Abstract
Epidemiological studies have shown a correlation between flavonoid-rich diets and improved cardiovascular prognosis. Cocoa contains large amounts of flavonoids, in particular flavanols (mostly catechins and epicatechins). Flavonoids possess pleiotropic properties that may confer protective effects to tissues during injury. We examined the ability of epicatechin to reduce short-and long-term ischemia-reperfusion (I/R) myocardial injury. Epicatechin (1 mg·kg−1·day−1) pretreatment (Tx) was administered daily via oral gavage to male rats for 2 or 10 days. Controls received water. Ischemia was induced via a 45-min coronary occlusion. Reperfusion was allowed until 48 h or 3 wk while Tx continued. We measured infarct (MI) size (%), hemodynamics, myeloperoxidase activity, tissue oxidative stress, and matrix metalloproteinase-9 (MMP-9) activity in 48-h groups. Cardiac morphometry was also evaluated in 3-wk groups. With 2 days of Tx, no reductions in MI size occurred. After 10 days, a significant ∼50% reduction in MI size occurred. Epicatechin rats demonstrated no significant changes in hemodynamics. Tissue oxidative stress was reduced significantly in the epicatechin group vs. controls. MMP-9 activity demonstrated limited increases in the infarct region with epicatechin. By 3 wk, a significant 32% reduction in infarct size was observed with Tx, accompanied with sustained hemodynamics and preserved chamber morphometry. In conclusion, epicatechin Tx confers cardioprotection in the setting of I/R injury. The effects are independent of changes in hemodynamics, are sustained over time, and are accompanied by reduced levels of indicators of tissue injury. Results warrant the evaluation of cocoa flavanols as possible therapeutic agents to limit ischemic injury.
Keywords: myocardial infarction, catechin, epigallocatechin, cocoa, chocolate
known risk factors for the development of cardiovascular disease (CVD) include hypertension, a poor diet, obesity, lack of physical activity, smoking, and diabetes. Diet has been established as one of the most important lifestyle factors that can strongly influence the incidence of CVD. Polyphenols are widely distributed in plants and are known as flavonoids (30). Flavonoids can be divided into four classes, as follows: flavones, flavanols, flavonols, and anthocyanins (34). All four classes have in common phenolic rings with variations in the number and arrangement of the hydroxyl groups as well as the nature and extent of alkylation and/or glycosylation of these groups (34). Flavonoids have been long recognized for their free radical scavenging activity (34). Epidemiological evidence indicates a negative correlation between consumption of flavonoid-rich foods or beverages and incidence of CVD (20, 23, 25, 26). Wine has garnered attention because its polyphenol concentration is relatively high and may be partly responsible for the prolonged longevity associated with the “French Paradox” (2, 13). However, cocoa powder contains by far the largest amount of flavonoids, in particular the subtype known as flavanols (up to 10% flavanols by weight) (11). Main flavanols in cocoa are catechins and epicatechins present in mono- or multimeric forms (11, 26). Interest in the beneficial effects of cocoa-derived flavonols emerged from observations of Kuna Indians living off Panama. Kuna islanders have a very low incidence of CVD, in particular, hypertension. The low incidence of CVD is related to environmental factors and not genetic, since protection is lost when Kuna Indians migrate to mainland (21, 29). Kuna islanders drink large amounts of cocoa rich in flavanols vs. mainland migrants. Studies relate the low indices of CVD to the consumption of cocoa beverages and not other factors such as fruit or protein, physical activity, or alcohol intake. In addition to their free radical scavenging activity, there are other clues as to the mechanisms that may explain cocoa effects. Consumption of flavanol-rich cocoa leads to vasodilation which can be reversed by the use of the NO synthesis inhibitors (12, 18). Other effects of flavanoids/flavanols include the inhibition of platelet adhesion, low-density lipoprotein oxidation, inflammation, reactive oxygen species (ROS) generation, eicosanoid synthesis, and insulin resistance (for reviews, see Refs. 10 and 33). Recent reports link the effects of cocoa to epicatechin. The ingestion of epicatechin in humans can reproduce the hypotensive, antioxidant, and insulin-sensitizing effects of cocoa (35).
In spite of the identification of many candidate compounds that have the potential to reduce infarct size, none have demonstrated the capacity to retain the effect over the long term (3, 24, 45). Most pharmacological agents studied to date have only managed to postpone cell death. On the basis of the reported pleiotropic actions of flavanols, it is reasonable to propose that, on the short-term, these agents may ameliorate tissue injury secondary to ischemia-reperfusion (I/R) and that in the long-term their continued actions may lead to improved outcomes. We hypothesize that the administration of epicatechin to rats leads to a sustained reduction in infarct size and that this phenomena is secondary to a suppression of mediators of reperfusion injury.
MATERIALS AND METHODS
Animal groups and epicatechin treatment.
