Abstract
Asymmetric dimethylarginine (ADMA), an endogenous inhibitor of nitric oxide (NO) synthase, has been proposed to be a mediator of vascular dysfunction during hyperhomocysteinemia. Levels of ADMA are regulated by dimethylarginine dimethylaminohydrolase (DDAH). Using both in vitro and in vivo approaches, we tested the hypothesis that hyperhomocysteinemia causes downregulation of the two genes encoding DDAH (Ddah1 and Ddah2). In the MS-1 murine endothelial cell line, the addition of homocysteine decreased NO production but did not elevate ADMA or alter levels of Ddah1 or Ddah2 mRNA. Mice heterozygous for cystathionine β-synthase (Cbs) and their wild-type littermates were fed either a control diet or a high-methionine/low-folate (HM/LF) diet to produce varying degrees of hyperhomocysteinemia. Maximal relaxation of the carotid artery to the endothelium-dependent dilator acetylcholine was decreased by ∼50% in Cbs+/− mice fed the HM/LF diet compared with Cbs+/+ mice fed the control diet (P < 0.001). Compared with control mice, hyperhomocysteinemic mice had lower levels of Ddah1 mRNA in the liver (P < 0.001) and lower levels of Ddah2 mRNA in the liver, lung, and kidney (P < 0.05). Downregulation of DDAH expression in hyperhomocysteinemic mice did not result in an increase in plasma ADMA, possibly due to a large decrease in hepatic methylation capacity (S-adenosylmethionine-to-S-adenosylhomocysteine ratio). Our findings demonstrate that hyperhomocysteinemia causes tissue-specific decreases in DDAH expression without altering plasma ADMA levels in mice with endothelial dysfunction.
Keywords: asymmetric dimethylarginine, endothelium, homocysteine, vascular function
asymmetric dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide (NO) synthase (NOS) (45). Elevation of plasma ADMA has been found to be associated with endothelial dysfunction due to decreased endothelial NO bioavailability in subjects with cardiovascular disease (44). Elevation of endogenous ADMA also may contribute to the “arginine paradox” in which NOS activity increases in response to administration of l-arginine at levels well above is its Km (4). Some recent clinical studies have suggested that the elevation of ADMA may be a biomarker predictive of adverse vascular outcomes in high-risk patients with diabetes mellitus, hypertension, or hypercholesterolemia (6, 30, 37, 39).
ADMA is produced from the proteolysis of certain proteins, such as histones and RNA-binding proteins, that contain dimethylated arginine residues (9, 47). Dimethylated arginine residues are formed posttranslationally by the transfer of methyl groups from S-adenosyl methionine (SAM) (4). A major byproduct of methyl transfer reactions is S-adenosyl homocysteine (SAH), which is subsequently hydrolyzed to adenosine and homocysteine. The rate of methyl transfer reactions is regulated by the intracellular concentrations of both SAM and SAH, and the SAM-to-SAH ratio is often referred to as the cellular “methylation capacity” (19). The accumulation of homocysteine from methyltransferase reactions leads to hyperhomocysteinemia, an established risk factor for cardiovascular disease and stroke (23).
Because of the metabolic link between ADMA and homocysteine, ADMA could be a potential mediator of endothelial dysfunction in hyperhomocysteinemia (5, 7, 38). It has been suggested that the elevation of ADMA in hyperhomocysteinemia might be mediated by an inhibitory effect of homocysteine on the expression or activity of dimethylarginine dimethylaminohydrolase (DDAH) (21, 38, 42), which hydrolyzes ADMA to citrulline and dimethylamine. Some recent studies, however, have questioned the association between plasma ADMA and plasma total homocysteine (tHcy) (2, 26, 29), and others have suggested that hyperhomocysteinemia can produce endothelial dysfunction without elevating plasma ADMA (14, 25). It is possible that ADMA may accumulate intracellularly and inhibit endothelial function even in the absence of elevation of plasma ADMA, since ADMA is actively taken up by endothelial cells (12).
Humans and mice contain two distinct Ddah genes that encode two separate DDAH proteins (DDAH-1 and DDAH-2). Previous surveys of human tissues by Northern blot analysis (32) or mRNA dot blot analysis (41) have suggested that DDAH-1 and DDAH-2 have distinct tissue distributions, with DDAH-2 expressed preferentially in highly vascularized tissues. The tissue distribution of DDAH-1 and DDAH-2 has not been examined in mice, and it is not known if hyperhomocysteinemia affects the expression of Ddah genes in vivo.
In the present study, we tested the hypothesis that homocysteine downregulates the expression of murine DDAH-1 and DDAH-2 in vitro and in vivo. We used a murine endothelial cell line and a mouse model of hyperhomocysteinemia that produces endothelial dysfunction. Our findings indicate that DDAH-1 and DDAH-2 have distinct tissue distributions in mice and that hyperhomocysteinemia causes tissue-specific downregulation of DDAH mRNA and protein. Interestingly, we found that homocysteine-associated endothelial dysfunction occurred without a significant elevation of ADMA in plasma or the liver.
METHODS
Cell culture.
The MS-1 murine pancreatic islet endothelial cell line was obtained from the American Type Culture Collection (CRL-2279). MS-1 cells were cultured to 80% confluence in DMEM containing either 70 or 500 μmol/l l-arginine (A8094, Sigma), 5% FBS, and 1% penicillin-streptomycin (no. 15140, GIBCO-BRL). Cells were washed with PBS and then cultured in the same medium in the absence or presence of various concentrations of dl-homocysteine (53510, Fluka) for 48 h with fresh medium added at 24 h. After 48 h, the medium was collected for measurement of nitrite and ADMA, and cells were harvested for measurements of the total protein concentration using the Bradford method (Bio-Rad protein assay, Hercules, CA).
Measurement of nitrite and ADMA.
Nitrite concentrations in conditioned medium or cell lysates were determined by the reductive generation of NO from nitrite in the University of Iowa Electron Spin Resonance Facility as previously described (52). The resulting NO was detected by the gas-phase chemiluminescent reaction of NO with ozone (O3) using a Sievers 280 NO Analyzer (Sievers Instrument, Boulder, CO). Reduction of nitrite was performed anaerobically using argon as the purge/carrier gas in the presence of 13.9 mol/l acetic acid and 60 mmol/l KI at room temperature. Typically, 20 μl of conditioned medium samples or 0.5–10 μmol/l of NaNO2 standards were analyzed on the instrument with the high sensitivity setting. Data were analyzed with the instrument's NOAnalysis Liquid Program (version 3.00.004) software. Samples were typically run in triplicate with a standard curve prepared for each analysis session.
