Abstract
Exposure to secondhand smoke (SHS), a major indoor air pollutant, is linked to increased cardiovascular morbidity and mortality, including cardiac arrhythmias. However, the mechanisms underlying the epidemiological findings are not well understood. Impaired cardiac autonomic function, indexed by reduced heart rate variability (HRV), may represent an underlying cause. The present study takes advantage of well-defined short-term SHS exposure (3 days, 6 h/day) on HRV and the susceptibility to arrhythmia in mice. With the use of electrocardiograph telemetry recordings in conscious mice, HRV parameters in the time domain were measured during the night after each day of exposure and 24 h after 3 days of exposure to either SHS or filtered air. The susceptibility to arrhythmia was determined after 3 days of exposure. Exposure to a low concentration of SHS [total suspended particle (TSP), 2.4 ± 3.2; and nicotine, 0.3 ± 0.1 mg/m3] had no significant effect on HRV parameters. In contrast, the exposure to a higher but still environmentally relevant concentration of SHS (TSP, 30 ± 1; and nicotine, 5 ± 1 mg/m3) significantly reduced HRV starting after the first day of exposure and continuing 24 h after the last day of exposure. Moreover, the exposed mice showed a significant increase in ventricular arrhythmia susceptibility and atrioventricular block. The data suggest that SHS exposure decreased HRV beyond the exposure period and was associated with an increase in arrhythmia susceptibility. The data provide insights into possible mechanisms underlying documented increases in cardiovascular morbidity and mortality in humans exposed to SHS.
Keywords: environmental tobacco smoke, indoor air pollution, autonomic function, cardiovascular diseases
secondhand smoke (SHS), a major source of indoor air pollution, poses a significant threat to the cardiovascular system (4). SHS is classified as a toxic air contaminant (43), and the American Heart Association has concluded that the risk of death due to heart disease is increased by 30% in those exposed to SHS in the home. The risk is thought to be higher in those exposed to SHS in the workplace (40). Epidemiological studies have shown that 70–80% of deaths attributable to SHS are caused by heart disease, suggesting that the cardiovascular system is vulnerable to SHS (7, 26). Despite the widely recognized association of cardiovascular morbidity and mortality with SHS exposure, only nineteen states in the United States require 100% smoke-free public places (2).
Epidemiological studies indicate significant associations between exposure to SHS and cardiovascular-related morbidity and mortality, including sudden cardiac death (17, 18, 28, 39, 41). A number of potential mechanisms have been proposed, including platelet aggregation, endothelial dysfunction, accelerated atherosclerotic lesions, increased circulating inflammatory mediators, oxidative stress, and changes in autonomic function, as indexed by reduced heart rate variability (HRV) (17, 18, 35, 36).
It is likely that multiple mechanisms contribute to SHS-induced cardiovascular-related morbidity and mortality; however, there is a particularly compelling association between exposure to SHS and decreased HRV. Pope and colleagues (35) provided the first direct evidence of SHS-induced decreased HRV. With the use of ambulatory electrocardiogram (ECG) monitoring, ECG signals were recorded in an airport setting while subjects moved between smoking and nonsmoking areas, spending 2 h in each area. The data showed that exposure to SHS was associated with decrements in all measures of HRV (35). Similarly, using 24-h ECG (Holter) recording, Dietrich and colleagues (13) examined 24-h HRV in nonsmokers with or without SHS exposure. The data showed that exposure to SHS either at home or at work for more then 2 h/day is associated with a higher heart rate and lower HRV.
Reduced exercise performance, including impaired heart rate responses, has also been observed when exercise is performed in the presence of SHS (33). The recovery time to return to preexercise heart rate, an indicator of vagal regulation of heart rate, has also been found to be significantly longer when subjects exercise in a smoking environment, suggesting that SHS impairs autonomic regulation of heart rate as well as HRV (29). In addition, a growing number of human studies suggest that decreased autonomic regulation of heart rate, including HRV, is an underlying cause of adverse cardiovascular consequences associated with particulate exposure (34), and SHS and particulate exposure have similar cardiovascular end points (29, 33, 35). These data suggest that SHS-induced reduction in HRV may be a mechanism linking SHS exposure and increased cardiovascular consequences, including arrhythmia vulnerability (8, 23, 27).
The present study used telemetry in conscious mice to determine directly the effects of short-term SHS exposure (3 days, 6 h/day) on HRV, at concentrations close to those that surround a person in the act of smoking or several people smoking in a confined space. Moreover, with the use of in vivo electrophysiological study, the effects of short-term SHS exposure in cardiac excitability and arrhythmia susceptibility was determined. This study is the first to provide evidence for short-term SHS exposure on the decrease in HRV and the associated increase in arrhythmia inducibility in an animal model mimicking the effects of SHS. The developed animal model will allow future studies to test directly the effects of SHS exposure and autonomic dysfunction to further understand the cellular mechanisms.
MATERIALS AND METHODS
All protocols were approved by the Institutional Animal Care and Use Committee in compliance with the Animal Welfare Act and Public Health Service Policy on Humane Care and Use of Laboratory Animals.
SHS exposure.