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250–300 g (∼8 wk of age) were used. All procedures were approved by the Institutional Animal Care and Use Committee and conform to published National Institutes of Health guidelines for animal research. Epicatechin (1 mg·kg−1·day−1; Sigma-Aldrich, St. Louis, MO) or vehicle (water) was administered by oral gavage one time per day beginning either 2 or 10 days before thoracotomy and continuing until the time of the terminal study (48 h or 3 wk). This dose was noted in humans to reproduce the vascular (i.e., hypotensive) effects of dark chocolate (35). Groups of normal (i.e., no surgery was performed in these groups) animals were treated with water (n = 6) or epicatechin (n = 6) for 10 days and were used solely to evaluate the effects of epicatechin on baseline hemodynamics. For 48-h studies, groups included I/R (n = 21), I/R + 2 days epicatechin (n = 7), and I/R + 10 days epicatechin (n = 15) and were used to measure infarct size. Subgroups of 10 days treatment (n = 8/each group) were used to measure hemodynamics. For 48-h biochemical determinations, subgroups included I/R (n = 8) and I/R + 10 days epicatechin (n = 7). The 48-h time point was selected to be able to clearly distinguish regions of necrotic tissue from viable myocardium. For 3-wk studies, groups included sham (n = 5), sham + 10 days epicatechin (n = 4), I/R (n = 7), and I/R + 10 days epicatechin (n = 8). Sham animals underwent a thoracotomy but were not subjected to I/R.
I/R surgery.
Animals were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), intubated, and positive-pressure ventilated. A left thoracotomy was then performed. In I/R animals, the left anterior descending coronary artery was ligated for 45 min and released, and the suture was left in place as a point of reference. The chest was closed in layers, and animals were allowed to recover. Successful occlusion and reperfusion was verified by visual inspection of left ventricle (LV) color.
Hemodynamics.
For hemodynamic measurements, animals were anesthetized with 5% isoflurane and maintained with 1–2% isoflurane. The right carotid artery was exposed via a neck dissection. Carotid and LV pressures were acquired using a micromanometer (2 French, 140 cm; Millar instruments, Houston, TX) introduced via the carotid artery. Hemodynamics were digitally recorded for subsequent analysis using WINDAQ software (version 2.15; DATAQ Instruments).
Terminal studies.
Hearts were excised and weighed. In 48-h hearts, the area at risk (AAR) was determined by the reocclusion of the snare and infusion of trypan blue in the cannulated aorta. Hearts was sectioned into five 2-mm rings and stained using triphenyltetrazolium chloride. Computer-assisted image analysis was used using blinded operators. Results are expressed as infarct area as a function of the AAR. For 3-wk studies, hearts were processed as above and sectioned to identify the infarct (i.e., scar) area. The images of unfixed, stained rings were also used to measure internal and external chamber diameters and anterior and septal wall thicknesses.
Tissue collection.
The 48-h excised hearts were perfused with cold saline to remove blood. LV free wall was separated and divided into two parts (the infarct region and the border zone). The right ventricle (RV) was also taken to be used as the remote or nonischemic region.
Gelatin zymography.
Heart samples (∼50 mg) were homogenized in 10 mM HEPES, pH 7.5, 150 mM NaCl, 0.2 mM EDTA, 25% glycerol, 100 μg/ml phenylmethylsulfonyl fluoride, and 0.2 kallikrein inhibitory units/ml aprotinin. Samples (10 μg of protein) were analyzed by SDS-PAGE as described (14). An internal control [human matrix metalloproteinase (MMP)-2/MMP-9; Chemicon, Temecula, CA] was loaded to normalize between gels. Bands of gelatinolytic activity were digitally quantified (Kodak 1D; Eastman Kodak, Rochester, NY).
Myeloperoxidase assay.
The myeloperoxidase (MPO) assay was performed as previously described with modification (14). Tissue samples were homogenized in MPO lysis buffer (50 mmol/l KH2PO4, pH 6.0, 0.5% hexadecyltrimethylammonium bromide) and incubated on ice for 30 min. After centrifugation, the supernatants were reacted with 0.4 mmol/l tetramethylbenzidine (Sigma) and 0.006% H2O2 in 50 mmol/l phosphate at pH 6.0. Absorbance was monitored, and MPO activity was expressed as relative units per minute.
Glutathione (reduced/oxidized) assay.
Tissue samples were homogenized in ice-cold homogenization buffer [154 mM KCl, 5 mM diethylenetriaminepentaacetic acid (DPTA), and 0.1 M potassium phosphate, pH 6.8]. After centrifugation, an aliquot was removed for protein determination using the bicinchoninic acid method (Pierce Chemical, Rockford, IL). Immediately after an aliquot was taken, one volume of cold acid buffer (40 mM HCl, 10 mM DPTA, 20 mM ascorbic acid, and 10% trichloroacetic acid) was added to one volume of homogenate. The suspension was centrifuged, and the resulting supernatant solution was centrifuged through a 0.45-μm microcentrifuge filter (Millipore, Bedford, MA). Reduced and oxidized glutathione, GSH and GSSG respectively, levels were determined as previously described by Senft et al. (37) using the fluorophore o-phthalaldehyde (OPA).
Statistical analysis.
