Abstract
Mechanical stretch, an important growth stimulus, results not only from pulsatile blood flow and diastolic stretch of the ventricles [cyclic stretch (CS)] but also from tissue expansion during growth [constant static stretch (SS)]. We compared growth factor receptor expression and vasculogenic/angiogenic responses of rat coronary microvascular endothelial cells (ECs) by exposing cells to CS (10% elongation at 30 cycles/min) and SS (constant 10% elongation). Both CS and SS increased VEGF receptor (VEGF-R)2 protein levels and the extent of tube formation and branching. Moreover, both CS and SS enhanced VEGF-induced cell proliferation and tube formation, indicating that both types of stretch increase the sensitivity of ECs to VEGF. Blockade of VEGF-R2 prevented the increases in EC proliferation and aggregate tube length. However, CS but not SS enhanced EC Tie-2 protein and migration. CS affected a greater increase in tube length and branch formation than did SS. A unique finding was that SS but not CS increased VEGFR-1 in ECs. Our study is the first to distinguish between the effects of CS and SS on growth factor receptor expression and rat coronary microvascular EC proliferation, migration, and tube formation. In conclusion, EC angiogenic responses to these two types of stretch display both differences and similarities, but both CS and SS are dependent on VEGF-R2 signaling for their vasculogenic/angiogenic effects.
Keywords: cyclic stretch, vascular endothelial growth factor/receptors, tie-2, coronary vasculogenesis/angiogenesis
blood vessel formation and growth are dependent on growth factors and their receptors. Endothelial cells (ECs) migrate, proliferate, and form vascular tubules, a process known as vasculogenesis. This process is followed by, or coexists with, branching or compartmentalization of the EC-lined tubes, i.e., angiogenesis (6, 30, 42, 49). An endothelium-specific growth factor, VEGF, plays an essential role in both the development and maintenance of blood vessels by binding to and stimulating receptor tyrosine kinases (RTKs) VEGF receptor 1 (VEGF-R1; Flt-1) and 2 (VEGF-R2; Flk-1) (2, 31, 47). The ensuing phosphorylation of RTKs activates intracellular signaling pathways that trigger EC migration, proliferation, and tube formation (4, 19, 33, 41, 55). Mice lacking VEGF-R2 die at embryonic day (E)8.5 because of a defect in vasculogenesis resulting from the failure of endothelial progenitor migration and expansion (34, 35), whereas VEGF-R1-null mice die between E8.5 to E9.0 because EC proliferation is excessive and tubule formation is disorganized (13). VEGF-R1 has been thought to function mainly by inhibiting VEGF-R2 signaling or by acting as a decoy receptor, whereas VEGF-R2 is known to mediate the major growth effects of VEGF (19, 25, 37). However, there is increasing evidence for VEGF-R1 signaling playing a role in angiogenesis (3, 43, 44). Tie-2, a receptor of angiopoietins expressed predominantly on ECs, is also a critical player in blood vessel formation (12). Functional disruption of Tie-2 in transgenic mice results in lethality by E9.5–10.5, and this correlates with reduced numbers of ECs and abnormalities of vascular morphogenesis (33). Thus, Tie-2 is also critical for vasculogenesis and angiogenesis during development.
The activation of each of the growth factors and receptors discussed above occurs in response to mechanical stimuli (27, 56). Mechanical stretch results not only from pulsatile blood flow and ventricular diastole [cyclic stretch (CS)] but also from tissue expansion during growth [constant static stretch (SS)]. Thus, ECs are subjected to mechanical forces that can activate intracellular signals for EC proliferation, migration, and tube formation as well as gene expression.
Our previous data have indicated that CS of rat coronary microvascular ECs (RCMEC) upregulates VEGF-R2 in an independent manner but that it does not alter the expression of VEGF-R1 (56). We and others have also shown that CS increases the expression of Tie-2 in ECs (8, 27, 56), However, the effect of SS on growth factor receptor expression and vasculogenic/angiogenic responses of RCMECs is unknown. This study is the first to 1) compare the effects of CS and SS on EC vasculogenic/angiogenic responses and 2) take into account all the key events in the angiogenic cascade: migration, proliferation, tube formation, and branching. Our study assessed the expression of VEGF-R1, VEGF-R2, and Tie-2 in RCMECs in response to both types of stretch to elucidate possible differences in the mechanisms that regulate vasculogenic/angiogenic events in the two types of stretch.
MATERIALS AND METHODS
All procedures were approved by the University of Iowa Animal Care and Use Committee and are in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Publication No. 85-23, Revised 1996).
Isolation and culture of RCMECs.
