Abstract
Recurrent and intermittent nocturnal hypoxia is characteristic of several diseases including chronic obstructive pulmonary disease, congestive heart failure, obesity-hypoventilation syndrome, and obstructive sleep apnea. The contribution of hypoxia to cardiovascular morbidity and mortality in these disease states is unclear, however. To investigate the impact of recurrent nocturnal hypoxia on hemodynamics, sympathetic activity, and vascular tone we evaluated 10 normal volunteers before and after 14 nights of nocturnal sustained hypoxia (mean oxygen saturation 84.2%, 9 h/night). Over the exposure, subjects exhibited ventilatory acclimatization to hypoxia as evidenced by an increase in resting ventilation (arterial Pco2 41.8 ± 1.5 vs. 37.5 ± 1.3 mmHg, mean ± SD; P < 0.05) and in the isocapnic hypoxic ventilatory response (slope 0.49 ± 0.1 vs. 1.32 ± 0.2 l/min per 1% fall in saturation; P < 0.05). Subjects exhibited a significant increase in mean arterial pressure (86.7 ± 6.1 vs. 90.5 ± 7.6 mmHg; P < 0.001), muscle sympathetic nerve activity (20.8 ± 2.8 vs. 28.2 ± 3.3 bursts/min; P < 0.01), and forearm vascular resistance (39.6 ± 3.5 vs. 47.5 ± 4.8 mmHg·ml−1·100 g tissue·min; P < 0.05). Forearm blood flow during acute isocapnic hypoxia was increased after exposure but during selective brachial intra-arterial vascular infusion of the alpha-blocker phentolamine it was unchanged after exposure. Finally, there was a decrease in reactive hyperemia to 15 min of forearm ischemia after the hypoxic exposure. Recurrent nocturnal hypoxia thus increases sympathetic activity and alters peripheral vascular tone. These changes may contribute to the increased cardiovascular and cerebrovascular risk associated with clinical diseases that are associated with chronic recurrent hypoxia.
Keywords: acclimatization, vascular resistance, pathophysiology, chronic obstructive pulmonary disease
chronic obstructive pulmonary disease (COPD) is a major health issue characterized by sustained hypoxemia in its advanced stages. In addition to respiratory failure that contributes to mortality in advanced COPD, there is also an increase in cardiovascular morbidity and mortality among these patients (22, 23, 25, 30). In these conditions there is recurrent sustained hypoxia lasting from several minutes to hours that may be a potential mediator of cardiovascular physiopathology.
Hypoxic exposures in normal subjects, lasting hours (9, 13) or days (7, 9, 13), results in a sustained increase in ventilation, which is termed ventilatory acclimatization to hypoxia (VAH). In contrast to the increase in ventilation during acute hypoxia, acclimatization is characterized by a sustained increase in ventilation that outlasts the exposure (13). Acclimatization is mediated through peripheral chemoreceptors and is associated with an increase in peripheral chemoreflex gain (7). The mechanism for acclimatization is thought to be altered neuromodulation of the carotid chemoreceptor, with endothelin (4), angiotensin (16), and other substances (2) believed to be contributors. Although most studies have employed continuous exposures to hypoxia or altitude to induce acclimatization, recently evidence has emerged that recurrent hypoxic exposures of as little as 4 h/day for 5 days/wk will also induce acclimatization in normal volunteers (15).
Increasing evidence suggests that exposure to hypoxia may also alter muscle sympathetic nervous system activity (MSNA) and arterial blood pressure. Several authors have documented an increase in sympathetic nervous system activation, both with and without significant increases in arterial blood pressure, that persists up to an hour after termination of various durations of sustained hypoxic exposure (3, 6, 12, 20, 27).
Interestingly, altered peripheral chemosensitivity is a proposed mechanism contributing to this sustained sympathoexcitation. Consistent with other investigators, we have demonstrated that exposures to sustained hypoxia for as short as 8 h lead to acclimatization and an increase in diastolic blood pressure (1, 10). After that exposure, there was altered vascular reactivity as evidenced by the presence of impaired hypoxic vasodilation (10). However, in a subsequent study MSNA showed a significant trend toward a decrease in activity after the 8-h exposure, pointing to a potential dissociation between changes at the level of the chemoreflex and MSNA after hypoxic exposure (28).
In the present study we have further investigated the time course of ventilatory, hemodynamic, MSNA, and vascular reactivity changes in response to recurrent sustained hypoxia. We hypothesized that normal volunteers exposed to a longer duration of sustained recurrent hypoxia would demonstrate VAH as well as sustained sympathoexcitation and altered hemodynamics and vascular reactivity consistent with the evolution of a milieu that would be consistent with increased risk for cardiovascular disease such as that seen in patients with COPD. To investigate this hypothesis we evaluated changes in normal volunteers exposed to 14 nights of 9 h of sustained hypoxia.
