Abstract
Neonatal hearts respond to stress and function in an environment quite different from adult hearts. There is evidence that these functional differences not only reflect modifications in the abundance and isoforms of sarcomeric proteins but also in the modulation of sarcomeric protein phosphorylation. Yet our understanding of changes in sarcomeric protein phosphorylation in development is incomplete. In the experiments reported here, we first quantified the intact sarcomeric protein phosphorylation status between neonatal and adult rat hearts by employing comparative two-dimensional (2-D) gel electrophoresis in conjunction with phosphoprotein-specific staining. Subsequently, we measured phosphorylation changes at the peptide level by employing high-resolution linear ion trap-Fourier transform (LTQ-FT) mass spectrometry analysis of titanium dioxide-enriched phosphopeptides differentially labeled with 16O/18O during in-gel digestion. We also employed Western blot analysis using phosphorylation site-specific antibodies to measure phosphorylation changes. Our data demonstrated the novel finding that phosphorylation levels of myosin-binding protein C (MyBP-C) at Ser295 and Ser315 as well as tropomyosin at Ser283 increased, whereas phosphorylation levels of MyBP-C at Ser320 and myosin light chain 2 at Ser15 decreased in neonatal hearts compared with the same sites in adult hearts. Although our data highlight the significant challenges in understanding relations between protein phosphorylation and cardiac function, they do support the hypothesis that developmental changes in the modulation of protein are functionally significant and correlate with the prevailing physiological state.
Keywords: neonatal, phosphorylation, 18O labeling, titanium dioxide, quantitative mass spectrometry, two-dimensional gel, tropomyosin, myosin light chain 2, myosin-binding protein C
the cellular regulation of cardiac contraction and relaxation is tuned to the changing hemodynamic demands associated with the development of neonatal to adult hearts (22). In the case of sarcomeric proteins, there are shifts in the isoform population as well as an altered modulation by phosphorylation (1, 21). Two well-studied examples of these modifications are the isoform switching of myosin heavy chains (MHCs), troponin I (TnI) from the slow skeletal variant (ssTnI) to the cardiac variant (cTnI), and shifts in the isoform population of cardiac troponin T (cTnT) from the neonatal isoform (cTnT1) to shorter adult isoforms (cTnT3 and cTnT4) (21, 29, 30). TnI is the inhibitory component of the troponin complex, and TnT is a tropomyosin (Tm)-binding component unit; both proteins are critical to the regulation of the actin-myosin reaction (17, 30). Understanding the modifications of sarcomeric function associated with these two changes has provided valuable insights into cardiac regulation and new concepts of regulation at the level of the sarcomere (for a review, see Ref. 17). Moreover, reexpression of fetal and neonatal proteins in disorders of the adult heart (16) makes it important to fully understand the developmental transitions in the structure and function of regulatory proteins of the sarcomere. Developmental modifications in sarcomere proteins are also relevant to the therapeutic use of stem cells.
Although it is clear that the advanced understanding of functional regulation during cardiac development requires the knowledge of changes to sarcomeric protein phosphorylation levels, this knowledge is lacking for key regulatory proteins other than TnT and TnI. There is strong evidence that modifications in myosin-binding protein C (MyBP-C), Tm, and myosin light chain 2 (MLC2) are functionally significant, yet how their phosphorylation changes with development is unknown. In the experiments reported here, we employed a proteomic approach to quantify phosphorylation changes to these sarcomeric proteins between neonatal and adult rat hearts.
An important aspect of our study was the use of cutting-edge proteomic techniques. These approaches include relative protein quantification with two-dimensional (2-D) difference gel electrophoresis (2D-DIGE) (24, 32) and relative peptide quantification with 16O/18O labeling (28). We used titanium dioxide (TiO2) (18, 25) to enrich phosphopeptides from in gel digestion. We employed high-resolution linear ion trap-Fourier transform (LTQ-FT) mass spectrometry, which offers two complementary peptide activation methods, collision-induced dissociation (CID) and electron capture dissociation (ECD) (6), to facilitate phosphorylation site determination. Using these techniques along with Western blot analysis, we successfully quantified changes to several phosphorylation sites from three differentially expressed proteins, MyBP-C, Tm, and MLC2. Our data indicate that developmentally regulated phosphorylation changes can occur in opposite directions between different myofilament proteins and between different sites within the same protein.
MATERIALS AND METHODS
Additional descriptions of materials and methods are reported as Supplemental Data.1
Isolation of myofilament proteins.
Animal care and use were performed in accordance with the Institutional Animal Care and Use Committee and National Institutes of Health (NIH) guidelines. The isolation of neonatal (1–2 days old) and adult (6–7 mo old, both genders) Sprague-Dawley rat hearts was performed as previously described (5, 38). Myofibrillar fractions were immediately prepared from excised hearts as previously described (19).
Gel electrophoresis and Western blot analysis.
