely blocked by 20 μM CNQX (Fig. 4A). The decrease in the frequency of sEPSCs occurred ∼2.0 ± 0.4 min after perfusion of baclofen into the recording chamber. In age-matched SHR, baclofen also dose-dependently decreased the frequency, but not the amplitude, of sEPSCs in all of the labeled PVN neurons (Fig. 4, n = 8 cells). In both WKY rats and SHR, the cumulative probability analysis of sEPSCs revealed that the distribution pattern of the interevent interval of sEPSCs was shifted toward the right in response to baclofen. However, the baclofen-induced inhibition of the frequency of sEPSCs at different concentrations was significantly greater in SHR than in WKY rats (Fig. 4F). In a separate group of labeled PVN neurons, blocking GABAB receptors with 2 μM CGP-55845 had no significant effect on the basal frequency and amplitude of sEPSCs in either WKY rats (n = 7 cells) or SHR (n = 8 cells) (Fig. 4G).
Fig. 4.
Effect of baclofen on glutamatergic spontaneous excitatory postsynaptic currents (sEPSCs) in labeled PVN neurons in WKY rats and SHR. A: raw tracings showing sEPSCs during control, bath application of 5, 10, and 20 μM baclofen, washout, and application of 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) in a labeled PVN neuron in SHR. B and C: cumulative plot analysis of sEPSCs of the same neuron showing the distribution of the interevent interval (B) and amplitude (C) during control and application of baclofen. D and E: dose-response effect of baclofen on the frequency (D) and amplitude (E) of sEPSCs in labeled PVN neurons of WKY rats and SHR. F: summary data showing the percentage of baclofen-induced reduction in frequency of sEPSCs in WKY rats and SHR. G: summary data showing the effects of bath application of 2 μM CGP on the basal frequency of sEPSCs in WKY rats and SHR. Data are means ± SE. *P < 0.05 compared with control. #P < 0.05 compared with the corresponding value in WKY rats.
Bath application of 1 μM tetrodotoxin had no significant effect on the frequency (2.5 ± 0.4 vs. 2.2 ± 0.5 Hz in WKY rats and 4.9 ± 0.6 vs. 4.6 ± 0.5 Hz in SHR) and amplitude (18.5 ± 5.1 vs. 17.4 ± 4.6 pA in WKY rats and 17.9 ± 4.5 vs. 18.1 ± 5.2 pA in SHR) of sEPSCs in these labeled PVN neurons in WKY rats (n = 7 cells) and SHR (n = 8 cells).
Effect of baclofen on sIPSCs in labeled PVN neurons in WKY rats and SHR.
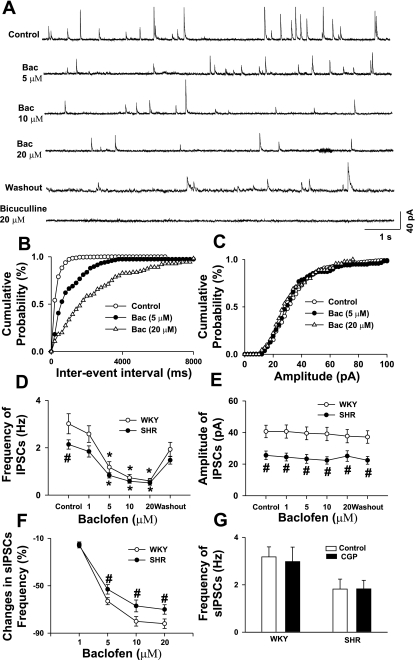
To further determine the function of presynaptic GABAB receptors on GABAergic terminals in the PVN in WKY rats and SHR, we tested the effect of baclofen on GABAergic sIPSCs. Inclusion of GDPβS (1 mM) in the recording pipette solution did not change the baseline frequency (3.2 ± 0.4 vs. 2.9 ± 0.6 Hz in WKY rats and 1.8 ± 0.4 vs. 1.9 ± 0.6 Hz in SHR) and amplitude (40.6 ± 4.9 vs. 41.3 ± 4.5 pA in WKY rats and 25.5 ± 3.2 vs. 24.4 ± 2.9 pA in SHR) of GABAergic sIPSCs in either WKY rats or SHR. Bath application of 1, 5, 10, and 20 μM baclofen reduced the frequency of sIPSCs in a dose-dependent fashion without an effect on the amplitude of sIPSCs in both WKY rats and SHR (Fig. 5, A–F). The sIPSCs were completely blocked by 10 μM bicuculline in both WKY rats and SHR (Fig. 5A). The cumulative probability analysis of sIPSCs showed that the distribution pattern of the interevent interval of sIPSCs was shifted toward the right in response to baclofen. The magnitude of inhibition of the sIPSC frequency by different concentrations of baclofen in labeled PVN neurons was significantly smaller in SHR than in WKY rats (Fig. 5F). In a separate group of labeled PVN neurons, blocking of GABAB receptors with 2 μM CGP-55845 had no significant effect on the basal frequency and amplitude of sIPSCs in either WKY rats or SHR (Fig. 5G).