METHODS
Subjects
Ten of eleven selected healthy, nonsmoking, normotensive subjects, free of vasoactive medications, completed the study (MSNA recording was not successfully obtained in 1 subject). Data analysis was completed only in the 10 subjects who completed the whole study. The subjects had a mean age of 27 ± 1.5 yr and a body mass index of 23 ± 0.9 kg/m2. All subjects underwent a screening history and physical examination to ensure they were free of significant cardiac, pulmonary, or neurological disease before providing written informed consent. Subjects who had journeyed to or lived at an altitude of ≥2,500 m in the 6 mo before enrolling in the study were excluded from participating. Six men and four women completed the study. All women began exposure during the week after menses to minimize the possible confounding effects of hormonal changes on vascular function, and all tested negative for pregnancy (urinary β-human chorionic gonadotropin test). This protocol was reviewed and approved by the Institutional Review Board at Beth Israel Deaconess Medical Center and conformed to the provisions of the Declaration of Helsinki.
Experimental Protocols
Subjects were admitted to the Clinical Research Center at Beth Israel Deaconess Medical Center at the start of the protocol. Subjects were studied in the morning (8 AM) after an overnight fast before and after a 14-night hypoxic exposure in an altitude tent. After baseline measurement of arterial pressure by brachial cuff and forearm blood flow (FBF) by venous occlusion plethysmography, reactive hyperemia (RH) was assessed. Subjects were then instrumented in the supine position. A brachial artery catheter was placed in the nondominant arm, and a microelectrode was placed in the peroneal nerve for recording of muscle sympathetic activity. After a 30-min recovery period following successful instrumentation, data collection was initiated with the subject breathing room air with continuous measurement of heart rate (HR), arterial pressure, and MSNA and measurement of FBF bilaterally in triplicate. Once baseline recordings were completed, intra-arterial vascular infusion of phentolamine was begun through the brachial artery catheter with a loading dose followed by a continuous infusion and all measurements were repeated. Next, progressive isocapnic hypoxia was induced with the technique of Rebuck and Campbell (21) while continuing the intra-arterial vascular phentolamine infusion. During hypoxia, HR, arterial pressure, and MSNA were again recorded continuously and FBF was measured bilaterally at regular intervals. Room temperature was maintained constant at ∼24°C throughout data collection. A schema of the testing protocol is shown in Fig. 1.
Fig. 1.
Time line of the experiment. Experiments started at 8 AM. Reactive hyperemia (RH) was performed before instrumentation [i.e., arterial line and muscle sympathetic nervous system activity (MSNA)]. After instrumentation 15 min of recovery was allowed, and then baseline recording, intra-arterial vascular phentolamine infusion in the experimental arm, and isocapnic hypoxia were performed.
Subjects were exposed to 9 h of continuous poikilocapnic hypoxia between the hours of 10 PM and 7 AM for 14 consecutive nights. Subjects underwent acclimation to the hypoxic exposure with graduated increases in “altitude” over three nights. Altitude levels started at sea level, followed by one night at 7,700 ft, one night at 10,000 ft, and then 13,000 ft for 14 consecutive nights. Independent evaluation of the inspired O2 fraction (FiO2) at this altitude setting with an oxygen sensor showed FiO2 to be 0.13 with the tent system set at 13,000 ft.
The hypoxic exposure was achieved with a commercially available normobaric “altitude tent” (Colorado Altitude Training, Colorado Springs, CO). Subjects slept in a standard hospital bed while in the tent, which measured 9 × 7 × 6 ft. Altitude was set and continuously monitored with a central controller with real-time output. Altitude and CO2 levels within the tent were monitored continuously throughout the exposure. CO2 was removed with soda-phosphate crystals and a fan-driven system within the tent to allow continuous passage of tent gas across the system to allow stable CO2 levels to be maintained. Independent verification of CO2 levels performed by an automated CO2 monitoring system (Realterm, Colorado Altitude Training) yielded 0.4% as an average value during the night (range: 0.1–0.52%). O2 saturation SaO2 was monitored continuously overnight, and mean SaO2 were 84.2% during the exposure.
Measurements
Respiratory variables.