Results are expressed as means ± SE. Comparisons between means were analyzed, as appropriate, by Student's t-tests or one-way ANOVA followed by Bonferroni t-test. A value of P < 0.05 was considered statistically significant.
RESULTS
Hemodynamics.
Hemodynamic parameters were measured in normal, and normal + 10 days epicatechin rats. Epicatechin treatment only raised heart rate vs. normal rat. Values recorded 48 h after I/R (Table 1) demonstrate no significant changes in either heart rate, LV end-diastolic/peak systolic pressure, or mean aortic pressure between I/R groups. In 3-wk studies (Table 2), hemodynamic parameters were recorded in sham and I/R animals, and results were comparable between untreated vs. treated groups.
Table 1.
Hemodynamic data obtained from either normal or 48 h I/R groups
| Normal | Normal + Epicatechin | I/R | I/R + Epicatechin | |
|---|---|---|---|---|
| Group size | 6 | 6 | 8 | 8 |
| HR, beats/min | 298±17 | 346±8 | 368±12* | 373±17* |
| LVPSP, mmHg | 125±8 | 112±14 | 102±6 | 110±5 |
| LVEDP, mmHg | 7.6±0.9 | 6.7±1.3 | 5±0.3 | 4.9±0.5 |
| MAP, mmHg | 101±7 | 99±10 | 89±3 | 90±5 |
Values are means ± SE. I/R, ischemia-reperfusion; HR, heart rate; LVPSP, left ventricular peak systolic pressure; LVEDP, left ventricular end diastolic pressure; MAP, mean arterial pressure. Epicatechin groups were pretreated for 10 days.
P < 0.05 vs. normal.
Table 2.
Hemodynamic and morphometry data obtained from either sham or 3-wk I/R groups
| Sham | Sham + Epicatechin | I/R | I/R + Epicatechin | ||||
|---|---|---|---|---|---|---|---|
| Hemodynamics | |||||||
| Group size | 5 | 4 | 7 | 8 | |||
| HR, beats/min | 318±12 | 328±11 | 290±6 | 302±18 | |||
| LVPSP, mmHg | 119±2.2 | 116±6.2 | 108±2 | 108±4 | |||
| LVEDP, mmHg | 6.9±1.1 | 2.6±0.2 | 6.1±0.8 | 5.5±1.5 | |||
| MAP, mmHg | 94±4.7 | 92±11 | 89±2 | 85±4 | |||
| Morphometry | |||||||
| Group size | 5 | 4 | 7 | 8 | |||
| HW/BW | 3.2±0.2 | 3.2±0.5 | 4.5±0.2* | 4.5±0.2* | |||
| Outer LV diameter, mm | 1.6±0.1 | 1.6±0.1 | 1.6±0.06 | 1.6±0.04 | |||
| Inner LV diameter, mm | 0.53±0.02 | 0.52±0.08 | 0.52±0.06 | 0.55±0.05 | |||
| AW thickness, mm | 0.52±0.05 | 0.53±0.05 | 0.4±0.03† | 0.41±0.02 | |||
| SW thickness, mm | 0.49±0.05 | 0.47±0.04 | 0.62±0.04 | 0.49±0.03 | |||
Values shown are mean ± SE. MAP, mean arterial pressure; HW/BW, heart weight-to-body weight ratio; LV, left ventricle; AW, anterior wall; SW, septal wall. Epicatechin groups were pretreated for 10 days.
P < 0.01 vs. sham
P < 0.01 vs. I/R SW thickness.
Infarct size and morphometry.
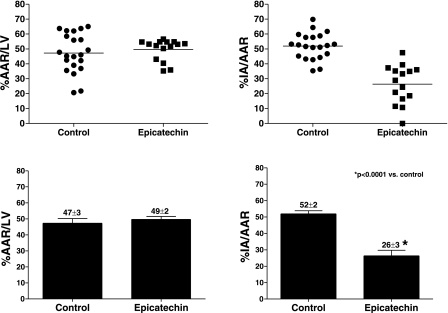
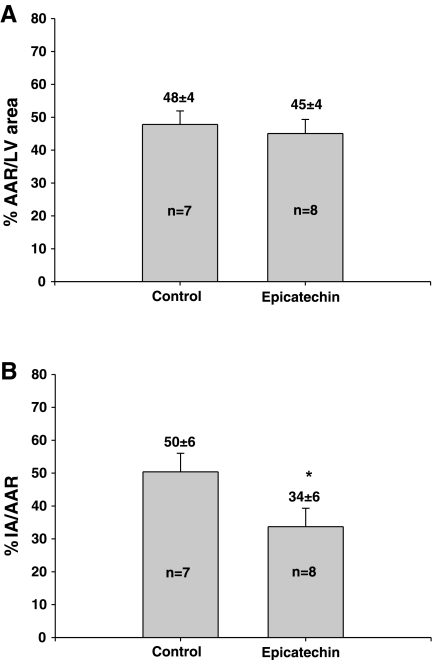
For the 2-day pretreatment study, the AAR were 50 ± 4 and 56 ± 2% and were statistically not different between the I/R and I/R + 2 days epicatechin groups, respectively. The I/R group and the I/R + 2 days epicatechin group also had comparable (P > 0.05) infarct areas (infarct area/AAR) of 41 ± 8 and 34 ± 4% respectively. In the 10 day pretreatment study (Fig. 1), infarct area was 52 ± 2% vs. 26 ± 3% (P < 0.0001). There was no difference in the AAR, 47 ± 3 and 49 ± 2%, between the two groups. Figure 2 summarizes the 3-wk results. The infarct area of I/R animals was 50 ± 6 vs. 34 ± 6% (P < 0.05). The two groups had similar AAR (48 ± 4 and 45 ± 4%). As shown in Table 2, I/R animals treated with epicatechin demonstrate comparable post-I/R morphometric changes to those of 3-wk untreated I/R animals. Anterior wall vs. septal wall thicknesses yielded a statistical difference only in the vehicle-treated I/R group. Changes in infarct size and morphometry appear independent from altered hemodynamics, since epicatechin did not modify these in a manner that would explain the observed results. No differences in survival rates were noted between any groups.
