Abstract
Ginseng botanicals are increasingly used as complementary or alternative medicines for a variety of cardiovascular diseases, yet little is known about their cellular actions in cardiac muscle. Electromechanical alternans (EMA) is a proarrhythmic cardiac abnormality that results from disturbances of intracellular Ca2+ homeostasis. This study sought to determine whether a purified ginsenoside extract of ginseng, Re, exerts effects to suppress EMA and to gain insight into its mechanism of action. Alternans was induced by electrically pacing cardiomyocytes at room temperature. Re (≥10 nM) reversibly suppressed EMA recorded from cat ventricular and atrial myocytes and Langendorff-perfused cat hearts. In cat ventricular myocytes, Re reversibly suppressed intracellular Ca2+ concentration ([Ca2+]i) transient alternans. Re exerted no significant effects on baseline action potential configuration or sarcolemmal L-type Ca2+ current (ICa,L), Na+ current, or total K+ conductance. In human atrial myocytes, Re suppressed mechanical alternans and exerted no effect on ICa,L. In cat ventricular myocytes, Re increased [Ca2+]i transient amplitude and decreased sarcoplasmic reticulum (SR) Ca2+ content, resulting in an increase in fractional SR Ca2+ release. In SR microsomes isolated from cat ventricles, Re had no effect on SR Ca2+ uptake. Re increased the open probability of ryanodine receptors (RyRs), i.e., SR Ca2+-release channels, isolated from cat ventricles and incorporated into planar lipid bilayers. We concluded that ginsenoside Re suppresses EMA in cat atrial and ventricular myocytes, cat ventricular muscle, and human atrial myocytes. The effects of Re are not mediated via actions on sarcolemmal ion channels or action potential configuration. Re acts via a subcellular mechanism to enhance the opening of RyRs and thereby overcome the impaired SR Ca2+ release underlying EMA.
Keywords: electrophysiology, Ca2+ transients, arrhythmias
panax ginseng is a botanical that has been used as a medicinal in China for centuries. In the United States, the use of botanicals as a complementary or alternative medicine has become increasing popular, including their use for various forms of cardiovascular disease (29). Ginseng also is now contained in a variety of dietary supplements as well as tea and soft drinks. However, the cellular actions of ginseng on the heart are not understood. The main biologically active components of ginseng are various ginsenoside (glycoside) compounds derived from two general groups: 20(S)-protopanaxatriol and 20(S)-protopanaxadiol. Re, a ginsenoside in the 20(S)-protopanaxatriol group, is a major component of ginseng.
Electromechanical alternans (EMA) is a proarrhythmic phenomena that is manifest as cyclic beat-to-beat alternations in action potential duration (APD; electrical alternans) and contraction amplitude (mechanical alternans) at a constant stimulation frequency (27). In electrocardiographic recordings, it appears as T wave alternans. An increase in the propensity for EMA has been associated with a wide variety of conditions, including hypothermia, hypocalcemia, hypercapnic acidosis, alcoholism, antiarrhythmic drug therapy, ischemia, hypertrophy, and congestive heart failure (6, 20). EMA is believed to be an important factor in the pathogenesis of arrhythmias (5, 10), including the development of reentry phenomena (3, 13, 15), ventricular fibrillation (13, 19), atrial fibrillation (7), and long QT syndrome (17). It is also considered a strong marker of susceptibility to sudden cardiac death (12). Alternans is a cellular phenomena that is thought to result primarily from impaired intracellular Ca2+ concentration ([Ca2+]i) handling (4, 8, 20).
The present study sought to determine whether the ginsenoside Re, a purified extract of panax ginseng, may exert beneficial effects on cardiac function by suppressing EMA and to gain insight into its mechanism of action. The present results indicate that ginsenoside Re effectively suppresses EMA in both cat and human cardiomyocytes through a subcellular mechanism to increase the opening of sarcoplasmic reticulum (SR) Ca2+-release channels. Portions of this work have been previously published in abstract form (11).
METHODS
Single myocytes were isolated from the cat heart as previously described (15, 28). Cats (n = 12) were obtained from R&R Research (Howard City, MI). The vendor and procedures for cell isolation were approved by the Institutional Animal Care and Use Committee of Stritch School of Medicine, Loyola University of Chicago (Maywood, IL). Briefly, adult mixed-breed cats of either sex were anesthetized with thiopental sodium (50 mg/kg ip). Once cats were fully anesthetized, a bilateral thoracotomy was performed, and the heart was rapidly excised and mounted on a Langendorff perfusion apparatus. Hearts were enzymatically (collagenase type II, Worthington Biochemical) digested to isolate both atrial and ventricular myocytes.
Electrophysiology.
Cardiomyocytes were transferred to a small tissue bath (0.3 ml) on the stage of an inverted microscope (Nikon Diaphot). Cells were superfused at 35 ± 1°C with a HEPES-buffered modified Tyrode solution containing (in mM) 145 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 5 HEPES, and 11 glucose and titrated with NaOH to pH 7.4. In general, voltage and ionic currents were recorded using a nystatin (150 μg/ml)-perforated patch whole cell recording method. The internal pipette solution contained (in mM) 100 Cs-glutamate, 40 KCl, 1 MgCl2, 4 Na2-ATP, 0.5 EGTA, and 5 HEPES and titrated with KOH to pH 7.2. CsCl (5 mM) also was added to all external solutions to block K+ conductances except in experiments designed to measure K+ conductances. A single suction pipette recorded either voltage (bridge mode) or ionic currents (discontinuous voltage-clamp mode) using an Axoclamp 2A amplifier (Axon Instruments/Molecular Devices, Sunnyvale, CA). Computer software (pCLAMP, Axon Instruments/Molecular Devices) was used to deliver voltage protocols and to acquire and analyze data. L-type Ca2+ current (ICa,L) was activated by depolarizing pulses from a holding potential of −40 to 0 mV for 200 ms every 5 s. The peak ICa,L amplitude was measured in relation to steady-state current. Total K+ conductance was measured by voltage-clamp ramps from −130 to +30 mV, as previously described (25). Na+ current (INa) was recorded by the ruptured patch whole cell method, as previously described (22). INa was measured at room temperature and with extracellular [Na+] reduced to one-third normal by substitution with equimolar tetraethylammonium (TEA)-Cl. The external recording solution contained (in mM) 50 NaCl, 67 TEA-Cl, 5.4 KCl, 1 MgCl2, 2 CaCl2, 20 CsCl, 5 HEPES, and 11 glucose and titrated with CsOH to pH 7.35. In addition, INa was isolated by blocking ICa,L with 5 μM verapamil and blocking transient outward current with 2 mM 4-aminopyridine. INa was activated by depolarizing voltage steps from a holding potential of −80 mV for 80 ms and measured with respect to steady-state current. In the ruptured patch method, the junction potential (10 mV) measured between the internal pipette and bath solutions was subtracted from all voltage measurements.