To measure the ventilatory response to hypoxia, subjects breathed from a closed circuit connected to a 7-liter bag-in-box. The box was connected to a 10-liter Wedge Spirometer (Med Science, St. Louis, MO). Linear displacement of the spirometer was recorded continuously and was proportional to volume. SaO2 was monitored with a pulse oximeter (Biox model 3740; Ohmeda, Louisville, KY). Subjects were allowed to breathe during >1 min through a mouthpiece connected to room air, wearing nose clips to be acclimatized to the device. CO2 fraction was measured continuously with an infrared gas analyzer connected to the mouthpiece (model 17630; Vacu-med, Ventura, CA). We used the last 20 s of this resting ventilation to measure resting end-tidal CO2 before and after exposure. The subject was then switched to the rebreathing circuit, filled with calibrated gas made up of 24% O2-7% CO2 balanced with N2 such that the bag volume was 60% of the subject's vital capacity (VC) + 1 liter. CO2 was removed as necessary from the circuit by directing a variable amount of the flow through a scrubber to maintain isocapnia. After the subject breathed on the circuit for 1 min, N2 was added to increase the bag volume to 1 liter above VC to hasten the decrease in SaO2. When SaO2 decreased to 92%, oxygen was added to the circuit at 0.1–0.2 l/min through a pediatric flowmeter to allow precise control of the rate of fall of saturation. Oxygen flow was adjusted to allow a progressive decrease in SaO2, so that at least 2 min of data could be collected between SaO2 90% and 85% and between SaO2 85% and 80%. Expiratory tidal volume was obtained by integration of the flow signal. Breath-by-breath respiratory frequency (fR) was obtained by the ratio 1/Ttot, where Ttot is the duration of each respiratory cycle. Breath-by-breath exhaled minute ventilation (V̇e) was calculated by multiplying tidal volume and fR. A linear correlation was used to obtain the slope of the SaO2-V̇e relationship.
Cardiovascular variables.
HR was taken from the electrocardiogram. Dominant arm arterial pressure was measured at 5-min intervals with an automated arm cuff sphygmomanometer (Dinamap model, Critikon, Tampa, FL). Nondominant arm intra-arterial pressure was continuously recorded through the arterial catheter with a catheter-transducer system (Transpac II, Abbott Critical Care Systems, Chicago, IL).
Forearm blood flow.
Blood flow was measured in both forearms by venous occlusion plethysmography (EC6 plethysmography, Hokanson, Bellevue, WA) and mercury-in-Silastic strain gauges. The arm was placed in a passive position above the level of the right atrium. The strain gauge was placed at the midpoint of the forearm with a distally placed occlusion cuff and a proximal venous occlusion cuff. Before data collection a series of occlusions was performed to determine the venous occlusion pressure that resulted in the steepest slope of the arterial inflow curve. This typically yielded venous pressure of 45–50 mmHg. The wrist arterial occlusion cuff was inflated to 200 mmHg. After 1 min, the collecting cuff positioned above the elbow was rapidly inflated above venous pressures for 8 s every 16 s. An average of four to six flow measurements was used in the computation of the results at each time point. FBF was expressed in milliliters per 100 ml of limb tissue per minute. Forearm vascular resistance (FVR; expressed in mmHg·ml−1·100 ml tissue·min) was obtained by dividing mean arterial pressure (MAP) by FBF. During the pharmacological trial, results were expressed as forearm vascular conductance calculated as (FBF/MAP) × 100 and expressed in arbitrary conductance units.
Brachial Artery Cannulation and Intra-Arterial Vascular Phentolamine Infusion
Brachial artery cannulation was obtained with a 5-cm, 20-gauge catheter that was placed in the nondominant arm under sterile conditions with local anesthesia (2–3 ml of 1% lidocaine). The catheter was continuously flushed (3 ml/h) with heparinized saline (2 U/ml). Intra-arterial pressure was continuously monitored, and intra-arterial vascular medication infusion was accomplished with a three-way stopcock (Baxter, Deerfield, IL), which was placed in series with the transducer system. Room air arterial blood gases were also obtained before and after exposure in these subjects. Intra-arterial vascular phentolamine infusion consisted of a 5-min loading dose (100 μg/min) followed by a continuous infusion (25 μg/min) until completion of the testing. Variables were measured sequentially in three conditions. The conditions were normoxia, followed by normoxia + phentolamine, followed by acute isocapnic hypoxia + phentolamine.
Reactive hyperemia.
RH was induced by inflating the upper cuff to 200 mmHg for 15 min, after which the cuff was deflated for 15 s and FBF was measured for 2.5 min as described above. Peak blood flow was evaluated as an average of the first three FBF measurements after release of cuff occlusion. Vascular reactivity was considered to be the total excess blood flow above baseline, calculated as the difference between the area under the curve (AUC) during RH and baseline.
Muscle sympathetic nerve activity.