After the rat had been heparinized and anesthetized [ketamine (50 units)-xylazine (10 mg/kg) intraperitoneally], the heart was removed and mounted on a Langendorff apparatus. RCMECs were isolated by collagenase perfusion as previously described (57). Briefly, collagenase (0.7 mg/ml) was introduced into the perfusate and allowed to circulate for 30–40 min, after which the ventricles were minced and the cells dispersed in collagenase-containing perfusate. Myocytes were separated from RCMECs following filtration of the perfusate. RCMEC identity was confirmed by positive staining for platelet/EC adhesion molecule (antibody from BD Biosciences)-related and von Willebrand factor (antibody from Dako)-related antigens and by negative staining for prolyl-4-hydroxylase-β (fibroblast marker, antibody from ACRIS Antibodies). RCMECs from three hearts were pooled into two 100-mm gelatin-coated culture dishes. Cells were cultured at 37°C under 5% CO2 in DMEM supplemented with 20% FBS, 2 mM l-glutamine, 20 mM d-glucose, 20 U/ml heparin, 1 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin. After RCMECs neared confluence, they were passaged by trypsinization and used for experiments at passages 2–3.
Application of stretch to cultured cells.
To produce CS and SS in vitro, we used a computerized Flexercell strain unit (Flexcell, McKeesport, PA). RCMECs were seeded on a Bioflex culture plate with a type I collagen substrate. After serum starvation for 16 h, cells were treated with 50 or 100 ng/ml VEGF (R&D Systems, Minneapolis, MN), 10 μM SU-1498 (Flk-1 inhibitor, Calbiochem, La Jolla, CA), and VEGF or SU-1498 combined with stretch, respectively, for 18 or 24 h. CS was accomplished by subjecting cultured cells to a 10% average surface elongation at 30 cycles/min (1 s of stretch followed by 1 s of relaxation); SS was induced at the same elongation but at constant stretch without intermittent relaxation. Similar stretch periods were used for both CS and SS. These protocols were selected to provide a dynamic, near-physiological strain stimulus. Controls consisted of cells seeded on the Bioflex culture plate but not subjected to CS or SS.
Western blot assay.
Cell lysates were immunoblotted as previously described (57). Protein extracts (50–75 μg) were separated by 7.5% SDS-PAGE. Membranes were incubated with anti-VEGF-R1, anti-VEGF-R2, and anti-Tie-2 rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) diluted 1:100–1:200 and anti-GAPDH monoclonal antibody (Chemicon, Temecula, CA) diluted 1:8,000 in 3% nonfat dry milk at 4°C overnight. Antigen-antibody complexes were visualized using an Odyssey Infrared Imaging System (LI-COR, Lincoln, NE).
Cell proliferation assay.
RCMEC proliferation was measured with an In Situ Cell Proliferation Kit (FLOUS, Roche Applied Science, Indianapolis, IN), which detects 5-bromo-2′-deoxyuridine (BrdU) incorporation into cellular DNA using fluorescein-conjugated monoclonal antibodies. RCMEC (5 × 104 cells/ml) were plated on Bioflex culture plates and starved with serum-free DMEM for 16 h. RCMECs were then exposed to CS or SS for 1–24 h in DMEM supplemented with 10% FBS in the absence or presence of recombinant VEGF protein (50 ng/ml, R&D Systems). At the end of the experiments, RCMECs were incubated with BrdU labeling solution for 1 h at 37°C and then fixed with a solution containing ethanol. After cells had been washed in PBS, anti-BrdU-FLUOS antibody working solution was added, and cells were mounted in Vectashield containing propidium iodide (Vector Laboratories, Burlingame, CA). Ten random microscope fields were photographed from each well, and the percentage of BrdU-positive cells was determined by Image ProPlus software (Media Cybernetics, Silver Spring, MD).
EC migration assay.
RCMEC migration was quantified on Bioflex culture plates by modification of a previously described method (11). A cloning cylinder (diameter: 3/16-in., Bel-Art Products, Pequannock, NJ) was placed in each culture plate well. A 100-μl RCMEC suspension (1 × 105 cells/ml) was added to the center of the cylinder. RCMECs were then incubated at 37°C and 5% CO2. After cells had attached to the plates, the cylinder was removed, and cells were starved in serum-free DMEM for 16 h and then exposed to stretch. After stretch, RCMEC were fixed in cold 100% methanol and mounted in Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI). To measure EC migration, a circle denoting the inside circumference of the cylinder (3/16-in., software generated) was superimposed on the images, and the mean distance from the edge of the circle to the front of the cells that had migrated in response to stimulation was measured with Image ProPlus software.
EC tube formation.
Three-dimensional cultures were established as previously described with minor modifications (57). Rat tail type I collagen (1.0 mg/ml) was mixed with 2× medium 199 (M199) and neutralized with 1 N NaOH. Collagen mixture (300 μl) was added to each well of a four-well plate and allowed to gel in a humidified incubator at 37°C. RCMECs (2 × 105 cells/ml) that had been subjected to stretch or control conditions were subcultured on the collagen in DMEM supplemented with 10% FBS. RCMECs were then allowed to form tube-like structures over a period of 6 days. Cells were rinsed in ice-cold PBS and fixed in 4% paraformaldehyde overnight at 4°C. They were then incubated in Griffonia simplificolia I Isolectin IB4 [Alexa labeled (10 μg/ml, 1:100) in PBS with Ca2+ and Mg2+] for 45 min at 37°C to visualize ECs. After cells had been washed with PBS containing Ca2+ and Mg2+, the gel was mounted in Vectashield mounting medium with DAPI and examined under fluorescence. Tube formation was quantified by measuring the aggregate tube length, and branching was quantified by counting the total number of tube segments in five fields at a magnification of ×40 power. We used a Nikon fluorescence microscope with Image ProPlus software to analyze the images.