We performed one-dimensional gel electrophoresis, Western blot analysis, 2D-DIGE (GE Healthcare, Piscataway, NJ), and ProQ Diamond staining (Invitrogen, Carlsbad, CA) as previously described (19, 38). 2D-DIGE data were analyzed with DeCyder software (GE Healthcare). Protein changes (in %) from adult to neonatal samples are expressed as means ± SE, and changes with P values of <0.05 were considered statistically significant. A phospho-Tm Ser283 antibody was manufactured by 21st Century Biochemicals (Marlborough, MA). A phospho-PKC substrate antibody was purchased from Cell Signaling (Danvers, MA). A total MLC2 antibody was purchased from Axxora (San Diego, CA). Western blot images were quantified with ImageJ software (NIH, Bethesda, MD). The intensity of bands detected by the phosphorylation-specific antibody was normalized against that detected by the pan antibody and compared between neonatal and adult cohorts using Student's t-test. Data are expressed as means ± SE, and P values of <0.05 were considered statistically significant.
In-gel digestion and peptide mass fingerprinting.
In-gel digestion of differentially expressed proteins identified from 2D-DIGE was performed as previously described (37, 38). Matrix-assisted laser desorption/ionization (MALDI) analysis was performed with an Applied Biosystems (Foster City, CA) Voyager DE-Pro mass spectrometer in reflector mode. We used α-cyano-4-hydroxycinnamic acid as the MALDI matrix. Peptide mass fingerprinting (PMF) was performed using the ProFound search engine. We used the following search parameters: missed cleavage = 1, mass tolerance < 30 ppm, National Center for Biotechnology Information (NCBI) mammalian database (2007/10/01), and cysteine treated with iodoacetamide. Identifications with Z scores over 1.5 were considered high confidence. All protein identifications were confirmed by comparing our data with an existing 2-D gel database for the rat heart (http://www.mpiib-berlin.mpg.de/2D-PAGE/RAT-HEART/2d/2d.html) and our previous 2-D gel analysis of canine hearts (38).
16O/18O labeling.
For differential 16O/18O labeling, in-gel digestion was performed in the presence of either 16O water (HPLC grade, Sigma-Aldrich, St. Louis, MI) or 18O water (95% pure, Sigma-Aldrich). A stock solution of NH4HCO3 (1.0 M) was added to the reaction to achieve a final concentration of 10 mM and a reaction pH of ∼6.5. This pH facilitated carboxyl oxygen exchange (11). Frozen stocks of sequencing grade trypsin (0.5 mg/ml, Promega, Madison, WI) were added directly to the reaction. A higher enzyme-to-protein ratio (1:4 instead of 1:20) was used to compensate for the nonoptimal amidase activity of trypsin at pH 6.5 (11), and the reaction was carried out for an extended time (20 h instead of 16 h) to ensure complete digestion. At the end of digestion, peptides were extracted with 50% acetonitrile (ACN)-0.1% trifluoroacetic acid (TFA), and equal amounts of 16O- and 18O-labeled in-gel digests were combined for TiO2 enrichment of phosphopeptides. We always labeled fewer phosphorylated samples with 16O as this enabled easy identification of COOH-terminal fragment ions (y and z) in MS2 spectra (see examples in Supplemental Fig. 3B).
TiO2 enrichment and MALDI-time of flight analysis of phosphopeptides.
TiO2 Nutips (Glygen, Columbia, MD) were washed with binding buffer (10% ACN-2% FA), then with 10 mg/ml dihydroxybenzoic (DHB; Sigma-Aldrich) freshly prepared in binding buffer, and finally with binding buffer again until removal of the yellow color of DHB. To ensure the maximum binding of phosphopeptides to TiO2 Nutips, we aspirated and dispensed samples for 3 min. Nutips were then washed as before, and bound peptides were eluted with 10 μl of 100 mM NH4OH (pH 11.5). To reduce the pH, 10 μl of 4% formic acid solution was added. To further clean up the enriched peptides, C18 Ziptips (Millipore, Billerica, MA) were used, and the bound peptides were eluted with 8 μl of 50% ACN-0.1% formic acid. About 0.5 μl of the Ziptip eluate was used for MALDI-time of flight (TOF) analysis, and the remaining solution was used for LTQ-FT analysis. During MALDI analysis, phosphopeptides were identified by characteristic −80- and −98-Da neutral loss peaks present in post-source decay (PSD) MALDI spectra (2). These peptides were further analyzed by LTQ-FT (Thermo Fisher, San Jose, CA) for phosphorylation site determination as described below.
LTQ-FT mass spectrometry.
TiO2-bound fractions were directly loaded into empty PicoTip emitters (1.2 mm outer diameter and 0.94 mm inner diameter, standard coating, NewObjective, Woburn, MA) for static spray analysis. Loaded emitters were mounted onto the static probe included in Finnigan's nano spray ionization kit (Thermo Fisher). Peptide peaks detected in LTQ-FT full scans were manually selected for MS2 analysis. FT was used for both full and MS2 scans. ECD was employed when CID failed to locate the phosphorylation site. CID and ECD parameters were experimentally determined to give the optimal peptide fragmentation pattern. Both full scans and MS2 scans were performed for 1 min at a resolution of 10,000. TiO2-unbound fractions of MLC2 digests were analyzed by static spray. TiO2-unbound fractions of MyBP-C digests were analyzed by liquid chromotography/mass spectroscopy/mass spectroscopy (LC/MS/MS) with a Michrom Paradigm HPLC system (Auburn, CA). Details of LC/MS/MS and the subsequent database search can be found in the Supplemental Data.
Mass spectrometry data analysis.