Fig. 1.
Infarct area (IA) as a function of area at risk (AAR) in ischemia-reperfusion (I/R) rats subjected to 10 days vehicle or epicatechin treatment and 48 h reperfusion. Infarct size was determined by staining hearts with trypan blue and triphenyltetrazolium chloride. A: dispersion plot of the AAR in I/R (n = 21) and I/R +10 days epicatechin (n = 15) in rats subjected to 48 h reperfusion. B: bar graphs of the AAR. C: dispersion plot of the IA in I/R (n = 21), and I/R + 2 days epicatechin (n = 15) subjected to 48 h reperfusion. D: bar graphs of the IA. Values are means ± SE.
Fig. 2.
IA as a function of AAR in I/R rats subjected to 10 days vehicle or epicatechin treatment and 3 wk reperfusion. Infarct size was determined by staining hearts with trypan blue and triphenyltetrazolium chloride. A: AAR in I/R (n = 7) and I/R + 10 days epicatechin (n = 8) in rats subjected to 3 wk reperfusion. B: IA in I/R (n = 7) and I/R + 10 days epicatechin (n = 8) subjected to 3 wk of reperfusion. Values are means ± SE. *P < 0.05, significant difference between groups.
MPO activity.
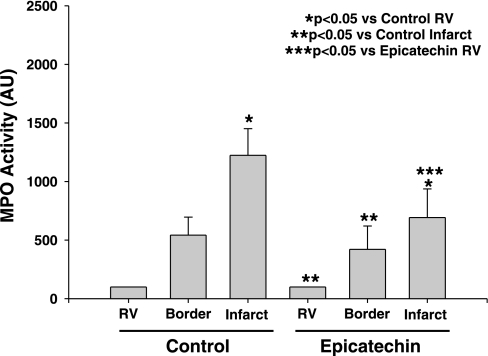
We assessed the capacity of epicatechin to alter inflammatory cell infiltration in the myocardium by measuring MPO activity using a colorimetric assay (14). As shown in Fig. 3, in both the 48-h I/R and I/R + 10 days epicatechin groups, MPO in the infarct region was higher when compared with the RV (P < 0.05), indicating infiltration of inflammatory cells in the border zone and ischemic regions. No differences in MPO levels were noted between I/R and I/R + 10 days epicatechin at any of the analyzed regions.
Fig. 3.
Myocardial myeloperoxidase (MPO) activity in I/R (n = 8) and I/R + 10 days epicatechin (n = 7) hearts was determined by a recently described colorimetric assay. Values are means ± SE.
Myocardial oxidative stress.
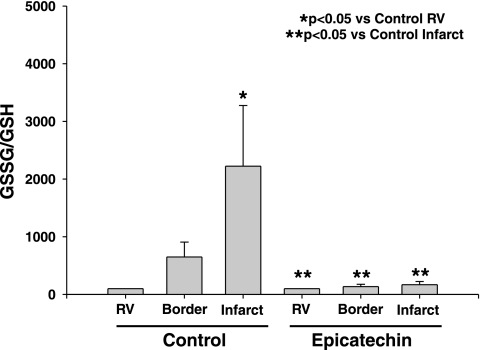
We determined if epicatechin ameliorates the generation of oxidative stress within the myocardium of rats subjected to I/R. Results are shown as the ratio GSSG/GSH, which is a measure of total tissue oxidative stress. Oxidative stress was increased significantly (P < 0.05) in the infarct region compared with the RV in the I/R group, and this increase was attenuated significantly (P < 0.05) in the border zone and ischemic regions of the I/R + 10 days epicatechin group (Fig. 4).
Fig. 4.
Myocardial levels of oxidative stress [oxidized glutathione (GSSG)/reduced glutathione (GSH)] in I/R (n = 8) and I/R + 10 days epicatechin (n = 7) infarcted hearts were detected in I/R hearts using the fluorophore o-phthalaldehyde. Values are means ± SE.
Zymography results.