ADMA in the conditioned medium was measured by liquid chromatography/mass spectrometry (LCMS). To prepare standards, ADMA was diluted with 10 mmol/l HCl to make a 1 mg/ml solution. This standard was serially diluted with 10 mmol/l HCl to 10 μg/ml and 1 μg/ml stock solutions. Calibration standards and controls were prepared in cell culture medium from the stock solutions. Standards, blanks, null blanks, controls, and experimental samples were prepared for solid-phase extraction by adding internal standard (50 μl of propyl arginine, 2.5 μg/ml), phosphoric acid (20 μl), and H2O (600 μl) to each sample (200 μl). All solutions were vortex mixed and subjected to solid-phase extraction with Waters Oasis MCX extraction cartridges (30 mg/1 ml, Waters, Milford, MA) The solid-phase extraction steps included the following: wash column with 1 ml methanol, wash column with 1 ml of 0.1 N HCl, load sample, wash column with 0.1 N HCl, wash column with 1 ml methanol, and elute with 1 ml of methanol-water-NH4OH (50:40:10). Samples were dried under nitrogen and reconstituted with 100 μl of 10 mmol/l ammonium formate buffer with 0.1% formic acid. The instrumentation system consisted of a Shimadzu LCMS-2010A mass spectrometer operated using atmospheric pressure chemical ionization (APCI) in positive-ion detection mode controlled using LCMS solutions software (version 2.04H3, Shimadzu, Columbia, MD). The APCI temperature was 425°C, the curved desolvation line temperature was 200°C, and the block temperature was 200°C. Data were collected in the selected ion monitoring mode at 203.2 (ADMA) and 217.1 amu (propyl arginine). The analytical column was a Phenomenex Luna Silica (1) (3 μm, 150 × 2 mm) preceded by a Phenomenex Luna Silica (1) securityGuard (2 × 4 mm) column. Separation conditions were as follows: sample temperature, 4°C; column temperature, 24°C (+3°C); and sample injection volume, 10 μl. The analysis was isocratic at a flow rate of 0.5 ml/min. Solvent A (70%) was methanol and was pumped postcolumn at a flow of 0.35 ml/min; solvent B (30%) was 10 mmol/l ammonium formate with 0.1% formic acid and 2% methanol and was pumped through the column at a flow rate of 0.15 ml/min. The total run time for LCMS analysis was 9 min. The retention time for ADMA was 5.6 min and for propyl arginine was 5.8 min. The retention time for symmetric dimethylarginine (SDMA) was 5 min, and it did not interfere with the ADMA measurement.
Mice and experimental diets.
Animal protocols were approved by the University of Iowa and Veterans Affairs Animal Care and Use Committees. Mice heterozygous for disruption of the Cbs gene (49) were crossbred to C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) for at least nine generations to generate heterozygous (Cbs+/−) and wild-type (Cbs+/+) littermates. Genotyping for wild-type and disrupted Cbs alleles was performed by PCR as previously described (17). Starting from the time of weaning, male and female mice were fed either a control diet (LM485, Harlan Teklad, Madison, WI), which contained 6.7 mg/kg folic acid and 4.0 g/kg l-methionine, or a high-methionine/low-folate (HM/LF) diet (TD00205, Harlan Teklad), which contained 0.2 mg/kg folic acid and 8.2 g/kg l-methionine (19). A total of 67 male and 51 female mice were studied.
At 5–11 mo of age, mice were anesthetized with pentobarbital sodium (75 mg/kg ip), blood was collected by cardiac puncture into EDTA (final concentration: 5 mmol/l), and plasma was flash frozen and stored at −80°C. Samples of the liver were immediately homogenized in ice-cold perchloric acid (0.4 mol/l) and centrifuged. The supernatant fraction was flash frozen and stored at −80°C for the later analysis of SAM, SAH, ADMA, and SDMA. Additionally, tissue samples of the liver, lung, brain, aorta, and kidney were harvested and flash frozen for RNA or protein analysis.
Vasomotor function.
The right and left carotid arteries were removed and cleaned of loose connective tissue. Each artery was cut into two equal rings of 3–5 mm and suspended in an organ chamber containing oxygenated Krebs buffer at 37°C. Rings were contracted submaximally using the thromboxane analog U-46619, and relaxation dose-response curves were generated by cumulative additions of the endothelium-dependent vasodilator acetylcholine (10−10–10−4 M) or the endothelium-independent vasodilator nitroprusside (10−10–10−4 M) as previously described (33).
Metabolite assays.
Plasma tHcy, defined as the total concentration of homocysteine after quantitative reductive cleavage of all disulfide bonds (35), was measured by HPLC using ammonium 7-fluorobenzo-2-oxa-1,3-diazole-4-sulfonate fluorescence detection (34). Plasma methionine was measured by HPLC coupled to fluorescence detection after precolumn derivatization using °-phthaldialdehyde as previously described (10). Plasma folate was measured with the Quantaphase II folate radioimmunoassay (Bio-Rad Laboratories). Plasma and tissue levels of ADMA and SDMA were determined by HPLC using precolumn derivatization with o-phthaldialdehyde (40). SAM and SAH concentrations in the liver were determined by HPLC using UV detection as previously described (11).
Real-time PCR.
Levels of mRNA for Ddah1, Ddah2, and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) were measured by quantitative real-time PCR as previously described (20). Total RNA was isolated from carotid, aorta, lung, kidney, liver, and brain tissues using TRIzol reagent (Invitrogen, Carlsbad, CA) and treated with DNAse I to remove contaminating genomic DNA. RNA from cultured cells was prepared using the Rneasy kit (Quiagen). RNA (1.5 μg from the liver, lung, brain, and kidney, 0.325 μg from the aorta and carotid artery, and 1.0 μg from MS-1 cells) was reverse transcribed using Taqman reverse transcriptase and random hexamer primers. PCR primers and 6-carboxy fluorescein-labeled probes for Gapdh (Mm99999915_g1), Ddah1 (Mm01319451_m1), and Ddah2 (Mm00516768_m1) were purchased from Applied Biosystems (Foster City, CA). Reverse-transcribed cDNA was incubated with TaqMan Universal PCR mix (Applied Biosystems) and PCR primers and probes at 50°C for 2 min and then at 95°C for 10 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min using the Applied Biosystems 7700 sequence detection system. Amplicon-specific standard curves generated by serial dilution of cloned cDNA were used to quantify the amount of Ddah1, Ddah2, and Gapdh cDNA in each sample. Data were analyzed using Sequence Detection software (version 1.6.3, Applied Biosystems) and expressed as a ratio to levels of Gapdh mRNA.
Immunoblot analysis.