Measurements of alternans.
Alternans was elicited by field stimulation of cardiomyocytes at room temperature. Microscopic visual inspection was used to select cells that displayed alternating mechanical activity. At a stimulation frequency of 1 Hz at room temperature, ∼10% of cat myocytes that responded to stimulation displayed alternans. Because alternans is frequency dependent, higher frequencies of stimulation significantly increase the number of cells displaying alternans. Cell shortening (contraction) was measured with a video edge detector (Crescent Electronics) using a raster line placed on one edge of the cell. Mechanical (or Ca2+ transient) alternans was quantified as the alternans ratio (AR), which was defined as follows: AR = 1 − S/L, where S/L is the ratio of the small (S) to large (L) contraction (or [Ca2+]i transient) amplitude. AR = 0 indicates no alternans, and AR = 1 indicates the maximum degree of alternans.
Intracellular Ca2+ measurements.
Fast one-dimensional (line scan) imaging was performed using a confocal scanning unit (Bio-Rad Radiance 2100 and Bio-Rad 2000 MP) equipped with an argon-ion laser and attached to an inverted microscope. Single cells were loaded with the fluorescent indicator fluo-4 (fluo-4 AM). Fluorescence was excited at 488 nm and simultaneously recorded at wavelengths > 515 nm. The line scan was positioned along the longitudinal axis of the cell, avoiding the nucleus. Fluo-4 fluorescence emission (F) was normalized to baseline fluorescence emission (Fo) to correct for loading differences. Changes in [Ca2+]i are presented as changes of F/Fo.
Measurements of Ca2+ uptake by SR microsomes.
SR microsomes were prepared from cat ventricular tissue as previously described (30). SR vesicles (50 μg) were added to a cuvette containing 1 ml of buffered phosphate medium, which contained (in mM) 100 KH2PO4, 3 MgCl2, 2 ATP, 0.01 ruthenium red, and 0.2 antipyrylazo III (APIII; Sigma); pH 7.0. Changes of [Ca2+] were measured as changes in absorbance between 710 and 790 nm of the Ca2+-sensitive dye APIII with the use of an ultraviolet-visible diode array spectrophotometer (Cory 50, Varian). Energized Ca2+ uptake was initiated by the addition of Ca2+ aliquots (10 μM CaCl2) to the cuvette. The rapid rise in Ca2+-dependent APIII absorbance was followed by a slower absorbance decrease due to ATP-dependent Ca2+ uptake by SR vesicles. The rate of Ca2+ loading of the vesicles, i.e., the net Ca2+ uptake, equaled the SR pumping rate minus the Ca2+ leak rate. The latter reflects the activity of ryanodine receptors (RyRs), which were blocked by ruthenium red. Thus, under our experimental conditions, net Ca2+ uptake by the vesicles is equal to the SR Ca2+-ATPase pumping rate.
Single-channel records of RyRs.
RyR (type 2) single-channel recordings were performed as previously described (30). Planar lipid bilayers were formed from a lipid mixture containing phosphatidylethanolamine, phosphatidylserine, and phosphatidylcholine (ratio: 5:4:1) dissolved in n-decane at a final lipid concentration of 45 mg/ml. SR vesicles were added to the cis chamber, which corresponded to the cytosolic side of the RyR channel. The trans chamber (luminal side of RyR) was connected to the virtual ground of the amplifier. During fusion, the cis and trans chambers contained solutions of the following composition (in mM): 400 (cis) and 40 (trans) CsCH3SO3, 0.1 CaCl2, and 20 HEPES; pH 7.3 (CsOH). After channel incorporation, the concentration of CsCH3SO3 in the trans chamber was increased to 400 mM and free [Ca2+] in the cis chamber was adjusted to 3 μM by the addition of EGTA. Free [Ca2+] in the experimental solutions was verified with a Ca2+-sensitive mini-electrode (2). Single-channel currents were recorded using an Axopatch 200B amplifier (Axon Instruments/Molecular Devices). All recordings were obtained at a holding potential of −20 mV. Currents were filtered at 1 kHz and digitized at 5 kHz.
Langendorff-perfused hearts.
Cat hearts were mounted on a Langendorff perfusion apparatus via cannulation of the aorta and perfused at a constant pressure of 45–50 mmHg at room temperature. The pulmonary artery was cut to decompress the right ventricle, and the left ventricle was vented through an apical stab wound. A latex fluid-filled balloon on the end of a polyethylene-100 catheter was inserted into the left ventricle through the mitral orifice and inflated using a 100-μl syringe to obtain an end-diastolic pressure (EDP) of 10 mmHg. The size of the balloon was selected (from balloon pressure-volume curves) to ensure that the EDP reflected left ventricular wall stiffness rather than balloon wall stiffness. To electrically pace the ventricles, the atrioventricular (AV) nodal region was ligated to produce complete AV block. Left ventricular balloon pressure was measured with a fluid-filled pressure transducer. Stainless steel hook electrodes on the right atrium and left ventricle were used to record bipolar electrograms, which represented a pseudo-“ECG” recording. The heart was electrically paced with paired bipolar hook electrodes attached to the ventricular wall. Data were recorded on a Grass polygraph chart recorder.