Peptide sequences and phosphorylation sites were determined manually using the static spray data. Quantification of 16O/18O mass spectrometry data was performed using the algorithms described in a previous report (8). Changes to selected phosphopeptides were normalized against protein loading, which was calculated as the average ratio of unphosphorylated peptides present in the TiO2-unbound fraction. Data from three separate experiments are reported, and changes are expressed as means ± SE. The obtained 16O-to-18O ratios were analyzed by Student's t-test, and P values of <0.05 were considered statistically significant.
RESULTS
Gel electrophoresis.
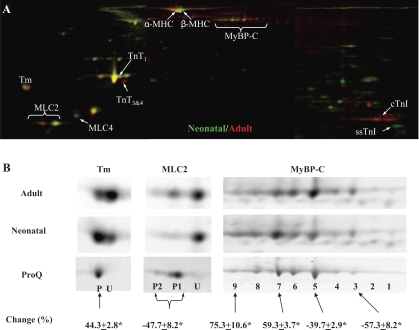
Sarcomeric proteins extracted from neonatal (n = 6) and adult (n = 6) rat hearts were analyzed by 2D-DIGE (Supplemental Fig. 1). A representative gel image is shown in Fig. 1. Differentially expressed proteins (shown in Fig. 1) were identified by MALDI-TOF analysis in conjunction with PMF (Supplemental Table 1). Changes to MHC (7), TnT (15), TnI (10), and fetal MLC (MLC4) (3) have been previously reported; therefore, these were not further investigated in the present study. The changes in MyBP-C, Tm, and MLC2 expressions were novel. To obtain phosphorylation information of individual spots, fluorescent Cy2-labeled protein were separated by 2-D electrophoresis and subsequently stained with ProQ Diamond (Supplemental Fig. 2) for the codetection of total protein (Cy2) and phosphoprotein (Pro-Q Diamond). Figure 1B shows the enlarged ProQ Diamond stains of MyBP-C, Tm, and MLC2 spots along with their total protein stains from the 2D-DIGE analysis described above. The degree of phosphorylation was calculated as the ratio of ProQ to total stain intensities. Quantitative comparison of selected spots was performed using the 2D-DIGE data and expressed as changes from adult to neonatal samples (Fig. 1B). As previously reported in canine heart tissue, rat MyBP-C resolved as multiple spots with increasing degrees of phosphorylation from the basic end to the acidic end of 2-D gels. Compared with adult samples, neonatal samples demonstrated a shift of the MyBP-C population toward the acidic end of 2-D gels, indicating increased phosphorylation levels of neonatal MyBP-C. Tm ran as two spots. ProQ Diamond staining indicated that the acidic spot (P) was phosphorylated and the basic one (U) was not. In neonatal samples, the phosphorylated Tm spot (P) increased significantly (44.3 ± 2.8%, n = 6) and the unphosphorylated Tm spot (U) decreased significantly (−73.4 ± 8.2%, n = 6) compared with the same spots in adult samples. Two of three MLC2 spots (P1 and P2) were phosphorylated, and their combined intensities decreased significantly (−47.7 ± 8.2%, n = 6) in neonatal hearts compared with adult hearts. To further characterize changes in these three phosphoproteins at peptide and amino acid residue levels, we performed detailed mass spectrometry and Western blot analysis as described below.
Fig. 1.
Two-dimensional (2-D) gel analysis of neonatal and adult rat myofilament proteins. Sarcomeric proteins extracted from neonatal (n = 6) and adult (n = 6) rat hearts were labeled with Cy3 (green) or Cy5 (red). A pool was created by mixing equal amount of all 12 samples, and this pool was labeled with Cy2. Then, 15 μg of differentially Cydye-labeled proteins were combined and separated by 2-D electrophoresis, with the isoelectric focusing (IEF) performed with 24-cm, pI range 3–11 IPG (immobilized pH gradient) strips (GE Healthcare). At the end of the IEF, IPG strips were cut into 3 pieces of 7 cm in length, and proteins were separated by molecular weight with three 4–12% bis-Tris gels (Invitrogen). A: representative stitched Cy3/Cy5 image overlay (pI 3–11) is shown. Consistently changed protein spots are indicated. MHC, myosin heavy chain; MyBP-C, myosin-binding protein C; Tm, tropomyosin; MLC, myosin light chain; TnT, troponin T; cTnI, cardiac isoform of troponin I (TnI); ssTnI, slow skeletal isoform of TnI. B: separate views of Tm, MLC2, and MyBP-C are shown. Additional 2-D gels were run using Cy2-labeled proteins (15 μg/gel) and stained with Diamond ProQ for the codetection of total protein (Cy2) and phosphoprotein (ProQ). ProQ staining of adult Tm, MLC2, and MyBP-C spots is also shown in B. Cy2 images (not shown) were used to align ProQ images with difference gel electrophoresis (DIGE) images. P, phosphorylated spot; U, unphosphorylated spot. Spot volume changes (in %) from adult to neonatal samples were calculated using DIGE data and are expressed as means ± SE. *P < 0.05.
MyBP-C: phosphorylation levels of Ser295 and Ser315 increased and phosphorylation levels of Ser320 decreased in neonatal hearts.