Sidestream smoke, a surrogate for SHS, was generated by a modified ADL/II (Little Cambridge, MA) system using 1R4F cigarettes from the University of Kentucky Tobacco and Health Research Institute (Lexington, KY). Two to five cigarettes at a time were smoked under Federal Trade Commission conditions in a staggered fashion at a rate of 1 puff/min (35 ml/puff, 2-s duration). The smoke was diluted with high-efficiency particulate air filters to the appropriate concentration in a mixing chamber and then passed into a stainless steel and glass Hinners-type exposure chamber that was 0.44 m3 in size. Nicotine was sampled daily for 15 min during each 6-h exposure period. Likewise, the total suspended particle (TSP) concentration was sampled with the piezobalance technique for 30 min every hour. Carbon monoxide concentration was measured using a carbon monoxide analyzer (Rosemount Analytical, Orrville, OH) taken every 30 min during each 6-h daily exposure period. During exposures, the mice were housed in their home cage with wire lids, rodent chow, and water ad libitum. The mice were randomly assigned to either a filtered air (FA) exposure, low-concentration SHS exposure (TSP, 2.42 ± 3.22 mg/m3; nicotine, 0.26 ± 0.06 mg/m3; and carbon monoxide, 4.8 ± 3.5 parts/million), or high-concentration SHS exposure (TSP, 30.10 ± 1.48 mg/m3; nicotine, 4.91 ± 1.48 mg/m3; and carbon monoxide, 107 ± 12 parts/million) group.
ECG telemetry implants.
Male C57BL/6 mice (10 wk old from Charles River, Wilmington, MA) were anesthetized with ketamine (90 mg/kg im; Phoenix Scientific, St. Joseph, MO) and xylazine (12.5 mg/kg im; Ben Venue, Bedford, OH). The criteria for adequacy of anesthesia include the following: 1) lack of eye blink reflex, 2) no whisker movement, 3) lack of paw withdraw pinch, and 4) no irregular or sudden changes in breathing frequency. An ECG telemetry device (TA10EA F-20, Data Sciences International, St. Paul, MN) was implanted in the peritoneal cavity via a midline abdominal incision and sutured to the inside of the abdominal muscle. The two ECG leads were tunneled subcutaneously. The negative lead of the transmitter was sutured to the upper right pectoris muscle near the shoulder, and the positive lead was sutured to the left lateral side of the xiphoid process for recording ECGs at lead II configuration. Animals were given carprofen (5 mg/kg sc) for pain control and allowed to recover from the surgery for 3 wk before the exposure protocol began. During the recovery, the mice were checked daily for signs of pain, such as excessive grooming and inactivity.
ECG recording protocols.
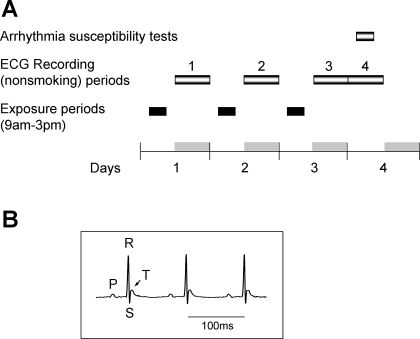
The mice were randomly assigned to either a FA exposure (n = 18), low-concentration SHS exposure (n = 18), or high-concentration SHS exposure (n = 17) group. The exposure and ECG recording protocol is shown in Fig. 1A. All mice were exposed to either FA or SHS for 3 days (6 h/day). Continuous ECG signals were recorded in freely moving mice in their home cage at the following time periods: 1) the night after the first day of exposure (6:00 pm to 6:00 am), 2) the night after the second day of exposure (6:00 pm to 6:00 am), and 3) the time period encompassing 24 h after the third and last day of exposure (6:00 pm to 6:00 pm). The mice remained in their home cage throughout the exposure and recording period. Figure 1B shows an example trace of the ECG signals recorded with the telemetry system from one conscious mouse.
Fig. 1.
Experimental protocol and an example of recorded traces. A: exposure and recording protocol. Mice were exposed for 3 days (6 h/day) from 9:00 am to 3:00 pm (black bar, exposure cycle). ECG signals were recorded for 12 h (6:00 pm to 6:00 am) during each night of exposure (recording periods 1, 2, and 3) and for additional 12 h (recording period 4) after the third night. The arrhythmia susceptibility test was performed after 3 days of exposure. Gray bars, dark cycle (6:00 pm to 6:00 am). B: an example trace of the recorded ECG signals from one mouse.
In vivo electrophysiological protocols.
In separate mice, the arrhythmia susceptibility and cardiac conduction properties were tested after 3 days of SHS exposure (Fig. 1A). Since the low concentration of SHS produced no effect on HRV, only the FA control (n = 15) and high concentration of SHS (n = 12) were included in this part of the studies. In vivo electrophysiological studies were performed as previously described in a blinded fashion (6, 46). Briefly, the animals were anesthetized with pentobarbital sodium (40 mg/kg ip). A 1.7-Fr octapolar catheter with an interelectrode spacing of 0.5 mm (CIBer mouse EP, NuMed, Hopkinton, NY) was inserted via the internal jugular vein into the right atrium and ventricle. Surface electrocardiograms and intracardiac electrograms were recorded using Recording System (CardioLab, Prucka, GE Medical System). Pacing protocols were performed via the catheter. Standard pacing protocols were used to determine the electrophysiological parameters, including sinus node recovery; atrial, atrioventricular (AV) nodal, and ventricular refractory periods; and AV nodal conduction properties. To induce atrial and ventricular tachycardia and fibrillation, programmed extrastimulation techniques and burst pacing were used. Programmed right atrial and right ventricular double and triple extrastimulation techniques were performed at 100-ms drive cycle length (CL), down to a minimum coupling interval of 10 ms. Right atrial and right ventricular burst pacing were performed as eight 50-ms and four 30-ms CL train episodes repeated several times, up to a maximum 1-min time limit of total stimulation. For heart conduction properties, standard pacing protocols were used to determine the electrophysiological parameters, including sinus node recovery time; atrial, AV nodal, and ventricular refractory periods; and AV nodal conduction properties. Each animal underwent an identical pacing and programmed stimulation protocol.