Human atrial myocytes.
Discarded human left atrial muscle was obtained from patients at the time of surgery. Approval from the Loyola University Medical Center Internal Review Board was determined to be exempt. Tissue was transported to the laboratory in Ca2+-free Tyrode solution containing 50 mM taurin. In the laboratory, the tissue was bubbled with 100% O2, minced into small pieces (1 mm2), and washed three times in Ca2+-free solution. Tissues were exposed to type I collagenase (350 U/ml) plus 4 U/ml protease in Ca2+-free solution for 30 min and then collagenase solution containing 30 μM Ca2+ for an additional 30 min at 37°C in a shaking water bath. The supernatant was collected, and the remaining tissue pieces were incubated in fresh collagenase solution containing 60 μM Ca2+. This last step was repeated every 10 min until no further cells were obtained. Supernatants containing cells were centrifuged at 1,000 rpm at 4°C for 2 min. The supernatant was removed, and the pellet was resuspended in 100 μM Ca2+. Extracellular [Ca2+] was slowly increased to 1 mM. Only quiescent rod-shaped cells showing regular striated patterns were used in these experiments.
Ginseng and ginsenosides.
Ginsenosides Re and Rb1 were obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). HPLC indicated that the purity of Re was 98%.
Statistics.
Data are presented as means ± SE. Measurements were analyzed using either paired or unpaired Student's t-tests for significance at P < 0.05.
RESULTS
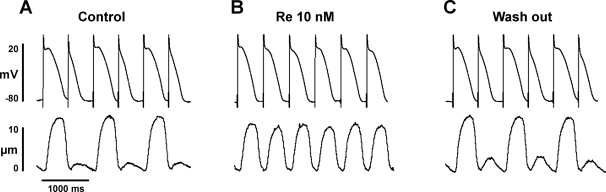
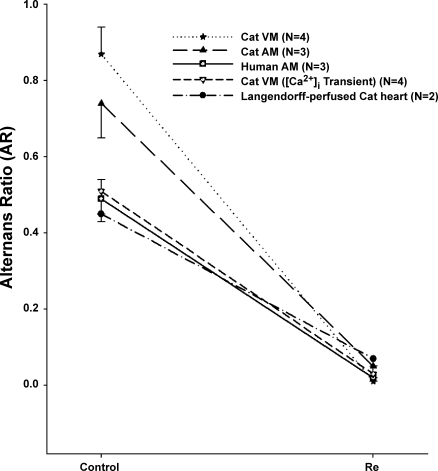
Figure 1 shows EMA recorded from a ventricular myocyte field stimulated (2 Hz) at room temperature. The longer action potentials triggered the larger contractions, and the shorter action potentials triggered the smaller contractions. Within ∼2 min of exposure, 10 nM Re markedly increased the smaller contraction (+249 ± 96%, n = 4, P < 0.05), whereas the larger contraction was decreased slightly (−23 ± 4%, n = 4, P < 0.05) in amplitude. At the same time, the longer action potentials shortened and the shorter action potentials lengthened. Figure 1B shows the steady-state (∼5 min) effect of Re to suppress EMA and normalize both electrical and mechanical activities. Figure 1C shows that the effects of Re reversed upon washout (∼5 min). EMA was quantified by measurements of contraction amplitudes and APDs at 90% repolarization (APD90) to determine the AR (see methods). Control contraction AR = 0.87 ± 0.07 versus Re AR = 0.01 ± 0.01 (n = 4, P < 0.001; see Fig. 5), and control APD90 AR = 0.25 ± 0.02 versus Re AR = 0.03 ± 0.02 (n = 4, P < 0.01). In addition, Re significantly decreased the cycle length at which EMA could be elicited (control: 543 ± 157 ms vs. Re: 440 ± 124 ms, −19%, P < 0.05). In other words, Re raised the pacing threshold at which EMA could be induced. As shown in Fig. 5, Re also exerted similar effects in cat atrial myocytes (control contraction AR = 0.74 ± 0.09 vs. Re AR = 0.05 ± 0.03, n = 3, P < 0.001). These findings indicate that nanomolar concentrations of Re suppress EMA in both atrial and ventricular myocytes and raise the threshold for the induction of EMA.
Fig. 1.
Re suppresses electromechanical alternans (EMA) in a cat ventricular myocyte (VM). A: action potentials (top) and cell shortenings (bottom) recorded during EMA induced by electrical pacing (2 Hz) at room temperature. B: steady-state effects of 10 nM Re to suppress EMA and normalize both electrical and mechanical activities. C: washout of Re restored EMA.
Fig. 5.
Summary of the effects of Re to suppress alternans. Re exerted similar effects to suppress alternans recorded in cat VMs, cat AMs, human AMs, [Ca2+]i transients recorded from cat VMs, and cat Langendorff-perfused cat hearts. Compared with control (Ctrl) alternans, the effects of Re were statistically significant in each series of experiments. The ordinate shows the alternans ratio (AR; see methods).
To determine whether the effects of Re are shared by other ginsenosides, we tested the effects of Rb1, a biologically active, major constituent of panax ginseng belonging to the protopanaxadiol group (16). Rb1 (10 μM) failed to exert any effects to suppress EMA (data not shown; n = 3). These findings suggest that the effects of Re are not shared by Rb1, another major ginsenoside component of ginseng.
