Abstract
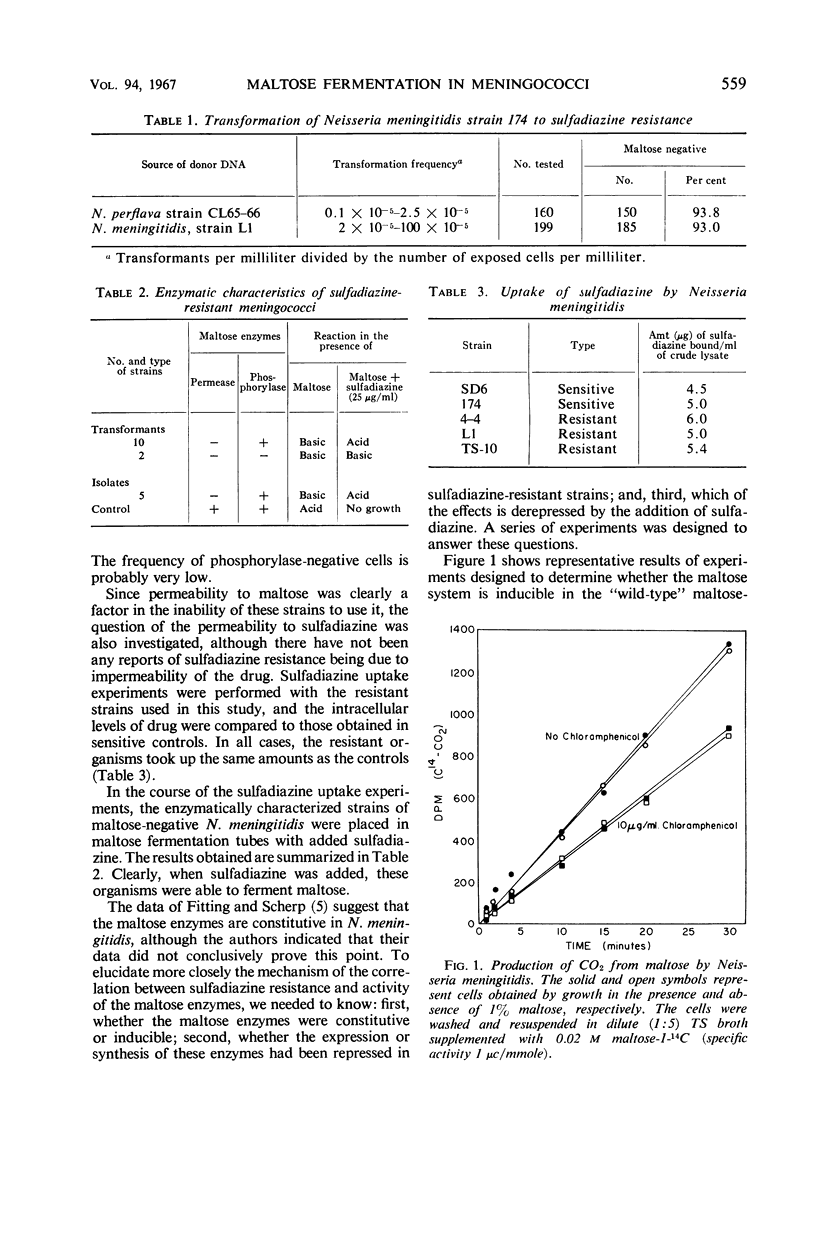
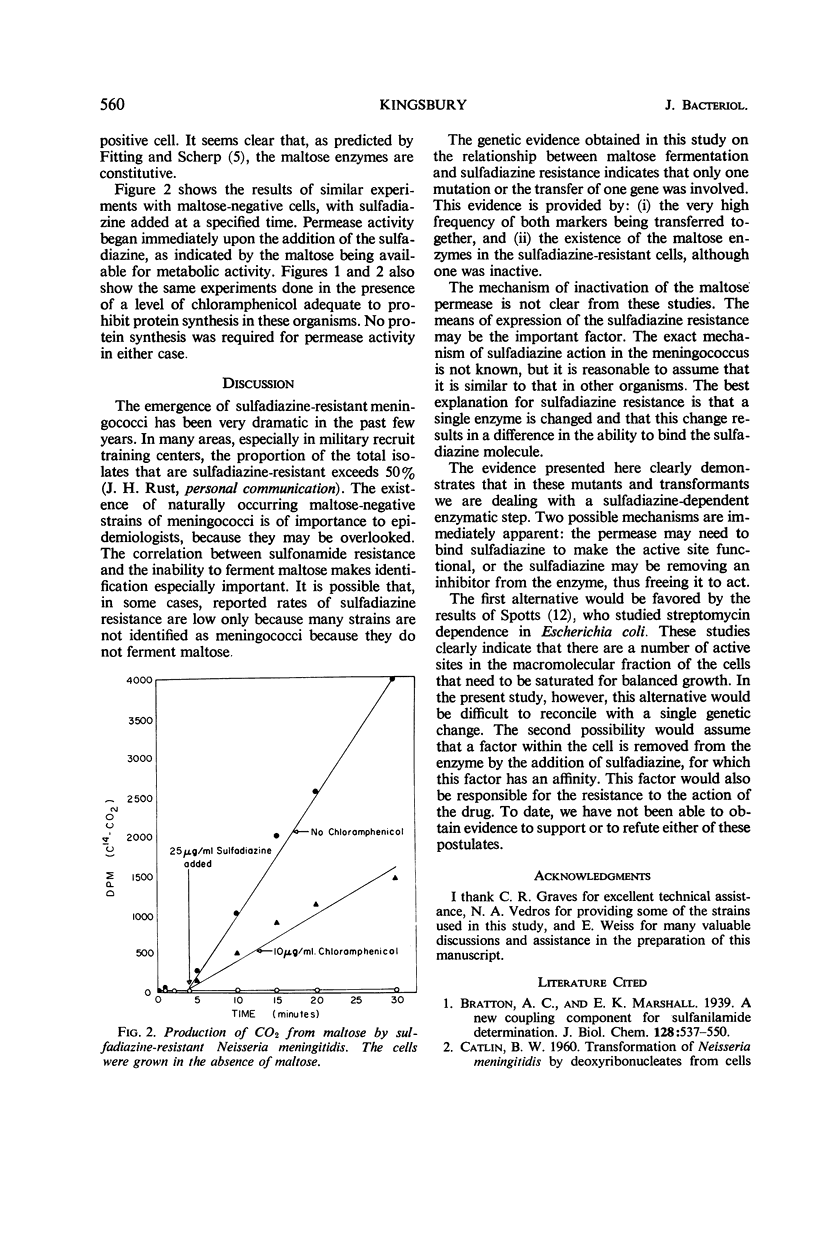
The genetic marker for sulfadiazine resistance was transferred by means of purified deoxyribonucleic acid from sulfadiazine-resistant Neisseria meningitidis and N. perflava to a sulfadiazine-sensitive strain of N. meningitidis. Over 80% of the isolates from these experiments, selected on the basis of sulfadiazine resistance, failed to produce acid from maltose. The same proportion of naturally occurring isolates that are sulfadiazine-resistant failed to ferment maltose. The enzymatic block in 17 isolates tested was the loss of maltose permease activity; in two cases, maltose phosphorylase activity was lost also. The permease was present in these cells, however, and could be activated by the addition of sulfadiazine. The results obtained support the hypothesis that these organisms, in becoming resistant to sulfadiazine, have undergone a single mutation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CATLIN B. W., CUNNINGHAM L. S. Transforming activities and base contents of deoxyribonucleate preparations from various Neisseriae. J Gen Microbiol. 1961 Oct;26:303–312. doi: 10.1099/00221287-26-2-303. [DOI] [PubMed] [Google Scholar]
- FITTING C., DOUDOROFF M. Phosphorolysis of maltose by enzyme preparations from Neisseria meningitidis. J Biol Chem. 1952 Nov;199(1):153–163. [PubMed] [Google Scholar]
- FITTING C., SCHERP H. W. Observations on a strain of Neisseria meningitides in the presence of glucose and maltose. II. Studies with washed cells1. J Bacteriol. 1952 Apr;63(4):545–560. doi: 10.1128/jb.63.4.545-560.