Data acquisition and analysis.
All values are means ± SE unless otherwise indicated. Differences were considered significant at P < 0.05. The ECG signals were recorded at 5 kHz with Dataquest A.R.T. (Data Sciences International) program. The raw data were converted to binary format with the MiniAnalysis program (Synaptosoft, Decatur, GA) and analyzed with the Nevorkard SA-HRV (Intellectual Services, Ljubljana, Slovenia) program. The accuracy of the R-wave detection was visually confirmed. All arrhythmic events, ectopics, and missing beats were excluded from data analysis. Only normal-to-normal R-R intervals were used for HRV analysis in the time domain. Five-minute CLs are commonly used for human HRV analysis, whereas most mouse studies used 2-min CLs. In a preliminary analysis in three mice, the standard deviation of all normal-to-normal R-R interval averages (SDANNs) obtained from 2-min CLs were similarly lower than that of 5-min CLs in control (4.9%) and exposed (4.4%) mice. Therefore, the 2-min CL was used because 2-min CL is most frequently used in mice. The standard HRV parameters determined are listed and defined in Table 1. Since the duration of the recording used for HRV analysis could affect the outcome of the HRV, the recordings taken over the 24-h period after the third day of exposure were divided into 12-h sections for HRV analysis to match the time length of recording periods 1 and 2. Three mice in each group only had recordings for the first 24 h after the third day of exposure. A two-way mixed-model ANOVA was used for analyzing the difference between the FA- and SHS-exposed mice with exposure (FA, low SHS, and high SHS) as one factor and time (recording periods) as the other factor and followed by Fisher least significant differences test when appropriate. The raw numbers of HRV were used for statistical analysis. Even though the statistical analysis was performed with the raw numbers, to demonstrate the SHS-induced changes, all exposure data in the figures are expressed as percent changes from the averages of the FA-exposed control group.
Table 1.
Time domain measures of HRV and definitions
| Definitions | |
|---|---|
| Overall HRV | |
| SDNN | SD of all normal-to-normal R-R intervals |
| CV% | Coefficient of variance, 100(SDNN/mean R-R) |
| HRV due to CL >2 min | |
| SDANN | SD of all 2-min R-R interval averages |
| HRV due to 2-min cycles | |
| SDNNIDX | Averages of SD of all 2-min R-R intervals |
| Short-term HRV | |
| rMSSD | Root mean square of successive difference |
Values are means ± SE. HRV, heart rate variability; CL, cycle length.
For in vivo electrophysiological studies, the programmed extrastimulation techniques and the stimulation duration of atrial and ventricular burst pacing were the same in all mice for the comparison of the inducibility in each mouse. The sinus node function was evaluated at the same pacing CL, and the sinus node recovery time (SNRT) and corrected SNRT were measured (6, 25, 45). Refractory periods at the same pacing CL were measured for the AV node and the right ventricle (6, 25, 45). Inducibility for atrial and ventricular arrhythmias was examined, as well as the presence of heart block or sinus arrest. Sustained atrial or ventricular arrhythmias were defined as atrial arrhythmias lasting longer than 30 s. Reproducibility was defined as greater than one episode of induced atrial or ventricular tachycardia.
Fisher exact test was used for noncontinuous variables, and Student's t-test was used for continuous variables. For atrial and ventricular arrhythmias and heart block, a Fisher exact test was used for comparisons between the FA control and SHS-exposed mice. For recovery time and refractory periods, a Student's t-test was used for a comparison between the FA control and SHS-exposed mice.
RESULTS
Table 2 shows the 12-h baseline day- and nighttime heart rate and HRV recorded after 3 days of FA exposure. The mice displayed a typical circadian rhythm having lower heart rate and higher HRV during the daytime when the vagal regulation is expected to be higher.
Table 2.
HR and HRV in filtered air control mice
| Daytime | Nighttime | |
|---|---|---|
| HR, beats/min | 538.7±9.0 | 624.3±8.9* |
| R-R, ms | 111.9±7.5 | 96.7±1.4* |
| SDNN, ms | 18.5±3.1 | 16.4±0.6* |
| CV% | 16.5±1.9 | 17.0±0.6 |
| SDANN, ms | 15.1±2.6 | 15.5±2.8 |
| SDNNIDX, ms | 9.8±1.9 | 7.1±1.4* |
| rMSSD, ms | 5.8±1.5 | 4.6±1.5* |
Values are means ± SE. HR, heart rate.
P < 0.05, daytime vs. nighttime (paired t-test).
Short-term exposure to SHS reduced measures of HRV.
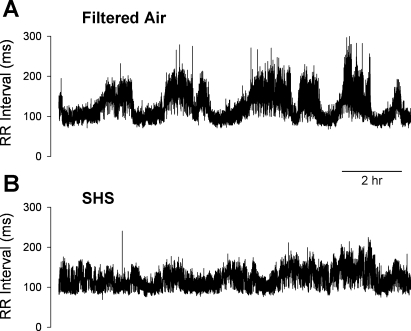
Exposure to SHS for 6 h/day for 3 days reduced measures of HRV in C57BL/6 mice. Figure 2 shows an example of a tachogram from a FA-exposed control mouse (Fig. 2A) and from a high SHS-exposed mouse (Fig. 2B) recorded during the night after the first day of exposure. The SHS-exposed mouse showed a reduced HRV as indicated by having less frequent and lower magnitude “fluctuations” in the R-R intervals.
