Abstract
Nitric oxide (NO) modulates vasodilation in cerebral cortex during sensory activation. NO is known to inhibit the synthesis of 20-HETE, which has been implicated in arteriolar constriction during astrocyte activation in brain slices. We tested the hypothesis that the attenuated cerebral blood flow (CBF) response to whisker stimulation seen after NO synthase (NOS) inhibition requires 20-HETE synthesis and that the ability of an epoxyeicosatrienoic acids (EETs) antagonist to reduce the CBF response is blunted after NOS inhibition but restored with simultaneous blockade of 20-HETE synthesis. In anesthetized rats, the increase in CBF during whisker stimulation was attenuated after the blockade of neuronal NOS with 7-nitroindazole. Subsequent administration of the 20-HETE synthesis inhibitor N-hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine (HET0016) restored the CBF response to control levels. After the administration of 7-nitroindazole, the inhibitory effect of an EETs antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) on the CBF response was lost, whereas the simultaneous administration of 7-nitroindazole and HET0016 restored the inhibitory effect of 14,15-EEZE. The administration of HET0016 alone had only a small effect on the evoked CBF response in rats. Furthermore, in neuronal NOS+/+ and NOS−/− mice, HET0016 administration did not increase the CBF response to whisker stimulation. In neuronal NOS+/+ mice, HET0016 also blocked the reduction in the response seen with acute NOS inhibition. These results indicate that 20-HETE synthesis normally does not substantially restrict functional hyperemia. Increased NO production during functional activation may act dynamically to suppress 20-HETE synthesis or downstream signaling and permit EETs-dependent vasodilation. With the chronic loss of neuronal NOS in mice, other mechanisms apparently suppress 20-HETE synthesis or signaling.
Keywords: eicosanoids, epoxyeicosatrienoic acid, functional activation, mouse, rat, vibrissae, 20-hydroxyeicosatetraenoic acid
when neurons in a specific brain region increase their activity, local blood flow increases in a temporally and spatially coordinated manner. The physiological mechanisms that underlie this functional coupling are complex and involve adenosine (13, 31, 39), nitric oxide (NO) (12, 29), arachidonic acid metabolites derived from cyclooxygenase (34) and cytochrome P-450 (CYP) epoxygenase (36) enzymes, Ca2+-activated K+ (KCa) channels, and inward-rectifier K+ channels (17).
Although NO is required for functional hyperemia in cerebellum (50), NO may not be an essential mediator in cerebral cortex. Neuronal NO synthase (nNOS) null mice (nNOS−/−) and endothelial NOS (eNOS) null mice retain normal cerebral blood flow (CBF) responses to whisker stimulation (6, 29). Further work demonstrated that NO may act in a permissive fashion in the cerebral cortex to enable vasodilation by other mediators. Inhibition of NOS decreases basal cGMP and increases vascular tone. Restoration of vascular tone after NOS inhibition with either an NO donor or a cGMP analog restores the CBF response to functional activation (28). These findings imply that an increase in NO during neuronal activation may act to ensure that cGMP levels in vascular smooth muscle (VSM) are maintained at a level that permits vasodilation by other mediators.
However, NO has important targets in VSM other than soluble guanylyl cyclase. For example, NO can inhibit CYP ω-hydroxylase activity that is responsible for the conversion of arachidonic acid to 20-hydroxyeicosatetraenoic acid (20-HETE) in cerebral arteries (43). Part of the cerebrovascular dilation to NO donors is blocked by 20-HETE synthesis inhibitors (3). 20-HETE typically produces cerebral constriction by inhibiting KCa channels (19, 23), although a cyclooxygenase metabolite of 20-HETE has also been found to induce the dilation of the mouse basilar artery (15). Thus an increase in NO during neuronal stimulation could also inhibit the synthesis of 20-HETE and thereby permit the opening of vascular KCa channels and promote vasodilation.
Studies in brain and retinal slices have reported that 20-HETE contributes to the arteriolar constriction that is sometimes seen when glia are activated (32, 33). Photolysis of caged Ca2+ in astrocytes elicited the constriction of adjacent arterioles that was blocked by a phospholipase A2 inhibitor or by the 20-HETE synthesis inhibitor N-hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine (HET0016) (32). The release of arachidonic acid from astrocyte endfeet was postulated to stimulate 20-HETE synthesis in arteriolar smooth muscle. Whether 20-HETE synthesis normally limits the increase in CBF during functional activation in vivo with physiological levels of vascular tone and shear stress is unclear.
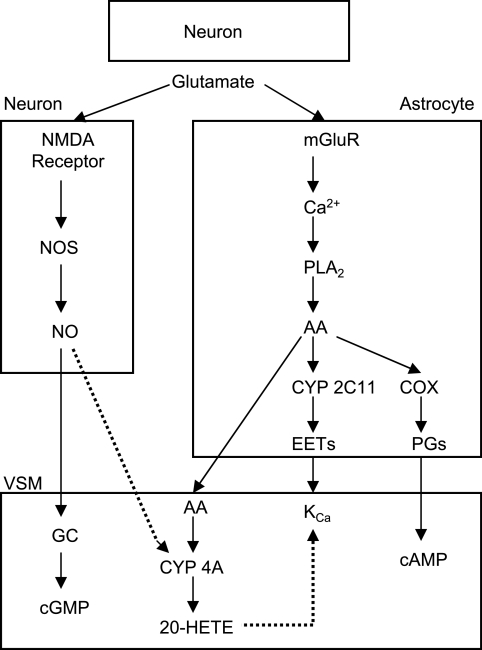
Work from our laboratories indicates that epoxyeicosatrienoic acids (EETs) are synthesized from arachidonic acid by CYP enzymes in astrocytes (2) and that the use of EETs synthesis inhibitors and antagonists attenuates functional hyperemia (36, 37, 39). Because EETs open KCa channels (20) and induce vasodilation (5, 27), the release of EETs from astrocytes during neuronal activation may act on vascular KCa to produce vasodilation. In this context, NO may facilitate EETs-mediated vasodilation by suppressing the 20-HETE-induced blockade of KCa channels in cerebral VSM. This concept is illustrated schematically in Fig. 1. To investigate this concept, whisker stimulation was used in rodents to evoke increased CBF in whisker barrel cortex. We examined whether the 20-HETE synthesis inhibitor HET0016 enhances the evoked CBF response, HET0016 has a larger effect on enhancing the evoked CBF response after NOS inhibition, and the decreased CBF response seen with an EETs antagonist is lost after NOS inhibition but restored with combined NOS and 20-HETE synthesis inhibition. Because previous neurovascular coupling studies in brain slices are often studied in both rat and mouse models, the effect of inhibiting 20-HETE synthesis on functional hyperemia was evaluated in both species. Studies were also performed on nNOS−/− mice to test the effect of a chronic loss of nNOS.
Fig. 1.
Schematic diagram of potential mechanisms by which nitric oxide (NO) can modulate cerebral vasodilation during neuronal activation. In addition to stimulating soluble guanylyl cyclase (GC) in vascular smooth muscle (VSM), NO generated by the Ca2+-dependent coupling of NO synthase (NOS) with N-methyl-d-aspartate (NMDA) receptor activation in neurons is postulated to inhibit synthesis of 20-HETE from arachidonic acid (AA) by cytochrome P-450 (CYP) 4A and thereby inhibit 20-HETE-mediated closure of Ca2+-activated K+ (KCa) channels. Presynaptic release of glutamate during neuronal activation is also thought to stimulate metabotropic glutamate receptors (mGluR) on astrocytes and generate an increase in Ca2+ that stimulates phospholipase A2 (PLA2). The consequent mobilization of AA could then provide substrate 1) for astrocyte cyclooxygenase (COX) to produce vasodilatory prostaglandins (PGs); 2) for astrocyte CYP 2C11 to generate epoxyeicosatrienoic acids (EETs), which can produce vasodilation by opening VSM KCa channels; or 3) for VSM CYP 4A. Arrows with solid lines denote stimulatory effects, and those with dotted lines denote inhibitory effects.
MATERIALS AND METHODS
All animal procedures were conducted in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Johns Hopkins University Animal Care and Use Committee.
Surgical preparation in rats.