Fig. 5.
Effect of baclofen on GABAergic spontaneous inhibitory postsynaptic currents (sIPSCs) in labeled PVN neurons in WKY rats and SHR. A: original tracings showing sIPSCs during control, bath application of 5, 10, and 20 μM baclofen, washout, and application of 10 μM bicuculline in a labeled PVN neuron in SHR. B and C: cumulative plot analysis of sIPSCs of the same neuron showing the distribution of the interevent interval (B) and amplitude (C) during control and application of baclofen. D and E: dose-response effect of baclofen on the frequency (D) and amplitude (E) of sIPSCs in labeled PVN neurons of WKY rats and SHR. F: summary data showing the percentage of baclofen-induced reduction of frequency in sIPSCs in WKY rats and SHR. G: summary data showing the effects of bath application of 2 μM CGP on the basal frequency of sIPSCs in WKY rats and SHR. Data are means ± SE. *P < 0.05 compared with control. #P < 0.05 compared with the corresponding value in WKY rats.
Bath application of 1 μM tetrodotoxin did not significantly change the frequency (3.1 ± 0.6 vs. 2.7 ± 0.6 Hz in WKY rats and 1.8 ± 0.4 vs. 1.6 ± 0.4 Hz in SHR) and amplitude (38.8 ± 4.2 vs. 37.6 ± 4.3 pA in WKY rats and 24.5 ± 3.8 vs. 22.7 ± 3.5 pA in SHR) of sIPSCs in these labeled PVN neurons in WKY rats (n = 7 cells) and SHR (n = 6 cells).
DISCUSSION
Our finding that baclofen produced a greater inhibition of the firing activity of PVN neurons in SHR than in WKY rats is consistent with previous studies showing that the hypothalamic GABAB receptor function is enhanced in the hypothalamus in SHR (28, 29, 51). To assess whether the postsynaptic GABAB receptor function is altered in spinally projecting PVN neurons in SHR, we compared postsynaptic GABAB receptor currents evoked by puff application of baclofen directly to labeled PVN neurons in WKY rats and SHR. We found that the amplitude of baclofen-elicited outward currents in labeled PVN neurons was significantly greater in SHR than in WKY rats. Bath application of CGP-55845, a specific GABAB receptor antagonist (37, 44), or inhibition of G proteins with intracellular dialysis of GDPβS completely blocked baclofen-elicited outward currents in all PVN neurons tested. These data suggest that the postsynaptic GABAB receptor function is upregulated in the PVN presympathetic neurons in SHR. It is likely that this upregulation of postsynaptic GABAB receptor function contributes to the potentiated inhibitory effect of baclofen on the firing activity of spinally projecting PVN neurons and sympathetic vasomotor tone in SHR (28, 29).