We obtained nerve recordings with standard tungsten microelectrodes inserted into the peroneal nerve posterior into the popliteal area, after localization by surface stimulation. Signals were filtered, amplified, and full-wave rectified. The rectified signal was integrated for display on an oscilloscope and for recording (Nerve Traffic Analyzer, model 662c-3, Bioengineering Dept., University of Iowa, Iowa City, IA). Electrode position in muscle fibers was confirmed by pulse synchronous bursts of activity occurring 1.2–1.4 s after the QRS complex, reproducible activation during the second phase of the Valsalva maneuver, elicitation of afferent nerve activity by mild muscle stretching, and the absence of response to startle. Sympathetic bursts were identified with a specific algorithm described by Hamner and Taylor (11) and Matlab software (The Mathworks, Natick, MA). For purposes of quantification MSNA was reported in 5-min periods and expressed as burst frequency (bursts/min) normalized by heart rate (bursts/100 heart beats).
Data Analysis
We averaged nerve activity parameters over a window of 5 min of data collection in normoxic conditions and 2-min windows during the end of hypoxic ventilatory response for acute isocapnic hypoxia (SaO2 between 85% and 80%). HR and MAP were averaged over the corresponding time intervals during which plethysmographic forearm flow measurements were made. An MSNA signal-to-noise ratio of 3/1 or greater was obtained in seven subjects both before and after exposure. The analysis of MSNA was performed on these seven subjects. For technical reasons RH was not obtained in one of the subjects. Overall analysis was completed on nine subjects. Brachial artery cannulation was obtained both before and after exposure in seven subjects; analysis was performed only in the subjects having successful cannulation at both time points.
Statistics
P values <0.05 were considered statistically significant. Baseline values were compared from the two different trials and preexposure to postexposure with a two-tail distribution paired t-test. Differences among multiple means were evaluated by ANOVA corrected for multiple measures; when overall differences were detected, individual means were tested by Bonferroni test, with a P value <0.003 necessary to be considered significant. Except where otherwise noted, data are reported as means ± SE.
RESULTS
Changes in Ventilation
Changes in resting ventilation were assessed by room air arterial blood gas measurements. There was a significant decrease in baseline arterial Paco2 after exposure [41.75 ± 1.5 (pre) vs. 37.50 ± 1.34 (post) mmHg, P < 0.05]. Peripheral chemoreceptor responsiveness, evaluated by the slope of the ventilatory response to progressive isocapnic hypoxia, revealed a significant increase after exposure [0.49 ± 0.06 (pre) vs. 1.32 ± 0.24 (post) l/min per 1% fall in SaO2, P < 0.05]. These results are summarized in Table 1.
Table 1.
Ventilatory changes after hypoxic exposure
| PaO2, mmHg | PaCO2, mmHg | pH | HCO3−, mmol/l | HVR Slope | |
|---|---|---|---|---|---|
| Baseline | 102.50±3.46 | 41.75±1.50 | 7.41±0.003 | 27.00±0.80 | 0.49±0.06 |
| Postexposure | 111.00±2.97 | 37.50±1.34* | 7.42±0.010 | 24.67±0.56† | 1.32±0.24* |
Values are means ± SE. PaO2, arterial Po2; PaCO2, arterial Pco2; HVR, hypoxic ventilatory response. HVR slope is expressed in liters per minute per 1% fall in O2 saturation.
P < 0.05,
P < 0.01 compared with baseline value.
Changes in Hemodynamic Variables
Heart rate and blood pressure.
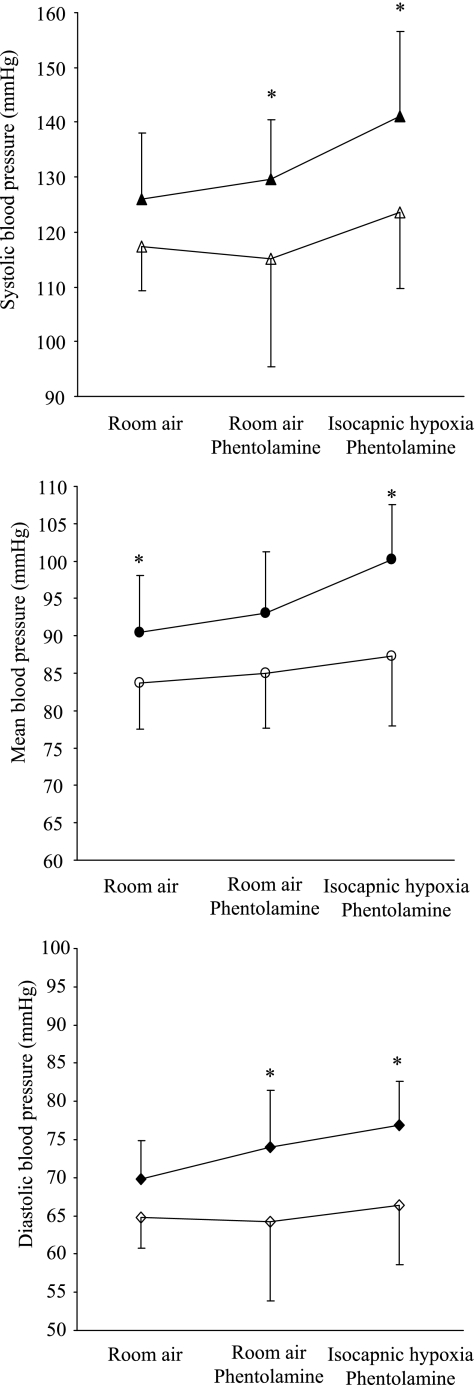
After exposure, there is a significant increase in MAP in room air conditions [86.7 ± 6.1 (pre) vs. 90.5 ± 7.6 (post) mmHg, ANOVA P < 0.001]. Although there is a small increase in blood pressure during isocapnic hypoxia, upon detailed analysis there is no significant change in blood pressure (systolic, diastolic, or mean) across testing conditions either before or after exposure. These results are summarized in Fig. 2. Resting HR did not change significantly after exposure [59.4 ± 2 (pre) vs. 61.55 ± 2.5 (post) beats/min, P = 0.15].