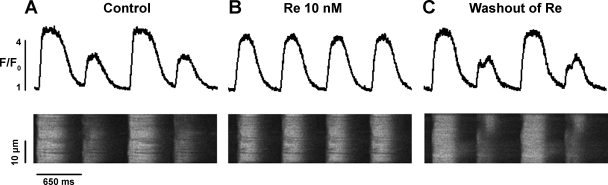
EMA is thought to result from disturbances in the gain of SR Ca2+ release (4). We therefore used confocal laser scanning microscopy to record [Ca2+]i release, i.e., the [Ca2+]i transient, during EMA. Figure 2 shows line scan profiles of [Ca2+]i transients (top) and corresponding line scan images of [Ca2+]i (bottom) obtained from a ventricular myocyte paced at room temperature. As shown in Fig. 2A, the cell exhibited alternations in [Ca2+]i transient amplitudes that were consistent with mechanical alternans. Exposure to Re markedly increased the amplitude of the smaller (+147 ± 21%, n = 7, P < 0.05) [Ca2+]i transient and slightly decreased the amplitude of the larger (−9 ± 1%, n = 7, P < 0.05) [Ca2+]i transient, resulting in normalization of [Ca2+]i transient amplitudes (Fig. 2B). Washout of Re restored [Ca2+]i transient alternans (Fig. 2C). These findings are consistent with the effects of Re to suppress EMA, as shown in Fig. 1. As shown in Fig. 5, control [Ca2+]i transient AR = 0.49 ± 0.04 vs. Re AR = 0.02 ± 0.01 (n = 7, P < 0.001). The fact that Re primarily increases the amplitudes of the smaller contractions and [Ca2+]i transients during alternans supports the idea that Re suppresses EMA by enhancing SR Ca2+ release.
Fig. 2.
Re suppresses intracellular Ca2+ concentration ([Ca2+]i) transient alternans in a cat VM. A: line scan profiles of [Ca2+]i transients (top) and line scan images of [Ca2+]i (bottom) recorded during alternans elicited by electrical pacing (650 ms) at room temperature. B: steady-state effects of 10 nM Re to suppress [Ca2+]i transient alternans and normalize [Ca2+]i transient amplitudes. C: washout of Re restored [Ca2+]i transient alternans.
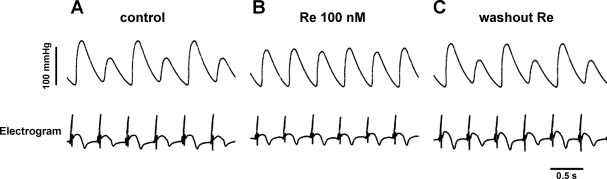
An important question is whether Re exerts similar actions in an intact heart preparation. We therefore determined the effects of Re in a Langendorff-perfused cat heart. The records shown in Fig. 3 show isovolumic pressure changes (top trace) and electrograms (bottom trace) recorded from a Langendorff-perfused heart electrically paced (2 Hz) at room temperature. As shown in Fig. 3A, electrical and mechanical activities displayed typical EMA. Note that in the electrogram tracings the repolarization waveform exhibits alternating amplitudes. Within minutes of exposure, Re (100 nM) markedly increased the smaller pressure pressure change and slightly decreased the larger pressure change. Figure 3B shows the steady-state effects of Re to suppress EMA and normalize both the electrical and mechanical activities. Washout of Re restored EMA (Fig. 3C). Results are shown in Fig. 5 (control pressure AR = 0.45 ± 0.02 vs. Re AR = 0.07 ± 0.02, n = 2). These findings were similar to those recorded from isolated ventricular and atrial myocytes and indicated that Re suppresses EMA in an intact heart preparation.
Fig. 3.
Re suppresses EMA recorded from a Langendorff-perfused cat heart. A: isovolumic pressure changes (top) and electrograms (bottom) recorded during EMA induced by electrical pacing (2 Hz) at room temperature. B: steady-state effects of 100 nM Re to suppress EMA and normalize both electrical and mechanical activities. C: washout of Re restored EMA.
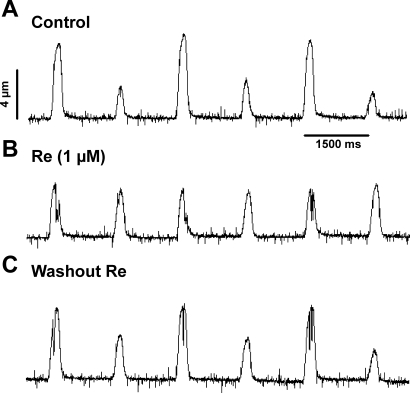
The records shown in Fig. 4 address the question of whether Re suppresses EMA in human cardiomyocytes. The traces show cell shortenings recorded from a single atrial myocyte isolated from human left atrial tissue. The cell was electrically paced (0.75 Hz) at room temperature. Figure 4A shows typical mechanical alternans. Figure 4B shows steady-state effects of Re (1 μM) to suppress mechanical alternans and normalize contraction amplitudes. The effects of Re were reversed upon washout (Fig. 4C). As shown in Fig. 5, control contraction AR = 0.51 ± 0.03 vs. Re AR = 0.03 ± 0.02 (n = 3, P < 0.01). These findings indicate that Re suppresses EMA in human cardiomyocytes, similar to its actions in cat heart.
Fig. 4.
Re suppresses mechanical alternans recorded from a human left atrial myocyte (AM). A: mechanical alternans induced by electrical pacing (0.75 Hz) at room temperature. B: steady-state effects of 1 μM Re to suppress alternans and normalize mechanical activity. C: washout of Re restored alternans.