However, little is known about how the postsynaptic GABAB receptor function is augmented in the PVN in hypertension. Previous studies have shown that NMDA receptor activation can increase the receptor gene expression and postsynaptic GABAB receptor function in the spinal cord and hippocampus (3, 13, 35). Since the glutamatergic input in the PVN is enhanced in SHR (27, 30), it is possible that the enhanced glutamatergic input in SHR causes more Ca2+ influx into postsynaptic PVN neurons, which could upregulate postsynaptic GABAB receptors through Ca2+-dependent phosphorylation or dephosphorylation mechanisms. However, we found that acutely blocking NMDA receptors with AP5 or MK-801 had no significant effect on the baclofen-elicited currents in labeled PVN neurons in SHR. Because acute blockade of NMDA receptors may not be sufficient to inhibit the downstream signaling cascade leading to increased expression of GABAB receptors by enhanced glutamatergic inputs in SHR, we cannot rule out the possibility that chronic blockade of NMDA receptor is required to affect GABAB receptors in SHR. Furthermore, increased angiotensin II concentration and angiotensin receptors in the PVN in SHR (18, 42) also could play a role in the upregulation of GABAB receptors. The physiological significance of increased function of postsynaptic GABAB receptors in PVN presympathetic neurons in hypertension is not fully known. The excitability of PVN presympathetic neurons is tonically controlled by the GABAergic input and both GABAA and GABAB receptors. We have shown previously that the GABAA receptor function in the PVN is attenuated, which contributes to increased firing activity of PVN presympathetic neurons in SHR (29). The GABAB receptor can function as a “brake” to reduce the NMDA action in CA1 neurons (40). Hence, upregulation of postsynaptic GABAB receptors in the PVN may represent a compensatory response to the reduced GABAA receptor function and enhanced glutamatergic input to these neurons in SHR and serve to dampen the increased sympathetic outflow in hypertension.
The firing activity of PVN neurons is regulated by excitatory and inhibitory synaptic inputs such as glutamatergic and GABAergic inputs from inside the PVN, suprachiasmatic nucleus, and subfornical organ (2, 14, 16, 52). Previous studies have shown that the GABAergic system may be impaired in the hypothalamus in hypertension (26, 28, 34, 51). Consistent with these previous studies, we found that the basal frequency of GABAergic sIPSCs of labeled PVN neurons was significantly lower in SHR than in WKY rats. In contrast, the basal frequency of glutamatergic sEPSCs of labeled PVN neurons was much higher in SHR than in WKY rats (30). It is possible that both increased glutamatergic input and decreased GABAergic input contribute to the high basal firing activity of PVN presympathetic neurons and increased sympathetic vasomotor tone in SHR (28–30). We found that tetrodotoxin had no significant effect on the basal frequency of sIPSCs and sEPSCs in both WKY rats and SHR, which is consistent with the findings from previous studies (10, 11, 21). These results suggest that changes in sIPSCs and sEPSCs in our thin slice preparation are probably caused by altered synaptic transmitter release at the terminal rather than the activity of glutamatergic and GABAergic neurons. However, it is uncertain whether altered synaptic release of glutamate and GABA is due to changes in the release probability or the density of presynaptic terminals in the PVN in SHR. Notably, blocking of GABAB receptors with CGP-55845 alone did not significantly alter the frequency and amplitude of sIPSCs and sEPSCs in labeled PVN neurons in either WKY rats or SHR. Thus it is unlikely that the observed difference in the baseline sIPSCs and sEPSCs between SHR and WKY rats is the result of differential activation of GABAB receptors at the GABAergic and glutamatergic terminals.