Statistical analysis.
All experiments were repeated at least three times. Results are expressed as means ± SE. For protein expression experiments, six wells of cultured cells from each group were combined. One-way ANOVA followed by Dunnet's test was used to determine intergroup significance differences for protein data, and Bonferroni's procedure was used for all other data. P ≤ 0.05 was considered significant.
RESULTS
Differential effects of CS and SS on the expression of growth factor receptors.
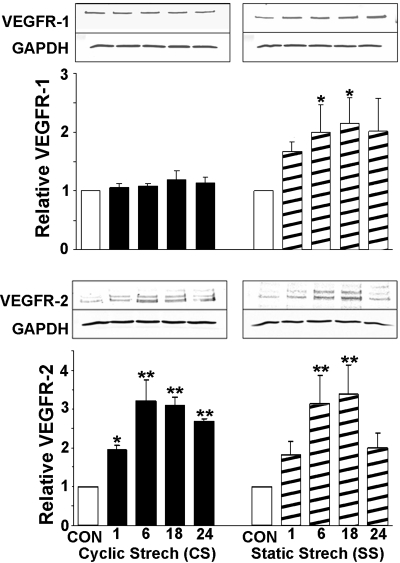
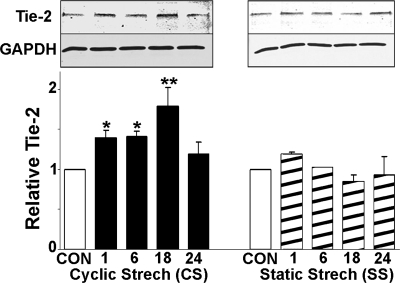
To compare the effect of CS and SS on the regulation of key growth factor receptors, RCMECs were exposed to either CS or SS for 1, 6, 18, and 24 h. The level of each receptor protein was assessed by Western blot analysis. RCMEC lysates were probed with anti-VEGF-R1, anti-VEGF-R2, or anti-Tie-2 antibodies. The application of SS caused VEGF-R1 protein to increase within 18 h (2.2-fold over the control value). In contrast, stimulation of RCMECs with CS did not alter VEGF-R1 expression (Fig. 1). Levels of VEGF-R2 protein increased in a time-dependent manner following CS initiation and peaked (3.22-fold) at 6 h (Fig. 1). Significant increases were maintained throughout the 24-h period. SS also significantly increased VEGF-R2 (3.38-fold at 18 h), but this increase declined to levels approaching control after 24 h. CS induced the upregulation of Tie-2 protein after only 1 h and reached a peak increase at 18 h (Fig. 2). However, SS did not significantly elevate Tie-2. These results suggest that CS and SS have similar effects on VEGF-R2 but dissimilar effects on VEGF-R1 and Tie-2 expression. Moreover, CS induces a more prolonged increase in VEGFR-2 than does SS.
Fig. 1.
Effect of cyclic stretch (CS) and static stretch (SS) on the expression of VEGF receptor 1 (VEGFR-1) and 2 (VEGFR-2). Rat coronary microvascular endothelial cells (RCMECs) were cultured under nonstretch control (CON) or stretch conditions for 1, 6, 18, and 24 h. Total protein (50–75 μg/lane) isolated from cells was analyzed for VEGF-Rs and GAPDH. Quantitative densitometry analysis is expressed as means ± SE (3–4 experiments/group). Expression levels of VEGFR-1 and VEGFR-2 were normalized to those of GAPDH and are shown as fold increases over control. Representative Western blot images are shown above each graph. *P ≤ 0.05 and **P ≤ 0.01, statistically significant increases compared with control.
Fig. 2.
Effects of 1–24 h of CS and SS on the expression of Tie-2 protein in RCMECs. Representative Western blots (top) correspond to graphs showing means ± SE (bottom). Each mean is based 3–4 experiments/group. *P ≤ 0.05 and **P ≤ 0.01, statistically significant increases compared with control.
CS and SS have similar effects on RCMEC proliferation.