MyBP-C is a relatively large protein (150 kDa, pI 6.4) with at least five known phosphorylation sites (see the peptide sequence in Fig. 2A) clustered in its NH2-terminal region (38). MyBP-C is believed to affect cross-bridge kinetics by acting as a “brake,” which is released by phosphorylation (9). There are two functional studies (26, 27) using transgenic mice, designated as AllP− and AllP+, in which three cardiac MyBP-C phosphorylation sites were simultaneously mutated to either unphosphorylatable or pseudophosphorylated residues. However, the phosphorylation status of individual sites on MyBP-C and their dynamic changes under different physiological or pathological conditions remain largely unknown.
Fig. 2.
Matrix-assisted laser desorption/ionization (MALDI) analysis of MyBP-C in-gel digests. Five known phosphorylation sites of MyBP-C are indicated in the peptide sequence shown in A. A: neonatal MyBP-C in-gel digests (∼3 μg) before and after TiO2 enrichment were analyzed by MALDI-time of flight. The number of detected peaks was dramatically reduced after TiO2 treatment. Each peak in the TiO2-enriched fraction was subjected to post-source decay (PSD) analysis, and the two detected phosphopeptides are indicated as P1 and P2. Their corresponding PSD-MALDI spectra are shown in B. Neutral loss peaks (−80 and −98 Da) are indicated. Trypsin digestion was performed at pH 8.0 with an protein-to-enzyme ratio of 4:1 to ensure complete digestion.
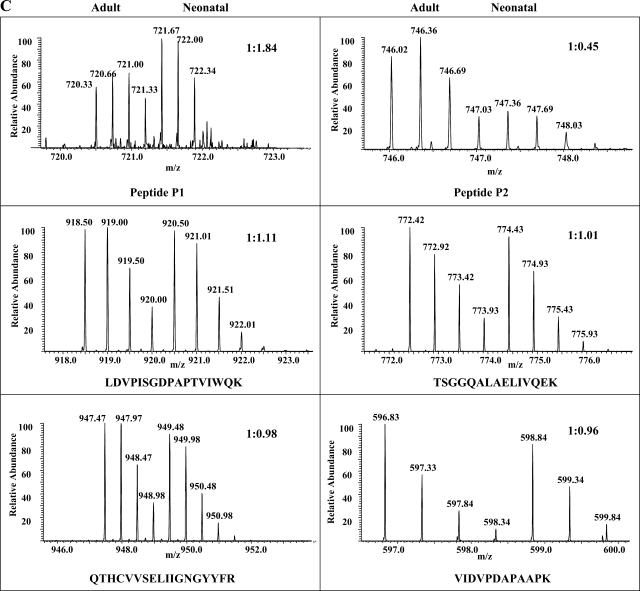
In this study, we performed MALDI analysis of total (Fig. 2A, top) and TiO2-bound (Fig. 2A, bottom) fractions of MyBP-C in-gel digests. Subsequently, we performed PSD-MALDI analysis and identified two phosphopeptides (indicated as P1 and P2 in Fig. 2A) by their characteristic neutral loss (−80 and −98 Da) peaks (Fig. 2B). These two phosphopeptides were undetectable before TiO2 enrichment. To map the phosphorylation sites and obtain quantification information, differentially 16O/18O-labeled P1 and P2 peptides were subjected to LTQ-FT analysis using the static spray mode. Initial CID analysis of peptide P1 failed to locate the phosphorylation site unambiguously (Supplemental Fig. 3); therefore, we performed ECD analysis, and the resulting MS2 spectrum (Fig. 3A) pinpointed the phosphorylation at Ser295. The subsequent analysis of 16O/18O data demonstrated a significant increase of phosphorylation levels at Ser295 (81.1 ± 14.4%, n = 3, P < 0.05) in the neonatal heart after normalization using 16O-to-18O ratios of unphosphorylated peptides present in the TiO2-unbound fraction (see representative spectra in Fig. 3C; a detailed description 18O labeling, mass spectroscopy, and data analysis can be found in Supplemental Figs. 3–6). Peptide P1 contains another potential phosphorylation site at Ser297, but fragment ions supporting its phosphorylation were not found in either CID or ECD MS2 spectra; therefore, we concluded that Ser297 is not phosphorylated in rat hearts under basal functional states. Phosphorylation of Ser297 was first reported in our previous study (38) using canine hearts after ischemia-reperfusion (I/R) injury. Therefore, our present data indicated that phosphorylation at Ser297 was likely species specific or I/R induced.
Fig. 3.
Identification and quantification of MyBP-C phosphorylation at Ser295 and Ser320. Two phosphopeptides, P1 and P2, identified by PSD-MALDI analysis were subjected to linear ion trap-Fourier transform (LTQ-FT) analysis, and they contained phospho-Ser295 and phospho-Ser320, respectively. A: electron capture dissociation (ECD)-MS2 spectrum of phosphopeptide P1 is shown along with its amino acid sequence. Matched ions are indicated. B: collision-induced dissociation (CID)-MS2 spectrum of peptide P2. C: FT full scans of P1 and P2 peptides as well as selected unphosphorylated peptides present in the TiO2-unbound fractions. The determined 16O-to-18O ratios and peptide sequences are indicated.