Adult male Wistar rats (250–350 g; Harlan) were maintained in a climate-controlled room on a 12-h:12-h light-dark cycle with food and water available ad libitum. Rats were anesthetized with 1.5% halothane during the surgical procedure and maintained by α-chloralose (25 mg/kg ip plus 12 mg·kg−1·h−1 iv) during the experimental protocol, which commenced 1 h after discontinuing halothane. A femoral artery and femoral vein were catheterized with polyethylene (PE)-50 tubes, and mean arterial blood pressure was monitored. A tracheostomy was performed for mechanical ventilation with 30–40% O2 and ∼1.5% halothane. Rectal temperature was maintained at 37°C with a heating blanket. Arterial pH, Pco2, and Po2 were measured with a blood gas analyzer (Chiron Diagnostics, Halstead, Essex, UK), and hemoglobin concentration was measured with a hemoximeter (OSM3; Radiometer, Copenhagen, Denmark).
The rat was placed in the prone position, and the head was fixed with a stereotaxic holder. An approximate 3 × 3-mm region of skull on the left side was thinned to translucency by careful drilling for placement of a laser-Doppler flow (LDF) probe, which was located 2 to 3 mm posterior and 7 mm lateral to the bregma. Some drugs were administered by subarachnoid superfusion over the cortical surface at a constant rate of 5 μl/min. A small drill hole was made superior to the LDF probe site to expose the dura. A PE-10 catheter, with the tip tapered to ∼120 μm, was carefully inserted subdurally (36). Another hole was made inferior to the probe site, and the dura was incised for the passive drainage of the superfused fluid. At a superfusion rate of 5 μl/min, drug outflow concentration can attain a quasi-steady state within 10 min (39).
CBF measurement and whisker stimulation.
Cortical red blood cell flux was monitored with a laser-Doppler flowmeter (PeriFlux System 5000; Stockholm, Sweden). The flow probe was mounted on a micromanipulator and positioned at the surface of the thinned skull that was devoid of large visible blood vessels and that generated maximal response to whisker stimulation. The probe position was kept fixed for the entire experiment, and background lighting was not changed.
Somatosensory stimulation was performed by the continuous mechanical deflection of whiskers on the right side through a plastic screen mesh connected to a custom-built stimulation device (36). The whiskers, cut to a length of 3 to 4 cm, were inserted through the plastic mesh, and the stimulation frequency was 10 Hz over a period of 60 s. The orientation of the plastic mesh was held fixed for the duration of the entire experiment. Relative changes of LDF were calculated as percentages of the baseline during the 60 s before stimulation. The responses to three trials spaced 5 min apart were averaged for each experimental condition at the end of every hour of the cortical superfusion for the 3-h duration of superfusion.
Experimental protocol for whisker stimulation in rats.
The LDF response to whisker stimulation in rats was recorded under three experimental conditions with different inhibitors and antagonists. For the first hour of the protocol, the cortical surface was superfused with artificial cerebrospinal fluid (CSF) starting 1 h after completion of the surgery at a constant rate of 5 μl/min. The artificial CSF constituents were (in mmol/l) 156 Na+, 3 K+, 1.25 Ca2+, 0.66 Mg2+, 133 Cl−, 25 HCO3−, 6.7 urea, and 3.7 dextrose. HET0016 was dissolved in ethanol and diluted in artificial CSF to a concentration of 1 μmol/l and 0.1% ethanol. This concentration of ethanol did not affect the LDF response to whisker stimulation (36). The 1 μmol/l concentration of HET0016 has previously been shown to specifically inhibit the 20-HETE formation in renal microsomes by 90% without inhibiting EETs formation (25). The EETs antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) was delivered at a concentration of 30 μmol/l in 0.1% ethanol in CSF. This concentration was found to inhibit the LDF response to whisker stimulation and is threefold greater than the concentration that inhibits the EETs-mediated dilation of cerebral and coronary arteries in vitro (18, 39). To inhibit nNOS, 7-nitroindazole (7-NI) was dissolved in peanut oil and injected intraperitoneally at a dose of 40 mg/kg, which is slightly greater than the dose required to produce 80% inhibition of brain NOS activity (30). The guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) was superfused over the cortical surface at a concentration of 100 μmol/l, which was previously reported to decrease basal cGMP and the LDF response to whisker stimulation (28).
The LDF response to whisker stimulation was recorded hourly in seven groups of rats (Table 1). Group 1 served as a time control group in which artificial CSF superfusion was continued for the second and third hour. In group 2, one of two vehicles was administered for the second hour and HET0016 was superfused for the third hour. One-half of the rats in this group received a vehicle subarachnoid superfusion of 0.1% ethanol (vehicle for HET0016 and 14,15-EEZE) in CSF during the second hour, and the remaining one-half received an intraperitoneal injection of peanut oil (vehicle for 7-NI) plus CSF subarachnoid superfusion. Because neither vehicle had any effect on the LDF response to whisker stimulation, the responses of these cohorts were pooled into a combined control group. In group 3, 7-NI was injected intraperitoneally at the beginning of the second hour, whereas the CSF superfusion continued during the second and third hour. In group 4, 7-NI was injected at the beginning of the second hour, and HET0016 was superfused over the cortex during the third hour. In group 5, ODQ was superfused during the second hour, and ODQ plus HET0016 was superfused during the third hour. In group 6, 7-NI was injected at the start of the second hour, whereas CSF continued to be superfused over the cortex. 14,15-EEZE was then superfused over the third hour. In group 7, 7-NI was injected at the start of the second hour and HET0016 was concurrently superfused during the second hour. During the third hour, 14,15-EEZE was added to the superfusate with HET0016.
Table 1.
Experimental design for whisker stimulation in rats and percent changes in resting LDF
| Group | n |
Hour (%) |
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| 1 | 8 | CSF | CSF | (1±3) | CSF | (5±5) |
| 2 | 8 | CSF | Vehicle + CSF | (0±2) | HET0016 | (7±7) |
| 3 | 5 | CSF | 7-NI + CSF | (−16±2*) | CSF | (−17±2*) |
| 4 | 8 | CSF | 7-NI + CSF | (−13±6*) | HET0016 | (13±7†) |
| 5 | 6 | CSF | ODQ | (−8±1*) | ODQ + HET0016 | (−4±1*†) |
| 6 | 8 | CSF | 7-NI + CSF | (−7±2*) | 14,15-EEZE | (−12±2*) |
| 7 | 8 | CSF | 7-NI + HET0016 | (4±6) | 14,15-EEZE + HET0016 | (0±6) |
Values are means ± SE; n, number of rats per group. Values in parentheses at hours 2 and 3 are percent changes in resting laser-Doppler flow (LDF) from baseline LDF during hour 1.
P < 0.05 from hour 1;
P < 0.05 from hour 2. CSF, cerebrospinal fluid; 7-NI, 7-nitroindazole; ODQ, 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one; HET0016, 20-HETE synthesis inhibitor N-hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine; EEZE, epoxyeicosa-5(Z)-enoic acid.
Vascular response to superfusion of acetylcholine.
To determine whether HET0016 restored the vascular response to another NO-dependent stimulus after NOS inhibition, LDF was measured in five rats during 5 min of superfusion of the cranial window with 10 μmol/l of acetylcholine (ACh). After the control response to ACh was obtained, 1 mmol/l of Nω-nitro-l-arginine (l-NNA), an inhibitor of both eNOS and nNOS, was superfused for 1 h followed by a repeat exposure to ACh in the presence of l-NNA. After washout of ACh, the cortical surface was superfused with l-NNA plus 1 μmol/l of HET0016 for an additional hour followed by a repeat exposure to ACh in the presence of l-NNA + HET0016. Finally, the response to 1 mmol/l of papaverine was tested in the presence of l-NNA + HET0016 to determine whether the vessels were still capable of dilating.
CBF response to an NO donor.
Three sets of rats were studied to determine the conditions under which the increase in LDF produced by an NO donor depended on the presence of 20-HETE synthesis. The LDF response to the superfusion of 1 μmol/l of sodium nitroprusside was measured three times in each rat: 1) with no inhibitor, 2) after 1-h superfusion of 1 μmol/l of HET0016, and 3) after an additional hour of superfusion of HET0016 plus ODQ (100 μmol/l). At the end of the experiment, the ability of cerebral vessels to respond to another vasodilator papaverine (1 mmol/l) was tested in the presence of HET0016 and ODQ. In one set of rats (n = 6), the experiment was conducted during arterial normotension. With the knowledge that increased myogenic tone is accompanied by increased 20-HETE synthesis (19), responses to vasodilators were tested during continuous arterial hypertension produced by intravenous phenylephrine infusion (∼10 μg·kg−1·min−1) in a second set of rats (n = 4). In a third set of rats (n = 5), the responses to vasodilators were tested after the administration of 7-NI (40 mg/kg ip) to decrease the basal levels of NO.