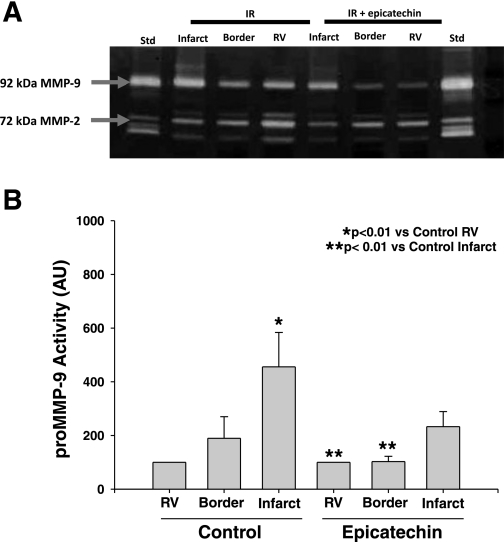
We determined if epicatechin decreases levels of MMP-2 and/or MMP-9 activity. Gelatin zymography of tissue homogenates from the RV, border, and infarct region only revealed bands corresponding to 92-kDa MMP-9 and 75-kDa MMP-2 (Fig. 5A). The 86-kDa MMP-9 and 72-kDa MMP-2 were not visible. Densitometric analysis revealed that 92-kDa MMP-9 levels were increased significantly (P < 0.01) in the infarct region compared with the RV in the I/R group, but not in the I/R + 10 days epicatechin group (Fig. 5B). As observed in the gel image and by densitometric analysis, no notable differences were identified in 75-kDa MMP-2 levels.
Fig. 5.
Myocardial matrix metalloproteinase (MMP)-2 and MMP-9 levels in I/R (n = 8) and I/R + 10 days epicatechin hearts (n = 7) as determined by gelatin zymography. A: representative gelatin zymograms of myocardial MMP-2 and MMP-9 levels in I/R and I/R + 10 days epicatechin. B: densitometric analysis of 92-kDa MMP-9 zymographic activity. Values are means ± SE. Std, human MMP-2/-9 standard.
DISCUSSION
Our results indicate that 10 days pre- and continuous epicatechin Tx significantly reduced infarct size in rats 48 h after I/R injury. The smaller infarct size observed in treated animals is accompanied by significant reductions in tissue oxidative stress at the infarct region. These results were accompanied by a preservation of local inflammation and lesser degrees of MMP-9 activity in treated, injured myocardium. More importantly, a sustained reduction in infarct size was observed 3 wk after I/R injury.
Because the length of exposure to cocoa flavanols appears to determine bioactivity, we first examined if short-term pretreatment with epicatechin could confer cardioprotective effects. Two days of epicatechin pretreatment did not significantly reduce myocardial infarct (MI) size. These results contrast to those of others where cardioprotection with flavanols can be generated immediately upon treatment. Wang et al. (42) demonstrated the capacity of a synthetic flavanol, administered at 5 mg/kg just before reperfusion to reduce MI size in sheep while also decreasing ROS generation, improving coronary blood flow and nitric oxide (NO) metabolite levels. Aneja et al. (1) reported similar effects with epigallocatechin-3-gallate (EGCG) at 10 mg/kg given just before reperfusion to rats. An important difference arises when comparing our 2-day results with these studies. Flavanol doses used by these investigators were significantly higher than those of our study (∼5- to 10-fold). It is well known that higher doses of flavanols attain greater antioxidant effects, thus effectively mitigating (with increased efficacy) the damaging effects of ROS during I/R. This observation is supported by data that indicates that serum concentrations of the order of ∼0.01 mM allow flavonoids to act as effective in vivo ROS scavengers (17). The serum concentration reported in animals treated with 1 mg·kg−1·day−1 would only approximate 1/10th of this. The fact that short-term treatment with 1 mg·kg−1·day−1 epicatechin does not confer cardioprotection suggests that changes in myocardial gene expression and/or protein levels may need to develop with longer Tx times (11, 40). Alternatively, changes in epicatechin metabolism may be required to generate I/R protection (11).
In studies performed in patients suffering from hypertension, sustained reductions in blood pressure were observed when Tx was given with high flavanol cocoa for at least 7 days (15). Our results demonstrate that, in animals with 10 days of pre- and continuous epicatechin Tx, a significant ∼50% reduction in infarct size was observed. A potential mechanism by which epicatechin may decrease MI size is a reduction in afterload. Ten days of epicatechin treatment in normal and I/R animals did not reduce blood pressure; thus, changes in afterload fail to explain the observed effects.