Activation of presynaptic GABAB receptors inhibits GABAergic and glutamatergic synaptic inputs to spinally projecting PVN neurons (11). We determined the presynaptic action of baclofen by using the following strategies. According to the quantal hypothesis, presynaptic action can affect the probability of neurotransmitter release. Analysis of the frequency and amplitude of IPSCs or EPSCs has been commonly used to determine pre- and postsynaptic loci of the agents (21, 41, 47). Furthermore, to determine the presynaptic effect of baclofen, we blocked the postsynaptic effect of baclofen by including GDPβS in the pipette internal solution (36). In this study, we observed that baclofen produced a greater inhibitory effect on the frequency of glutamatergic sEPSCs in SHR than in WKY rats. In contrast, baclofen caused a smaller inhibitory effect on the frequency of sIPSCs in SHR than in WKY rats. These findings suggest that the presynaptic GABAB receptor function in the control of glutamatergic and GABAergic synaptic inputs to the PVN presympathetic neurons is differentially affected in SHR. The increased GABAB receptor function on glutamatergic terminals and reduced GABAB receptor function on GABAergic terminals could contribute to the enhanced inhibitory effect of baclofen on the firing activity of PVN neurons and sympathetic vasomotor tone in SHR. We have shown that microinjection of the GABAB receptor antagonist into the PVN produces a sympathoexcitatory effect in SHR (29). However, we found that GABAB receptor antagonist had no significant effect in the in vitro experiments. It is possible that the lack of the GABAB receptor antagonist effect on synaptic glutamate and GABA release is because tonic activation of GABAB receptors by endogenously released GABA is reduced in the slice preparation.
It is not clear whether different GABAB receptor subunits (i.e., GABAB1 and GABAB2) are differentially expressed at glutamatergic and GABAergic terminals in the PVN in SHR. For example, in the hippocampus, both GABAB1 and GABAB2 subtypes are mainly present at glutamatergic axon terminals (25). Also, in the dorsal cochlear nucleus, GABAB1 is primarily located at glutamatergic synapse but not GABAergic terminals (31). Furthermore, GABAB receptor subunits are differentially localized in the subcellular structures in glutamatergic and GABAergic synapses in the globus pallidus (9). Alternatively, changes in the downstream signaling also can preferentially alter the GABAB receptor function in glutamatergic and GABAergic terminals in the PVN in SHR.
In summary, data from our study strongly suggest an upregulation of postsynaptic GABAB receptor function in PVN presympathetic neurons in SHR, an enhancement of presynaptic GABAB receptor control of glutamatergic inputs, and an attenuation of presynaptic GABAB receptor control of GABAergic inputs in the PVN in SHR. It has been shown that GABAB receptor-mediated depressor response and the GABAB mRNA level are increased in the nucleus of the solitary tract in SHR and renal-wrap model of hypertension (8, 17, 48, 53). However, the mechanisms underlying the plasticity of pre- and postsynaptic GABAB receptor function in hypertension remain to be determined. The enhanced GABAB control of the firing activity of the PVN neurons may represent a potential new target for the treatment of neurogenic hypertension.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL60026 and HL77400 and Scientist Development Grant 0635402N from the American Heart Association National Center.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Allen AM Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39: 275–280, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bains JS, Ferguson AV. Paraventricular nucleus neurons projecting to the spinal cord receive excitatory input from the subfornical organ. Am J Physiol Regul Integr Comp Physiol 268: R625–R633, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Benardo LS N-methyl-d-aspartate transmission modulates GABAB-mediated inhibition of rat hippocampal pyramidal neurons in vitro. Neuroscience 68: 637–643, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev 84: 835–867, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Blake JF, Cao CQ, Headley PM, Collingridge GL, Brugger F, Evans RH. Antagonism of baclofen-induced depression of whole-cell synaptic currents in spinal dorsal horn neurones by the potent GABAB antagonist CGP55845. Neuropharmacology 32: 1437–1440, 1993. [DOI] [PubMed] [Google Scholar]