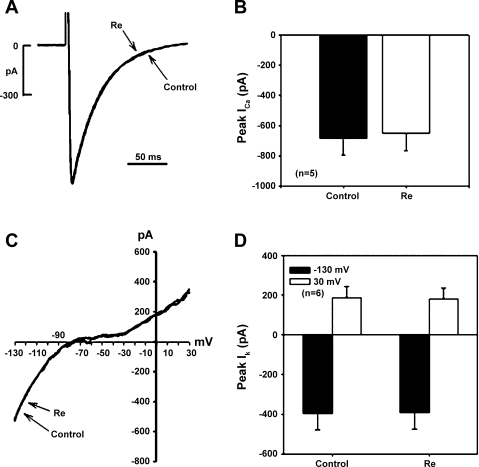
To gain insight into the potential mechanism of Re action, we determined the effects of Re on ICa,L, fast INa, and total K+ conductances as well as baseline APD90. In paced (1 Hz) cells that did not display EMA, APD90 was unchanged by 10 nM Re (−0.8 ± 2.9%, n = 5) and slightly shortened by 10 μM Re (−15 ± 4%, n = 7). Figure 6A shows original recordings of ICa,L from a single ventricular myocyte in the absence (control) and presence of Re. Compared with control, 10 μM Re had no effect on the holding current or ICa,L (traces superimposed). The results shown in Fig. 6B indicate that Re had no significant effect on peak ICa,L (control: 685 ± 110 pA vs. Re: 649 ± 117 pA, n = 5). Importantly, Re also had no significant effects on ICa,L recorded from human atrial myocytes (control: 876 ± 218 pA vs. Re: 847 ± 193 pA, n = 3). The fact that Re had no significant effect on peak ICa,L suggests that Re does not exert significant effects on second messenger signaling pathways that regulate ICa,L. As shown in Fig. 6C, we also analyzed the effects of Re on total K+ conductance recorded from cat ventricular myocytes using voltage ramps from −130 to +30 mV (40 mV/s), as previously described (24, 25). Compared with control, 10 μM Re had no effect on total K+ conductance throughout the voltage range (traces superimposed). The results shown in Fig. 6D indicate that Re had no effect on K+ conductance measured at −130 mV (control: 396 ± 84 pA vs. Re: 392 ± 82 pA) and +30 mV (control: 188 ± 56 pA vs. Re: 182 ± 53 pA, n = 6). Similarly, lower (10 nM) concentrations of Re exerted no effect on ICa,L (n = 3) or total K+ conductances (n = 3). Additional experiments used ruptured whole cell patch recordings in low (50 mM) extracellular [Na+] at room temperature to study INa, as previously described (22). Re (10 μM) had no effect on peak INa (control: 2,815 ± 643 pA vs. Re: 2,768 ± 617 pA, n = 5; data not shown). Together, these results indicate that nanomolar concentrations of Re that suppressed EMA failed to affect baseline APD90, consistent with their lack of effect on ICa,L or total K+ conductance. Even micromolar concentrations of Re had no significant effects on surface membrane ion channels. The fact that nanomolar concentrations of Re had no effect on action potential configuration, ICa,L, or total K+ conductance and yet suppressed EMA supports the idea that the effects of Re to suppress EMA are not mediated via changes in sarcolemmal ion channels or second messenger signaling pathways that regulate these channels.
Fig. 6.
Effects on L-type Ca2+ current (ICa,L) or K+ conductance [peak K+ current (IK)]. A: original recordings of ICa,L obtained from a cat VM. Compared with control, Re (10 μM) had no effect on ICa,L. B: compared with control (solid bar), Re (open bar) had no significant effects on peak ICa,L in cat VMs. C: original recordings of total K+ conductance as measured by a voltage ramp between −130 and +30 mV obtained from a cat VM. Compared with control (solid trace), Re (dashed trace) had no effect on total K+ conductance. D: total K+ conductance measured at −130 (solid bar) and +30 mV (open bar) showed that Re had no effect on total K+ conductance.
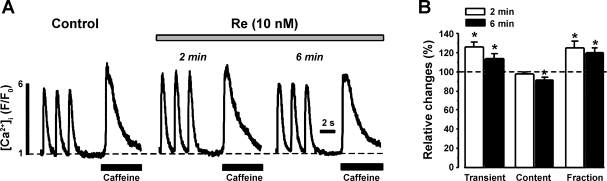
As shown in Fig. 7, we determined the effects of Re on SR Ca2+ release (i.e., [Ca2+]i transients) and SR Ca2+ content (load). [Ca2+]i transients were elicited by field stimulation (1 Hz), and SR Ca2+ content was assessed by brief, rapid exposure to 10 mM caffeine. Figure 7A shows original recordings of stimulated [Ca2+]i transients and caffeine-induced [Ca2+]i transients from a single ventricular myocyte before (control) and during exposure to 10 nM Re. Within the first 2 min of Re exposure, [Ca2+]i transient amplitude increased with little change in SR Ca2+ content. At 6 min (steady state), [Ca2+]i transient amplitude was reduced but still larger than control, and SR Ca2+ content was reduced. Figure 7B shows normalized data and demonstrates that Re elicited a small but significant increase in [Ca2+]i transient amplitude and a small but significant (6 min) decrease in SR Ca2+ content (n = 6). Because Re had no significant effect on peak ICa,L (the trigger for SR Ca2+ release), Re significantly increased fractional SR Ca2+ release (the ratio of [Ca2+]i transient/content).
Fig. 7.
Effects of Re on [Ca2+]i transients and sarcoplasmic reticulum (SR) Ca2+ content. A: original recordings of electrically stimulated [Ca2+]i transients and caffeine-induced [Ca2+]i transients recorded from a cat VM. Within 2 min of exposure to 10 nM Re, stimulated [Ca2+]i transient amplitude increased without significant changes in SR Ca2+ content. However, at 6 min of Re exposure (steady state), [Ca2+]i transient amplitude was reduced but still larger than control, whereas SR Ca2+ content was reduced. B: graph summarizing normalized data (dashed line, 100%) measured at 2 min (open bars) and 6 min (solid bars). The data show that Re increased [Ca2+]i transient amplitudes and decreased SR Ca2+ content, resulting in an increase in fractional SR Ca2+ release. *P < 0.05.