Liver and lung samples were homogenized in ice-cold HEMGN buffer [100 mmol/l KCl, 25 mmol/l HEPES (pH 7.6), 0.1 mM EDTA (pH 8.0), 10% glycerol (vol/vol), and 0.1% Triton X-100 (vol/vol)] containing protease inhibitor cocktail (Complete Mini EDTA-free, Roche). Homogenates were centrifuged at 14,000 g for 30 min at 4°C. Protein concentrations of supernatants were determined using the Bradford colorimetric assay. Protein (10 μg) was run on 12% Tris·HCl gels (Bio-Rad), and membranes were probed with 1 μg/ml monoclonal antibody raised against rat DDAH-1 (27) or with 0.5 μg/ml monoclonal antibody against β-actin (ab8226, Abcam, Cambridge, MA) for 2 h at room temperature. This rat DDAH-1 antibody cross reacts with both mouse and human DDAH-1. Horseradish peroxidase-conjugated goat anti-mouse antibody (no. 1858413, Pierce) was used as the secondary antibody (10 ng/ml, 1 h at room temperature). Immunoreactive bands were visualized using the Supersignal West Femto (Pierce) detection system and quantified by densitometry.
DDAH activity.
DDAH enzyme activity was measured in liver homogenates by determining the formation of citrulline from ADMA as previously described (38). Briefly, fresh liver samples were homogenized in 0.1 mol/l sodium phosphate buffer (pH 6.9) containing protease inhibitor cocktail (Complete Mini EDTA-free, Roche). Supernatants were incubated with 5 mmol/l ADMA for 2 h, and the product, citrulline, was measured by a chromogenic reaction (28). Standards were prepared by serial dilution of citrulline. One unit of enzyme activity was defined as the amount of enzyme required to catalyze the formation of 1 μmol/l citrulline in 1 min at 37°C.
Statistical analysis.
Two-way ANOVA with the Tukey post hoc test for multiple comparisons was used to analyze the effects of the Cbs genotype and diet on plasma and liver metabolites and mRNA levels. Reponses to vasodilators in carotid arteries were analyzed using two-way repeated-measures ANOVA with Bonferroni post hoc analysis. Correlation analysis was performed by linear regression followed by one-way ANOVA. The two-tailed Student's t-test was used to analyze Western blot data and DDAH activity. Statistical significance was defined as a P value of <0.05. Values are reported as means ± SE.
RESULTS
Effects of homocysteine on cultured murine endothelial cells.
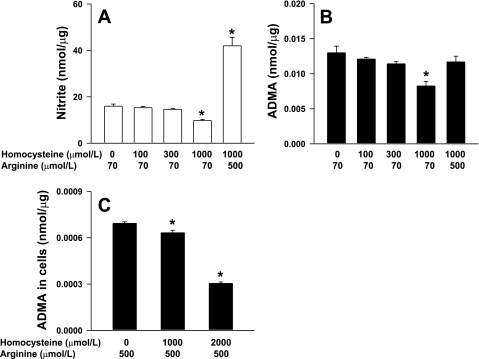
We first explored the effects of homocysteine in the MS-1 murine endothelial cell line in vitro. Exogenous homocysteine (100–1,000 μmol/l) was added to the cell culture medium, and MS-1 cells were incubated for 48 h. The addition of 1,000 μmol/l homocysteine caused a 40% decrease in the levels of nitrite, a marker of NO production, in the conditioned medium (P < 0.001; Fig. 1A). The addition of lower concentrations of homocysteine had no effect on nitrite levels. The addition of excess amounts of the NOS substrate l-arginine (500 μmol/l) produced a large increase in nitrite levels even in the presence of 1,000 μmol/l homocysteine. Incubation of MS-1 cells with 1,000 μmol/l homocysteine also caused a significant decrease in ADMA levels in the conditioned medium (P < 0.05), and this effect was also reversed by the addition of excess l-arginine (Fig. 1B). In a second set of experiments, MS-1 cells were treated with homocysteine for 48 h in the presence of 500 μmol/l arginine. We found that ADMA levels were decreased significantly in cell lysates (Fig. 1C). These observations demonstrate that incubation of MS-1 cells with homocysteine results in a decrease, rather than an increase, in both intracellular and extracellular ADMA.
Fig. 1.
Nitrite (A) and asymmetric dimethylarginine (ADMA; B) levels in the conditioned medium of cultured MS-1 cells treated with 0–1,000 μmol/l of exogenously added dl-homocysteine (Hcy) in the presence of 70 or 500 μmol/l l-arginine for 48 h. C: in a second set of experiments, ADMA levels were measured in cell lysates of MS-1 cells treated with Hcy in the presence of 500 μmol/l l-arginine for 48 h. Levels were normalized to cellular protein. Each data set is representative of 3 experiments. Values are means ± SE. *P < 0.05 vs. 0 μmol/l homocysteine.
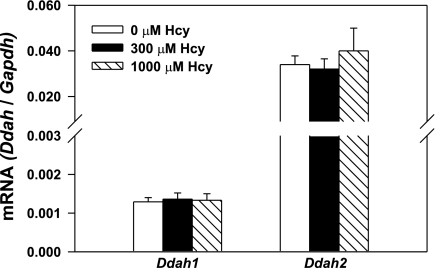
We next measured mRNA levels of Ddah1 and Ddah2 by quantitative PCR in MS-1 cells incubated for 48 h in the presence or absence of homocysteine. The addition of 300 or 1,000 μmol/l homocysteine to MS-1 cells did not cause any changes in mRNA levels for either Ddah1 or Ddah2 (Fig. 2).
Fig. 2.
Levels of dimethylarginine dimethylaminohydrolase (Ddah)1 and Ddah2 mRNA normalized to Gapdh mRNA in cultured MS-1 cells treated with 0, 300, or 1,000 μmol/l of exogenously added Hcy for 48 h. Each data set is representative of 6 experiments. Values are means ± SE.
Plasma tHcy, methionine, and folate in mice.