In the setting of myocardial ischemia, the lack of oxygen and associated ATP production can lead to cell death (necrosis). Upon reperfusion, the reintroduction of blood flow to the previously ischemic area leads to the activation of an injury pathway associated with reoxygenation (4, 45). Reperfusion injury activates an inflammatory response, which can lead to loss of contractile function, arrythmias, and may promote cell death in ischemic myocytes (4). Major mediators of I/R injury are neutrophils, oxygen radicals, proteases, and calcium overload (4, 45). Compounds that target “single” mediators of I/R-related injury, such as antioxidants, have shown to improve indicators of tissue injury in animal models. However, the translation of these improvements into the clinical setting has not been successful (3, 24, 45). Similar failures have been observed with strategies that target tissue inflammation (i.e., neutrophil infiltration) or protease activity where, again, promising results in animal models did not translate into the clinical setting (3, 24, 45). This leads to the question of: Are small molecules that possess pleiotropic actions potentially a better strategy to limit I/R injury? Studies have demonstrated that flavonoids, including flavanols, are pleiotrophic. Flavonoids possess antioxidant (17, 43, 44), anti-inflammatory (1, 9, 36, 39), and antithrombotic properties (19, 31). Flavonoids can also induce NO-mediated vasodilation (12, 22, 35). Interestingly, several flavonoid properties mirror those of adenosine, the only agent that has shown some promise as a cardioprotectant (3, 24). It is interesting to note that, for adenosine to act as a prophylactic cardioprotector, the doses required would likely yield hypotension and thus be impractical for daily use. However, studies using flavonoids have yet to identify any toxic or significant side effects, including notable hypotension in normotensive individuals (6, 12).
It is well established that, during the acute phase of I/R injury, inflammatory cells are recruited to the site of injury either infiltrating the tissue and/or causing microvascular plugging (i.e., no-reflow phenomenom). Inflammatory cells release proteases, such as MMPs, and generate an oxidative burst (4, 45). Previous studies have investigated the effects of catechins on tissue inflammation. Dona et al. (9) examined the effects of EGCG to modulate in vitro and in vivo lung inflammatory responses. EGCG reduced ROS activity, inhibited apoptosis, and reduced chemokine-induced neutrophil chemotaxis in vitro. EGCG significantly reduced pulmonary inflammatory responses and fibrosis. Aneja et al. (1) also investigated the effects of EGCG on neutrophil activation in rats subjected to I/R. Cardioprotection was associated with decreased interleukin-6 production and neutrophil infiltration, as measured by MPO activity. In our study, epicatechin treatment did not reduce in a significant manner MPO activity in the infarct region, indicating that, at 48 h post-I/R, there is a preservation of acute inflammation. There is continuing controversy as to the wisdom of the potential benefit to be derived from therapies intended to suppress acute inflammation in the setting of MI. Although the reduction of neutrophil infiltration can reduce I/R injury in animals, the use of anti-inflammatory agents in patients has led to adverse outcomes, including ventricular rupture (38). In a study performed in our laboratory, the continuous use of steroids after coronary occlusion led to post-MI adverse remodeling (14).
In the setting of I/R injury, oxidative stress has a number of deleterious biochemical effects on the heart. ROS produced in I/R injury can contribute to mitochondrial damage, which can lead to cell death by necrosis and apoptosis (4, 45). ROS can also activate proenzymes, such as pro-MMPs, and promote calcium overload (4, 45). There are several endogenous antioxidants found in tissues, including GSH. It is known that GSH plays a central role in intracellular endogenous antioxidant defenses, since it is involved in all lines of protection against ROS (28). In the course of I/R, GSH is converted to GSSG, and the level of ROS exceeds the antioxidant capacity of the cell, leading to oxidative stress (28). A study by Chen et al. (5) investigated the effect of tea catechin on the Pb2+-induced change of intracellular thiol levels. They demonstrated that Pb2+ significantly increased tissue oxidative stress in a concentration-dependent manner. Supplementation of the cells with (−)-epicatechin or (−)-epicatechin gallate resulted in reduced oxidative stress. These results match ours in that we showed a significant increase in the GSSG-to-GSH ratio in the infarct regions compared with the noninfarct regions in control hearts, with epicatechin significantly attenuating this increase. In our study, we used doses of epicatechin that are low and would appear on the basis of expected blood concentrations (∼0.002 mM) to yield limited antioxidant activity (17). Thus the manner in which low doses of epicatechin significantly reduce tissue oxidative stress needs further investigation, since it raises a wide spectrum of possibilities. One such possibility is that epicatechin over the course of several days is concentrated intracellularly severalfold vs. blood levels and allows it to act as an effective antioxidant.
The activation of MMPs with I/R injury has also been described. We and others have documented the upregulation of MMP-2 and MMP-9 activity in infarcted myocardium (16, 27). MMPs contribute to the loss of contractile function and damage the integrity of the extracellular matrix. Our results showed significant increases in MMP-9 activity in the infarct region compared with the noninfarct region in the vehicle group, and a lack of a significant increase in the necrotic region with epicatechin Tx. This correlates with the findings of Dell'agli et al. (8) who studied the effects of EGCG on MMP-9. Results indicate that EGCG directly inhibited MMP-9 activity (8). Interestingly, the reduction in tissue oxidative stress observed in our study may have also led to decreased levels of MMP-9 activity via inhibition of the cysteine switch activation mechanism, which can be ROS dependent (32).