Fig. 2.
Examples of 12-h tachogram. Data were from individual mice after 6 h of exposure to filtered air (FA; A) or a high concentration of secondhand smoke (SHS; B).
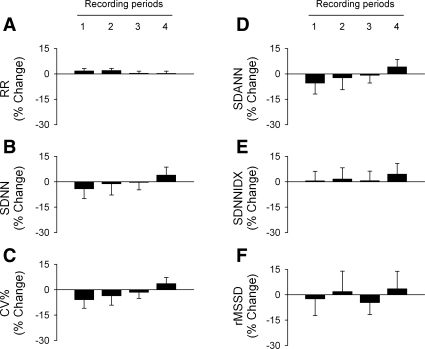
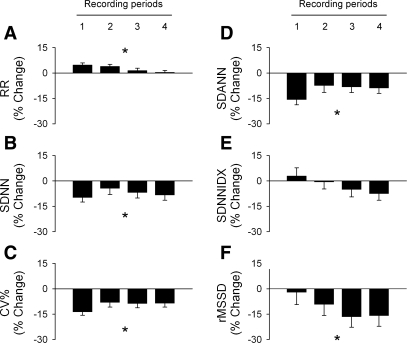
The group data show that short-term exposures to the low concentration of SHS had no significant effect on R-R interval or measures of HRV during the nonsmoking periods (Fig. 3). Exposure to the higher concentration of SHS resulted in a physiologically small but statistically significant increase in R-R interval (Fig. 4A, P < 0.05 high SHS vs. FA) compared with FA-exposed controls. Exposure to the higher concentration of SHS also significantly decreased measures of overall HRV: SDNN (Fig. 4B, P < 0.05, high SHS vs. FA and low SHS) and coefficient of variance (Fig. 4C, P < 0.05, high SHS vs. FA and low SHS). The HRV component longer than 2 min in CL (SDANN, Fig. 4D, P < 0.05, high SHS vs. FA and low SHS) and short-term HRV (rMSSD, Fig. 4F, P < 0.05, high SHS vs. FA and low SHS) were also significantly decreased. There was no significant difference in the HRV component due to the 2-min CL (SDNNIDX, Fig. 4E). The SHS-induced reduction on SDANN and rMSSD were greater when the baseline R-R interval was taken into account (data not shown).
Fig. 3.
Group data of R-R interval and heart rate variability (HRV) in mice exposed to a low concentration of SHS. All data are expressed as percent changes from the average values of the FA-exposed control group. Exposure to the low concentration of SHS had no significant effect on R-R interval and HRV. The standard HRV parameters are shown (RR, A; SDNN, B; CV%, C; SDANN, D; SDNNIDX, E; rMSSD, F) and defined in Table 1.
Fig. 4.
Group data of R-R interval and HRV in mice exposed to a high concentration of SHS. All data are expressed as percent changes from the average values of the FA-exposed control group. High concentration of SHS exposure significantly decreased overall HRV beginning after the first day of exposure, reflecting decreased HRV due to cycle length longer than 2 min. The short-term HRV gradually decreased over the exposure period, with a maximum reduction after the third day of exposure. The standard HRV parameters are shown (RR, A; SDNN, B; CV%, C; SDANN, D; SDNNIDX, E; rMSSD, F) and defined in Table 1. *P < 0.05, high SHS vs. FA, Fisher least significant differences test.
The overall HRV (SDNN, Fig. 4B, left) and the HRV due to CL longer than 2 min (SDANN, Fig. 4D) show the greatest decrease on the night after the first day of exposure (10–19% decrease). The decrease in these HRV measures persisted to a smaller extent (6–10% decline) after the second and third day of exposure and 24 h after the third days of exposure (recording periods 2, 3, and 4, respectively). In contrast, short-term HRV (rMSSD, Fig. 4F) gradually decreased over the 3 days of exposure and reached a maximum reduction (16–18%) after the cessation of the 3-day exposure (recording periods 3 and 4). The data suggest that the SHS-induced decrease in HRV can extend to the nonsmoking period.
Short-term exposure to SHS increased arrhythmia inducibility in mice.
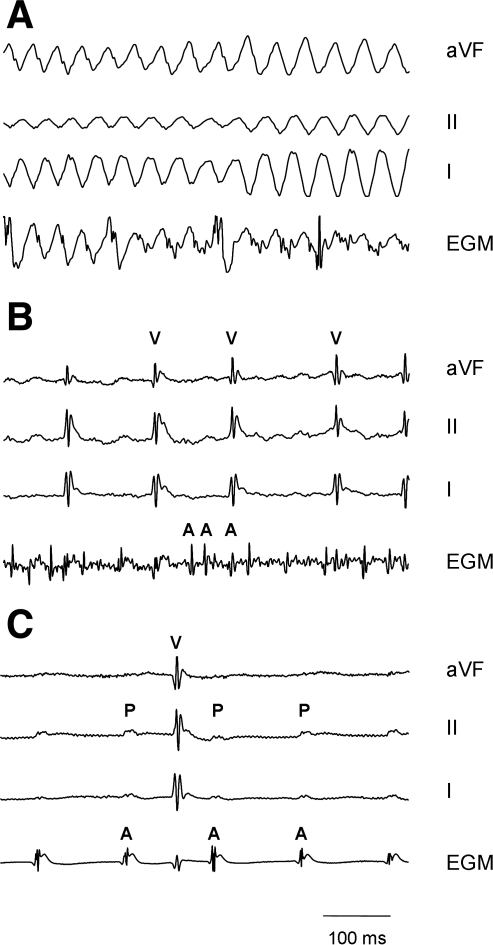
Short-term SHS exposure increased the arrhythmia inducibility, including atrial fibrillation and ventricular tachyarrhythmia/fibrillation. Figure 5 shows examples of sustained ventricular tachycardia/fibrillation (Fig. 5A), atrial fibrillation (Fig. 5B), and intermittent AV block (Fig. 5C) from SHS-exposed mice. Under the standard stimulation protocol, there was no arrhythmia induced in FA control mice (Table 3, P < 0.05, Fisher exact test). Short-term SHS exposure also altered the cardiac electrical properties of the intact mice (Table 3). SHS resulted in a significant prolongation of the recovery of the sinus node function (SNRT, P < 0.05, Student's t-test).