Liquid chromatography/mass spectroscopy measurement of CYP metabolites of arachidonic acid.
To demonstrate that HET0016 decreases 20-HETE synthesis in brain, the formation of 20-HETE was measured in rat brain homogenates incubated with 42 μmol/l arachidonic acid in the presence of 1 mM NADPH. Measurements were made in control brains (n = 3) and brains harvested 1 h after an intravenous injection of 1 mg/kg of HET0016 (n = 3). Systemic administration, rather than subarachnoid superfusion, was used so that measurements could be performed on tissue with a homogeneous distribution of the drug and because the analysis requires the sampling of a large portion of the cerebral hemisphere. The systemic dose of 1 mg/kg has been shown to reduce 20-HETE levels in CSF and to ameliorate cerebral hypoperfusion after an intracisternal injection of blood (9, 25).
Measurements of 20-HETE, EETs, and the dihydroxyeicosatetraenoic acid (DiHETE) products of EETs were made with a liquid chromatography/mass spectroscopy, as previously described (47). Samples of the cerebral cortex (0.3 g) were homogenized in 2 ml of a 10 mmol/l KPO4 buffer containing 250 mmol/l sucrose and 1 mmol/l EDTA (pH 7.7). The homogenate was centrifuged at 4,000 g for 5 min. The supernatant was extracted twice with 3 ml of ethyl acetate after the addition of 10 ng of an internal standard, d6 20-hydroxyeicosatetranoic acid, and the organic phase was dried under nitrogen. The metabolites of arachidonic acid were separated by high-performance liquid chromatography on a Betabasic C18 column (150 × 2.1 mm, 3 μm; Thermo Hypersil-Keystone, Bellefonte, PA) at a flow rate of 0.2 ml/min using an isocratic elution with a 51:9:40:0.01 mixture of acetonitrile:methanol:water:acetic acid for 30 min, followed by a step gradient to 68:13:19:01 acetonitrile:methanol:water:acetic acid and water for 15 min. The effluent was ionized using a negative ion electrospray, and the peaks eluting with a mass-to-charge ratio of 319 > 245 (20-HETE), 319 > 301 (HETEs and EETs), 337 > 319 (DiHETEs), or 325 > 251 (internal standard) were monitored using an Applied Biosystems 3000 triple quadrupole mass spectrometer (AME Bioscience, Foster City, CA). The ratio of ion abundances in the peaks of interest versus that seen in the internal standard was determined and compared with standard curves generated over a range from 0.2 to 1.0 ng for 20-HETE and from 1.0 to 10 ng for the other metabolites. CYP activity was expressed as picomoles of product formed per minute per milligram of protein.
Surgical preparation and protocol in mice.
Modifications were made for similar experiments performed in mice. After the induction of anesthesia with 5% isoflurane, anesthesia was maintained with 2% isoflurane via a face mask during the initial surgery for the placement of an airway tube via a tracheostomy and the placement of catheters in the femoral artery and peritoneal cavity. The lungs were then mechanically ventilated with 25–30% O2, urethane was injected (500 mg/kg ip), and a continuous infusion of urethane (250 mg·kg−1·h−1 ip) was maintained for the duration of the experiment. After the administration of urethane, the inspired isoflurane concentration was decreased to 1%. The mouse was placed in a head holder, a small craniotomy (∼3 mm) was performed over the whisker barrel cortex, and a plastic ring with inlet and outlet ports and a thermistor was glued to the skull around the craniotomy. The dura was gently cut and retracted, the window was filled with artificial CSF, and the window was sealed with a glass coverslip. The LDF probe was placed on the glass coverslip with a drop of mineral oil for optical continuity. Isoflurane was discontinued, and the closed cranial window was continuously perfused with warmed artificial CSF at a rate of 100 μl/min. The window fluid temperature and rectal temperature were maintained at ∼37°C. Three 60-s epochs of whisker stimulation were performed ∼90 min after completing surgery and discontinuing isoflurane. After the control LDF response to whisker stimulation was obtained, the window was superfused with drugs for 1 h, and three epochs of whisker stimulation were repeated. Arterial blood pressure was continuously monitored to ensure that no changes occurred during the stimulation period. To minimize blood loss during the experiment, arterial blood gas measurements were limited to before the first set and after the last set of whisker-stimulation trials.
Experiments were performed with 1 μmol/l superfusion of HET0016 for 1 h through closed cranial windows in C57Bl/6 (nNOS+/+) mice and in nNOS−/− mice on a C57Bl/6 genetic background. Furthermore, in nNOS+/+ mice, the interaction of acute NOS inhibition with 20-HETE synthesis inhibition was tested with l-NNA, which was superfused for 1 h at a concentration of 1 mmol/l. This NOS inhibitor has been shown to inhibit the LDF response to whisker stimulation in mice at this concentration (6, 29) and to inhibit NOS activity after 1 h of administration (24).
Statistical analysis.
Data are presented as means ± SE. The significance of differences in mean values at 1, 2, and 3 h was analyzed by repeated-measures ANOVA and the Newman-Keuls multiple range test. A P value of <0.05 was considered to be significant.
RESULTS
Inhibition of 20-HETE synthesis.
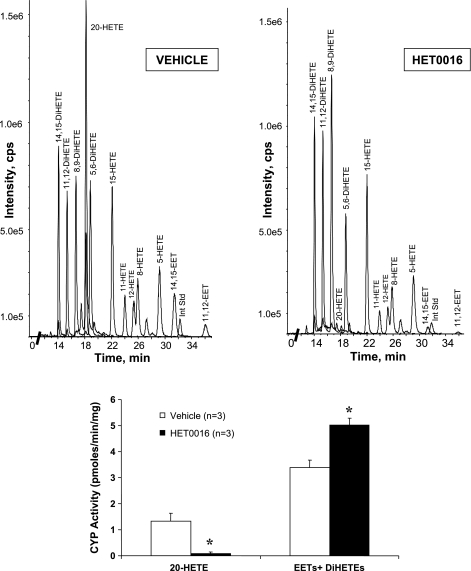
20-HETE, EETs, and DiHETEs were all produced by homogenates of the brain incubated with arachidonic acid (Fig. 2), but most of the EETs were converted to their corresponding DiHETEs. The systemic administration of HET0016 (1 mg/kg) inhibited the synthesis of 20-HETE in the brain by >90% as previously reported by others (38). HET0016 did not inhibit, but rather increased, epoxygenase activity slightly, as defined by the sum of the formation of EETs and DiHETEs metabolites.
Fig. 2.
Top: representative liquid chromatography/mass spectroscopy chromatograms of homogenized rat forebrain incubated with AA showing the presence of 20-HETE and the various regioisomers of EETs and dihydroxyeicosatetraenoic acids (DiHETEs). The chromatogram of the rat brain after treatment in vivo with 20-HETE synthesis inhibitor N-hydroxy-N′-(4-n-butyl-2-methylphenyl)formamidine (HET0016; 1 mg/kg) showed suppressed production of 20-HETE but sustained production of other metabolites. Bottom: bar graph of CYP activity (pmoles·min−1·mg−1 protein) for formation of 20-HETE and EETs + DiHETEs (as a measure of epoxygenase activity) of 3 rat brains from vehicle- and HET0016-treated groups. *P < 0.05 from vehicle group. Int Std, internal standard; cps, cycles per second. n, Number of rats.
Whisker stimulation response in rats.
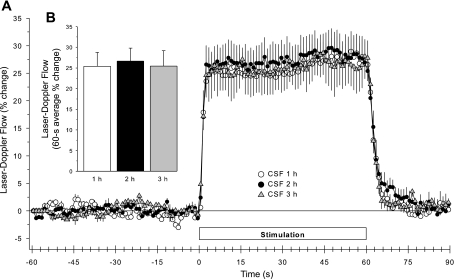
In the time-control group, baseline LDF was unchanged (Table 1) over the 3-h course of the experiment. Whisker stimulation produced a brisk and sustained increase in LDF (Fig. 3A). The averaged time course of the on-and-off response as well as the magnitude of the LDF response to whisker stimulation was stable over the 3-h course of these experiments (Fig. 3B).