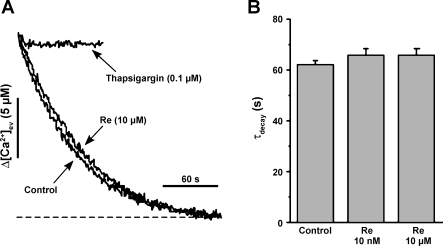
SR Ca2+ content can be affected by either changes in SR Ca2+ release or uptake. To assess the latter, we determined the effects of a relatively high concentration of Re (10 μM) on Ca2+ uptake by SR microsomes isolated from cat ventricles (see methods). Figure 8A shows the decrease in extravesicular [Ca2+] resulting from Ca2+ uptake into SR microsomes after the addition of 10 μM Ca2+. SR Ca2+ uptake in the absence (control) and presence of 10 μM Re were essentially the same. Similar results were obtained with 10 nM Re. In contrast, 0.1 μM thapsigargin, an agent that specifically inhibits SR Ca2+ uptake, abolished Ca2+ uptake. The data were fit by a single exponential and quantified as the time constant of uptake (Fig. 8B). As shown in Fig. 8B, there were no differences among control, 10 nM Re, and 10 μM Re. These findings indicated that Re has no effect of SR Ca2+ uptake (Ca2+-ATPase) and that the effect of Re to decrease SR Ca2+ content is likely due to an increase in SR Ca2+ release (SR Ca2+ leak).
Fig. 8.
Re fails to affect Ca2+ uptake into SR microsomes isolated from cat ventricular muscle. A: compared with control, Re (10 μM) had no effect on the rate of decay of extravesicular Ca2+ concentration ([Ca2+]ev), i.e., Ca2+ uptake into SR microsomes. As a positive control, thapsigargin (0.1 μM) blocked Ca2+ uptake. The dashed line represents the signal level before the addition of Ca2+. B: bar graph summarizing the lack of effect of 10 nM or 10 μM Re on the rate of [Ca2+]ev decay (τdecay; n = 5).
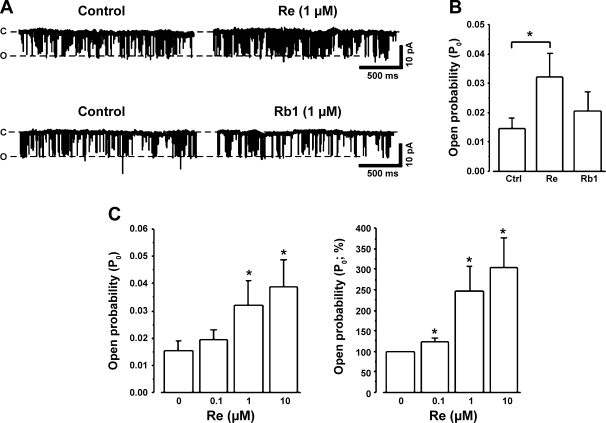
So far, the results suggested that Re increased the opening of SR Ca2+-release channels, i.e., RyRs. Therefore, in the next experiments, we determined the direct effects of Re on RyRs isolated from cat ventricles and incorporated into planar lipid bilayers (see methods). Figure 9A, top, shows original single RyR channel recordings before (control) and during an exposure to 1 μM Re. The addition of Re to the cis chamber markedly increased the frequency of RyR channel openings without affecting single-channel conductance. The addition of Re to the trans chamber had no effect (data not shown). The graph in Fig. 9B shows the effects of 1 μM Re to significantly increase the open probability of RyRs (n = 6). Fig. 9A, bottom, shows that the addition of 1 μM Rb1 to the cis chamber failed to increase RyR channel openings (n = 3). The effects of Rb1 are shown in the graph of Fig. 9B. Figure 9C, left, shows the dose-dependent increase in open probability of RyRs exerted by three different concentrations of Re (0.1, 1, and 10 μM). Figure 9C, right, shows the same data calculated as percent changes normalized against control (100%). These data show that each concentration of Re caused a significant dose-dependent increase in RyR channel opening. Re concentrations below 0.1 μM were not tested because their effects would be below the resolution of the single-channel recording method. Nevertheless, the present findings support the idea that Re suppresses EMA by directly increasing RyR channel opening. They also indicate that the effects of Re are not shared by Rb1.
Fig. 9.
Re increases single ryanodine receptor (RyR) channel activity. A, top: compared with control, Re (1 μM) increased the opening of RyRs without changing single-channel conductance. Bottom, compared with control, Rb1 (1 μM) failed to affect RyR channel opening. c, Closed; o, open. B: bar graph summarizing the effect of Re to significantly increase RyR open probability (Po) and the lack of effect of Rb1 to affect RyR Po. C: bar graphs summarizing the dose-dependent effects of Re to increase Po (left) and the percent increase in Po normalized against the control (right). n = 7 for 0 μM Re (control), 7 for 0.1 μM Re, 5 for 1 μM Re, and 5 for 10 μM Re. *P < 0.05.
DISCUSSION
The present study demonstrates that the ginsenoside Re suppresses alternans in cat and human cardiomyocytes as well as in Langendorff-perfused cat hearts. Re exerts no significant effects on the major sarcolemmal ion channel currents or action potential configuration. Instead, Re directly increases the open probability of RyRs, i.e., increases SR Ca2+ release.
Several of the present findings indicate that Re suppresses EMA by increasing the opening of RyR channels and thereby enhancing SR Ca2+ release. First, Re suppressed alternans in a variety of different preparations by consistently causing a marked increase in the smaller contraction or [Ca2+]i transient amplitude. This is consistent with direct measurements of intra-SR Ca2+ content during alternans, which indicated that the smaller Ca2+ transient is due to less SR Ca2+ release and that SR Ca2+ release is enhanced when the smaller Ca2+ transient amplitude is increased (14). In the present study, Re also elicited a relatively small decrease in the larger contraction or [Ca2+]i transient amplitudes, consistent with a decrease in available SR Ca2+ that resulted from the enhanced efflux of SR Ca2+ induced by Re during the smaller beat. In other words, the total steady-state SR Ca2+ content is slightly reduced. This is, in fact, consistent with the slight but significant reduction in steady-state SR Ca2+ content as measured by exposure to caffeine (Fig. 7). The fact that Re had little effect on the trigger (ICa,L) for SR Ca2+ release and yet increased stimulated Ca2+ transient amplitudes and decreased SR Ca2+ content indicates that Re increased fractional SR Ca2+ release. Finally, direct measurements of RyR channel function showed that Re increased the open probability of RyR channels without affecting single-channel conductance. These bilayer experiments were performed on native channels in the absence of ATP or Mg2+, indicating that the effects of Re were not dependent on either. Moreover, additional bilayers experiments (unpublished observations) performed in the presence of ATP and Mg2+ yielded similar results. It is also important to note that another biologically active ginseng extract, ginsenoside Rb1, failed to suppress EMA and failed to affect RyR channel opening. These findings provide an important negative control that supports a cause-and-effect relationship between Re-induced opening of RyR channels and suppression of EMA. Moreover, they indicate that Re exerts an effect not shared by all ginsenoside compounds. Taken together, the present study suggests that Re suppresses EMA by directly increasing the opening of RyRs and adds further support to the idea that the ultimate cause of EMA is a disturbance in SR Ca2+ release.