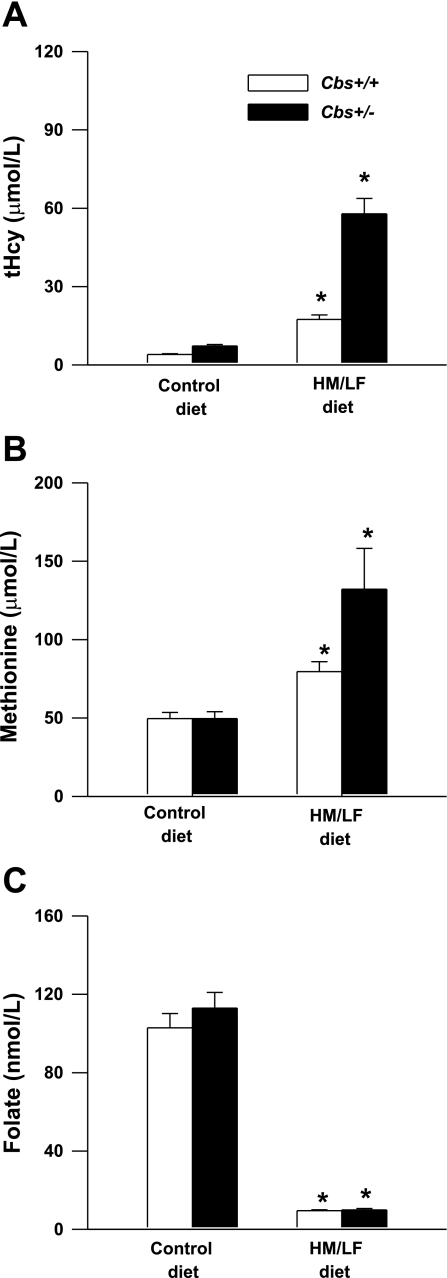
To examine the effects of elevated homocysteine on DDAH expression under pathophysiological conditions in vivo, we utilized the Cbs-deficient mouse model of hyperhomocysteinemia (49). Cbs+/+ or Cbs+/− mice were fed either a control diet or a HM/LF diet for 5–11 mo. Plasma levels of tHcy were influenced significantly by diet (P < 0.001) as well as Cbs genotype (P < 0.001; Fig. 3A). Cbs+/+ mice had plasma tHcy levels of 4.1 ± 0.2 μmol/l when they were fed the control diet and 17 ± 1.7 μmol/l when they were fed the HM/LF diet (P < 0.01). Plasma tHcy levels in Cbs+/− mice were 7.3 ± 0.6 μmol/l when they were fed the control diet and 58 ± 6 μmol/l when they were fed the HM/LF diet (P < 0.001). Plasma levels of methionine were also influenced significantly by diet (P < 0.001; Fig. 3B). However, an effect of Cbs genotype on plasma methionine was observed only in mice fed the HM/LF diet (P < 0.05). Both Cbs+/+ and Cbs+/− mice fed the HM/LF diet had significantly lower levels of plasma folate than mice of the same genotypes fed the control diet (P < 0.001; Fig. 3C).
Fig. 3.
Plasma levels of total Hcy (tHcy; A), methionine (B), and folate (C) in cystathione β-synthase (Cbs)+/+ and Cbs+/− mice fed either the control diet or the high-methionine/low-folate (HM/LF) diet (n = 12–29 in each group). Values are means ± SE. *P < 0.05 vs. mice of the same genotype fed the control diet.
ADMA, SDMA, and methylation capacity.
Plasma ADMA levels were similar in Cbs+/+ and Cbs+/− mice fed the control diet (P = 0.9; Table 1). Interestingly, Cbs+/+ and Cbs+/− mice fed the HM/LF diet did not show any elevation in plasma ADMA levels compared with mice fed the control diet (P = 0.4) despite having a moderate increase in plasma tHcy levels (Fig. 1). Plasma levels of SDMA were ∼75% lower than plasma levels of ADMA in all four groups of mice. No significant effects of diet (P = 0.5) or genotype (P = 0.7) on plasma SDMA levels were observed (Table 1).
Table 1.
Plasma and hepatic levels of various metabolites in Cbs+/+ and Cbs+/− mice fed either the control of HM/LF diet
| Metabolites |
Cbs+/+ Mice |
Cbs+/− Mice
|
||||||
|---|---|---|---|---|---|---|---|---|
| Control Diet | HM/LF Diet | Control Diet | HM/LF Diet | |||||
| Plasma | ||||||||
| ADMA, μmol/l | 0.62±0.04 | 0.59±0.03 | 0.60±0.05 | 0.56±0.03 | ||||
| n | 24 | 20 | 17 | 23 | ||||
| SDMA, μmol/l | 0.15±0.01 | 0.15±0.02 | 0.16±0.02 | 0.15±0.02 | ||||
| n | 17 | 17 | 11 | 14 | ||||
| Liver | ||||||||
| ADMA, μmol/l | 12.4±2.3 | 7.4±2.4 | 10.6±3.7 | 11.4±2.4 | ||||
| n | 9 | 7 | 6 | 7 | ||||
| SDMA, μmol/l | <0.1 | <0.1 | <0.1 | <0.1 | ||||
| n | 9 | 7 | 6 | 7 | ||||
| SAM, nmol/g | 43.3±5.0 | 48.1±3.1 | 36.4±6.6 | 54.8±3.2 | ||||
| n | 11 | 7 | 6 | 5 | ||||
| SAH, nmol/g | 27.3±6.6 | 38.5±3.6 | 30.8±9.1 | 65.4±8.1*† | ||||
| n | 10 | 7 | 6 | 5 | ||||
| SAM/SAH | 3.0±0.8 | 1.3±0.1 | 2.3±0.9 | 0.9±0.1 | ||||
| n | 10 | 7 | 6 | 5 | ||||
Values are means ± SE; n, no. of animals. Cbs, cystathione β-synthase gene; HM/LF, high methionine/low folate; ADMA, asymmetric dimethylarginine; SDMA, symmetric dimethylarginine; SAM, S-adenosyl methionine; SAH, S-adenosyl homocysteine.
P < 0.05 vs. Cbs+/− mice fed the control diet;
P < 0.05 vs. Cbs+/+ mice fed the HM/LF diet.
We also measured tissue levels of ADMA and SDMA in the liver. Hepatic levels of ADMA were not influenced by either diet (P = 0.4) or Cbs genotype (P = 0.7; Table 1). There was a trend toward a lower hepatic level of ADMA in Cbs+/+ mice fed the HM/LF diet (7.4 ± 2.4 nmol/g) compared with Cbs+/+ mice fed the control diet (12.4 ± 2.3 nmol/g), but this difference did not reach statistical significance (P = 0.1). Hepatic levels of SDMA were below the limit of detection (<0.1 nmol/g) in all groups of mice (Table 1).
Hepatic levels of SAM did not differ significantly between groups, but, as expected (15), hepatic levels of SAH were higher in Cbs+/− mice fed the HM/LF diet than in the other groups of mice (Table 1). The SAM-to-SAH ratio, an indicator of methylation capacity, was similar in Cbs+/+ and Cbs+/− mice fed the control diet (P = 0.5). However, Cbs+/+ and Cbs+/− mice fed the HM/LF diet showed a significant overall decrease in hepatic methylation capacity compared with Cbs+/+ and Cbs+/− mice fed the control diet (P = 0.03).
Vasomotor responses in the carotid artery.