Fig. 3.
Time course of cortical laser-Doppler flow (LDF; ± SE), expressed as a percent change from a 60-s baseline recording, during and after 60 s of whisker stimulation at 1, 2, and 3 h of subarachnoid superfusion with artificial cerebrospinal fluid (CSF) in 8 time-control rats (A). B: percent change in flow during whisker stimulation averaged over the 60-s stimulation period.
Mean arterial blood pressure was unchanged during whisker stimulation and remained stable over the 3-h recording period (supplementary Table 1; all supplemental material can be found with the online version of this article). Furthermore, arterial pH, Pco2, Po2, and arterial hemoglobin concentration were also stable in all groups of rats (supplementary Table 2).
Effect of 20-HETE synthesis inhibition.
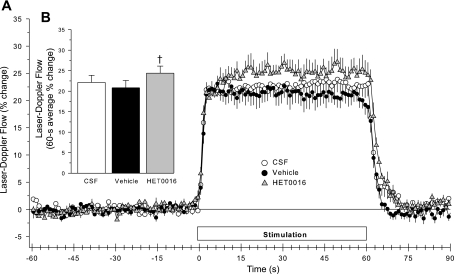
The superfusion of the 0.1% ethanol vehicle for HET0016 or an intraperitoneal injection of the peanut oil vehicle for 7-NI did not change baseline LDF or the percent change in LDF during whisker stimulation. Thus the results from the two vehicle control groups were combined into a single vehicle group in Fig. 4. The LDF response to whisker stimulation increased by a small amount (21 ± 3% to 24 ± 2%; n = 8) after the superfusion of the brain with HET0016 compared with vehicle (Fig. 4B). However, an inspection of the time course of the response reveals that the early phase of the response was unchanged by HET0016 superfusion (Fig. 4A).
Fig. 4.
Time course of cortical LDF (± SE), expressed as a percent change from a 60-s baseline recording, during and after 60 s of whisker stimulation at 1 h of subarachnoid superfusion with artificial CSF, 1 h of vehicle administration, and 1 h of HET0016 superfusion in 8 rats (A). B: percent change in flow during whisker stimulation averaged over the 60-s stimulation period. Superfusion of the 0.1% ethanol vehicle for HET0016 did not change baseline LDF (100 ± 4%) or the LDF response to whisker stimulation (21 ± 1% with CSF vs. 20 ± 2% with 0.1% ethanol). Likewise, administration of the peanut oil vehicle for 7-nitroindazole (7-NI) did not change resting LDF (100 ± 3%) or the response to whisker stimulation (23 ± 3% with CSF superfusion vs. 21% ± 3% 1 h after peanut oil + CSF superfusion). Thus the results from the 2 vehicle control groups were combined into a single vehicle group. †P < 0.05 from vehicle value.
Effect of nNOS inhibition.
7-NI has been reported to decrease the LDF response to whisker stimulation (10, 28), but the duration of action of this compound on the response has not been determined. We found that baseline LDF decreased to the same extent at 1 versus 2 h after the administration of 7-NI (40 mg/kg; Table 1). The percent increase in LDF during whisker stimulation averaged 23 ± 2% before 7-NI administration and 16 ± 1% and 16 ± 1% 1 and 2 h after 7-NI administration, respectively. This stable reduction in the LDF response is consistent with the known prolonged inhibitory effect of a large dose of 7-NI (30) and permitted an experimental design in which the effect a second drug could be superimposed in the presence of sustained nNOS inhibition.
Interaction of NO and 20-HETE.
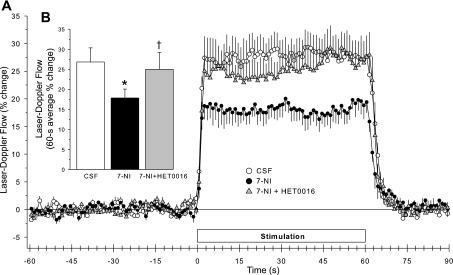
HET0016 superfusion had no significant effect on resting LDF in control animals (Table 1); however, HET0016 restored resting LDF back to the original baseline level (Table 1) in animals treated with 7-NI. The superfusion of HET0016 after 7-NI administration also increased the early and steady-state LDF response to whisker stimulation compared with the response seen after 7-NI alone (Fig. 5A). This effect of HET0016 on the early response was in contrast with the small, delayed effect of HET0016 seen after vehicle (Fig. 4A). The 60-s average response 1 h after HET0016 superfusion (2 h after 7-NI injection) was significantly greater than the response to whisker stimulation seen 1 h after 7-NI injection but was not different from the control response (Fig. 5B). The restoration of the LDF response after HET0016 superfusion in 7-NI-treated rats was not simply the result of a loss of NOS inhibition with time because the inhibition of the LDF response to whisker stimulation produced by 7-NI administration alone was sustained over a 2-h period.
Fig. 5.
Time course of cortical LDF (± SE), expressed as a percent change from a 60-s baseline recording, during and after 60 s of whisker stimulation at 1 h of subarachnoid superfusion with artificial CSF, 1 h after 7-NI administration, and 1 h of HET0016 superfusion in 8 rats (A). B: percent change in flow during whisker stimulation averaged over the 60-s stimulation period. *P < 0.05 from CSF value; †P < 0.05 from 7-NI value.
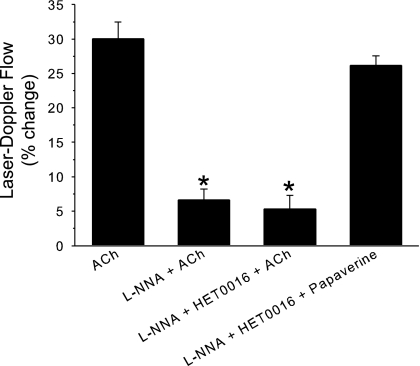
To determine whether the restoration of the response by HET0016 after NOS inhibition was specific for whisker stimulation, the effect of HET0016 was examined on ACh-induced vasodilation. In this experiment, the superfusion of ACh increased LDF, and the increase was markedly reduced by l-NNA to inhibit NOS (Fig. 6). HET0016 had no effect on the LDF response to ACh in rats pretreated with l-NNA. In contrast, cerebral vessels were still responsive to papaverine in the presence of l-NNA and HET0016. Therefore, the reversal of NOS inhibitory effects by HET0016 was specific for whisker stimulation-induced vasodilation.
Fig. 6.
Percent change of LDF (± SE; n = 5 rats) during subarachnoid superfusion of 10 μmol/l of acetylcholine (ACh) under control conditions, after superfusion of Nω-nitro-l-arginine (l-NNA) to inhibit NOS, and after superfusion of l-NNA + HET0016 to inhibit both NOS and 20-HETE synthesis. Ability to retain vasodilatory capacity after l-NNA and HET0016 was tested by superfusion of 1 mmol/l of papaverine. *P < 0.05 of ACh responses after inhibitors from control ACh response.