In the present study, EMA was elicited by electrical pacing at room temperature (hypothermia). Our previous work has also indicated that disturbances of metabolic activity can precipitate alternans (4, 8). This raises the question of whether the alternans seen here is elicited by a hypothermia-mediated impairment of metabolic activity and whether Re is acting to suppress alternans by somehow rectifying the impaired metabolic activity. The present experiments cannot eliminate this possibility. However, it should be noted that alternans can be precipitated by a wide range of abnormal conditions (6, 20), and it is not clear how these different conditions actually cause alternans. There is, however, compelling evidence that the ultimate cause of alternans is a subcellular disturbance of [Ca2+]i handling (4, 8, 20). More specifically, we reported that in cat cardiomyocytes, inhibition of SR Ca2+ release by ryanodine abolishes electrical alternans (15), indicating that abnormal SR Ca2+ handling is the primarily mechanism responsible for electrical alternans. Moreover, alternans is not associated with alternations of ICa,L or intracellular SR Ca2+ content but rather with alterations in the gain of SR Ca2+ release, i.e., the efficiency of a given trigger to activate SR Ca2+ release (8). This is consistent with direct measurements of intra-SR Ca2+ content, which showed that alternations in SR Ca2+ content are not required for Ca2+ alternans to occur and that RyR channel availability may be of greater importance (14). In light of these considerations, the simplest interpretation of the present experiments is that Re suppresses EMA by directly opening RyR channels.
The effects of Re to increase open probability of RyR channels without changing single-channel conductance appear similar to those of caffeine. However, exposure to caffeine also induces rapid diastolic Ca2+ release that can result in arrhythmic activity (18), effects not shared by Re. In other words, in the present study, Re did not induce Ca2+-mediated arrhythmic activity. Further experiments will be required to determine whether the molecular mechanism of Re action on RyR channels is similar to that of caffeine.
In rat ventricular myocytes, Re decreased [Ca2+]i transients and contraction strength, and these effects were blocked by inhibition of nitric oxide (NO) synthase activity, leading to the conclusion that the negative inotropic effects of Re were mediated by intracellular NO production (16). In the present study, Re failed to significantly affect ICa,L, which is regulated by NO production (23, 26). Moreover, the antiarrhythmic effects of Re to suppress EMA were unaffected by exposure to l-N5-(1-iminoethyl)-ornithine, a potent inhibitor of constitutive NO synthase activity (unpublished observations). Therefore, in cat cardiomyocytes, the effects of Re are not mediated via NO production. In fact, in paced ventricular myocytes exhibiting normal excitation-contraction coupling, nanomolar concentrations of Re had no effect on baseline action potential configuration and yet suppressed EMA by normalizing the Ca2+ transient amplitude. It is worth noting that nanomolar concentrations are compatible with those found in human plasma after the oral administration of ginsenoside (21).
The present results also indicate that in cat cardiomyocytes, Re does not affect sarcolemmal ICa,L, INa, or K+ conductances. These findings differ from those reported for guinea pig (1) and rat (9) ventricular myocytes, where Re decreased ICa,L and single-channel recordings of ICa,L, respectively. In guinea pig ventricular myocytes, Re also shortened APD and increased the activation of delayed rectifier K+ current (1). In the present study, we found no evidence that Re significantly affected ICa,L or K+ conductance. This is consistent with the fact that concentrations of Re (≤1 μM) that suppress EMA had no effect on APD90. The apparent discrepancies between the present findings and published reports could result from several factors, including differences in animal species, the purity of ginsenoside compounds, and/or the recording methods used. It should be noted, however, that in the present study Re failed to affect ICa,L in either cat or human cardiomyocytes and yet suppressed EMA in both cat and human cardiomyocytes. Therefore, any potential effect of Re on ICa,L is unrelated to the ability of Re to suppress EMA. In addition, these findings establish the relevance of the present study in cat to human cardiac physiology.
In summary, this study demonstrates that a main biologically active component of ginseng, ginsenoside Re, acts via a specific subcellular mechanism that targets RyR channels. Further experiments are needed to determine how Re acts to open RyRs and whether Re may serve as a safe, inexpensive, and effective drug therapy to reduce the development of arrhythmic activities arising from EMA. On the other hand, this study also demonstrates that ginseng extracts exert significant cardiac actions that have the potential to interact with other therapeutic and nontherapeutic cardiotonic drugs. For example, the addition of ginseng to sports drinks or soft drinks that already contain relatively high concentrations of caffeine may have the potential to significantly alter cardiac Ca2+ homeostasis and adversely affect cardiac function.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-079038 (to S. L. Lipsius) and HL-62231 and HL-80101 (to L. A. Blatter), American Heart Association Grants AHA0530309Z (to A. V. Zima) and AHA05501707 (to L. A. Blatter), and a Loyola University Medical Center, Cardiovascular Institute, Dr. Ralph and Marian Falk Medical Research Trust Foundation Fellowship Grant (to X. Ji).