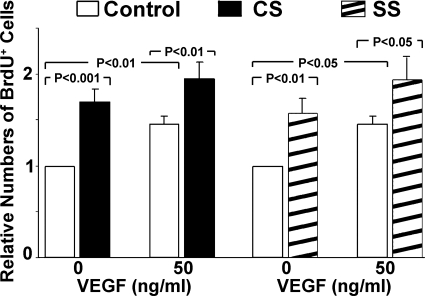
To evaluate cell proliferation, BrdU was added to the culture medium for 1 h after stretch for 23 h. BrdU-positive cells were identified by anti-BrdU antibodies conjugated to fluorescein. As shown in Fig. 3, RCMEC proliferation was significantly increased by CS and SS as well as by the presence of VEGF (50 ng/ml). CS and SS increased BrdU-positive cells by 1.7- and 1.6- fold, respectively. The addition of exogenous VEGF enhanced the effects of both CS and SS on cell proliferation.
Fig. 3.
CS, SS, and exogenous VEGF each increase the proliferation of RCMECs. The addition of exogenous VEGF to the medium of cells exposed to CS or SS further enhanced RCMEC proliferation. 5-Bromo-2′-deoxyuridine (BrdU) immunofluorescent staining was used to measure cell proliferation in RCEMCs stretched for 24 h in the absence or presence of recombinant VEGF protein (50 ng/ml). The percentage of BrdU-positive cells was determined with Image Proplus software. Values are means ± SE; n = 3–5 per group.
CS is more effective than SS in increasing RCMEC migration.
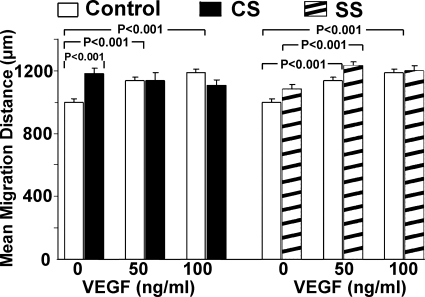
RCMEC migration was assessed by measuring the distance between the edge of and the front of the migrating cells after 24 h of stretch. Both VEGF (50–100 ng/ml) and CS significantly increased RCMEC migration (Fig. 4). However, the addition of VEGF to the medium did not further increase the effects of CS. In contrast, migration was not significantly increased by SS but was enhanced in the presence of 50 ng/ml VEGF. Increasing the dose of VEGF to 100 ng/ml in the presence of SS did not increase migration further. These results indicate that 1) CS has a stronger effect than SS on RCMEC migration, and 2) exogenous VEGF enhances migration induced by SS but not that induced by CS.
Fig. 4.
Differential effects of CS and SS on the migration of RCMECs. Cells were seeded in a cloning cylinder and allowed to attach to the surface. After removal of the cylinder, confluent RCMEC cultures were subjected to CS or SS for 24 h in the absence or presence of VEGF (50 or 100 ng/ml). Mean migration distance was measured from the edge of the cylinder to the front of the migrated cells after stimulation. Values are means ± SE (n = 3–5 per group).
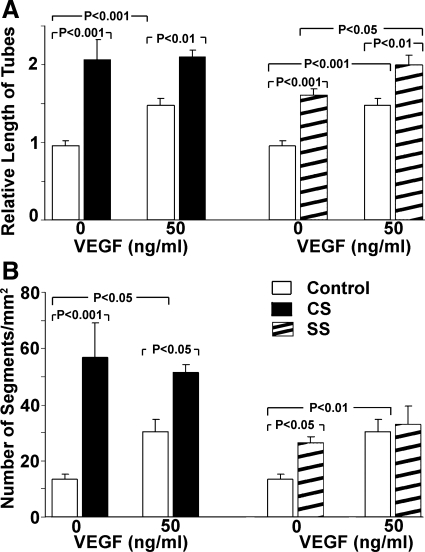
CS induces a greater magnitude of tube formation and branching than SS.
RCMEC that had been subjected to stretch for 18 h were incubated on collagen gels in the absence or presence of VEGF for 6 days. CS, SS, and the presence of VEGF each significantly increased total tube length, but CS was the most effective (total tube length increased 2.16-fold vs. 1.69-fold in SS; Fig. 5A). Although both CS and SS enhanced VEGF-induced tube formation, exogenous VEGF increased only tube formation induced by SS. The number of tube segments was higher in cultures subjected to either CS or SS compared with nonstretched controls, but CS increased the number of segments to a greater degree than SS (4.24-fold vs. 1.97-fold; Fig. 5B).
Fig. 5.
Effect of CS and SS on tube formation of RCMECs. Cells that had been subjected to stretch or control conditions for 24 h were subcultured on three-dimensional collagen gel. RCMECs were then allowed to form tubes for 6 days. A: tube formation was quantified by measuring the total tube length in 5 fields at a magnification of ×40. B: numbers of tube segments. Note that CS induced a greater magnitude of tube branching than SS.