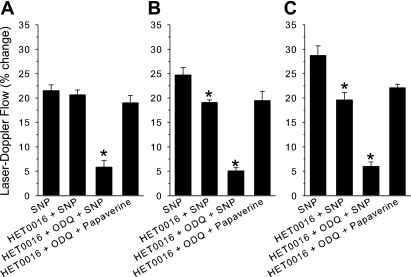
If an increase in NO contributes to vasodilation during whisker stimulation by inhibiting 20-HETE synthesis or signaling, an NO donor may then produce vasodilation, in part, by inhibiting 20-HETE synthesis or its downstream actions as well as by activating guanylyl cyclase. The relative contribution of 20-HETE inhibition by an NO donor will depend on the baseline levels of 20-HETE synthesis. To test this hypothesis, a submaximal dose of sodium nitroprusside was used to produce an increase in LDF that was comparable in magnitude with the increase in LDF obtained with whisker stimulation. The superfusion of HET0016 did not significantly change the increase in LDF seen with the nitroprusside superfusion (Fig. 7A). The combined superfusion of HET0016 and ODQ nearly blocked the response to nitroprusside. Because HET0016 has little effect on baseline LDF (Table 1), the basal levels of 20-HETE synthesis may have been too low for the NO donor to demonstrate an inhibitory effect on 20-HETE synthesis or downstream signaling. The experiment was thus repeated in the presence of phenylephrine-induced hypertension because increasing myogenic tone has been shown to increase vascular 20-HETE levels (19). Phenylephrine increased mean arterial pressure from 99 ± 5 to 146 ± 7 mmHg. Baseline LDF increased by 13 ± 2%. Cerebrovascular resistance was estimated to increase by ∼27 ± 3%, and the increase was assumed to be the result of increased myogenic tone. Although baseline LDF was slightly elevated, the initial response to nitroprusside was maintained. Under these conditions, HET0016 inhibited the increase in LDF to 77% of the initial response to nitroprusside (Fig. 7B). The combined treatment with HET0016 and ODQ inhibited the response further to <20% of the control response. The lack of effect of HET0016 in normotensive rats may have been due to endogenous NO production that was sufficient to inhibit endogenous 20-HETE synthesis or signaling and did not require additional NO from an NO donor. To test this possibility further, 7-NI was administered to inhibit basal neuronal NO production before testing nitroprusside reactivity. Mean arterial blood pressure was stable at 97 ± 4 mmHg and was similar to the 97 ± 2 mmHg value seen in the first normotensive group without 7-NI treatment. The increase in LDF during nitroprusside superfusion after 7-NI administration (29 ± 2%) was greater than the response in the untreated, normotensive group (21 ± 1%). Treatment with HET0016 after 7-NI administration inhibited the increase in LDF to 68% of the initial response (Fig. 7C). The combined treatment with HET0016 and ODQ inhibited the response to 21% of the initial response. In contrast with the increase in the control response to nitroprusside after 7-NI, the response to papaverine after HET0016 and ODQ was similar among groups. Thus increasing myogenic tone or inhibiting basal NO production selectively augments the ability of HET0016 to inhibit vasodilation evoked by an NO donor.
Fig. 7.
Percent change of LDF (± SE) during 1 μmol/l of nitroprusside under control conditions, after superfusion of the 20-HETE synthesis inhibitor HET0016, and after superfusion of HET0016 + the GC inhibitor 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ). Ability to retain vasodilatory capacity after HET0016 and ODQ was tested by superfusion of 1 mmol/l of papaverine. *P < 0.05 of nitroprusside responses after inhibitors from the initial nitroprusside response. A: responses during normotension (n = 6 rats). B: responses during phenylephrine-induced hypertension (n = 4 rats). C: responses after systemic administration of 7-NI (n = 5 rats). SNP, sodium nitroprusside.
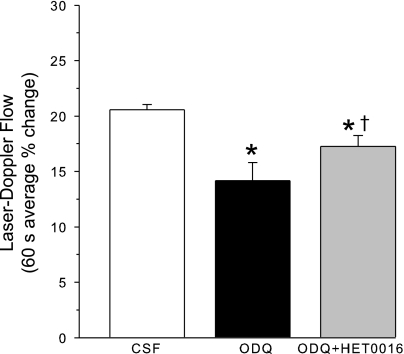
ODQ without NOS inhibition is known to decrease the LDF response to whisker stimulation (28). To determine whether 20-HETE synthesis inhibition restores the whisker response in the presence of ODQ to the same extent as it did in the presence of NOS inhibition, the whisker response was measured after superfusing ODQ alone and ODQ plus HET0016. As expected, the superfusion of ODQ decreased the LDF response to whisker stimulation (Fig. 8). The cosuperfusion of ODQ and HET0016 significantly increased the LDF response compared with that of ODQ alone, but the response remained significantly less than the control response with CSF superfusion. Thus HET0016 was less effective in restoring the whisker response in the presence of ODQ than in the presence of 7-NI.
Fig. 8.
Cortical LDF (± SE), expressed as a percent change from a 60-s baseline recording, averaged over 60 s of whisker stimulation at 1 h of subarachnoid superfusion with artificial CSF, 1 h of ODQ superfusion, and 1 h of ODQ + HET0016 superfusion in 6 rats. *P < 0.05 from CSF value; †P < 0.05 from ODQ value.
Interaction with EETs.
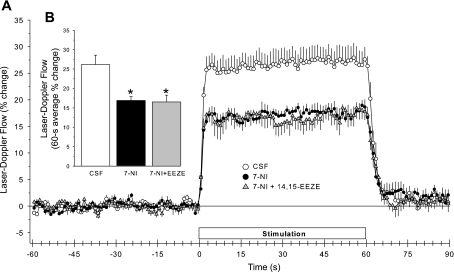
Previous work has shown that the superfusion of the EET antagonist 14,15-EEZE for 1 h did not significantly change baseline LDF but reduced the LDF response to whisker stimulation (39). To determine whether the EET antagonist could still reduce the response in the presence of nNOS inhibition, 14,15-EEZE was superfused for 1 h starting 1 h after the administration of 7-NI. As in previous groups of rats, the administration of 7-NI decreased the LDF response to whisker stimulation (Fig. 9). The superfusion of 14,15-EEZE in the presence of 7-NI failed to produce any additional inhibition of the whisker-stimulation response. The decrease in resting LDF by 7-NI was also unaffected by 14,15-EEZE (Table 1).
Fig. 9.
Time course of cortical LDF (± SE), expressed as a percent change from a 60-s baseline recording, during and after 60 s of whisker stimulation at 1 h of subarachnoid superfusion with artificial CSF, 1 h after 7-NI administration, and 1 h of 14,15-epoxyeicosa-5(Z)-enoic acid (14,15-EEZE) superfusion in 8 rats (A). B: percent change in flow during whisker stimulation averaged over the 60-s stimulation period. *P < 0.05 from CSF value.
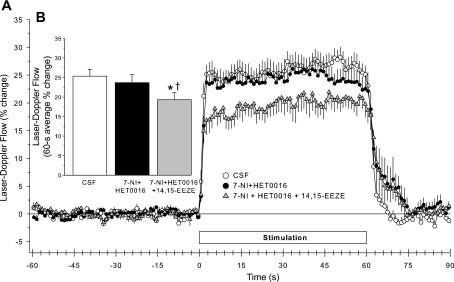
To determine whether the inhibitory effect of 14,15-EEZE could be restored if 20-HETE synthesis and nNOS were simultaneously inhibited, 14,15-EEZE was administered 1 h after the coadministration of 7-NI and HET0016 in another group of rats. When HET0016 superfusion was started immediately after an injection of 7-NI, resting LDF (Table 1), the initial response to whisker stimulation (Fig. 10A), and the 60-s averaged response (Fig. 10B) measured 1 h after initiating drug administration were unaffected. Thus the effect of the simultaneous drug administration was the same as that seen in the previous group with a 1-h delay in the HET0016 administration after 7-NI. The cosuperfusion of 14,15-EEZE and HET0016 after 7-NI administration decreased the LDF response to whisker stimulation compared with both the control response and the response after 7-NI plus HET0016 (Fig. 9B). The inhibitory effect was evident by 3 s of stimulation (Fig. 10A). Resting LDF was not changed by the addition of 14,15-EEZE to the superfusate (Table 1).
Fig. 10.
Time course of cortical LDF (± SE), expressed as a percent change from a 60-s baseline recording, during and after 60 s of whisker stimulation at 1 h of subarachnoid superfusion with artificial CSF, 1 h after 7-NI administration + 1 h of HET0016 superfusion, and 1 h of HET0016 + 14,15-EEZE superfusion in 8 rats (A). B: percent change in flow during whisker stimulation averaged over the 60-s stimulation period. *P < 0.05 from CSF value; †P < 0.05 from 7-NI + HET0016 value.
Whisker-stimulation response in mice.