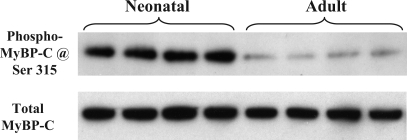
Figure 3B shows CID-MS2 spectrum of peptide P2. Phosphorylation was unambiguously located at Ser320. Surprisingly, compared with the adult sample, there was a significant decrease (−59.5 ± 7.4%, n = 3, P < 0.05) in the phosphorylation at Ser320 of neonatal MyBP-C (see the selected spectrum in Fig. 3C). Peptides containing the remaining two potential phosphorylation sites, Ser286 and Ser315 (indicated with dashed lines in the peptide sequence shown in Fig. 2A), were not detected in either TiO2-bound or -unbound fractions, likely due to their smaller sizes. However, a commercially available antibody, the phospho-PKC substrate antibody from Cell Signaling, can detect the phosphorylation of Ser315 specifically, and we validated this motif antibody's specificity with synthesized peptides phosphorylated in vitro at Ser315 (36). Using this motif antibody and total MyBP-C antibody, we demonstrated that phosphorylation levels of Ser315 were higher (7.1 ± 1.2-fold, n = 8, P < 0.01) in neonatal hearts compared with adults hearts (Fig. 4).
Fig. 4.
Western blot analysis MyBP-C phosphorylation at Ser315. One-dimensional gel-separated myofilament proteins (10 μg) isolated from neonatal and adult rat hearts were probed for phospho-Ser315 (top) and total MyBP-C (bottom).
Tm: phosphorylation levels of Ser283 increased in neonatal hearts.
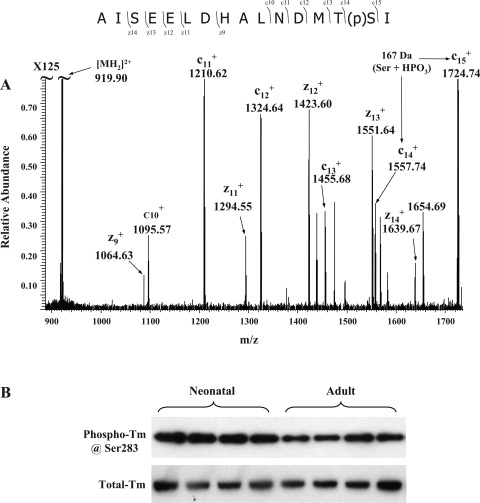
Tm (38 kDa, pI 4.5) is a major regulatory myofilament protein. During the diastolic stage of the cardiac cycle, Tm strands block myosin-binding sites. When Ca2+ binds to the troponin complex, a series of conformational changes leads to the movement of Tm away from myosin-binding sites, attachment and reaction of myosin heads with the thin filament, and eventually force generation driven by ATP hydrolysis. Striated muscle Tm has only one previously reported phosphorylation site at the penultimate COOH-terminal amino acid (20). Currently, the function of Tm phosphorylation remains poorly understood, although there is in vitro evidence from studies using reconstituted thin filament preparations that phosphorylation of Tm affects its affinity for TnT and actin and also affects end-to-end interactions between contiguous Tm strands along the thin filament (13). These effects of Tm phosphorylation would be expected to enhance the sensitivity of myofilaments to Ca2+. In this report, we detected the phosphorylated COOH-terminal tryptic peptide as well as its Met-oxidized form from the TiO2-enriched fraction of Tm in-gel digests using PSD-MALDI-TOF (Supplemental Fig. 7). The location of phosphate group was mapped to the penultimate COOH-terminal amino acid, Ser283, using LTQ-FT and ECD (Fig. 5A). Because COOH-terminal peptides of Tm cannot be 18O labeled during trypsin digestion (data not shown), we quantified Tm phosphorylation changes using a custom-made phosphorylation site-specific antibody (see its specificity in Supplemental Fig. 8). After being normalized to the total Tm intensity, phospho-Tm signals were significantly higher (47.7% ± 7.9%, n = 8; see representative data in Fig. 5B) in neonates, and this quantification was consistent with our 2D-DIGE (44.3 ± 2.8%) data. Although predicted to occur in data from an earlier study (12), our data explicitly demonstrated an increase of Tm phosphorylation in neonatal hearts and pinpointed the change at residue Ser283.
Fig. 5.
Phosphorylation levels of Tm at Ser283 are increased. Neonatal and adult Tm in-gel digests (∼2 μg each) were subjected to TiO2 Nutip enrichment and LTQ-FT analysis. A: representative ECD-MS2 spectrum. The phosphorylation site was determined to be at Ser283. B: Western blots using antibodies specific to phospho-Ser283 and total Tm confirmed increased levels of phosphorylation of Tm at Ser283 in neonatal samples.
MLC2: phosphorylation levels of Ser15 decreased in neonatal hearts.