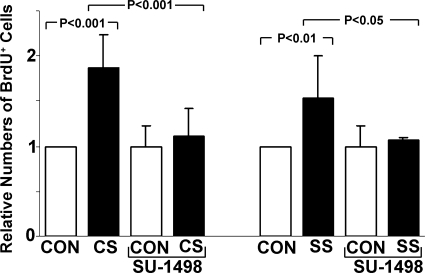
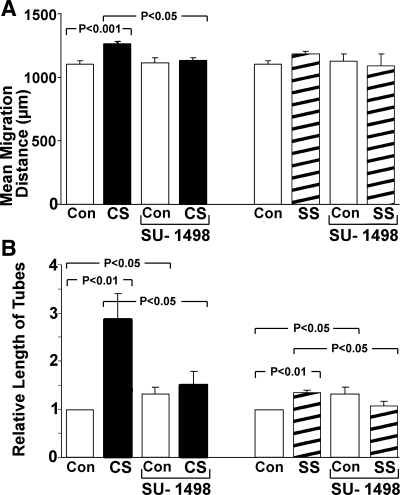
VEGFR-2 mediates stretch-stimulated vasculogenic/angiogenic events.
To determine if VEGF-R2 signaling is a requirement for angiogenic events triggered by CS and SS, we inhibited this receptor with SU-1498. EC proliferation activated by both CS and SS was abolished with VEGF-R2 inhibition (Fig. 6). Similarly, neither CS nor SS were able to increase mean migration distance (Fig. 7A) and relative tube length (Fig. 7B) in the presence of SU-1498. Accordingly, this experiment documents VEGF-R2 signaling as a requirement for the vasculogenic/angiogenic cascade that is activated by either CS or SS.
Fig. 6.
EC proliferation by CS or SS was completely inhibited by VEGF-R2 blockade with SU-1498. Proliferation was assessed by BrdU labeling. Means ± SE are shown (each group was based on 3–5 experiments).
Fig. 7.
A: the increase in mean migration distance of ECs after CS was prevented by VEGF-R2 blockade with SU-1498. B: moreover, the increase in relative tube length stimulated by CS or SS was virtually negated when VEGF-R2 was blocked by SU-1498. Each group mean ± SE value is based on 3–5 experiments.
DISCUSSION
This study, the first to compare EC angiogenic responses to CS and SS, documents that cells respond differently to these mechanical fences in some respects and similarly in others. Our experiments provide several novel and significant findings. First, CS elicits a more rapid and longer upregulation of VEGF-R2. Second, CS but not SS affects increases in Tie-2 protein. Third, VEGF-R1 is enhanced by SS but not by CS. However, despite the increase in VEGF-R1 in response to SS, our data show that VEGF-R2 signaling is the critical component of enhanced vasculogenesis/angiogenesis in response to either CS or SS, since its inhibition abolishes the enhanced EC proliferation, migration, and tube formation. Finally, while SS increases EC proliferation to a similar magnitude as CS, the latter induces a more robust vascular tube formation and branching. Thus, the cyclic component of CS contributes significantly to coronary tubulogenesis.
Mechanical forces activate/upregulate RTKs.
Most RTKs can be activated by either shear stress or CS in the absence of ligand activation (9, 36, 56). Shear stress upregulates VEGFR-2, Tie-2, and angiopoietins in ECs (9, 21, 39). Similarly, our data and those published by others have shown that CS upregulates VEGFR-2 and Tie-2 mRNA and protein levels in ECs (8, 39, 56). These data imply that these mechanical forces associated with shear stress and CS share some common signaling pathways that converge on VEGFR-2 and Tie-2. Our data document that SS, like CS, triggers increases in VEGFR-2. However, Tie-2 was significantly enhanced by CS but not by SS (Fig. 2). Thus, the cyclic component of stretch plays a role in the upregulation of Tie-2 protein. Another notable difference between CS and SS is the fact that SS upregulated VEGFR-1 but CS did not. This finding is consistent with the evidence that VEGFR-1 plays a role in embryogenesis (13) when tissue growth may constitute a SS. A previous report (28) has shown that VEGFR-1 signals are required for VEGF-B-mediated angiogenesis. VEGF-B, a VEGF-R1 ligand, is most abundant in the heart, with especially strong expression in the developing heart (1, 29). Antibodies to VEGF-B, but not VEGF-A, have been found to have a strong inhibitory effect on coronary artery development in vivo and tubulogenesis in vitro (43, 44). The presence of VEGFR-1 is also critical for VEGFR-2 abundance. In bovine ECs, the application of short interfering RNA targeting VEGFR-1 led to a significant reduction in both VEGF-R1 and VEGF-R2 at protein and mRNA levels (20). Despite the implication that VEGF-R1 contributes to SS-induced tubulogenesis, our data clearly document that VEGF-R2 is required for tubulogenesis induced by either CS or SS.
The mechanism by which cellular stretch is detected and translated into intracellular signaling is not completely understood. However, it is clear that mechanical forces can modulate the expression of numerous genes through the activation of various intracellular pathways. Such genes include those encoding specific ion channels, G proteins, cAMP, cGMP, PKC, inositol triphosphate, MAPKs, RTKs, and focal adhesion kinase (7, 9, 22, 23, 40, 55). For instance, Hu et al. (14) suggested that changes in cellular morphology induced by stretch led to alterations in growth factor receptor conformation and that this results in exposure of the kinase domain and subsequent autophosphorylation. A previous study (50) indicated that interplay between integrins and VEGFR-2 plays a key role in the transduction of shear stress into chemical signals.