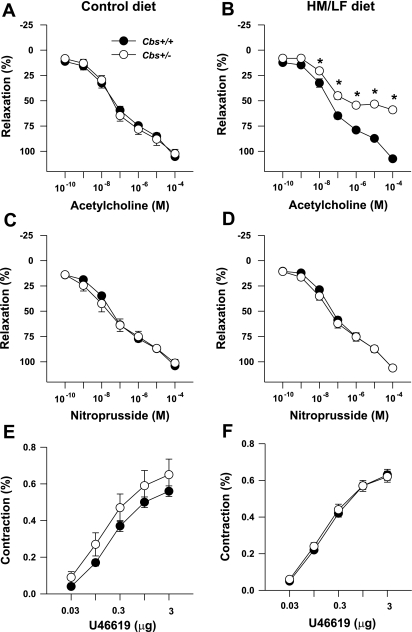
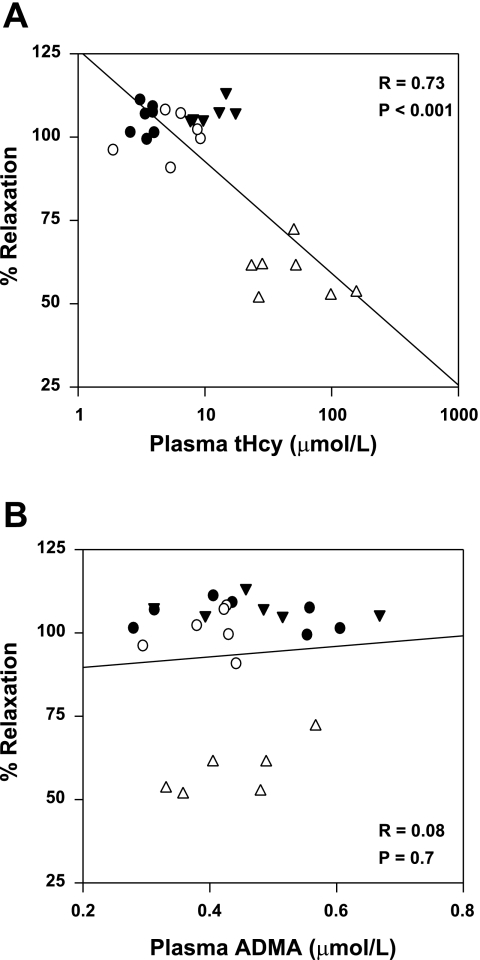
The endothelium-dependent dilator acetylcholine as well as the endothelium-independent dilator nitroprusside produced dose-dependent relaxation of carotid artery rings (Fig. 4). Maximal relaxation to acetylcholine was similar in Cbs+/+ and Cbs+/− mice fed the control diet (105 ± 2% vs. 102 ± 4%, respectively; Fig. 4A). Relaxation responses to acetylcholine were completely preserved in Cbs+/+ mice fed the HM/LF diet (maximal relaxation 107 ± 1%; Fig. 4B). In contrast, Cbs+/− mice fed the HM/LF diet had markedly impaired relaxation responses to acetylcholine, with maximal relaxation decreased by ∼50% compared with Cbs+/+ mice fed same diet (P < 0.001; Fig. 4B). Relaxation responses to nitroprusside were similar in Cbs+/+ and Cbs+/− mice fed control or HM/LF diets (Fig. 4, C and D), and vessels from all groups of mice contracted similarly to the thromboxane analog U-46619 (Fig. 4, E and F). Linear regression analysis of all mice demonstrated a significant inverse correlation between the maximal relaxation to acetylcholine and plasma tHcy levels (R = 0.73, P < 0.001; Fig. 5A). No significant correlation was observed with plasma ADMA levels (Fig. 5B).
Fig. 4.
Relaxation of carotid artery rings to acetylcholine (A and B), nitroprusside (C and D), or U-46619 (E and F) in mice fed the control diet (n = 6–7; A, C, and E) or the HM/LF diet (n = 6–7; B, D, and F). *P < 0.01 vs. Cbs+/+ mice fed the same diet.
Fig. 5.
Relationship between maximal relaxation of carotid artery rings in response to acetylcholine and plasma tHcy (A) or ADMA (B) in Cbs+/+ and Cbs+/− mice fed the control or HM/LF diet (n = 6–7). •, Cbs+/+ mice fed control diet; ○, Cbs+/− mice fed the control diet; ▾, Cbs+/+ mice fed the HM/LF diet; ▵, Cbs+/− mice fed HM/LF diet.
Expression of Ddah1 and Ddah2 mRNA in mice.
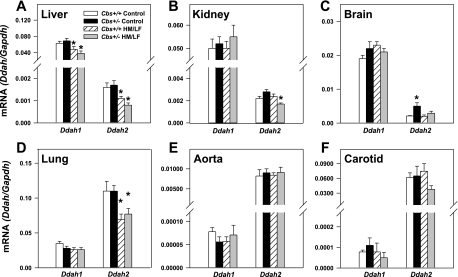
To examine effects of hyperhomocysteinemia on the expression of DDAH, we measured mRNA levels of Ddah1 and Ddah2 in several tissues by quantitative PCR. In control mice (Cbs+/+ mice fed the control diet), levels of Ddah2 mRNA were much higher than levels of Ddah1 mRNA in the lung, aorta, and carotid artery (Fig. 6). In contrast, the liver, kidney, and brain contained higher levels of Ddah1 mRNA than Ddah2 mRNA. The highest levels of Ddah1 mRNA were found in the liver, and the highest levels of Ddah2 mRNA were detected in the lung.
Fig. 6.
Levels of Ddah1 and Ddah2 mRNA in the liver (A), kidney (B), brain (C), lung (D), aorta (E), and carotid artery (F) of Cbs+/+ and Cbs+/− mice fed either the control or HM/LF diet (n = 6–10 in each group). Levels are normalized to levels of Gapdh mRNA. Values are means ± SE. *P < 0.05 vs. mice of the same genotype fed the control diet.
The HM/LF diet produced tissue-specific decreases in Ddah1 and Ddah2 mRNA in both Cbs+/+ and Cbs+/− mice. In the liver, Cbs+/+ and Cbs+/− mice fed the HM/LF diet showed decreases of 25% and 45%, respectively, in Ddah1 mRNA compared with mice of the same genotype fed the control diet (P < 0.001; Fig. 6A). Cbs+/+ and Cbs+/− mice fed the HM/LF diet also had 30–50% lower levels of Ddah2 mRNA in the liver than mice of the same genotype fed the control diet (P < 0.001; Fig. 6A). In the lung, the HM/LF diet did not significantly affect levels of Ddah1 mRNA but did produce a 30% decrease in levels of Ddah2 mRNA in both Cbs+/+ and Cbs+/− mice (P < 0.05 vs. mice of the same genotype fed the control diet; Fig. 6D). In the kidney, a significant decrease in Ddah2 mRNA was observed in Cbs+/− mice fed the HM/LF diet (P < 0.05 vs. Cbs+/− mice fed the control diet). No significant decreases in levels of Ddah1 or Ddah2 mRNA in the aorta or carotid artery were seen in mice fed the HM/LF diet compared with mice fed the control diet.
Expression of DDAH-1 protein.
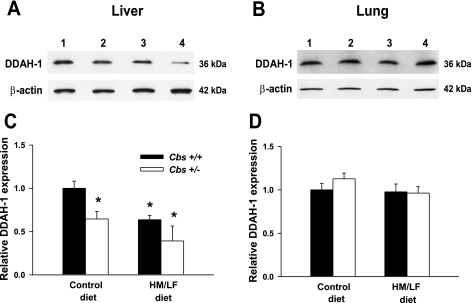
Using a monoclonal antibody against DDAH-1 (27), immunoblot analysis was performed to assess the effects of the Cbs genotype and diet on the expression of DDAH-1 protein in the liver and lung (Fig. 7). Hepatic levels of DDAH-1 protein were decreased by 50% in Cbs+/− mice fed the HM/LF diet compared with Cbs+/+ mice fed the control diet (Fig. 7, A and C, P < 0.05). In contrast, the expression of DDAH-1 protein in the lung did not differ between the four groups of mice (Fig. 7, B and D). These findings are consistent with the observed levels of Ddah1 mRNA in the liver (Fig. 6A) and lung (Fig. 6D).