Fig. 5.
Example traces of atrial fibrillation and ventricular tachyarrhythmia/fibrillation. A: sustained ventricular tachycardia/fibrillation in a male mouse exposed to SHS. The traces shown are surface ECG recordings from leads aVF, II, and I and the intracardiac electrogram (EGM) recordings from the atrium illustrating the sinusoidal-appearing ventricular tachycardia. This arrhythmia would generally lead to sudden death in humans, although the mouse did recover. B: induction of sustained atrial fibrillation in a male mouse exposed to SHS. Note the very fine atrial fibrillatory waves (A) that were induced after pacing. C: intermittent heart block in a male mouse exposed to SHS. Note that there are P waves (P) that failed to be conducted to the ventricle (V). This finding was not seen in control animals.
Table 3.
Electrophysiological changes and arrhythmia inducibility in SHS-exposed mice
| Control | SHS Exposure | |
|---|---|---|
| n | 15 | 12 |
| Atrial fibrillation (%) | 0/15 (0) | 2/12 (17) |
| VT/VF (%) | 0/15 (0) | 4/12 (33)* |
| AV block (%) | 0/15 (0) | 4/12 (33)* |
| SNRT (at CL 80 ms), ms | 145±35 | 191±50† |
| CSNRT (at CL 80 ms), ms | 50±12 | 48±45 |
| AVNERP, ms | 73±8 | 62±8 |
| VERP, ms | 47±8 | 33±5 |
Values are means ± SE; n, number of mice. SHS, secondhand smoke; VT/VF, ventricular tachycardia or ventricular fibrillation; SNRT, sinus node recovery time; CSNRT, corrected SNRT; AVNERP, atrioventricular (AV) node refractory period; VERP, right ventricular refractory period.
P < 0.05 (Fisher exact test);
P < 0.05 (Student's t-test).
DISCUSSION
The major findings of the present study are that the short-term SHS exposure can result in a significant reduction in HRV that persists after the exposure ceases. Moreover, the reduction in HRV is associated with an increase in arrhythmia susceptibility (atrial fibrillation, ventricular fibrillation or tachycardia), altered cardiac electrical conduction system, and AV block. The initial drop in HRV, after 1 day of exposure, was predominately associated with measures of long-term components (SDANN) rather than the short-term components (rMSSD) of HRV. Our data also suggest that SHS effects on short-term HRV components may be time dependent and accumulative, having a slower onset than SDANN and accumulated over the 3 days of exposure.
It is well accepted that HRV is a marker for cardiac autonomic function. In time-domain measurements, the short-term component (rMSSD) is believed to be an indicator for the cardiac parasympathetic modulation of the heart periods, whereas both cardiac sympathetic and parasympathetic modulate the long-term and overall HRV (16). In mice, the blocking the cardiac parasympathetic modulation with atropine had no effect on the heart rate but significantly decreased both long- and short-term HRV parameters (16). On the other hand, the blockade of cardiac sympathetic modulation significantly increased R-R interval as well as the HRV parameters. When we take the changes in baseline R-R interval into account, the effects of sympathetic blockade were on overall and long-term HRV parameters and only had a minimal effect on the short-term HRV (16). The data suggest that, in mice, although sympathetic had greater influence on the baseline heart rate, the short-term HRV is mostly under cardiac parasympathetic regulation and the long-term HRV is under dual regulation as in humans.
In humans, exposure to SHS for 2 h in an airport smoking area significantly decreased long-term measures of HRV (35). Similarly, SHS exposure for longer than 2 h/day significantly decreased the long-term HRV as well as HR analyzed over a 24-h period (13). The data suggest that SHS exposure may change the autonomic balance toward sympathetic modulation. Similar to these studies in humans (13, 35), the initial drop in HRV in mice, after 1 day of exposure, was predominately associated with a decrease in long-term components (SDANN) rather than the short-term components (rMSSD) of HRV. The data suggest that the activation of the cardiovascular sympathetic nervous system during exposure period and immediately after exposure might contribute to increase the risk factor for arrhythmias in both humans and mice. In addition, with repeated exposure for 3 days, the accumulative effect of SHS on short-term HRV suggested that a reduced cardiac parasympathetic modulation may play a more important role with repeated exposures.