Mechanical forces stimulate angiogenic responses in ECs.
EC are equipped with numerous receptors that allow them to detect and respond to mechanical forces and thereby lead to functional changes. In the present study, exposure to CS or SS resulted in similar magnitudes of RCMEC proliferation. The ability of mechanical forces to increase cellular growth in the absence of exogenous VEGF is consistent with previous reports in other cell types (5, 39). Previous studies have reported that VEGFR-2 is a positive modulator of angiogenesis and that it mediates VEGF-induced cell proliferation (54), whereas Tie-2 is not involved in EC proliferation (17, 26). These findings are consistent with our data, which reveal that CS and SS induce similar magnitudes of RCMEC proliferation but that CS is more effective in enhancing tubular branching than is SS. Since VEGF-R2 inhibition abolishes the enhanced EC proliferation, migration, and tube formation, signaling via this receptor is necessary for the vasculogenic/angiogenic events stimulated by either CS or SS. This finding is consistent with those of Yamamoto et al. (52), who found that blockade of the shear stress-induced increase in VEGFR-2 abolished the shear stress-induced proliferation and differentiation of Flk-1-positive embryonic stem cells. Our data also fit with the finding that capillary growth in ischemic skeletal muscle is dependent on VEGF-R2 (24). The upregulation of VEGF-R1 noted after SS may play some additional, yet undefined, role in vasculogenesis or angiogenesis under conditions involving SS, such as occur during embryonic growth.
Previous studies have documented a role for mechanical forces in EC migration and tube formation (16, 45, 46, 48). The importance of VEGF-R2 in EC proliferation, migration, and tubulogenesis (4, 32) and the chemoattractant role of angiopoietin-1 for ECs (51) have also been documented. However, the role of VEGF-R1 in vasculogenesis/ angiogenesis is controversial. Results reported by Kearney et al. (18) have suggested that VEGF-R1 (Flt-1) is a positive mediator of angiogenesis since sprout formation and migration were found to be decreased in Flt-1−/− mice. However, Yang et al. (53) found that Flk-1 but not Flt-1 stimulation is responsible for EC tubulogenesis. Therefore, these growth factor receptors play important roles in vasculogenesis/angiogenesis through complex processes, i.e., direct or indirect actions, interactions, or cross-talk.
The finding that exogenous VEGF enhanced tubulogenesis (tube length) after SS but not CS may be due to the fact that CS had a more profound effect on VEGF-R2 and also increased Tie-2. Thus, it is possible that the VEGFR-2 and Tie-2 proteins are maximally increased by CS, in contrast to the smaller VEGFR-2 elevation after SS and the failure of SS to significantly increase Tie-2. The supposition that EC stretch, per se, enhances capillary growth during development is supported by recent data from fetal mice in which lung airspaces were experimentally distended (10). These mice demonstrated accelerated microvascular growth and increased in VEGF-A, VEGFR-1, and angiopoietin-1.
In conclusion, the results reported here support the hypothesis that both CS and SS evoke vasculogenic/angiogenic responses in coronary microvascular EC. However, SS is less effective in this role because it has little effect on EC migration and is less effective in evoking tubular branching than CS. Two major differences at the receptor level were noted for CS and SS: 1) Tie-2 is significantly increased only by CS, and 2) VEGFR-1 is increased only by SS. However, the vasculogenic/angiogenic responses are dependent on VEGFR-2 since the responses are negated by blockade of VEGFR-2. Thus, the study reveals that while SS clearly plays a role in vascular tubulogenesis, the cyclic component of stretch is an important dimension that maximizes the effect. While it is recognized that mechanical forces have an important influence on angiogenic regulation (15, 38, 56), the precise signaling mechanisms involved are unclear. Since gene expression and cell function vary from one vascular network to another, our conclusions are limited to coronary microvascular ECs.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-075446.