MLC2 (18 kDa, pI 5.2), also known as the regulatory light chain, is partially phosphorylated in hearts during basal levels of function (35). Ser15 of cardiac MLC2 was thought to be phosphorylated because its counterpart on smooth muscle MLC2, Ser19, could be readily phosphorylated in vitro (14). In the present report, we confirmed the phosphorylation of Ser15 in rat cardiac MLC2 after TiO2 enrichment and CID-MS2 analysis (Fig. 6A). To quantify changes to this site, differentially 16O/18O-labeled peptides were analyzed by LTQ-FT. Compared with unphosphorylated peptides present in TiO2-unbound fractions, the amount of phosphopeptide bearing phospho-Ser15 was significantly reduced in neonatal hearts (−63.1 ± 5.2%, n = 3, P < 0.05; see selected spectra in Fig. 6B and Supplemental Fig. 9). To validate this change, we obtained a newly developed cardiac MLC2 phospho-Ser15 antibody (see its specificity in Supplemental Fig. 10). We then used this antibody and a total MLC2 antibody to probe one-dimensional electrophoresis-separated sarcomeric proteins (see representative images in Fig. 6C). Quantification of the resulting Western blots showed a significant decrease of phosphorylation levels at MLC2 Ser15 (−60.8 ± 4.6%, n = 8, P < 0.01) in neonatal samples, and these data were consistent with our mass spectrometry data.
Fig. 6.
Phosphorylation levels of MLC2 at Ser15 are decreased. Only one phosphopeptide was detected from the TiO2-enriched fraction of MLC2 in-gel digests, and its CID-MS2 spectrum is shown along with the determined sequence in A. Phosphorylation is indicated by “p” and oxidation is indicated by “o.” Analysis O16/O18 data indicated that the peptide bearing phospho-Ser15 decreased significantly from adult to neonatal samples, whereas the total protein load was about same, as indicated by the near 1:1 ratio of unphosphorylated peptides present in the TiO2-unbound fraction (see representative spectra in B; additional spectra are shown in Supplemental Fig. 9). The detected lower level of phosphorylation of MLC2 at Ser15 in neonatal samples was confirmed by Western blots (C) using a total MLC2 antibody and an anti-phospho-MLC2 Ser15 antibody.
The presence of two phosphorylated MLC2 spots on our 2-D gels (Fig. 2) indicated the likelihood of a second phosphorylation site. Moreover, in the case of smooth muscle MLC2, the use of a relatively high kinase concentration induced an additional phosphorylation at an unidentified Thr residue in addition to phosphorylation of Ser19 (14). However, we were unable to detect a second phosphorylation site in rat cardiac MLC2, even when we used additional proteases (Lys-C and Glu-C) for in-gel digestion to increase the sequence coverage (data not shown). Interestingly, when our samples were subjected to higher resolution 2-D electrophoresis analyses, the larger phosphospot (spot P1) was separated into two spots, and one of them was not phosphorylated (Supplemental Fig. 11). Similar results have been obtained in a previous study (35) on human MLC2, in which this extra spot was attributed to posttranslational modifications other than phosphorylation. Therefore, whether there is a second phosphorylation site in cardiac MLC2 remains unknown.
DISCUSSION
Our data add to the increasing information on the functional significance of the multiple-protein phosphorylation that occurs in the cardiac sarcomere and emphasize the complexity of analytical approaches and interpretation of the data.
Our hypothesis is that the constellation of sarcomeric protein phosphorylation represents a balance appropriate to the prevailing physiological situation. In the case of the developing embryonic/neonatal heart versus the adult heart, in which the prevailing physiological state is different (22), it is not surprising that there are differences in the phosphorylation of sarcomeric proteins, as reported here. Moreover, the relative proportions of intracellular organelles differ between the two age groups, and there are differences in the expression of proteins regulating the contraction/relaxation cycle, including a lower myofibrillar mass and sarcoplasmic reticulum membrane area/cross-sectional area (22). Owing to this lack of fully developed membrane systems, the transient increase in intracellular Ca2+ is smaller in amplitude and slower in duration in neonatal cardiac myocytes compared with adults (31). Moreover, especially during embryonic life and during the stress of being born, neonatal myocytes function in relatively hypoxic environments.
There is substantial evidence that the expression of ssTnI in the neonate serves to render myofilaments sensitive to Ca2+ and insensitive to hypoxia/acidosis (4, 31, 33). This isoform switching makes sense in that the relatively high sensitivity to Ca2+ and low sensitivity to hypoxia/acidosis is important in the embryonic/neonatal heart to maintain systolic pressure development. The data reported here emphasize that these specialized properties are due not only to differences in isoforms but also to differences in sarcomeric protein phosphorylation. The lack of phosphorylation sites on ssTnI also appears significant in that the phosphorylation of cTnI depresses myofilament sensitivity to Ca2+ (17, 30). Our determination of phosphorylation levels of MyBP-C, Tm, and MLC2 also indicates that modifications in the neonatal heart likely provide a mechanism for the sustaining of systolic force.
The complexity in sites and levels of phosphorylation as well as a lack of functional information with regard to specific sites make it difficult to speculate as to the functional significance of the alterations in MyBP-C with development. However, the relative higher levels of phosphorylation at Ser295 and Ser315 generally fit with our hypothesis that posttranslational modifications and the isoform population of neonatal sarcomeric proteins protects the heart from acidosis and hypoxia. Hearts of transgenic mice expressing a phosphorylation mimetic mutant of MyBP-C in which three Ser residues were replaced with Asp demonstrate significant protection against I/R injury (27). Among these three sites, we only found that two sites, Ser295 and Ser315, were increasingly phosphorylated in the neonatal heart, suggesting a role in the protection from hypoxia and acidosis. The potential significance of phosphorylation at the other sites (Ser286, Ser297, and Ser320) awaits further investigation.