Several factors should be taken into consideration when comparing the current study to the previous human studies. First, there is the species difference in heart rate regulation between humans and mice. Second, heart rate and HRV responses to SHS exposure in the present study were not recorded during the exposure period and no direct comparison can be made. Finally, the particle levels, nicotine concentrations, and carbon monoxide levels were higher in the present study. In this regard, we wished to study the effects of SHS within a range of concentrations that could be generated under a wide variety of conditions. The high dose of SHS is a concentration that can be easily achieved in poorly ventilated, small enclosed spaces (such as automobiles) (24). Although the “high” concentration of SHS would not be typically encountered for prolonged periods of time, this provides a range to begin to elucidate whether a dose-response effect might be seen for HRV following short-term exposure to SHS. Although testing the exposure effects with a lower concentration over a longer period of time is also important, the present study provides the first evidence for the potential mechanisms mediating the SHS-induced cardiovascular effects. It is conceivable that a more extended exposure, such as when working in a smoky bar, might result in a more prolonged effect with a lower exposure concentration. This is particularly important when considering lifetime exposures; it is estimated that 63% of nonsmokers are exposed to SHS for more than 1 h/wk, 35% of nonsmokers are exposed to SHS for more than 10 h/wk, and 16% of nonsmokers are exposed for at least 40 h/wk (40).
Studies have shown that a decreased HRV is associated with deleterious health outcomes and an increased risk for cardiovascular-related sudden death, particularly in individuals with cardiovascular disease (5, 10), diabetes (37), Parkinson's disease (20), and depression (1). More importantly, the risks are not just limited to susceptible groups. Reduced HRV may predict mortality even in the absence of overt disease (12). Although it is well recognized that a reduced HRV is a marker for a reduced autonomic function, the mechanism by which the reduced autonomic function contributes to cardiovascular-related mortality is not well understood. The present study suggests that the increase in arrhythmias inducibility associated with a reduced autonomic function in SHS-exposed mice may account for the findings in the epidemiologic data linking SHS to cardiac arrhythmias and sudden cardiac death. Indeed, previous published studies have documented that control mice do not show evidence of inducible atrial fibrillation without pharmacological provocation (0% incidence of atrial fibrillation), and wild-type mice have only a 2% incidence of ventricular tachycardia or fibrillation (6, 25, 45). Recently, Smith and colleagues (38) presented the most direct evidence showing the link between autonomic modulation and ventricular repolarization that is independent of heart rate. In humans, autonomic blockade exaggerated drug-induced prolongation of the Q-T interval, a known substrate for polymorphic ventricular tachycardia. Although the exact cellular mechanisms underlying the effect are unclear, the study demonstrated the link between a reduced autonomic function and the susceptibility to arrhythmia.
In addition to the increase in arrhythmia inducibility, the present study showed that the incidence of AV block was increased from near 0% in control mice to 33% after SHS exposure. Because of the nature of the experiment, although desirable, the 24-h HRV and the cardiac electrophysiology were not performed in the same animals. Additional studies are required to further delineate the underlying mechanisms for the observed AV block. For example, the use of autonomic blockade on the SHS-induced changes in cardiac electrophysiology will help to reveal the contribution of each autonomic limb in the modulation of cardiac electrophysiology after exposure.
Short-term SHS exposure alters the cardiac electrical properties of the intact mouse (Table 3). The SHS exposure results in the prolongation of the recovery of the sinus node function, which is one mechanism speculated to cause atrial fibrillation and sick sinus syndrome in humans. Additionally, SHS exposure shortened the refractory period over the AV node, which could accelerate the conduction to the ventricular and promote the development of ventricular arrhythmias. Finally, SHS shortened the ventricular refractory period, which may result in the increase in ventricular susceptibility to the development of ventricular arrhythmias as the electrical wavelets have more time to synergize and regenerate.
With more than 4,000 components in SHS, the exposure-induced cardiovascular consequences are unlikely to be caused by a single SHS component. Suspect components affecting cardiovascular health include carbon monoxide, nicotine, and particulate matter. The main concern with increased carbon monoxide levels resides in the carboxyhemoglobin levels. Although not measured in this study, the carboxyhemoglobin levels were below 1% immediately following exposure to the low concentration of SHS (unpublished observation). Based on the prediction models, it is estimated that the carboxyhemoglobin levels increased gradually over the 6 h of exposure and reached a maximal of 7.6–10% at the end of 6 h of exposure (19, 42). At these carboxyhemoglobin levels, the available studies on the effect of increased carboxyhemoglobin on the cardiovascular regulation are mixed; some showed no change in the occurrence of arrhythmias (9, 15, 44) and no change in heart rate and blood pressure (14, 22, 44), whereas others showed a decrease in ventricular fibrillation threshold (3, 11). Importantly, the carboxyhemoglobin levels after high-concentration SHS exposure are expected to decrease down to near control level within an hour after the exposure (42). Therefore, the carboxyhemoglobin level during the HRV recording periods is not expected to be elevated. In addition, Pope et al. (35) found that the individuals consistently showed a lower HRV in the smoking areas of an airport even though the carbon monoxide levels were extremely low. Taken together, carbon monoxide does not seem to play a major role in SHS-induced reduction of HRV, arrhythmias, and heart block.
In terms of the contribution from nicotine, in the present study, the SHS environment is generated in a glass and stainless steel chamber of small capacity. Because of the limited surface found in such a chamber, nicotine can rapidly saturate this surface and lead to the off-gassing of nicotine from the surfaces into the air, resulting in a higher nicotine concentration. This is a limitation of the exposure system but is consistent with the properties of SHS and a small enclosed space in human exposure. This would not be considered as active smoking since the gases and particulates emitted arise predominately from the smoke being emitted off the end of the cigarette between puffs, and the particle concentrations are well below any that is designed to simulate active smoking (21, 31, 32). Importantly, Lucini and colleagues (30) showed that nicotine patches produce a much smaller reduction in HRV compared with smoking, suggesting that nicotine is not the only component causing the reduction in HRV.