Acknowledgments
The authors thank Dr. Eduard D. Dedkov for developing methods to measure migration with computer software.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aase K, von Euler G, Li X, Ponten A, Thoren P, Cao R, Cao Y, Olofsson B, Gebre-Medhin S, Pekny M, Alitalo K, Betsholtz C, Eriksson U. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation 104: 358–364, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA 95: 548–553, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad S, Hewett PW, Wang P, Al-Ani B, Cudmore M, Fujisawa T, Haigh JJ, le Noble F, Wang L, Mukhopadhyay D, Ahmed A. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ Res 99: 715–722, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bernatchez PN, Soker S, Sirois MG. Vascular endothelial growth factor effect on endothelial cell proliferation, migration, and platelet-activating factor synthesis is Flk-1-dependent. J Biol Chem 274: 31047–31054, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Brunette DM Mechanical stretching increases the number of epithelial cells synthesizing DNA in culture. J Cell Sci 69: 35–45, 1984. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P Angiogenesis in life, disease and medicine. Nature 438: 932–936, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103: 15463–15468, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang H, Wang BW, Kuan P, Shyu KG. Cyclical mechanical stretch enhances angiopoietin-2 and Tie2 receptor expression in cultured human umbilical vein endothelial cells. Clin Sci (Lond) 104: 421–428, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem 274: 18393–18400, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Cloutier M, Maltais F, Piedboeuf B. Increased distension stimulates distal capillary growth as well as expression of specific angiogenesis genes in fetal mouse lungs. Exp Lung Res 34: 101–113, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Dolle JP, Rezvan A, Allen FD, Lazarovici P, Lelkes PI. Nerve growth factor-induced migration of endothelial cells. J Pharmacol Exp Ther 315: 1220–1227, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev 8: 1897–1909, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Bock G, Wick G, Xu Q. Activation of PDGF receptor alpha in vascular smooth muscle cells by mechanical stress. FASEB J 12: 1135–1142, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Jones EA, le Noble F, Eichmann A. What determines blood vessel structure? Genetic prespecification vs. hemodynamics. Physiology (Bethesda) 21: 388–395, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Joung IS, Iwamoto MN, Shiu YT, Quam CT. Cyclic strain modulates tubulogenesis of endothelial cells in a 3D tissue culture model. Microvasc Res 71: 1–11, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Kanda S, Miyata Y, Mochizuki Y, Matsuyama T, Kanetake H. Angiopoietin 1 is mitogenic for cultured endothelial cells. Cancer Res 65: 6820–6827, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Kearney JB, Ambler CA, Monaco KA, Johnson N, Rapoport RG, Bautch VL. Vascular endothelial growth factor receptor Flt-1 negatively regulates developmental blood vessel formation by modulating endothelial cell division. Blood 99: 2397–2407, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Koolwijk P, Peters E, van der Vecht B, Hornig C, Weich HA, Alitalo K, Hicklin DJ, Wu Y, Witte L, van Hinsbergh VW. Involvement of VEGFR-2 (kdr/flk-1) but not VEGFR-1 (flt-1) in VEGF-A and VEGF-C-induced tube formation by human microvascular endothelial cells in fibrin matrices in vitro. Angiogenesis 4: 53–60, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Kou R, SenBanerjee S, Jain MK, Michel T. Differential regulation of vascular endothelial growth factor receptors (VEGFR) revealed by RNA interference: interactions of VEGFR-1 and VEGFR-2 in endothelial cell signaling. Biochemistry 44: 15064–15073, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Koh GY. Shear stress activates Tie2 receptor tyrosine kinase in human endothelial cells. Biochem Biophys Res Commun 304: 399–404, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto T, Mugishima H. Signal transduction via vascular endothelial growth factor (VEGF) receptors and their roles in atherogenesis. J Atheroscler Thromb 13: 130–135, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci 119: 508–518, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Milkiewicz M, Hudlicka O, Verhaeg J, Egginton S, Brown MD. Differential expression of Flk-1 and Flt-1 in rat skeletal muscle in response to chronic ischaemia: favourable effect of muscle activity. Clin Sci (Lond) 105: 473–482, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 72: 835–846, 1993. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki Y, Nakamura T, Kanetake H, Kanda S. Angiopoietin 2 stimulates migration and tube-like structure formation of murine brain capillary endothelial cells through c-Fes and c-Fyn. J Cell Sci 115: 175–183, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Morrow D, Cullen JP, Cahill PA, Redmond EM. Cyclic strain regulates the Notch/CBF-1 signaling pathway in endothelial cells: role in angiogenic activity. Arterioscler Thromb Vasc Biol 27: 1289–1296, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Olofsson B, Korpelainen E, Pepper MS, Mandriota SJ, Aase K, Kumar V, Gunji Y, Jeltsch MM, Shibuya M, Alitalo K, Eriksson U. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA 95: 11709–11714, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Pettersson RF, Alitalo K, Eriksson U. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA 93: 2576–2581, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patan S Vasculogenesis and angiogenesis. Cancer Treat Res 117: 3–32, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Roberts DM, Kearney JB, Johnson JH, Rosenberg MP, Kumar R, Bautch VL. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol 164: 1531–1535, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rousseau S, Houle F, Kotanides H, Witte L, Waltenberger J, Landry J, Huot J. Vascular endothelial growth factor (VEGF)-driven actin-based motility is mediated by VEGFR2 and requires concerted activation of stress-activated protein kinase 2 (SAPK2/p38) and geldanamycin-sensitive phosphorylation of focal adhesion kinase. J Biol Chem 275: 10661–10672, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376: 70–74, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Schuh AC, Faloon P, Hu QL, Bhimani M, Choi K. In vitro hematopoietic and endothelial potential of flk-1−/− embryonic stem cells and embryos. Proc Natl Acad Sci USA 96: 2159–2164, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66, 1995. [DOI] [PubMed] [Google Scholar]