Fig. 7.
A and B: expression of DDAH-1 protein in the liver (A) and lung (B) of Cbs+/+ and Cbs+/− mice fed the control or HM/LF diet. Levels are normalized to the expression of β-actin. C and D: immunoreactive bands were quantified by densitometry [liver (C) and lung (D)], and values are expressed relative to Cbs+/+ mice fed the control diet (n = 4 in each group). Lane 1, Cbs+/+ mice fed the control diet; lane 2, Cbs+/− mice fed the control diet; lane 3, Cbs+/+ mice fed the HM/LF diet; lane 4, Cbs+/− mice fed the HM/LF diet. *P < 0.05 vs. Cbs+/+ mice fed the control diet.
Hepatic DDAH activity.
Total DDAH activity was measured in liver homogenates. Compared with Cbs+/+ mice fed the control diet, both Cbs+/+ and Cbs+/− mice fed the HM/LF diet showed a trend toward decreased DDAH activity (1.1 ± 0.5 and 0.9 ± 0.3 U/mg, respectively, vs. 1.6 ± 0.9 U/mg in Cbs+/+ mice fed the control diet, n = 5–9). Although this trend did not reach statistical significance (P > 0.05), the data are consistent with the finding of decreased DDAH-1 protein and decreased Ddah1 and Ddah2 mRNA in the liver of mice fed the HM/LF diet.
DISCUSSION
In the present study, we investigated the effects of elevated homocysteine on endothelial function and DDAH expression using both in vitro and in vivo approaches. We found that the Ddah2 gene is preferentially expressed in the lung, aorta, and carotid artery, whereas the liver, brain, and kidney predominantly express the Ddah1 gene. We also found that hyperhomocysteinemia produces tissue-specific decreases in the expression of Ddah1 or Ddah2 in the liver, kidney, and lung. Interestingly, downregulation of DDAH expression in hyperhomocysteinemia did not result in a parallel increase in plasma ADMA, a finding that may be explained by the large decrease in hepatic methylation capacity in these mice. Finally, we found that elevation of homocysteine caused endothelial dysfunction in the carotid artery in mice and decreased production of NO in cultured endothelial cells without increasing intracellular or extracellular ADMA levels.
In the MS-1 murine endothelial cell line, the addition of 1,000 μmol/l exogenous homocysteine resulted in decreased accumulation of nitrite in the conditioned medium (Fig. 1A). This finding, which suggests that homocysteine at high concentrations decreases NO production, agrees with previous findings in endothelial cells (38, 43). Unlike some other studies (8, 38), however, we did not observe elevated levels of ADMA in MS-1 cells or their conditioned medium after an incubation with homocysteine. In fact, we found that ADMA levels were actually decreased after an incubation with 1,000 μmol/l homocysteine (Fig. 1, B–D). This decrease in ADMA was probably not due to an increase in DDAH, since exogenous homocysteine had no effect on the expression of Ddah1 or Ddah2 mRNA in MS-1 cells (Fig. 2).
We used the Cbs-deficient mouse model of hyperhomocysteinemia to examine the effects of elevated homocysteine on DDAH expression under pathophysiological conditions in vivo. Cbs+/+ and Cbs+/− mice were fed either a control diet or a HM/LF diet to produce a wide range of plasma tHcy concentrations. Cbs+/− mice fed the HM/LF diet had impaired relaxation of carotid artery rings in response to the endothelium-dependent dilator acetylcholine. The finding of impaired carotid artery relaxation during hyperhomocysteinemia in mice has potential clinical relevance, because hyperhomocysteinemia has been associated with an increased incidence of carotid artery intima-media thickness (1) and stroke (23). Moreover, some recent clinical studies have suggested that primary prevention of hyperhomocysteinemia may decrease the incidence of stroke in certain populations (48, 51).
Since there was no impairment of carotid artery relaxation in response to the exogenous NO donor nitroprusside, we infer that the primary defect is related to the decreased bioavailability of endothelium-derived NO rather than an intrinsic defect in vascular muscle relaxation or response to NO. The degree of impairment in response to acetylcholine correlated strongly with plasma tHcy concentration (Fig. 5A) but not with plasma ADMA concentration (Fig. 5B). These observations suggest that ADMA is not a major mediator of hyperhomocysteinemia-induced endothelial dysfunction in this model. It is perhaps more likely that other vascular effects of hyperhomocysteinemia, such as oxidative stress (16), endoplasmic reticulum stress (3), or hypomethylation of the endothelial cyclin A gene (24), may be responsible for producing endothelial dysfunction.
We further used this murine model to examine the role of altered DDAH expression and ADMA in endothelial dysfunction of hyperhomocysteinemia. Using quantitative PCR to analyze tissue levels of Ddah1 and Ddah2 mRNA in control mice (Cbs+/+ mice fed the control diet), we observed that Ddah2 is primarily expressed in the aorta and lung and that Ddah1 is predominantly expressed in the brain, liver, and kidney. These findings are in general agreement with previous surveys of DDAH expression performed by hybridization (32, 41). We further observed that mice with moderate hyperhomocysteinemia (Cbs+/+ or Cbs+/− mice fed the HM/LF diet) had tissue-specific decreases in the expression of Ddah1 and/or Ddah2. Ddah1 mRNA was decreased by 25–45% in the liver of hyperhomocysteinemic mice compared with control mice (Fig. 6C) but was not altered by hyperhomocysteinemia in the lung, aorta, carotid artery, kidney, or brain. Decreased expression of DDAH-1 protein was confirmed in the liver by immunoblot analysis (Fig. 7). Ddah2 mRNA was decreased by 30–50% in the lung and liver of both Cbs+/+ and Cbs+/− mice fed the HM/LF diet (Fig. 6, A and D) but was not altered in the brain, aorta, or carotid artery. In the kidney, a significant decrease in Ddah2 mRNA was seen only in mice with the highest levels of plasma tHcy (Cbs+/− mice fed the HM/LF diet; Fig. 6B). One potential mechanism for the decreased expression of Ddah2 in hyperhomocysteinemic mice is hypermethylation of the Ddah2 promoter, which has been reported to occur in human umbilical vein endothelial cells exposed to high concentrations of homocysteine (53).