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITTING C., SCHERP H. W. Observations on a strain of Neisseria meningitidis in the presence of glucose and maltose. III. Cell-free extracts and the phosphorolysis of maltose. J Bacteriol. 1952 Sep;64(3):287–298. doi: 10.1128/jb.64.3.287-298.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T. Bacteriocin production by strains of Neisseria meningitidis. J Bacteriol. 1966 May;91(5):1696–1699. doi: 10.1128/jb.91.5.1696-1699.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIE S. STUDIES ON THE PHENOTYPIC EXPRESSION OF COMPETENCE IN NEISSERIA MENINGITIDIS. Acta Pathol Microbiol Scand. 1965;64:119–129. doi: 10.1111/apm.1965.64.1.119. [DOI] [PubMed] [Google Scholar]
- MILLAR J. W., SIESS E. E., FELDMAN H. A., SILVERMAN C., FRANK P. IN VIVO AND IN VITRO RESISTANCE TO SULFADIAZINE IN STRAINS OF NEISSERIA MENINGITIDIS. JAMA. 1963 Oct 12;186:139–141. doi: 10.1001/jama.1963.63710020008016. [DOI] [PubMed] [Google Scholar]
- MITCHELL M. S., RHODEN D. L., KING E. O. LACTOSE-FERMENTING ORGANISMS RESEMBLING NEISSERIA MENINGITIDIS. J Bacteriol. 1965 Aug;90:560–560. doi: 10.1128/jb.90.2.560-560.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPOTTS C. R. Physiological and biochemical studies on streptomycin dependence in Escherichia coli. J Gen Microbiol. 1962 Jun;28:347–365. doi: 10.1099/00221287-28-2-347. [DOI] [PubMed] [Google Scholar]
- WATSON R. G., SCHERP H. W. The specific hapten of group C (group II alpha) meningococcus. I. Preparation and immunological behavior. J Immunol. 1958 Oct;81(4):331–336. [PubMed] [Google Scholar]
- Weiss E. Transaminase activity and other enzymatic reactions involving pyruvate and glutamate in Chlamydia (psittacosis-trachoma group). J Bacteriol. 1967 Jan;93(1):177–184. doi: 10.1128/jb.93.1.177-184.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