- 36.Shay-Salit A, Shushy M, Wolfovitz E, Yahav H, Breviario F, Dejana E, Resnick N. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc Natl Acad Sci USA 99: 9462–9467, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibuya M Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol 39: 469–478, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Shiu YT, Weiss JA, Hoying JB, Iwamoto MN, Joung IS, Quam CT. The role of mechanical stresses in angiogenesis. Crit Rev Biomed Eng 33: 431–510, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Suzuma I, Hata Y, Clermont A, Pokras F, Rook SL, Suzuma K, Feener EP, Aiello LP. Cyclic stretch and hypertension induce retinal expression of vascular endothelial growth factor and vascular endothelial growth factor receptor-2: potential mechanisms for exacerbation of diabetic retinopathy by hypertension. Diabetes 50: 444–454, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Suzuma I, Suzuma K, Ueki K, Hata Y, Feener EP, King GL, Aiello LP. Stretch-induced retinal vascular endothelial growth factor expression is mediated by phosphatidylinositol 3-kinase and protein kinase C (PKC)-zeta but not by stretch-induced ERK1/2, Akt, Ras, or classical/novel PKC pathways. J Biol Chem 277: 1047–1057, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J 20: 2768–2778, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tomanek RJ Formation of the coronary vasculature during development. Angiogenesis 8: 273–284. 16308734, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Tomanek RJ, Holifield JS, Reiter RS, Sandra A, Lin JJ. Role of VEGF family members and receptors in coronary vessel formation. Dev Dyn 225: 233–240, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Tomanek RJ, Ishii Y, Holifield JS, Sjogren CL, Hansen HK, Mikawa T. VEGF family members regulate myocardial tubulogenesis and coronary artery formation in the embryo. Circ Res 98: 947–953. 16527987, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Ueda A, Koga M, Ikeda M, Kudo S, Tanishita K. Effect of shear stress on microvessel network formation of endothelial cells with in vitro three-dimensional model. Am J Physiol Heart Circ Physiol 287: H994–H1002, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Urbich C, Dernbach E, Reissner A, Vasa M, Zeiher AM, Dimmeler S. Shear stress-induced endothelial cell migration involves integrin signaling via the fibronectin receptor subunits alpha(5) and beta(1). Arterioscler Thromb Vasc Biol 22: 69–75, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res 60: 203–212, 2000. [PubMed] [Google Scholar]
- 48.Von Offenberg Sweeney N, Cummins PM, Cotter EJ, Fitzpatrick PA, Birney YA, Redmond EM, Cahill PA. Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochem Biophys Res Commun 329: 573–582, 2005. [DOI] [PubMed] [Google Scholar]
- 49.Wada AM, Willet SG, Bader D. Coronary vessel development: a unique form of vasculogenesis. Arterioscler Thromb Vasc Biol 23: 2138–2145, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Miao H, Li S, Chen KD, Li YS, Yuan S, Shyy JY, Chien S. Interplay between integrins and FLK-1 in shear stress-induced signaling. Am J Physiol Cell Physiol 283: C1540–C1547, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Witzenbichler B, Maisonpierre PC, Jones P, Yancopoulos GD, Isner JM. Chemotactic properties of angiopoietin-1 and -2, ligands for the endothelial-specific receptor tyrosine kinase Tie2. J Biol Chem 273: 18514–18521, 1998. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol 288: H1915–H1924, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Yang S, Xin X, Zlot C, Ingle G, Fuh G, Li B, Moffat B, de Vos AM, Gerritsen ME. Vascular endothelial cell growth factor-driven endothelial tube formation is mediated by vascular endothelial cell growth factor receptor-2, a kinase insert domain-containing receptor. Arterioscler Thromb Vasc Biol 21: 1934–1940, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Zeng H, Dvorak HF, Mukhopadhyay D. Vascular permeability factor (VPF)/vascular endothelial growth factor (VEGF) peceptor-1 down-modulates VPF/VEGF receptor-2-mediated endothelial cell proliferation, but not migration, through phosphatidylinositol 3-kinase-dependent pathways. J Biol Chem 276: 26969–26979, 2001. [DOI] [PubMed] [Google Scholar]
- 55.Zeng H, Zhao D, Mukhopadhyay D. KDR stimulates endothelial cell migration through heterotrimeric G protein Gq/11-mediated activation of a small GTPase RhoA. J Biol Chem 277: 46791–46798, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Zheng W, Christensen LP, Tomanek RJ. Stretch induces upregulation of key tyrosine kinase receptors in microvascular endothelial cells. Am J Physiol Heart Circ Physiol 287: H2739–H2745, 2004. [DOI] [PubMed] [Google Scholar]
- 57.Zheng W, Seftor EA, Meininger CJ, Hendrix MJ, Tomanek RJ. Mechanisms of coronary angiogenesis in response to stretch: role of VEGF and TGF-beta. Am J Physiol Heart Circ Physiol 280: H909–H917, 2001. [DOI] [PubMed] [Google Scholar]