In the case of Tm, there is evidence that the relatively higher levels of Tm phosphorylation in the neonate promote increased force generation by myofilaments. In a study comparing wild-type mouse hearts to hearts expressing an upstream activator of p38 MAPK, we (34) reported a strong correlation between levels of Tm phosphorylation and maximum force generation and the acto-myosin ATPase rate. Our data indicated that relatively low levels of phosphorylation of Tm reduce the number of cross-bridges reacting with the thin filament at any given level of Ca2+ (34). A solution study (13) of the ATPase rate of myosin reacting with reconstituted thin filaments also indicated an increase in the relative ATPase rate with relative increases in Tm phosphorylation. Evidence that neonatal hearts express a relative abundance of Tm kinase fits with our findings, and indirect evidence of the effect of Tm phosphorylation on force generation indicated an important role in the maintenance of function in the immature heart.
The relative lower phosphorylation level of MLC2 in neonatal versus adult hearts is likely due to relatively low levels of Ca2+ flux during the beat of the neonatal heart and thus relatively low levels of activation of MLC kinase activity by Ca2+-calmodulin. Phosphorylation of MLC2 has been reported to increase myofilament sensitivity to Ca2+ (23), and the relatively low level of MLC2 phosphorylation in the neonatal versus adult hearts would tend to reduce systolic force generation. However, it is evident that the isoform switching and altered phosphorylation of thin filament regulatory proteins offsets the lack of influence of MLC2 phosphorylation on myofilaments.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute (NHLBI) Grants PO1-HL-62426 (project 1) (to R. J. Solaro), RO1-HL-22231 (to R. J. Solaro), and RO1-HL-64035 (to R. J. Solaro). C. Yuan was supported by NHLBI Training Grant T32-HL-07692. Q. Sheng and H. Tang are supported by the National Center for Glycomics and Glycoproteomics funded by National Center for Research Resources. Usae of LTQ-FT was provided by the Chicago Biomedical Consortium (CBC)-University of Illinois at Chicago Research Resources Center Proteomics and Informatics Services Facility, which was established by a grant from The Searle Funds at the Chicago Community Trust to the CBC.
Acknowledgments
We thank Dr. Anne M. Murphy for the usage of DeCyder software. The anti-phospho-MLC2 Ser15 antibody was from Dr. Neil L. Epstein. The total MyBP-C antibody was from Dr. Richard L. Moss.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Supplemental data for this article is available online at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Anderson PA, Greig A, Mark TM, Malouf NN, Oakeley AE, Ungerleider RM, Allen PD, Kay BK. Molecular basis of human cardiac troponin T isoforms expressed in the developing, adult, and failing heart. Circ Res 76: 681–686, 1995. [DOI] [PubMed] [Google Scholar]
- 2.Annan RS, Carr SA. Phosphopeptide analysis by matrix-assisted laser desorption time-of-flight mass spectrometry. Anal Chem 68: 3413–3421, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Arnold HH, Lohse P, Seidel U, Bober E. A novel human myosin alkali light chain is developmentally regulated. Expression in fetal cardiac and skeletal muscle and in adult atria. Eur J Biochem 178: 53–60, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol 289: H2183–H2192, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Boateng SY, Hartman TJ, Ahluwalia N, Vidula H, Desai TA, Russell B. Inhibition of fibroblast proliferation in cardiac myocyte cultures by surface microtopography. Am J Physiol Cell Physiol 285: C171–C182, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HJ, Hakansson K, Marshall AG. The role of electron capture dissociation in biomolecular analysis. Mass Spectrom Rev 24: 201–222, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Cummins P, Lambert SJ. Myosin transitions in the bovine and human heart. A developmental and anatomical study of heavy and light chain subunits in the atrium and ventricle. Circ Res 58: 846–858, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Eckel-Passow JE, Oberg AL, Therneau TM, Mason CJ, Mahoney DW, Johnson KL, Olson JE, Bergen HR 3rd. Regression analysis for comparing protein samples with 16O/18O stable-isotope labeled mass spectrometry. Bioinformatics 22: 2739–2745, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Flashman E, Redwood C, Moolman-Smook J, Watkins H. Cardiac myosin binding protein C: its role in physiology and disease. Circ Res 94: 1279–1289, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Gao L, Kennedy JM, Solaro RJ. Differential expression of TnI and TnT isoforms in rabbit heart during the perinatal period and during cardiovascular stress. J Mol Cell Cardiol 27: 541–550, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Hajkova D, Rao KC, Miyagi M. pH dependency of the carboxyl oxygen exchange reaction catalyzed by lysyl endopeptidase and trypsin. J Proteome Res 5: 1667–1673, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heeley DA, Moir AJ, Perry SV. Phosphorylation of tropomyosin during development in mammalian striated muscle. FEBS Lett 146: 115–118, 1982. [DOI] [PubMed] [Google Scholar]
- 13.Heeley DH, Watson MH, Mak AS, Dubord P, Smillie LB. Effect of phosphorylation on the interaction and functional properties of rabbit striated muscle αα-tropomyosin. J Biol Chem 264: 2424–2430, 1989. [PubMed] [Google Scholar]