- 6.Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma-aminobutyric acidB receptors: structure and function. Pharmacol Rev 54: 247–264, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Cabassi A, Vinci S, Cantoni AM, Quartieri F, Moschini L, Cavazzini S, Cavatorta A, Borghetti A. Sympathetic activation in adipose tissue and skeletal muscle of hypertensive rats. Hypertension 39: 656–661, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Catelli JM, Sved AF. Enhanced pressor response to GABA in the nucleus tractus solitarii of the spontaneously hypertensive rat. Eur J Pharmacol 151: 243–248, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Boyes J, Yung WH, Bolam JP. Subcellular localization of GABAB receptor subunits in rat globus pallidus. J Comp Neurol 474: 340–352, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Chen Q, Li DP, Pan HL. Presynaptic alpha1 adrenergic receptors differentially regulate synaptic glutamate and GABA release to hypothalamic presympathetic neurons. J Pharmacol Exp Ther 316: 733–742, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Pan HL. Regulation of synaptic input to hypothalamic presympathetic neurons by GABAB receptors. Neuroscience 142: 595–606, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Ciriello J, Kline RL, Zhang TX, Caverson MM. Lesions of the paraventricular nucleus alter the development of spontaneous hypertension in the rat. Brain Res 310: 355–359, 1984. [DOI] [PubMed] [Google Scholar]
- 13.Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature 340: 474–476, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Cui LN, Coderre E, Renaud LP. Glutamate and GABA mediate suprachiasmatic nucleus inputs to spinal-projecting paraventricular neurons. Am J Physiol Regul Integr Comp Physiol 281: R1283–R1289, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Dampney RA Functional organization of central pathways regulating the cardiovascular system. Physiol Rev 74: 323–364, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Decavel C, van den Pol AN. Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J Comp Neurol 316: 104–116, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Durgam VR, Vitela M, Mifflin SW. Enhanced gamma-aminobutyric acid-B receptor agonist responses and mRNA within the nucleus of the solitary tract in hypertension. Hypertension 33: 530–536, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Gutkind JS, Kurihara M, Castren E, Saavedra JM. Increased concentration of angiotensin II binding sites in selected brain areas of spontaneously hypertensive rats. J Hypertens 6: 79–84, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Hardy SG Hypothalamic projections to cardiovascular centers of the medulla. Brain Res 894: 233–240, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Judy WV, Watanabe AM, Henry DP, Besch HR Jr, Murphy WR, Hockel GM. Sympathetic nerve activity: role in regulation of blood pressure in the spontaneously hypertensive rat. Circ Res 38: 21–29, 1976. [DOI] [PubMed] [Google Scholar]
- 21.Kabashima N, Shibuya I, Ibrahim N, Ueta Y, Yamashita H. Inhibition of spontaneous EPSCs and IPSCs by presynaptic GABAB receptors on rat supraoptic magnocellular neurons. J Physiol 504: 113–126, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature 386: 239–246, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature 396: 683–687, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Kolaj M, Bai D, Renaud LP. GABAB receptor modulation of rapid inhibitory and excitatory neurotransmission from subfornical organ and other afferents to median preoptic nucleus neurons. J Neurophysiol 92: 111–122, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Kulik A, Vida I, Lujan R, Haas CA, Lopez-Bendito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABAB receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus. J Neurosci 23: 11026–11035, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunkler PE, Hwang BH. Lower GABAA receptor binding in the amygdala and hypothalamus of spontaneously hypertensive rats. Brain Res Bull 36: 57–61, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49: 916–925, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Li DP, Pan HL. Plasticity of GABAergic control of hypothalamic presympathetic neurons in hypertension. Am J Physiol Heart Circ Physiol 290: H1110–H1119, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther 320: 615–626, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol 586: 1637–1647, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lujan R, Shigemoto R, Kulik A, Juiz JM. Localization of the GABAB receptor 1a/b subunit relative to glutamatergic synapses in the dorsal cochlear nucleus of the rat. J Comp Neurol 475: 36–46, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19: 687–695, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABAB receptors in the rat central nervous system. J Comp Neurol 405: 299–321, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Martin DS, Haywood JR. Reduced GABA inhibition of sympathetic function in renal-wrapped hypertensive rats. Am J Physiol Regul Integr Comp Physiol 275: R1523–R1529, 1998. [DOI] [PubMed] [Google Scholar]
- 35.McCarson KE, Enna SJ. Nociceptive regulation of GABAB receptor gene expression in rat spinal cord. Neuropharmacology 38: 1767–1773, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Miller LD, Petrozzino JJ, Connor JA. G protein-coupled receptors mediate a fast excitatory postsynaptic current in CA3 pyramidal neurons in hippocampal slices. J Neurosci 15: 8320–8330, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol 46: 423–462, 1995. [DOI] [PubMed] [Google Scholar]
- 38.Mouginot D, Kombian SB, Pittman QJ. Activation of presynaptic GABAB receptors inhibits evoked IPSCs in rat magnocellular neurons in vitro. J Neurophysiol 79: 1508–1517, 1998. [DOI] [PubMed] [Google Scholar]
- 39.Nicoll RA The coupling of neurotransmitter receptors to ion channels in the brain. Science 241: 545–551, 1988. [DOI] [PubMed] [Google Scholar]
- 40.Otmakhova NA, Lisman JE. Contribution of Ih and GABAB to synaptically induced afterhyperpolarizations in CA1: a brake on the NMDA response. J Neurophysiol 92: 2027–2039, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki M, Shibuya I, Kabashima N, Isse T, Noguchi J, Ueta Y, Inoue Y, Shigematsu A, Yamashita H. Preferential potentiation by nitric oxide of spontaneous inhibitory postsynaptic currents in rat supraoptic neurones. J Neuroendocrinol 12: 273–281, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Phillips MI, Kimura B. Brain angiotensin in the developing spontaneously hypertensive rat. J Hypertens 6: 607–612, 1988. [DOI] [PubMed] [Google Scholar]
- 43.Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience 100: 549–556, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Riley RC, Trafton JA, Chi SI, Basbaum AI. Presynaptic regulation of spinal cord tachykinin signaling via GABAB but not GABAA receptor activation. Neuroscience 103: 725–737, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Slugg RM, Zheng SX, Fang Y, Kelly MJ, Ronnekleiv OK. Baclofen inhibits guinea pig magnocellular neurones via activation of an inwardly rectifying K+ conductance. J Physiol 551: 295–308, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491: 156–162, 1989. [DOI] [PubMed] [Google Scholar]
- 47.Sulzer D, Pothos EN. Regulation of quantal size by presynaptic mechanisms. Rev Neurosci 11: 159–212, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Sved JC, Sved AF. Cardiovascular responses elicited by gamma-aminobutyric acid in the nucleus tractus solitarius: evidence for action at the GABAB receptor. Neuropharmacology 28: 515–520, 1989. [DOI] [PubMed] [Google Scholar]
- 49.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6: 269–324, 1983. [DOI] [PubMed] [Google Scholar]
- 50.Takeda K, Bunag RD. Augmented sympathetic nerve activity and pressor responsiveness in DOCA hypertensive rats. Hypertension 2: 97–101, 1980. [DOI] [PubMed] [Google Scholar]
- 51.Takenaka K, Sasaki S, Uchida A, Fujita H, Nakamura K, Ichida T, Itoh H, Nakata T, Takeda K, Nakagawa M. GABAB-ergic stimulation in hypothalamic pressor area induces larger sympathetic and cardiovascular depression in spontaneously hypertensive rats. Am J Hypertens 9: 964–972, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Tasker JG, Dudek FE. Local inhibitory synaptic inputs to neurones of the paraventricular nucleus in slices of rat hypothalamus. J Physiol 469: 179–192, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsukamoto K, Sved AF. Enhanced gamma-aminobutyric acid-mediated responses in nucleus tractus solitarius of hypertensive rats. Hypertension 22: 819–825, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Zhou HY, Zhang HM, Chen SR, Pan HL. Increased nociceptive input rapidly modulates spinal GABAergic transmission through endogenously released glutamate. J Neurophysiol 97: 871–882, 2007. [DOI] [PubMed] [Google Scholar]