Despite the relatively large decreases in DDAH expression seen in the liver and lung, no significant increase in plasma ADMA was observed in hyperhomocysteinemic mice (Table 1). This observation is consistent with two previous studies in which hyperhomocysteinemia also did not lead to an increase in plasma ADMA in mice (14, 25). It is possible that these findings reflect a species difference between mice and humans, since several early studies in humans and primates found that plasma ADMA was elevated during hyperhomocysteinemia (5, 7, 38). However, some recent human studies have demonstrated that hyperhomocysteinemia is not always accompanied by an elevation of ADMA (2, 26, 29, 50). It is likely, therefore, that in some patients elevation of ADMA is not directly caused by hyperhomocysteinemia but is instead a marker of another common condition such as renal impairment (36, 50).
We considered the possibility that decreased DDAH expression might increase tissue levels of ADMA without altering plasma levels of ADMA. However, Cbs+/+ and Cbs+/− mice fed the HM/LF diet did not have increased hepatic levels of ADMA (Table 1) despite having a 25–50% decrease in hepatic expression of Ddah1 and Ddah2 (Fig. 6A). We also considered the possibility that the functional consequences of decreased DDAH expression may be masked by a parallel decrease in the synthesis of ADMA. ADMA is formed from the methylation of arginine residues in proteins in a reaction that uses SAM as the methyl donor and produces SAH as a byproduct. The rate of the protein arginine methyltransferase reaction is regulated by intracellular concentrations of SAM and SAH (13). We estimated the cellular methylation capacity by measuring the ratio of SAM to SAH in the liver. Our findings demonstrate a significant elevation in SAH and a consequent decrease in the SAM-to-SAH ratio in mice fed the HM/LF diet (Table 1). These findings suggest that the decreased hepatic methylation capacity of hyperhomocysteinemic mice may lead to decreased protein arginine methylation, potentially providing an explanation for the unaltered levels of ADMA in the face of decreased DDAH expression.
In summary, our findings demonstrate that hyperhomocysteinemia causes tissue-specific decreases in DDAH expression without altering plasma ADMA levels in mice with endothelial dysfunction. Additionally, murine endothelial cells treated with exogenous homocysteine had decreased NO production without a corresponding increase in intracellular or extracellular ADMA. These findings suggest that elevated plasma ADMA may not be a major mediator of hyperhomocysteinemia-induced endothelial dysfunction. Interestingly, a discrepancy between plasma ADMA and endothelial function was also observed recently by Wang et al. (46), who used short interfering RNA to selectively downregulate DDAH-1 or DDAH-2 in rats. They observed that selective downregulation of DDAH-1 caused an increase in serum ADMA but did not affect endothelial function. In contrast, selective downregulation of DDAH-2 caused endothelial dysfunction without altering serum ADMA. On the other hand, mice heterozygous for a targeted disruption of the Ddah1 gene were observed to have both endothelial dysfunction and elevation of plasma ADMA (31). Therefore, it will be necessary in future studies to examine the effect of elevated homocysteine in mice with altered expression of DDAH (18, 22, 31) to fully exclude the possibility that ADMA acts as a mediator of vascular dysfunction during hyperhomocysteinemia.
GRANTS
This work was supported by the Office of Research and Development, United States Department of Veterans Affairs, National Institutes of Health Grants HL-63943 and NS-24621 (to S. R. Lentz), a Postdoctoral Fellowship Award from the American Heart Association (to S. Dayal), and a Predoctoral Fellowship Award from the American Heart Association (to R. N. Rodionov).
Acknowledgments
We thank Dale Kinzenbaw and Brett Wagner for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Adachi H, Hirai Y, Fujiura Y, Matsuoka H, Satoh A, Imaizumi T. Plasma homocysteine levels and atherosclerosis in Japan: epidemiological study by use of carotid ultrasonography. Stroke 33: 2177–2181, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Antoniades C, Tousoulis D, Marinou K, Vasiliadou C, Tentolouris C, Bouras G, Pitsavos C, Stefanadis C. Asymmetrical dimethylarginine regulates endothelial function in methionine-induced but not in chronic homocystinemia in humans: effect of oxidative stress and proinflammatory cytokines. Am J Clin Nutr 84: 781–788, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ 11, Suppl 1: S56–S64, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Boger RH Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “l-arginine paradox” and acts as a novel cardiovascular risk factor. J Nutr 134: 2842S–2853S, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Boger RH, Bode-Boger SM, Sydow K, Heistad DD, Lentz SR. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in monkeys with hyperhomocyst(e)inemia or hypercholesterolemia. Arterioscler Thromb Vasc Biol 20: 1557–1564, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Boger RH, Bode-Boger SM, Szuba A, Tsao PS, Chan JR, Tangphao O, Blaschke TF, Cooke JP. Asymmetric dimethylarginine (ADMA): a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation 98: 1842–1847, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Boger RH, Lentz SR, Bode-Boger SM, Knapp HR, Haynes WG. Elevation of asymmetrical dimethylarginine may mediate endothelial dysfunction during experimental hyperhomocyst(e)inaemia in humans. Clin Sci (Lond) 100: 161–167, 2001. [PubMed] [Google Scholar]
- 8.Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, Schubert B, Tsikas D, Bode-Boger SM. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res 87: 99–105, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Boisvert FM, Cote J, Boulanger MC, Richard S. A proteomic analysis of arginine-methylated protein complexes. Mol Cell Proteomics 2: 1319–1330, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Bottiglieri T The effect of storage on rat tissues and human plasma amino acid levels determined by HPLC. Biomed Chromatogr 2: 195–196, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Bottiglieri T Isocratic high performance liquid chromatographic analysis of S-adenosylmethionine and S-adenosylhomocysteine in animal tissues: the effect of exposure to nitrous oxide. Biomed Chromatogr 4: 239–241, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem 282: 879–887, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Caudill MA, Wang JC, Melnyk S, Pogribny IP, Jernigan S, Collins MD, Santos-Guzman J, Swendseid ME, Cogger EA, James SJ. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine β-synthase heterozygous mice. J Nutr 131: 2811–2818, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Dayal S, Arning E, Bottiglieri T, Boger RH, Sigmund CD, Faraci FM, Lentz SR. Cerebral vascular dysfunction mediated by superoxide in hyperhomocysteinemic mice. Stroke 35: 1957–1962, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Dayal S, Bottiglieri T, Arning E, Maeda N, Malinow MR, Sigmund CD, Heistad DD, Faraci FM, Lentz SR. Endothelial dysfunction and elevation of S-adenosylhomocysteine in cystathionine β-synthase-deficient mice. Circ Res 88: 1203–1209, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Dayal S, Lentz SR. Role of redox reactions in the vascular phenotype of hyperhomocysteinemic animals. Antioxid Redox Signal 9: 1899–1909, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR. Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood 108: 2237–2243, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang BY, Tsao PS, Kimoto M, Vallance P, Patterson AJ, Cooke JP. Dimethylarginine dimethylaminohydrolase regulates nitric oxide synthesis: genetic and physiological evidence. Circulation 108: 3042–3047, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Devlin AM, Arning E, Bottiglieri T, Faraci FM, Rozen R, Lentz SR. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood 103: 2624–2629, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Devlin AM, Bottiglieri T, Domann FE, Lentz SR. Tissue-specific changes in H19 methylation and expression in mice with hyperhomocysteinemia. J Biol Chem 280: 25506–25511, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Frey D, Braun O, Briand C, Vasak M, Grutter MG. Structure of the mammalian NOS regulator dimethylarginine dimethylaminohydrolase: a basis for the design of specific inhibitors. Structure 14: 901–911, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hasegawa K, Wakino S, Tatematsu S, Yoshioka K, Homma K, Sugano N, Kimoto M, Hayashi K, Itoh H. Role of asymmetric dimethylarginine in vascular injury in transgenic mice overexpressing dimethylarginie dimethylaminohydrolase 2. Circ Res 101: e2–e10, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 288: 2015–2022, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Jamaluddin MD, Chen I, Yang F, Jiang X, Jan M, Liu X, Schafer AI, Durante W, Yang X, Wang H. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin A gene. Blood 110: 3648–3655, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang X, Yang F, Tan H, Liao D, Bryan RM Jr, Randhawa JK, Rumbaut RE, Durante W, Schafer AI, Yang X, Wang H. Hyperhomocystinemia impairs endothelial function and eNOS activity via PKC activation. Arterioscler Thromb Vasc Biol 25: 2515–2521, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonasson TF, Hedner T, Hultberg B, Ohlin H. Hyperhomocysteinaemia is not associated with increased levels of asymmetric dimethylarginine in patients with ischaemic heart disease. Eur J Clin Invest 33: 543–549, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Kimoto M, Whitley GS, Tsuji H, Ogawa T. Detection of NG,NG-dimethylarginine dimethylaminohydrolase in human tissues using a monoclonal antibody. J Biochem (Tokyo) 117: 237–238, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Knipp M, Vasak M. A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal Biochem 286: 257–264, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Korandji C, Zeller M, Guilland JC, Vergely C, Sicard P, Duvillard L, Gambert P, Moreau D, Cottin Y, Rochette L. Asymmetric dimethylarginine (ADMA) and hyperhomocysteinemia in patients with acute myocardial infarction. Clin Biochem 40: 66–72, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Krzyzanowska K, Mittermayer F, Krugluger W, Schnack C, Hofer M, Wolzt M, Schernthaner G. Asymmetric dimethylarginine is associated with macrovascular disease and total homocysteine in patients with type 2 diabetes. Atherosclerosis 189: 236–240, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O'Hara B, Rossiter S, Anthony S, Madhani M, Selwood D, Smith C, Wojciak-Stothard B, Rudiger A, Stidwill R, McDonald NQ, Vallance P. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med 13: 198–203, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, Vallance P. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J 343: 209–214, 1999. [PMC free article] [PubMed] [Google Scholar]
- 33.Lentz SR, Erger RA, Dayal S, Maeda N, Malinow MR, Heistad DD, Faraci FM. Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine β-synthase-deficient mice. Am J Physiol Heart Circ Physiol 279: H970–H975, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Martin SC, Hilton AC, Bartlett WA, Jones AF. Plasma total homocysteine measurement by ion-paired reversed-phase HPLC with electrochemical detection. Biomed Chromatogr 13: 81–82, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Mudd SH, Finkelstein JD, Refsum H, Ueland PM, Malinow MR, Lentz SR, Jacobsen DW, Brattstrom L, Wilcken B, Wilcken DE, Blom HJ, Stabler SP, Allen RH, Selhub J, Rosenberg IH. Homocysteine and its disulfide derivatives: a suggested consensus terminology. Arterioscler Thromb Vasc Biol 20: 1704–1706, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Rodionov RN, Lentz SR. The homocysteine paradox. Arterioscler Thromb Vasc Biol 28: 1031–1033, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schnabel R, Blankenberg S, Lubos E, Lackner KJ, Rupprecht HJ, Espinola-Klein C, Jachmann N, Post F, Peetz D, Bickel C, Cambien F, Tiret L, Munzel T. Asymmetric dimethylarginine and the risk of cardiovascular events and death in patients with coronary artery disease: results from the AtheroGene Study. Circ Res 97: e53–59, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Stuhlinger MC, Tsao PS, Her JH, Kimoto M, Balint RF, Cooke JP. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation 104: 2569–2575, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Takiuchi S, Fujii H, Kamide K, Horio T, Nakatani S, Hiuge A, Rakugi H, Ogihara T, Kawano Y. Plasma asymmetric dimethylarginine and coronary and peripheral endothelial dysfunction in hypertensive patients. Am J Hypertens 17: 802–808, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Teerlink T, Nijveldt RJ, de Jong S, van Leeuwen PA. Determination of arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in human plasma and other biological samples by high-performance liquid chromatography. Anal Biochem 303: 131–137, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Tran CT, Fox MF, Vallance P, Leiper JM. Chromosomal localization, gene structure, and expression pattern of DDAH1: comparison with DDAH2 and implications for evolutionary origins. Genomics 68: 101–105, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol 289: H2649–H2656, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Upchurch GR, Welch GN, Fabian AJ, Freedman JE, Johnson JL, Keaney JF Jr, Loscalzo J. Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem 272: 17012–17017, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol 24: 1023–1030, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 339: 572–575, 1992. [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Gill PS, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, Dennehy K, Li M, Modlinger P, Leiper J, Vallance P, Adler O, Leone A, Tojo A, Welch WJ, Wilcox CS. Isoform-specific regulation by NG-NG-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res 101: 627–635, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Huang ZQ, Xia L, Feng Q, Erdjument-Bromage H, Strahl BD, Briggs SD, Allis CD, Wong J, Tempst P, Zhang Y. Methylation of histone H4 at arginine 3 facilitating transcriptional activation by nuclear hormone receptor. Science 293: 853–857, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet 369: 1876–1882, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, Maeda N. Mice deficient in cystathionine β-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA 92: 1585–1589, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilcken DE, Wang J, Sim AS, Green K, Wilcken B. Asymmetric dimethylarginine in homocystinuria due to cystathionine β-synthase deficiency: relevance of renal function. J Inherit Metab Dis 29: 30–37, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Yang Q, Botto LD, Erickson JD, Berry RJ, Sambell C, Johansen H, Friedman JM. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation 113: 1335–1343, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Zhang HJ, Zhao W, Venkataraman S, Robbins ME, Buettner GR, Kregel KC, Oberley LW. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem 277: 20919–20926, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Zhang JG, Liu JX, Li ZH, Wang LZ, Jiang YD, Wang SR. Dysfunction of endothelial NO system originated from homocysteine-induced aberrant methylation pattern in promoter region of DDAH2 gene. Chin Med J (Engl) 120: 2132–2137, 2007. [PubMed] [Google Scholar]