The purpose behind the study of 3 wk post-I/R animals was to determine the extent to which there was a sustained reduction in tissue injury with treatment. Results yield a reduction in scar (infarct) size of ∼32%. Our 3-wk results are encouraging in that most studies have failed to demonstrate the capacity of candidate cardioprotective agents to yield sustained (long-term) effects (3, 24, 45). The observed preservation of post-MI chamber morphometry is also important, since little is known about what impact the pleiotropic effects of flavonoids may have on post-MI wound healing/remodeling. To our knowledge, as noted above, only the use of the pleiotrophic agent adenosine has shown promise as a possible cardioprotector and promoter of either preserved or reduced post-MI remodeling (7, 41).
Because the sustained beneficial effects of cocoa flavanols depend on their continued intake, it implies that their possible use as cardioprotective agents at the time of reperfusion or soon afterward would appear unlikely. However, cocoa-induced vasodilation can be reproduced by a select group of metabolites (35); thus, there is the possibility that future studies using flavanol derivatives may allow for their examination as “immediate” cardioprotectors. Alternatively, there is the possibility that higher does of flavanols may yield immediate cardioprotection. This possibility will need to be explored. The concept of feasible prophylactic cardioprotection has been proposed. Bolli et al. (3) have noted that the most practical strategy for limiting infarct size and improving clinical outcome may be to induce a chronically protected cardiac phenotype where this property would be operative at the onset of ischemia. The observation that Kuna Indians have low incidences of CVD is supportive of this idea. However, this population is not representative of the poorer health status observed in developed countries' general population. The concept of suggesting that humans consume traditional cocoa-based products high in flavanol content is not a practical one given their high caloric content. Alternatively, it is more likely to conceive of the production of cocoa-based “supplements” devoid of calories to be used for daily prophylactic use. This is a concept that has been proposed as viable on the basis of observations derived from the use of the flavonoid resveratrol on animals exposed to unhealthy diets (2).
On the basis of the results presented, more work needs to be performed to validate these results and identify key underlying mechanisms of action of epicatechin. The reproducibility of these observations by independent groups is warranted. In addition, further preclinical studies need to be performed using small and large animal models of human disease such as those with hypertension, diabetes, stroke prone, aging and, importantly, to verify the variability of the effects on the basis of gender. Nonetheless, the results presented provide support toward the consideration of cocoa flavanols as possible therapeutic agents intended to prevent and/or limit the development of ischemic heart disease.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-43617 and HL-67922 to Dr. F. Villarreal, a Consejo Nacional de Ciencia y Tecnologia— University of California Institute for Mexico and the United States MEXUS doctoral fellowship to D. Romero-Perez, an undergraduate diversity supplement to B. Cortez-Gomez (HL-80049), and a predoctoral NHLBI fellowship (HL-07444) to K. Yamazaki.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aneja R, Hake PW, Burroughs TJ, Denenberg AG, Wong HR, Zingarelli B. Epigallocatechin, a green tea polyphenol, attenuates myocardial ischemia reperfusion injury in rats. Mol Med 10: 55–62, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo R, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 444: 337–342, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolli R, Becker L, Gross G, Mentzer R Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res 95: 125–134, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Buja LM, Entman ML. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation 98: 1355–1357, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Yang X, Jiao H, Zhao B. Effect of tea catechins on the change of glutathione levels caused by Pb(++) in PC12 cells. Chem Res Toxicol 17: 922–928, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Chengelis CP, Kirkpatrick JB, Regan KS, Radovsky AE, Beck MJ, Morita O, Tamaki Y, Suzuki H. 28-Day oral (gavage) toxicity studies of green tea catechins prepared for beverages in rats. Food Chem Toxicol 46: 978–989, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Claeys MJ, Bosmans J, De Ceuninck M, Beunis A, Vergauwen W, Vorlat A, Vrints CJ. Effect of intracoronary adenosine infusion during coronary intervention on myocardial reperfusion injury in patients with acute myocardial infarction. Am J Cardiol 94: 9–13, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Dell'agli M, Bellosta S, Rizzi L, Galli GV, Canavesi M, Rota F, Parente R, Bosisio E, Romeo S. A structure-activity study for the inhibition of metalloproteinase-9 activity and gene expression by analogues of gallocatechin-3-gallate. Cell Mol Life Sci 62: 2896–2903, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dona M, Dell'Aica I, Calabrese F, Benelli R, Morini M, Albini A, Garbisa S. Neutrophil restraint by green tea: inhibition of inflammation, associated angiogenesis, and pulmonary fibrosis. J Immunol 170: 4335–4341, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Engler MB, Engler MM. The emerging role of flavonoid-rich cocoa and chocolate in cardiovascular health and disease. Nutr Rev 64: 109–118, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Fisher ND, Hollenberg NK. Flavanols for cardiovascular health: the science behind the sweetness. J Hypertens 23: 1453–1459, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Fisher ND, Hughes M, Gerhard-Herman M, Hollenberg