Fig. 2.
Blood pressure changes after 2-wk exposure. Open symbols, before recurrent hypoxia; closed symbols, after 2 wk of recurrent hypoxia. Significant differences were found between measurement for systolic, mean, and diastolic blood pressure (ANOVA P < 0.001 for all). *Significant differences in postexposure compared with preexposure values in post hoc analysis by Bonferroni test. Although across testing conditions arterial blood pressure tended to increase during isocapnic hypoxia, this change did not reach statistical significance.
Limb heart rate and blood pressure.
There was a significant increase in blood pressure after exposure. MAP [93.3 ± 4.8 (pre) vs. 99.4 ± 3.5 (post) mmHg, P < 0.05], diastolic blood pressure [69.2 ± 2.8 (pre) vs. 74.8 ± 2.3 (post) mmHg, P < 0.05], and systolic blood pressure [103.5 ± 8.4 (pre) vs. 112.17 ± 10.2 (post) mmHg, P < 0.05] all showed a significant increase after exposure. Resting HR did not change significantly after exposure [59.4 ± 2 (pre) vs. 61.55 ± 2.5 (post) beats/min, P = 0.15].
Vascular control.
After exposure, there was a significant increase in baseline (normoxic) FVR in the control forearm [39.6 ± 3.5 (pre) vs. 47.5 ± 4.8 (post) mmHg·ml−1·100 ml tissue·min, P < 0.05].
Nonspecific α-Adrenergic Blockade with Intra-Arterial Phentolamine Infusion
Subjects had FBF measured in both the arterial catheter instrumented arm (experimental forearm) and the noninstrumented arm (control forearm).
There was significant difference in both control and experimental FBF measurements across conditions (Fig. 3, ANOVA P < 0.01 and P < 0.001, respectively). In the control forearm, across testing conditions a significant increase in blood flow compared with room air breathing occurred only during isocapnic hypoxia after exposure. This value is not significantly higher than that seen during isocapnic hypoxia before exposure. However, in the experiment forearm, a significant increase in blood flow occurred with intra-arterial vascular phentolamine infusion and a further significant increase was seen with isocapnic hypoxia in the setting of continued intra-arterial vascular phentolamine infusion. However, for the experimental forearm there was no difference in these blood flow values compared in the same condition before and after exposure. When experimental FBF is normalized for control FBF in the same condition, there is a trend toward lower values after exposure that does not reach statistical significance.
Fig. 3.
Control and experimental forearm blood flow (FBF) and the ratio of experimental to control flow before (○) and after (•) 2 wk of recurrent hypoxia. Significant changes occurred in all parameters by ANOVA analysis: P < 0.001, P < 0.01, and P < 0.001, respectively. No significant differences in postexposure compared with preexposure values were found. *Significant differences in values across testing conditions in post hoc analysis by Bonferroni test.
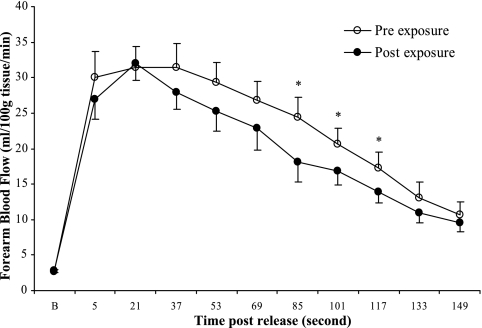
Reactive Hyperemia
The peak FBF after release of cuff occlusion was not significantly different after 2 wk of hypoxic exposure. Compared with preexposure values, FBF during RH was decreased after 2 wk of exposure. The AUC for RH shown in Fig. 4 exhibited a decrease that did not reach significance (P = 0.06). Analysis of the entire temporal response to RH during the 2.5-min interval of recording showed that FBF was decreased significantly at the time points 85, 101, and 117 s after cuff release (P < 0.05 for all) (Fig. 4).