Finally, suspended particles from a burning cigarette may play an important role in SHS-induced reduced HRV (35). Particulate matter exposure has the same characteristic health consequences as SHS exposure, such as cardiovascular mortality and morbidity (34). More importantly, a number of studies show that exposure to particulate matter, particularly in the fine and ultrafine range, is associated with a decreased HRV (34).
In summary, the present study shows that SHS exposure results in a reduced HRV and increased arrhythmia susceptibility, suggesting that the mouse is a suitable research model for future studies investigating the biochemical, cellular, and physiological mechanisms of SHS-induced cardiovascular morbidity and mortality. Further electrophysiological investigations will help elucidate any neuroplastic changes that may occur in the regions responsible for autonomic cardiovascular control, whereas biochemical experiments may help explain the neuroplasticity.
GRANTS
This work is supported by Environmental Protection Agency Grants RD-83191801 and R-832414; a National Institute of Environmental Health Grant ES-012957; and National Heart, Lung, and Blood Institute Grants HL-75274 and HL-85844.
Acknowledgments
We gratefully acknowledge Tam Do, Matthew Hoffman, Hai Pham, Suresh Raman, and Rick Tham for assistance with data analysis and Suzette Smiley-Jewell for editing the manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Agelink MW, Majewski T, Wurthmann C, Postert T, Linka T, Rotterdam S, Klieser E. Autonomic neurocardiac function in patients with major depression and effects of antidepressive treatment with nefazodone. J Affect Disord 62: 187–198, 2001. [DOI] [PubMed] [Google Scholar]
- 2.American Lung Association Government Relations Office. State Legislated Actions on Tobacco Issues. New York: American Lung Association, 2007.
- 3.Aronow WS, Stemmer EA, Zweig S. Carbon monoxide and ventricular fibrillation threshold in normal dogs. Arch Environ Health 34: 184–186, 1979. [DOI] [PubMed] [Google Scholar]
- 4.Barnoya J, Glantz SA. Cardiovascular effects of second-hand smoke help explain the benefits of smoke-free legislation on heart disease burden. J Cardiovasc Nurs 21: 457–462, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J Am Coll Cardiol 27: 1053–1060, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Berul CI, Aronovitz MJ, Wang PJ, Mendelsohn ME. In vivo cardiac electrophysiology studies in the mouse. Circulation 94: 2641–2648, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Bhatnagar A Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res 99: 692–705, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Billman GE A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol Ther 111: 808–835, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Chaitman BR, Dahms TE, Byers S, Carroll LW, Younis LT, Wiens RD. Carbon monoxide exposure of subjects with documented cardiac arrhythmias. Res Rep Health Eff Inst 52: 1–26, 1992. [PubMed] [Google Scholar]
- 10.Collier DJ, Bernardi L, Angell-James JE, Caulfield MJ, Sleight P. Baroreflex sensitivity and heart rate variability as predictors of cardiovascular outcome in hypertensive patients with multiple risk factors for coronary disease. J Hum Hypertens 15, Suppl 1: S57–S60, 2001. [DOI] [PubMed] [Google Scholar]
- 11.DeBias DA, Banerjee CM, Birkhead NC, Greene CH, Scott SD, Harrer WV. Effects of carbon monoxide inhalation on ventricular fibrillation. Arch Environ Health 31: 42–46, 1976. [DOI] [PubMed] [Google Scholar]
- 12.Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen Study. Am J Epidemiol 145: 899–908, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich DF, Schwartz J, Schindler C, Gaspoz JM, Barthelemy JC, Tschopp JM, Roche F, von Eckardstwein A, Brandli O, Leuenberger P, Gold DR, Akermann-Liebrich U. Effects of passive smoking on heart rate variability, heart rate and blood pressure: an observational study. Int J Epidemiol 36: 834–840, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Favory R, Lancel S, Tissier S, Mathieu D, Decoster B, Neviere R. Myocardial dysfunction and potential cardiac hypoxia in rats induced by carbon monoxide inhalation. Am J Respir Crit Care Med 174: 320–325, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Foster JR Arrhythmogenic effects of carbon monoxide in experimental acute myocardial ischemia: lack of slowed conduction and ventricular tachycardia. Am Heart J 102: 876–882, 1981. [DOI] [PubMed] [Google Scholar]
- 16.Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol 279: H733–H740, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Glantz SA, Parmley WW. Passive smoking and heart disease. Epidemiology, physiology, and biochemistry. Circulation 83: 1–12, 1991. [DOI] [PubMed] [Google Scholar]
- 18.Glantz SA, Parmley WW. Passive smoking and heart disease. Mechanisms and risk. JAMA 273: 1047–1053, 1995. [PubMed] [Google Scholar]
- 19.Goldsmith JR, Aronow WS. Carbon monoxide and coronary heart disease: A review. Environ Res 10: 236–248, 1975. [DOI] [PubMed] [Google Scholar]