NK. Flavanol-rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J Hypertens 21: 2281–2286, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Formica JV, Regelson W. Review of the biology of Quercetin and related bioflavonoids. Food Chem Toxicol 33: 1061–1080, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Garcia RA, Go KV, Villarreal FJ. Effects of timed administration of doxycycline or methylprednisolone on post-myocardial infarction inflammation and left ventricular remodeling in the rat heart. Mol Cell Biochem 300: 159–169, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension 46: 398–405, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Griffin MO, Jinno M, Miles LA, Villarreal FJ. Reduction of myocardial infarct size by doxycycline: a role for plasmin inhibition. Mol Cell Biochem 270: 1–11, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic Biol Med 16: 845–850, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Heiss C, Dejam A, Kleinbongard P, Schewe T, Sies H, Kelm M. Vascular effects of cocoa rich in flavan-3-ols. JAMA 290: 1030–1031, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Heptinstall S, May J, Fox S, Kwik-Uribe C, Zhao L. Cocoa flavanols and platelet and leukocyte function: recent in vitro and ex vivo studies in healthy adults. J Cardiovasc Pharmacol 47, Suppl 2: S197–S205, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, Giampaoli S, Jansen A, Menotti A, Nedeljkovic SI, Pekkarinen M, Simic BS, Toshima H, Feskens EJ, Hollman PC, Katan MB. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch Intern Med 155: 381–386, 1995. [PubMed] [Google Scholar]
- 21.Hollenberg NK, Martinez G, McCullough M, Meinking T, Passan D, Preston M, Rivera A, Taplin D, Vicaria-Clement M. Aging, acculturation, salt intake, and hypertension in the Kuna of Panama. Hypertension 29: 171–176, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Karim M, McCormick K, Kappagoda CT. Effects of cocoa extracts on endothelium-dependent relaxation. J Nutr 130: 2105S–2108S, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Keen CL, Holt RR, Oteiza PI, Fraga CG, Schmitz HH. Cocoa antioxidants and cardiovascular health. Am J Clin Nutr 81: 298S–303S, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Kloner RA, Rezkalla SH. Cardiac protection during acute myocardial infarction: where do we stand in 2004? J Am Coll Cardiol 44: 276–286, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. Br Med J 312: 478–481, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kris-Etherton PM, Keen CL. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipidol 13: 41–49, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Lindsey M, Wedin K, Brown MD, Keller C, Evans AJ, Smolen J, Burns AR, Rossen RD, Michael L, Entman M. Matrix-dependent mechanism of neutrophil-mediated release and activation of matrix metalloproteinase 9 in myocardial ischemia/reperfusion. Circulation 103: 2181–2187, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 16: 577–586, 2005. [DOI] [PubMed] [Google Scholar]
- 29.McCullough ML, Chevaux K, Jackson L, Preston M, Martinez G, Schmitz HH, Coletti C, Campos H, Hollenberg NK. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol 47, Suppl 2: S103–S121, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Middleton E Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol 439: 175–182, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Murphy KJ, Chronopoulos AK, Singh I, Francis MA, Moriarty H, Pike MJ, Turner AH, Mann NJ, Sinclair AJ. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am J Clin Nutr 77: 1466–1473, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med 37: 768–784, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr 74: 418–425, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20: 933–956, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA 103: 1024–1029, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selmi C, Mao TK, Keen CL, Schmitz HH, Eric Gershwin M. The anti-inflammatory properties of cocoa flavanols. J Cardiovasc Pharmacol 47, Suppl 2: S163–171, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Senft AP, Dalton TP, Shertzer HG. Determining glutathione and glutathione disulfide using the fluorescence probe o-phthalaldehyde. Anal Biochem 280: 80–86, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Silverman HS, Pfeifer MP. Relation between use of anti-inflammatory agents and left ventricular free wall rupture during acute myocardial infarction. Am J Cardiol 59: 363–364, 1987. [DOI] [PubMed] [Google Scholar]
- 39.Steffen Y, Schewe T, Sies H. Myeloperoxidase-mediated LDL oxidation and endothelial cell toxicity of oxidized LDL: attenuation by (−)-epicatechin. Free Radic Res 40: 1076–1085, 2006. [DOI] [PubMed] [Google Scholar]
- 40.van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, Gage FH. Plant-derived flavanol (−)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci 27: 5869–5878, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakeno M, Minamino T, Seguchi O, Okazaki H, Tsukamoto O, Okada K, Hirata A, Fujita M, Asanuma H, Kim J, Komamura K, Takashima S, Mochizuki N, Kitakaze M. Long-term stimulation of adenosine A2b receptors begun after myocardial infarction prevents cardiac remodeling in rats. Circulation 114: 1923–1932, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Wang S, Dusting GJ, May CN, Woodman OL. 3′,4′-Dihydroxyflavonol reduces infarct size and injury associated with myocardial ischaemia and reperfusion in sheep. Br J Pharmacol 142: 443–452, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waterhouse AL, Shirley JR, Donovan JL. Antioxidants in chocolate (Abstract). Lancet 348: 834, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Wippel R, Rehn M, Gorren AC, Schmidt K, Mayer B. Interference of the polyphenol epicatechin with the biological chemistry of nitric oxide- and peroxynitrite-mediated reactions. Biochem Pharmacol 67: 1285–1295, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007. [DOI] [PubMed] [Google Scholar]