Fig. 4.
FBF across time during RH plotted as individual values. *P < 0.05 compared with preexposure value.
Muscle Sympathetic Nerve Activity
Baseline room air MSNA, evaluated as burst frequency, was significantly elevated after exposure [n = 7, 20.8 ± 2.8 (pre) vs. 28.2 ± 3.3 (post) bursts/min and 35.6 ± 5.3 (pre) vs. 46.1 ± 5.2 (post) bursts/100 heartbeats, P < 0.01; Figs. 5 and 6] There was no significant change in MSNA response to isocapnic hypoxia after exposure (n = 4; Fig. 7). Isocapnic hypoxic HR did not change significantly after exposure [87.29 ± 6.34 (pre) vs. 93.23 ± 5.04 (post) beats/min, P = 0.5].
Fig. 5.
MSNA before (Pre) and after (Post) exposure for individual subjects (n = 7). hb, Heartbeat. *P < 0.01 compared with Pre value.
Fig. 6.
These 1-min records of 3 subjects were chosen as representative of the magnitude of increase in MSNA with exposure. MSNA analysis was performed on 5-min samples. Subject 1 went from 23.5 to 25.2 bursts/min, subject 4 went from 25.1 to 41.0 bursts/min, and subject 5 went from 9.0 to 13.9 bursts/min.
Fig. 7.
Changes in MSNA during isocapnic hypoxia before and after exposure. *P < 0.01 compared with Pre value.
DISCUSSION
The main findings of the present study are that chronic recurrent nocturnal hypoxia lasting 14 nights results in elevation of systemic arterial blood pressure with an increase in FVR and MSNA and there is a trend toward decreased forearm vascular vasodilation during RH and increased vasodilation in response to isocapnic hypoxic following exposure. These findings must be considered in the setting of the significant VAH demonstrated in our subjects. Although many studies have evaluated the effects of shorter-duration hypoxia, little is known about the effects of chronic recurrent hypoxia that characterizes such prevalent disorders as COPD and obesity-hypoventilation syndrome.
Consistent with the findings of others with shorter-duration exposures to hypoxia, subjects exhibited significant VAH after the hypoxic exposure in this study (8, 13). Interestingly, whereas the acute response to hypoxia has a significant contribution from both the central and peripheral chemoreceptors, short-term acclimatization is mediated primarily through peripheral chemoreceptors at the level of the carotid body (8, 29).
Hypoxic exposures from as brief as 2 h to long-term altitude exposures have been shown to contribute to the development of systemic blood pressure elevation that outlasts the exposure itself (3, 27). Here, the evidence of significant elevation in systemic blood pressure following 2 wk of nocturnal recurrent hypoxia, in the absence of a significant change in resting HR, would suggest that changes in peripheral vascular resistance are playing a primary role in the evolution of these changes. This potential mechanism is also suggested by the increase in baseline normoxic FVR seen after exposure in the noninstrumented forearm in our subjects.
The increases in MSNA seen with exposure, potentially mediated through increased chemoresponsiveness at the level of the carotid body, account for a substantial proportion of the increased peripheral vascular resistance (18). It is, however, clear that sympathetic nervous system-mediated changes in peripheral vascular tone are not sufficient to explain the vascular changes seen after our chronic recurrent hypoxic exposure.
Vasodilation during isocapnic hypoxia was enhanced after exposure. However, FBF during isocapnic hypoxia in the setting of nonspecific α-adrenergic blockade by intra-arterial vascular phentolamine infusion was not different from the preexposure value. Our results thus are not consistent with an enhanced vasodilatory mechanism but rather a lesser vasoconstrictor mechanism. With exposure to acute isocapnic hypoxia, we were not able to demonstrate a specific change of MSNA in the four subjects we were able to analyze. Interestingly, there was a suggestion of a decrease in the magnitude of the elevation in MSNA on exposure to acute isocapnic hypoxia after 2-wk exposure when expressed in bursts per 100 heartbeats. However, this last finding is of questionable significance. First, this decrease may be an artifact due to the increase in HR during isocapnic hypoxia, although this increase does not reach statistical significance. Second, there is no study that carefully looks at the significance of sympathetic activity normalized to HR for limb blood flow control. An alternative hypothesis would be that a change in sympathetic vascular coupling occurs after exposure to 2 wk of nocturnal hypoxia. This hypothesis is deserving of further study.