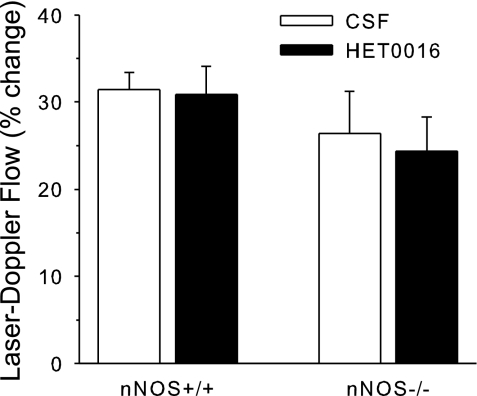
To determine whether the lack of a major effect of HET0016 on the normal LDF response to whisker stimulation was specific for rat, similar experiments were also performed in mice. Arterial blood gases were maintained within the physiological range over the course of the experiments in the mechanically ventilated mice, and arterial pressure was stable between the first and second trials of whisker stimulation (supplementary Table 3). The superfusion of HET0016 over the cortex of nNOS+/+ mice had no significant effect on the increase in LDF during whisker stimulation (Fig. 11). To test whether an effect of HET0016 would be augmented with a chronic loss of nNOS, responses were measured in nNOS−/− mice. The control LDF response to whisker stimulation in nNOS−/− mice was similar to that of nNOS+/+ mice. The superfusion of HET0016 in nNOS−/− mice did not increase the LDF response to whisker stimulation.
Fig. 11.
Percent change (± SE) in LDF during 60 s of whisker stimulation in 7 neuronal (n)NOS+/+ mice and 7 nNOS−/− mice after 1 h of cranial window superfusion with CSF followed by 1-h superfusion of HET0016. Response was not significantly changed by HET0016 in either group.
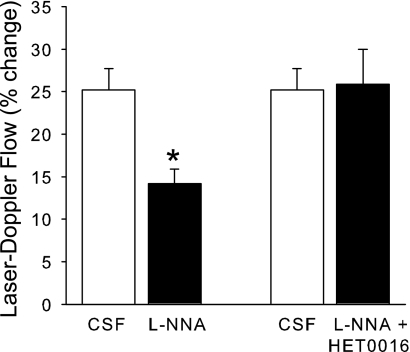
l-NNA is known to decrease the LDF response to whisker stimulation in mice (6, 29). This decrease in the response was confirmed in a group of nNOS+/+ mice after 1 h of superfusion of l-NNA (Fig. 12). To determine whether this decrease required 20-HETE synthesis, l-NNA and HET0016 were coadministered in the superfusate in another group of C57Bl/6 mice. The LDF response to whisker stimulation was unaltered from the control response with the coadministration. Thus HET0016 blocked the reduction in the evoked LDF response seen with two different NOS inhibitors in two different species.
Fig. 12.
Percent change (± SE) in LDF during 60 s of whisker stimulation in 2 groups of nNOS+/+ mice: 7 mice after 1 h superfusion with CSF followed by 1-h superfusion of l-NNA and 8 mice after 1-h superfusion with CSF followed by 1-h superfusion of l-NNA + HET0016. *P < 0.05 from CSF response.
DISCUSSION
The key findings of the present study are as follows. First, the administration of the 20-HETE synthesis inhibitor HET0016 produces only a small and delayed enhancement of the CBF response to whisker stimulation in the rat and no significant effect in the mouse. Second, the effect of 7-NI to induce a fall in resting cortical CBF and to attenuate the CBF response to sensory activation is blocked by inhibition of the formation of 20-HETE. Third, because 20-HETE synthesis inhibition failed to restore the vascular response to ACh after NOS inhibition or the vascular response to whisker stimulation after guanylyl cyclase inhibition, the restoration of the response to whisker stimulation by 20-HETE synthesis inhibition after NOS inhibition is specific for the effect of NOS inhibition on functional hyperemia. Fourth, the reduction in the vascular response to an NO donor by a 20-HETE synthesis inhibitor is enhanced by decreasing baseline NO production or raising arterial pressure, which has been report to augment vascular 20-HETE production (19). Fifth, the administration of an EETs antagonist in the presence of nNOS inhibition does not reduce the CBF response to whisker stimulation beyond that seen following inhibition of nNOS alone. Finally, the ability of the EETs antagonist to reduce the response to whisker stimulation in the presence of nNOS inhibition is restored when 20-HETE synthesis is concurrently inhibited.
Effect of 20-HETE synthesis inhibition.
In rat and mouse brain slices, Mulligan and MacVicar (32) reported that two-photon photolysis of caged Ca2+ in astrocyte cell bodies elicited a wave of increased intracellular Ca2+. If that wave reached the astrocyte endfeet on blood vessels, a constriction of nonpressurized vessels occurred. This constriction was blocked by a phospholipase A2 inhibitor and by a high concentration (100 μmol/l) of HET0016. They suggested that arachidonic acid released from astrocyte endfeet (1) served as a substrate for CYP 4A, known to be expressed in adjacent VSM, where 20-HETE formation produces vasoconstriction (19). To determine whether 20-HETE synthesis limits the integrated vasodilatory response to somatosensory activation in vivo, we applied HET0016 to the cortical surface. In mice, HET0016 had no significant effect on the CBF response to whisker stimulation. In rats, HET0016 produced a 17% enhancement of the response. Thus 20-HETE appears to play only a minor modulatory role in the CBF response to activation under basal conditions.
However, the influence of 20-HETE in neurovascular coupling depends on the basal level of vascular tone (7). When vascular tone is increased in brain slices, the dilation of vessels in response to astrocyte Ca2+ uncaging (32) or to electrical or pharmacological activation of astrocytes (16, 52) is more frequently observed than constriction. Furthermore, astrocyte Ca2+ uncaging in vivo in mice at normal perfusion pressure and endothelial shear rates reproducibly produces adjacent vascular dilation rather than constriction (44). Hence, during astrocyte activation, low vascular tone appears to be required to reveal a constrictor response that is dependent on 20-HETE.
One limitation of the present study is that multiple whiskers were stimulated simultaneously to elicit a spatially broad increase in CBF. With more selective activation of discrete cortical columns, regions of increased inhibitory synaptic activity associated with arteriolar constriction and decreased CBF can sometimes be observed adjacent to regions of increased CBF (11). A possible role of 20-HETE in decreasing CBF in inhibitory-surround regions cannot be excluded.
Interaction of NO and 20-HETE.
In addition to activating soluble guanylyl cyclase, our laboratory (4) and others (35) have shown that NO directly inhibits the formation of 20-HETE in kidney and in renal arteries (42), which express the same CYP 4As that are expressed in cerebral arteries (19). In cerebral arteries, NO donors can induce vasodilation by at least two mechanisms. First, the resulting increase in cGMP acts to inhibit the constriction induced by intracellular Ca2+ (51). Second, by inhibiting the CYP 4A that is responsible for 20-HETE synthesis in some cerebral vessels (3, 19), NO can indirectly inhibit the 20-HETE-induced closure of large conductance KCa channels by a mechanism that does not require an increase in cGMP (43). For example, an inhibitor of 20-HETE synthesis attenuated the NO donor-induced increase in the diameter of isolated middle cerebral arteries preconstricted with serotonin (43), and ODQ was less effective in inhibiting the response in these arteries than in isolated basilar arteries (3). Furthermore, a 20-HETE synthesis inhibitor blunted the increase in LDF to an NO donor (3). In the present study, the role of 20-HETE in the LDF response to nitroprusside depended on basal NO production and arterial pressure. Under normal conditions, HET0016 had little effect on the increase in LDF evoked by a submaximal vasodilatory concentration of nitroprusside, whereas ODQ nearly blocked the response, which is consistent with studies of others (40). However, when 7-NI was first administered to reduce the basal levels of NO, HET0016 then attenuated the response to nitroprusside. This observation implies that the lack of effect of HET0016 on the nitroprusside response under normal conditions was due to the effective inhibition of 20-HETE synthesis or downstream signaling by endogenous NO. This interpretation is supported by the finding that the 20-HETE synthesis inhibitor had little effect on increasing baseline LDF unless NOS was inhibited. Furthermore, when arterial pressure was increased by phenylephrine infusion, HET0016 was also found to attenuate the LDF response to nitroprusside. Because the increase in CBF during phenylephrine infusion was proportionately less than the increase in arterial pressure and because intracranial arteries have low α1-adrenergic receptor density, the resulting increase in vascular resistance associated with autoregulation is presumed to represent an increase in myogenic tone. An increase in myogenic tone increases 20-HETE synthesis (19). Thus exogenous NO is required to fully inhibit 20-HETE-dependent increases in myogenic tone associated with autoregulation.