Acknowledgments
We thank Dr. Joseph Akar for assistance in obtaining human cardiac tissue and Dr. Kathrin Banach for assistance with the isolation of human atrial myocytes.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bai CX, Sunami A, Namiki T, Sawanobori T, Furukawa T. Electrophysiological effects of ginseng and ginsenoside Re in ginea pig ventricular myocytes. Eur J Pharmacol 476: 35–44, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Baudet S, Hove-Madsen L, Bers DM. How to make and use a calcium-specific mini- and microelectrode. Methods Cell Biol 40: 93–113, 1994. [PubMed] [Google Scholar]
- 3.Berger Repolarization alternans RD. Toward a unifying theory of reentrant arrhythmia induction. Circ Res 87: 1083–1084, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Blatter LA, Kockskamper J, Sheehan KA, Zima AV, Hüser J, Lipsius SL. Local calcium gradients during excitation-contraction coupling and alternans in atrial myocytes. J Physiol 546: 19–31, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dilly SG, Lab MJ. Electrophysiological alternans and restitution during acute regional ischaemia in myocardium of anaesthetized pig. J Physiol 402: 315–333, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Euler DE Cardiac alternans: mechanisms and pathophysiological significance. Cardiovasc Res 42: 583–590, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Hiromoto K, Shimizu H, Furukawa Y, Kanemori T, Mine T, Masuyama T, Ohyanagi M. Discordant repolarization alternans-induced atrial fibrillation is suppressed by verapamil. Circulation 69: 1368–1373, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Hüser J, Wang YG, Sheehan KA, Cifuentes F, Lipsius SL, Blatter LA. Functional coupling between glycolysis and excitation-contraction coupling underlies alternans in cat heart cells. J Physiol 524: 795–806, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang Y, Liu W, Wang XM, Zhong GG, Zhang WJ, Chen L, Zhan S, Qi H, Zhao CY, Ma XY, Yang SJ, Li H. Calcium channel blockade and anti-free-radical actions of panaxatriol saponins in cultured myocardiocytes. Zhongguo Yao Li Xue Bao 17: 138–141, 1996. [PubMed] [Google Scholar]
- 10.Konta T, Ikeda K, Yamaki M, Nakamura K, Honma K, Kubota I, Yasui S. Significance of discordant ST alternans in ventricular fibrillation. Circulation 82: 2185–2189, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Lipsius SL, Ji X, Blatter LA, Wang YG. Ginsenoside Re acts via subcellular mechanisms to suppress electro-mechanical alternans in cat cardiomyocytes (Abstract). Circulation 112: 152, 2005. [Google Scholar]
- 12.Nearing Huang AH B, Verrier RL. Dynamic tracking of cardiac vulnerability by complex demodulation of the T-waves. Science 252: 437–440, 1991. [DOI] [PubMed] [Google Scholar]
- 13.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanisms linking T-wave alternans to the genesis of cardiac fibrillation. Circulation 99: 1385–1394, 1999. [DOI] [PubMed] [Google Scholar]
- 14.Picht E, Desantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res 99: 740–748, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Rubenstein DS, Lipsius SL. Premature beats elicit a phase reversal of mechanoelectrical alternans in cat ventricular myocytes. Circulation 91: 201–214, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Scott GI, Colligan PB, Ren BH, Ren J. Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. Br J Pharmocol 134: 1159–1165, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shimizu W, Antzelevitch C. Cellular and ionic basis for T-wave alternans under long-QT conditions. Circulation 99: 1499–1507, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res 100: 105–111, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Verrier RL, Nearing BD. Electrocardiographic basis for T wave alternans as an index of vulnerability to ventricular fibrillation. J Cardiovasc Electrophysiol 5: 445–461, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Walker ML, Rosenbaum DS. Repolarization alternans: implications for the mechanism and prevention of sudden cardiac death. Cardiovasc Res 57: 599–614, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Zou H, Kong L, Zhang Y, Pang H, Su C, Liu G, Hui M, Fu L. Determination of ginsenoside Rg3 in plasma by solid-phase extraction and high performance liquid chromatography for pharmacokinetic study. J Chromatography B 731: 403–409, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Wang YG, Dedkova EN, Fiening JP, Ojamaa K, Blatter LA, Lipsius SL. Acute exposure to thyroid hormone increase Na+ current and intracellular Ca2+ in cat atrial myocytes. J Physiol 546: 491–499, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang YG, Dedkova EN, Ji X, Blatter LA, Lipsius SL. Phenylephrine acts via IP3-dependent NO release to stimulate L-type Ca2+ current in atrial myocytes. J Physiol 567: 143–157, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YG, Lipsius SL. Acetylcholine activates a glibenclamide-sensitive K+ current in cat atrial myocytes. Am J Physiol Heart Circ Physiol 268: H1322–H1334, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Wang YG, Lipsius SL. Acetylcholine potentiates acetylcholine-induced increases in K+ current in cat atrial myocytes. Am J Physiol Heart Circ Physiol 268: H1313–H1321, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Wang YG, Rechenmacher CE, Lipsius SL. Nitric oxide signaling mediates stimulation of L-type Ca2+ current elicited by withdrawal of acetylcholine in cat atrial myocytes. J Gen Physiol 111: 113–125, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wohlfart B Analysis of mechanical alternans in rabbit papillary muscle. Acta Physiol Scand 115: 405–414, 1982. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Vereecke J, Carmeliet E, Lipsius SL. Ionic currents activated during hyperpolarization of single right atrial myocytes from cat heart. Circ Res 68: 1059–1069, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Yeh GY, Davis RB, Phillips RS. Use of complementary therapies in patients with cardiovascular disease. Am J Cardiol 98: 673–680, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Zima AV, Kockskamper J, Meija-Alvarez R, Blatter LA. Pyruvate modulates cardiac sarcoplasmic reticulum Ca2+ release in rats via mitochondria-dependent and independent mechanisms. J Physiol 550: 765–783, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]