Although there was no significant change in the peak blood flow response to cuff occlusion during RH testing, there is a suggestion of a decrease in total flow response after exposure. Specifically, compared with preexposure values, there was a significant decrease in FBF at time points 85, 101, and 117 s after cuff release. This would suggest a significant change in the balance between vasoconstrictor and vasodilator tone likely driven by the increase in MSNA. This increased sympathetic tone may contribute to a more rapid rate of return toward baseline after peak vasodilation following RH. However, we have to acknowledge that compared to preexposure values the amount of decrease in forearm vascular flow during hyperemia is mild. In our study, we cannot conclude that there is a change in mechanisms of vascular dilatation rather than a change in sympathetic vasoconstriction tone. Although it remains an active area of investigation, there is reasonable evidence that the peak response to RH with a 15-min occlusion may be mediated primarily by factors other than nitric oxide (NO) (26). It has been shown convincingly that NO does play a prominent role in the middle to late phases of the response to RH (24). The findings here would suggest that chronic recurrent hypoxia of this duration may lead to a significant impairment of vasodilation in addition to increased sympathetic nervous system-mediated vasoconstriction. The relative contribution to the specific NO-mediated vasodilation is deserving of further investigation.
Although altered vasodilation and altered vasoconstriction are both reasonable to consider in accounting for the changes seen here, regardless of the mechanisms our findings are potentially important from a clinical perspective because these changes may represent an initial step in atherosclerosis and cardiovascular morbidity (5, 17). Interesting data from the National Health and Nutrition Examination Survey revealed that after smoking and other known risk factors for cardiovascular disease were included in the multivariate model, increasing severity of COPD was associated with increased mortality (19). The possible mechanisms by which COPD may independently increase cardiovascular risk remain to be defined. A leading mechanism by which this may occur is generation of proinflammatory cytokines leading to endothelial cell activation and a prothrombotic state in addition to promotion of hepatic production of C-reactive protein (14). The nature of the proinflammatory state that characterizes COPD is an active area of investigation. The mechanism by which the chronic intermittent hypoxia that characterizes severe COPD may add to this risk has not yet been defined.
Our findings of increased arterial blood pressure mediated through sustained sympathetic activation and increased peripheral resistance do demonstrate that chronic intermittent hypoxia can alter systemic control of blood pressure, in part through alterations in chemoreflex sensitivity. In addition, the vasodilation in response to RH is likely to be altered, which is consistent with significant endothelial dysfunction. These findings provide important insight into the physiological changes in response to chronic intermittent hypoxia in humans and may provide a foundation for future investigations to determine the mechanisms by which this type of hypoxic exposure that characterizes disease states such as COPD and obesity independently contributes to an increase in cardiovascular and cerebrovascular morbidity and mortality.
Finally, several limitations of this study must be acknowledged. Although we have demonstrated a clear increase in systemic blood pressure and FVR we cannot generalize these findings to all vascular beds. FBF is a reasonable estimate of skeletal muscle blood flow changes, but other vascular beds that have a substantial contribution to total peripheral resistance such as the splanchnic circulation were not evaluated. In addition, there was no direct measurement of cardiac output to further define potential sources of the elevation in blood pressure seen, although HR was unchanged. This measurement should be incorporated into future studies.
In addition, there was a nonsignificant increase in FBF in the control forearm, which was not receiving intra-arterial vascular phentolamine infusion, during intra-arterial vascular infusion in the contralateral arm after exposure. The intra-arterial vascular phentolamine infusion protocol has been utilized extensively in this lab and others and has been shown to have only local effects in the infusion forearm. Subjects were studied after a minimum of 30 min of recovery following termination of hypoxic exposure. In addition, there was further delay in the baseline measurements for the intra-arterial vascular phentolamine infusion protocol because of the time required for insertion of the arterial catheter and RH testing. Despite this fact there may be some contribution to this increase in FBF based on increasing time from the termination of the exposure between baseline measurements and measurements made during the intra-arterial vascular phentolamine infusion. Variability of the measurement technique is unlikely to completely explain the finding because the decrease was seen across virtually all subjects. One also can hypothesize that the response to phentolamine may have changed because of a change in sympathetic transduction if phentolamine was indeed systemically active.