In the case of neuronal activation, an increase in endogenous NO production may be required to inhibit a potential increase in 20-HETE synthesis brought about by increased arachidonic acid availability or to counteract the effect of 20-HETE on potassium channels. Microelectrode recordings of NO in rat somatosensory cortex during forepaw stimulation demonstrated an increase in tissue NO lasting only 2 s and preceding the maximal LDF response at 4 s (8). This rapid burst of NO may act to inhibit CYP 4A localized proximal to neurons from the intraluminal hemoglobin sink for NO scavenging. Because astrocyte Ca2+ increases progressively over 6–9 s of whisker stimulation (46), the maximal activation of astrocyte phospholipase A2 likely occurs after the increase in NO during sensory activation. Our observation that 20-HETE synthesis inhibition had no effect on the LDF response to whisker stimulation over the first 12 s of stimulation is consistent with the possibility that an early burst of NO already inhibits 20-HETE synthesis or signaling.
Inhibition of 20-HETE synthesis or its actions by NO during the early phase of activation is also supported by results with 7-NI. After the inhibition of nNOS with 7-NI, HET0016 had a greater effect on increasing the LDF response both before the 12-s time point of stimulation and during the steady-state response. Indeed, HET0016 fully reversed the inhibition of the LDF response seen with the nNOS inhibitor. With simultaneous administration, HET0016 blocked the inhibitory effect of 7-NI on the hyperemic response to whisker stimulation. These results indicate that the inhibitory effect of 7-NI requires 20-HETE synthesis and that the suppression of 20-HETE synthesis and signaling is involved in the permissive role of NO in the cortical flow response. In contrast, 20-HETE synthesis inhibition had no effect on the ACh vascular response where NO plays a nonpermissive role.
Others have shown that ODQ, as well as 7-NI, can attenuate the LDF response to whisker stimulation (28). Interestingly, applying a cGMP analog at a dose sufficient to restore baseline LDF also restored the LDF response to whisker stimulation after 7-NI or ODQ. Thus a minimal amount of cGMP appears to be required for a normal vascular response to activation. Endothelial-derived NO induced by shear stress may maintain an adequate level of cGMP in the presence of 7-NI and enable HET0016 to fully restore the response. In the presence of ODQ, in contrast, HET0016 failed to fully restore the response to whisker stimulation. Apparently, a minimal amount of cGMP is required for the full response even when 20-HETE synthesis is inhibited and NOS is permitted to be activated. The small increase in the response seen when HET0016 is combined with ODQ compared with ODQ alone (17% vs. 14%; Fig. 8) is comparable in magnitude with the increase seen with HET0016 alone (24% vs. 21%; Fig. 4) and is less than the increase seen when HET0016 is given after 7-NI (25% vs. 18%; Fig. 5). These observations are consistent with the concept that endogenously produced NO largely suppresses the synthesis or downstream 20-HETE effects independent of the level of cGMP. It should also be appreciated that the interactions among signaling pathways described in the present study may differ in magnitude in the unanesthetized state where NOS inhibition exerts a relatively small effect on the evoked CBF response (22).
One might anticipate that HET0016 would enhance the LDF response to whisker stimulation in the absence of nNOS expression. Interestingly, HET0016 had little effect on the evoked LDF response in nNOS−/− mice. In agreement with others (29), the LDF response in nNOS−/− mice was not decreased compared with that of nNOS+/+ mice. Apparently, loss of nNOS throughout development results in alterations of pathways involved in 20-HETE signaling. Because l-NNA has no effect on the LDF response in nNOS−/− mice (29), the potential upregulation of eNOS is unlikely to be responsible for suppressing 20-HETE synthesis in nNOS−/− mice.
The kinetics of the NO inhibition of CYP ω-hydroxylase activity also needs to be considered. Dissociation of NO from the heme on CYPs and the reversal of CYP inhibition appears to occur on a time scale of minutes at physiological concentrations of NO (48). In the absence of other active mechanisms to regulate enzyme activity or to rapidly remove NO, increases in NO during activation could suppress CYP ω-hydroxylase activity for significant durations relative to the time constants for the on and off responses to whisker stimulation. Consequently, we cannot exclude that part of the interaction between 20-HETE and nNOS inhibitors is related to the effects of 7-NI on increasing 20-HETE-dependent baseline vascular tone rather than a dynamic regulation of 20-HETE synthesis during each episode of neuronal activation.
EETs interactions.
Previous work has demonstrated that epoxygenase inhibitors reduced the LDF response to electrical forepaw stimulation but that combining epoxygenase inhibition with the NOS inhibitor l-NNA produced no additive effect in reducing the LDF response (37). The present study confirmed the lack of an additive effect of blocking the epoxygenase and NOS pathways by using a more selective nNOS inhibitor (7-NI), an EETs antagonist (14,15-EEZE) rather than an EETs synthesis inhibitor, and a different modality of activation (vibrissae stimulation). Thus the EETs-dependent component of neurovascular coupling appears to require the activation of nNOS rather than eNOS, and the interaction of NO with EETs signaling appears to occur in regions of cerebral cortex activated by both forepaw and whisker stimulation.
Of interest in the present study is the finding that the inhibition of the LDF response to whisker stimulation by the EETs antagonist could be restored in the presence of nNOS inhibition if 20-HETE synthesis was concurrently inhibited. This result indicates that the loss of the ability of the EETs antagonist to reduce the LDF in the presence of nNOS inhibition requires the synthesis of 20-HETE. One explanation for these findings is that a dynamic increase in NO production during neuronal activation normally suppresses 20-HETE synthesis and downstream signaling, which permits EETs to evoke vasodilation, whereas after nNOS inhibition, 20-HETE synthesis and signaling may be increased during activation and limit EETs-dependent dilation. Because EETs open VSM KCa channels (20) and 20-HETE closes KCa channels (19), 20-HETE might oppose the vasodilator effect of EETs. Alternatively, EETs have also been found to open KCa channels in astrocytes (21, 49). Perhaps increased 20-HETE generated following nNOS inhibition limits VSM hyperpolarization following neuronal activation by closing VSM KCa channels and thereby limits the effect of K+ signaling between astrocyte endfeet and nearby VSM (17).
Previous studies indicate that NO has a different affinity to bind to the heme of different families of CYPs, and high levels of NO inhibit enzyme activity and the transcription expression of different hepatic CYPs to different extents (14, 26, 41, 45). In the kidney, the inhibition of NOS was found to augment ω-hydroxylase activity without affecting epoxygenase activity (35). Our observation that blocking the epoxygenase pathway with a synthesis inhibitor or an antagonist exerts a large effect on the LDF response to whisker stimulation, in contrast with the minor effect of ω-hydroxylase inhibition, suggests that NO generated during activation does not significantly inhibit epoxygenase activity. However, the dynamic response to activation may normally depend on the release of EETs from preformed storage pools in membrane phospholipids. The reduction in the LDF response, which occurred after EETs synthesis inhibition, was monitored after 1 h of inhibitor administration, and preformed EETs storage pools might have become depleted by that time.
In summary, the results of the current study indicate that inhibition of the synthesis of 20-HETE does not prevent the increase in cortical CBF during the physiological activation of the whisker barrel cortex in either rats or mice. This lack of effect of 20-HETE synthesis inhibition depends on the normal production of NO by nNOS, which itself can inhibit 20-HETE formation. When nNOS is inhibited, 20-HETE then exerts a greater influence on the CBF response to whisker stimulation, whereas the contribution of the EETs pathway is diminished. When NO and 20-HETE synthesis are simultaneously inhibited, the contribution of EETs becomes more apparent. The data are consistent with the hypothesis, outlined in Fig. 1, that NO permits EETs-dependent vasodilation by suppressing 20-HETE synthesis or signaling during functional activation in the cerebral cortex.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-59996, the National General Medical Sciences Institute Grant GM-31278, and the Robert A. Welch Foundation.