- 14.Ikebe M, Hartshorne DJ. Phosphorylation of smooth muscle myosin at two distinct sites by myosin light chain kinase. J Biol Chem 260: 10027–10031, 1985. [PubMed] [Google Scholar]
- 15.Jin JP, Lin JJ. Isolation and characterization of cDNA clones encoding embryonic and adult isoforms of rat cardiac troponin T. J Biol Chem 264: 14471–14477, 1989. [PubMed] [Google Scholar]
- 16.Kim SH, Kim HS, Lee MM. Re-expression of fetal troponin isoforms in the postinfarction failing heart of the rat. Circ J 66: 959–964, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol 67: 39–67, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJ. Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics 4: 873–886, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Layland J, Cave AC, Warren C, Grieve DJ, Sparks E, Kentish JC, Solaro RJ, Shah AM. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J 19: 1137–1139, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Mak A, Smillie LB, Barany M. Specific phosphorylation at serine-283 of alpha tropomyosin from frog skeletal and rabbit skeletal and cardiac muscle. Proc Natl Acad Sci USA 75: 3588–3592, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAuliffe JJ, Gao LZ, Solaro RJ. Changes in myofibrillar activation and troponin C Ca2+ binding associated with troponin T isoform switching in developing rabbit heart. Circ Res 66: 1204–1216, 1990. [DOI] [PubMed] [Google Scholar]
- 22.Nassar R, Reedy MC, Anderson PA. Developmental changes in the ultrastructure and sarcomere shortening of the isolated rabbit ventricular myocyte. Circ Res 61: 465–483, 1987. [DOI] [PubMed] [Google Scholar]
- 23.Olsson MC, Patel JR, Fitzsimons DP, Walker JW, Moss RL. Basal myosin light chain phosphorylation is a determinant of Ca2+ sensitivity of force and activation dependence of the kinetics of myocardial force development. Am J Physiol Heart Circ Physiol 287: H2712–H2718, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Pan S, Aebersold R. Quantitative proteomics by stable isotope labeling and mass spectrometry. Methods Mol Biol 367: 209–218, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Pinkse MW, Uitto PM, Hilhorst MJ, Ooms B, Heck AJ. Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-NanoLC-ESI-MS/MS and titanium oxide precolumns. Anal Chem 76: 3935–3943, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Sadayappan S, Gulick J, Osinska H, Martin LA, Hahn HS, Dorn GW Jr, Klevitsky R, Seidman CE, Seidman JG, Robbins J. Cardiac myosin-binding protein-C phosphorylation and cardiac function. Circ Res 97: 1156–1163, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadayappan S, Osinska H, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Seidman CE, Seidman JG, Robbins J. Cardiac myosin binding protein C phosphorylation is cardioprotective. Proc Natl Acad Sci USA 103: 16918–16923, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnolzer M, Jedrzejewski P, Lehmann WD. Protease-catalyzed incorporation of 18O into peptide fragments and its application for protein sequencing by electrospray and matrix-assisted laser desorption/ionization mass spectrometry. Electrophoresis 17: 945–953, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Siedner S, Kruger M, Schroeter M, Metzler D, Roell W, Fleischmann BK, Hescheler J, Pfitzer G, Stehle R. Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart. J Physiol 548: 493–505, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solaro RJ Modulation of cardiac myofilament activity by protein phosphorylation. In: Handbook of Physiology. The Cardiovascular System. The Heart. New York: Oxford Univ. Press, 2001, sect. 2, vol. 1, p. 264–300. [Google Scholar]
- 31.Solaro RJ, Lee JA, Kentish JC, Allen DG. Effects of acidosis on ventricular muscle from adult and neonatal rats. Circ Res 63: 779–787, 1988. [DOI] [PubMed] [Google Scholar]
- 32.Unlu M, Morgan ME, Minden JS. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis 18: 2071–2077, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Urboniene D, Dias FA, Pena JR, Walker LA, Solaro RJ, Wolska BM. Expression of slow skeletal troponin I in adult mouse heart helps to maintain the left ventricular systolic function during respiratory hypercapnia. Circ Res 97: 70–77, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Vahebi S, Ota A, Li M, Warren CM, de Tombe PP, Wang Y, Solaro RJ. p38-MAPK induced dephosphorylation of α-tropomyosin is associated with depression of myocardial sarcomeric tension and ATPase activity. Circ Res 100: 408–415, 2007. [DOI] [PubMed] [Google Scholar]
- 35.van der Velden J, Narolska NA, Lamberts RR, Boontje NM, Borbely A, Zaremba R, Bronzwaer JG, Papp Z, Jaquet K, Paulus WJ, Stienen GJ. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res 69: 876–887, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Xiao L, Zhao Q, Du Y, Yuan C, Solaro RJ, Buttrick PM. PKCɛ increases phosphorylation of the cardiac myosin binding protein C at serine 302 both in vitro and in vivo. Biochemistry 46: 7054–7061, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Wu H, Kobayashi T, Solaro RJ, van Breemen RB. Enhanced ionization of phosphorylated peptides during MALDI TOF mass spectrometry. Anal Chem 76: 1532–1536, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Yuan C, Guo Y, Ravi R, Przyklenk K, Shilkofski N, Diez R, Cole RN, Murphy AM. Myosin binding protein C is differentially phosphorylated upon myocardial stunning in canine and rat hearts–evidence for novel phosphorylation sites. Proteomics 6: 4176–4186, 2006. [DOI] [PubMed] [Google Scholar]