- 20.Haapaniemi TH, Pursiainen V, Korpelainen JT, Huikuri HV, Sotaniemi KA, Myllyla VV. Ambulatory ECG and analysis of heart rate variability in Parkinson's disease. J Neurol Neurosurg Psychiatry 70: 305–310, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hahn FF, Gigliotti AP, Hutt JA, March TH, Mauderly JL. A review of the histopathology of cigarette smoke-induced lung cancer in rats and mice. Int J Toxicol 26: 307–313, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hausberg M, Somers VK. Neural circulatory responses to carbon monoxide in healthy humans. Hypertension 29: 1114–1118, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256–262, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Knight-Lozano CA, Young CG, Burow DL, Hu ZY, Uyeminami D, Pinkerton KE, Ischiropoulos H, Ballinger SW. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation 105: 849–854, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Kovoor P, Wickman K, Maguire CT, Pu W, Gehrmann J, Berul CI, Clapham DE. Evaluation of the role of I(KACh) in atrial fibrillation using a mouse knockout model. J Am Coll Cardiol 37: 2136–2143, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Kritz H, Schmid P, Sinzinger H. Passive smoking and cardiovascular risk. Arch Intern Med 155: 1942–1948, 1995. [PubMed] [Google Scholar]
- 27.La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. A prospective study. Circulation 78: 816–824, 1988. [DOI] [PubMed] [Google Scholar]
- 28.Lam TH, He Y. Passive smoking and coronary heart disease: a brief review. Clin Exp Pharmacol Physiol 24: 993–996, 1997. [DOI] [PubMed] [Google Scholar]
- 29.Leone A, Mori L, Bertanelli F, Fabiano P, Filippelli M. Indoor passive smoking: its effect on cardiac performance. Int J Cardiol 33: 247–251, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. J Cardiovasc Pharmacol 31: 714–720, 1998. [DOI] [PubMed] [Google Scholar]
- 31.March TH, Bowen LE, Finch GL, Nikula KJ, Wayne BJ, Hobbs CH. Effects of strain and treatment with inhaled all-trans-retinoic acid on cigarette smoke-induced pulmonary emphysema in mice. COPD 2: 289–302, 2005. [PubMed] [Google Scholar]
- 32.March TH, Wilder JA, Esparza DC, Cossey PY, Blair LF, Herrera LK, McDonald JD, Campen MJ, Mauderly JL, Seagrave J. Modulators of cigarette smoke-induced pulmonary emphysema in A/J mice. Toxicol Sci 92: 545–559, 2006. [DOI] [PubMed] [Google Scholar]
- 33.McMurray RG, Hicks LL, Thompson DL. The effects of passive inhalation of cigarette smoke on exercise performance. Eur J Appl Physiol Occup Physiol 54: 196–200, 1985. [DOI] [PubMed] [Google Scholar]
- 34.Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 56: 709–742, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Pope CA, Eatough DJ, Gold DR, Pang Y, Nielsen KR, Nath P, Verrier RL, Kanner RE. Acute exposure to environmental tobacco smoke and heart rate variability. Environ Health Perspect 109: 711–716, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raupach T, Schafer K, Konstantinides S, Andreas S. Secondhand smoke as an acute threat for the cardiovascular system: a change in paradigm. Eur Heart J 27: 386–392, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Singh JP, Larson MG, O'Donnell CJ, Wilson PF, Tsuji H, Lloyd-Jones DM, Levy D. Association of hyperglycemia with reduced heart rate variability (The Framingham Heart Study). Am J Cardiol 86: 309–312, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Smith AH, Norris KJ, Roden DM, Kannankeril PJ. Autonomic tone attenuates drug-induced QT prolongation. J Cardiovasc Electrophysiol 18: 960–964, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Steenland K, Thun M, Lally C, Heath C Jr. Environmental tobacco smoke and coronary heart disease in the American Cancer Society CPS-II cohort. Circulation 94: 622–628, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Taylor AE, Johnson DC, Kazemi H. Environmental tobacco smoke and cardiovascular disease. A position paper from the Council on Cardiopulmonary and Critical Care, American Heart Association. Circulation 86: 699–702, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Thun M, Henley J, Apicella L. Epidemiologic studies of fatal and nonfatal cardiovascular disease and ETS exposure from spousal smoking. Environ Health Perspect 107, Suppl 6: 841–846, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyuma I, Ueda Y, Imaizumi K, Kosaka H. Prediction of the carbonmonoxyhemoglobin levels during and after carbon monoxide exposures in various animal species. Jpn J Physiol 31: 131–143, 1981. [DOI] [PubMed] [Google Scholar]
- 43.US Environmental Protection Agency. Respiratory health effects of passive smoking: lung cancer and other disorders. EPA/600/6-90/006F. Washington, DC: Office of Research and Development, US Environmental Protection Agency, 1992.
- 44.Vanoli E, De Ferrari GM, Stramba-Badiale M, Farber JP, Schwartz PJ. Carbon monoxide and lethal arrhythmias in conscious dogs with a healed myocardial infarction. Am Heart J 117: 348–357, 1989. [DOI] [PubMed] [Google Scholar]
- 45.Wakimoto H, Maguire CT, Kovoor P, Hammer PE, Gehrmann J, Triedman JK, Berul CI. Induction of atrial tachycardia and fibrillation in the mouse heart. Cardiovasc Res 50: 463–473, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, He Y, Tuteja D, Xu D, Timofeyev V, Zhang Q, Glatter KA, Xu Y, Shin HS, Low R, Chiamvimonvat N. Functional roles of Cav1.3(alpha1D) calcium channels in atria: insights gained from gene-targeted null mutant mice. Circulation 112: 1936–1944, 2005. [DOI] [PubMed] [Google Scholar]