Supplementary Material
Acknowledgments
We thank Kathleen Kibler for technical assistance and Tzipora Sofare for editorial assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alkayed NJ, Birks EK, Narayanan J, Petrie KA, Kohler-Cabot AE, Harder DR. Role of P-450 arachidonic acid expoygenase in the response of cerebral blood flow to glutamate in rats. Stroke 28: 1066–1072, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke 27: 971–979, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Alonso-Galicia M, Hudetz AG, Shen H, Harder DR, Roman RJ. Contribution of 20-HETE to vasodilator actions of nitric oxide in the cerebral microcirculation. Stroke 30: 2727–2734, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Alonzo-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension 29: 320–325, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Amruthesh SC, Falck JR, Ellis EF. Brain synthesis and cerebrovascular action of epoxygenase metabolites of arachidonic acid. J Neurochem 58: 503–510, 1992. [DOI] [PubMed] [Google Scholar]
- 6.Ayata C, Ma J, Meng W, Huang P, Moskowitz MA. l-NA-sensitive rCBF augmentation during vibrissal stimulation in type III nitric oxide synthase mutant mice. J Cereb Blood Flow Metab 16: 539–541, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Blanco VM, Stern JE, Filosa JA. Tone-dependent vascular responses to astrocyte-derived signals. Am J Physiol Heart Circ Physiol 294: H2855–H2863, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buerk DG, Ances BM, Greenberg JH, Detre JA. Temporal dynamics of brain tissue nitric oxide during functional forepaw stimulation in rats. Neuroimage 18: 1–9, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke 34: 1269–1275, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Cholet N, Bonvento G, Seylaz J. Effect of neuronal NO synthase inhibition on the cerebral vasodilatory response to somatosensory stimulation. Brain Res 708: 197–200, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci 27: 4452–4459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dirnagl U, Lindauer U, Villringer A. Role of nitric oxide in the coupling of cerebral blood flow to neuronal activation in rats. Neurosci Lett 149: 43–46, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Dirnagl U, Niwa K, Lindauer U, Villringer A. Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am J Physiol Heart Circ Physiol 267: H296–H301, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Eum HA, Yeom DH, Lee SM. Role of nitric oxide in the inhibition of liver cytochrome P450 during sepsis. Nitric Oxide 15: 423–431, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Fang X, Faraci FM, Kaduce TL, Harmon S, Modrick ML, Hu S, Moore SA, Falck JR, Weintraub NL, Spector AA. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse basilar artery: role of cyclooxygenase. Am J Physiol Heart Circ Physiol 291: H2301–H2307, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res 95: e73–e81, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci 9: 1397–1403, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier KM, Deeter C, Krishna UM, Reddy YK, Bondlela M, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ Res 90: 1028–1036, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res 87: 60–65, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Gebremedhin D, Ma YH, Falck JR, Roman RJ, VanRollins M, Harder DR. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol Heart Circ Physiol 263: H519–H525, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Gebremedhin D, Yamaura K, Zhang C, Bylund J, Koehler RC, Harder DR. Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated K+ channels in rat hippocampal astrocytes. J Neurosci 23: 1678–1687, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotoh J, Kuang TY, Nakao Y, Cohen DM, Melzer P, Itoh Y, Pak H, Pettigrew K, Sokoloff L. Regional differences in mechanisms of cerebral circulatory response to neuronal activation. Am J Physiol Heart Circ Physiol 280: H821–H829, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, Roman R. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol Heart Circ Physiol 266: H2098–H2107, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Irikura K, Maynard KI, Moskowitz MA. Importance of nitric oxide synthase inhibition to the attenuated vascular responses induced by topical l-nitroarginine during vibrissal stimulation. J Cereb Blood Flow Metab 14: 45–48, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Kehl F, Cambj-Sapunar L, Maier KG, Miyata N, Kametani S, Okamoto H, Hudetz AG, Schulte ML, Zagorac D, Harder DR, Roman RJ. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol 282: H1556–H1565, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Khatsenko O, Kikkawa Y. Nitric oxide differentially affects constitutive cytochrome P450 isoforms in rat liver. J Pharmacol Exp Ther 280: 1463–1470, 1997. [PubMed] [Google Scholar]
- 27.Leffler CW, Fedinec AL. Newborn piglet cerebral microvascular responses to epoxyeicosatrienoic acids. Am J Physiol Heart Circ Physiol 273: H333–H338, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Lindauer U, Megow D, Matsuda H, Dirnagl U. Nitric oxide: a modulator, but not a mediator, of neurovascular coupling in rat somatosensory cortex. Am J Physiol Heart Circ Physiol 277: H799–H811, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Ma J, Ayata C, Huang PL, Fishman MC, Moskowitz MA. Regional cerebral blood flow response to vibrissal stimulation in mice lacking type I NOS gene expression. Am J Physiol Heart Circ Physiol 270: H1085–H1090, 1996. [DOI] [PubMed] [Google Scholar]
- 30.MacKenzie GM, Rose S, Bland-Ward PA, Moore PK, Jenner P, Marsden CD. Time course of inhibition of brain nitric oxide synthase by 7-nitro indazole. Neuroreport 5: 1993–1996, 1994. [DOI] [PubMed] [Google Scholar]
- 31.Meno JR, Crum AV, Winn HR. Effect of adenosine receptor blockade on pial arteriolar dilation during sciatic nerve stimulation. Am J Physiol Heart Circ Physiol 281: H2018–H2027, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431: 195–199, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Newman EA Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci 25: 5502–5510, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci 20: 763–770, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oyekan AO, Youseff T, Fulton D, Quilley J, McGiff JC. Renal cytochrome P450 omega-hydroxylase and epoxygenase activity are differentially modified by nitric oxide and sodium chloride. J Clin Invest 104: 1131–1137, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng X, Carhuapoma JR, Bhardwaj A, Alkayed NJ, Falck JR, Harder DR, Traystman RJ, Koehler RC. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol Heart Circ Physiol 283: H2029–H2037, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Peng X, Zhang C, Alkayed NJ, Harder DR, Koehler RC. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab 24: 509–517, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Poloyac SM, Zhang Y, Bies RR, Kochanek PM, Graham SH. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. J Cereb Blood Flow Metab 26: 1551–1561, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, Liu X, Gebremedhin D, Falck JR, Harder DR, Koehler RC. Interaction of mechanisms involving epoxyeicosatrienoic acids, adenosine receptors, and metabotropic glutamate receptors in neurovascular coupling in rat whisker barrel cortex. J Cereb Blood Flow Metab 28: 111–125, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sobey CG, Faraci FM. Effects of a novel inhibitor of guanylyl cyclase on dilator responses of mouse cerebral arterioles. Stroke 28: 837–842, 1997. [DOI] [PubMed] [Google Scholar]
- 41.Stadler J, Trockfeld J, Schmalix WA, Brill T, Siewert JR, Greim H, Doehmer J. Inhibition of cytochromes P4501A by nitric oxide. Proc Natl Acad Sci USA 91: 3559–3563, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun CW, Alonso-Galicia M, Taheri MR, Falck JR, Harder DR, Roman RJ. Nitric oxide-20-hydroxyeicosatetraenoic acid interaction in the regulation of K+ channel activity and vascular tone in renal arterioles. Circ Res 83: 1069–1079, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Sun CW, Falck JR, Okamoto H, Harder DR, Roman RJ. Role of cGMP versus 20-HETE in the vasodilator response to nitric oxide in rat cerebral arteries. Am J Physiol Heart Circ Physiol 279: H339–H350, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 9: 260–267, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Vernia S, Beaune P, Coloma J, Lopez-Garcia MP. Differential sensitivity of rat hepatocyte CYP isoforms to self-generated nitric oxide. FEBS Lett 488: 59–63, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9: 816–823, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Williams JM, Sharma M, Anjaiahh S, Falck JR, Roman RJ. Role of endogenous CYP450 metabolites of arachidonic acid in maintaining the glomerular protein permeability barrier. Am J Physiol Renal Physiol 293: F501–F505, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wink DA, Osawa Y, Darbyshire JF, Jones CR, Eshenaur SC, Nims RW. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys 300: 115–123, 1993. [DOI] [PubMed] [Google Scholar]
- 49.Yamaura K, Gebremedhin D, Zhang C, Narayanan J, Hoefert K, Jacobs ER, Koehler RC, Harder DR. Contribution of epoxyeicosatrienoic acids to the hypoxia-induced activation of Ca2+-activated K+ channel current in cultured rat hippocampal astrocytes. Neuroscience 143: 703–716, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Yang G, Iadecola C. Obligatory role of NO in glutamate-dependent hyperemia evoked from cerebellar parallel fibers. Am J Physiol Regul Integr Comp Physiol 272: R1155–R1161, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Yu M, Sun CW, Maier KG, Harder DR, Roman RJ. Mechanism of cGMP contribution to the vasodilator response to NO in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 282: H1724–H1731, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 6: 43–50, 2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.