In conclusion, chronic intermittent nocturnal hypoxia of 2-wk duration in normal volunteers leads to VAH, MSNA activation, and elevations of systemic blood pressure that outlast the exposure. It is likely that flow-dependent endothelial dysfunction as evidenced by RH testing is also altered. These findings suggest that hypoxic exposure of this type may lead to systemic changes that represent reasonable mediators of a significantly increased risk for cardiovascular and cerebrovascular morbidity in disease states characterized by intermittent hypoxia.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant HL-072648.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arabi Y, Morgan BJ, Goodman B, Puleo DS, Xie A, Skatrud JB. Daytime blood pressure elevation after nocturnal hypoxia. J Appl Physiol 87: 689–698, 1999. [DOI] [PubMed] [Google Scholar]
- 2.Bisgard GE Carotid body mechanisms in acclimatization to hypoxia. Respir Physiol 121: 237–246, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Calbet JA Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J Physiol 551: 379–386, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in adaptation of the carotid body to chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 282: L1314–L1323, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Cosentino F, Volpe M. Hypertension, stroke, endothelium. Curr Hypertens Rep 7: 68–71, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol 96: 754–761, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Dempsey JA, Forster HV. Mediation of ventilatory adaptations. Physiol Rev 62: 262–346, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Dwinell MR, Janssen PL, Pizarro J, Bisgard GE. Effects of carotid body hypocapnia during ventilatory acclimatization to hypoxia. J Appl Physiol 82: 118–124, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Forster HV, Bisgard GE, Klein JP. Effect of peripheral chemoreceptor denervation on acclimatization of goats during hypoxia. J Appl Physiol 50: 392–398, 1981. [DOI] [PubMed] [Google Scholar]
- 10.Gilmartin G, Tamisier R, Anand A, Cunnington D, Weiss JW. Evidence of impaired hypoxic vasodilation after intermediate-duration hypoxic exposure in humans. Am J Physiol Heart Circ Physiol 291: H2173–H2180, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Hamner JW, Taylor JA. Automated quantification of sympathetic beat-by-beat activity, independent of signal quality. J Appl Physiol 91: 1199–1206, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol 546: 921–929, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard LS, Robbins PA. Alterations in respiratory control during 8 h of isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol 78: 1098–1107, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Hunninghake DB Cardiovascular disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 44–49, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Katayama K, Sato Y, Ishida K, Mori S, Miyamura M. The effects of intermittent exposure to hypoxia during endurance exercise training on the ventilatory responses to hypoxia and hypercapnia in humans. Eur J Appl Physiol Occup Physiol 78: 189–194, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Lam SY, Fung ML, Leung PS. Regulation of the angiotensin-converting enzyme activity by a time-course hypoxia in the carotid body. J Appl Physiol 96: 809–813, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation 111: 363–368, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia—influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Mannino DM, Buist AS, Petty TL, Enright PL, Redd SC. Lung function and mortality in the United States: data from the First National Health and Nutrition Examination Survey follow up study. Thorax 58: 388–393, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol 79: 205–213, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Rebuck AS, Campbell EJ. A clinical method for assessing the ventilatory response to hypoxia. Am Rev Respir Dis 109: 345–350, 1974. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond WD, Heiss G. Lung function and incident coronary heart disease: The Atherosclerosis Risk in Communities Study. Am J Epidemiol 158: 1171–1181, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Schroeder EB, Welch VL, Evans GW, Heiss G. Impaired lung function and subclinical atherosclerosis. The ARIC Study. Atherosclerosis 180: 367–373, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Shipley RD, Kim SJ, Muller-Delp JM. Time course of flow-induced vasodilation in skeletal muscle: contributions of dilator and constrictor mechanisms. Am J Physiol Heart Circ Physiol 288: H1499–H1507, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases? The potential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation 107: 1514–1519, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Tagawa T, Imaizumi T, Endo T, Shiramoto M, Harasawa Y, Takeshita A. Role of nitric oxide in reactive hyperemia in human forearm vessels. Circulation 90: 2285–2290, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Tamisier R, Anand A, Nieto LM, Cunnington D, Weiss JW. Arterial pressure and muscle sympathetic nerve activity are increased after two hours of sustained but not cyclic hypoxia in healthy humans. J Appl Physiol 98: 343–349, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Tamisier R, Hunt BE, Gilmartin GS, Curley M, Anand A, Weiss JW. Hemodynamics and muscle sympathetic nerve activity after 8 h of sustained hypoxia in healthy humans. Am J Physiol Heart Circ Physiol 293: H3027–H3035, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Tansley JG, Fatemian M, Howard LS, Poulin MJ, Robbins PA. Changes in respiratory control during and after 48 h of isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol 85: 2125–2134, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Truelsen T, Prescott E, Lange P, Schnohr P, Boysen G. Lung function and risk of fatal and non-fatal stroke. The Copenhagen City Heart Study. Int J Epidemiol 30: 145–151, 2001. [DOI] [PubMed] [Google Scholar]