Abstract
Heat stress (HS)-induced cardioprotection is associated with increased paxillin localization to the membrane fraction of neonatal rat ventricular myocytes (NRVM). The purpose of this study was 1) to examine the subcellular signaling pathways activated by HS; 2) to determine whether myocardial stress organizes and activates an integrated survival pathway; and 3) to investigate potential downstream cytoprotective proteins activated by HS. After HS, NRVM were subjected to chemical inhibitors (CI) designed to simulate ischemia by inhibiting both glycolysis and mitochondrial respiration. Protein kinase B (AKT) expression (wild type) was increased selectively with an adenoviral vector. Cell signaling was analyzed with Western blot analysis, while oncosis/apoptosis was assayed by measuring Trypan blue exclusion and/or terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining, respectively. HS increased phosphorylation of focal adhesion kinase (FAK) at tyrosine 397 but did not adversely affect the viability of NRVM before CI. HS increased association between FAK and phosphatidylinositol 3-kinase as well as causing a significant increase in AKT activity. Increased expression of wild-type AKT protected myocytes from both oncotic and apoptotic cell death. Increased expression of a FAK inhibitor, FRNK, reduced AKT phosphorylation in response to HS both at time 0 and after 10 min of CI compared with myocytes expressing empty virus. We conclude that myocardial stress activates cytoskeleton-based signaling pathways that are associated with protection from lethal cell injury.
Keywords: cell signaling, protection, ischemia
sustained periods of myocardial ischemia eventually result in irreversible myocyte injury and cell death that manifests in myocardium as coagulation necrosis. In contrast, brief episodes of ischemia (i.e., generally <20 min) result in a mild injury pattern that is reversible on restoration of normal arterial blood flow. Despite many years of active research, the exact series of events underlying the transition from reversible to irreversible injury remains elusive. It is known that certain interventions are capable of modulating or delaying the onset of irreversible injury in experimental model systems such as hypothermia (2, 14, 15), calcium channel blockade (18, 24, 37), heat stress (HS) (19), and ischemic preconditioning (IP) (17, 21, 29, 33). However, even in experimental model systems, the mechanisms responsible for protection are not fully known.
IP is known to provide the most dramatic and consistent protection against lethal cell injury, but the mechanism of protection has remained controversial. During investigations of the mechanism of IP, a wide range of pharmacological agents were described that could mimic the protective effect of IP, giving rise to the concept of “pharmacological preconditioning.” These studies showed that activation of a variety of membrane receptors, including adenosine, α1-adrenergic, muscarinic, angiotensin, and bradykinin receptors, induced a cardioprotective effect similar in magnitude to IP and gave rise to suggestions that the subcellular signaling pathways utilized by these receptors may underlie the mechanism of IP (3, 23, 30, 41). The earliest studies suggested one specific protein kinase; protein kinase C (PKC) may be the final common pathway. However, further investigation showed that the subcellular signaling pathways triggered by receptor occupancy activated additional signaling proteins that may be equally important in providing cardioprotection. As a result, there is no unifying hypothesis to explain the diverse proximal activators of protection described in pharmacological IP.
Focal adhesion kinase (FAK) is a nonreceptor protein tyrosine kinase that normally exists in nonmuscle cells at cell-to-matrix junctions known as focal adherens or focal adhesion junctions and is activated in response to cell adhesion, integrin clustering, and growth factor stimulation (28). FAK binds to integrins (cell surface receptors) as well as several intracellular proteins that are important in signal transduction including paxillin, tensin, p130CAS, talin, and vinculin (27, 34). Paxillin (cytoskeletal/adapter protein) and p130CAS (docking protein) concentrate and facilitate cell signaling proteins at the focal adhesion site (27). FAK plays a critical homeostatic/survival role because it transduces extracellular matrix-derived survival signals to the inside of the cell (4). Consequently, this cell signaling complex (integrin-FAK-paxillin), localized at the focal adhesion, has been shown to play an important role in maintaining cell survival/viability in nonmuscle cells (11–13, 25, 35, 36).
Although myocytes are not thought to contain focal adherens junctions proper (except when in culture), they contain an analogous structure called the costamere. Costameres are vinculin-containing bandlike structures that encircle myocytes perpendicular to the long axis and localized to the Z disk/I band region (see Fig. 1 in Ref. 26). Studies have shown that mechanical loads are transmitted bidirectionally through costameres, and therefore signaling molecules localized at the costameric junction, such as FAK, have been investigated primarily in relation to myocardial hypertrophy (26). However, immunohistochemistry studies have shown that virtually all of the proteins present in costameres are localized at focal adhesions when cells attach and spread in culture, making our model system utilizing neonatal rat ventricular myocytes (NRVM) ideal for the study of FAK-related signaling pathways. Interestingly, it is possible that FAK activation may play an important role in acute cellular protection, since FAK has been linked with downstream activation of the well-known antiapoptotic kinase protein kinase B (PKB)/AKT (11). However, to our knowledge FAK-related signaling pathways have not been investigated in acute ischemic cell injury.
Fig. 1.
Effect of heat stress (HS) on focal adhesion kinase (FAK) activation in cultured neonatal rat ventricular myocytes (NRVM). A representative immunoblot demonstrating phosphorylation of FAK at tyrosine 397 (indicative of activation) from lysates of control (C) myocytes and myocytes subjected to HS is shown (bottom). HS induced increased activation of FAK at time 0 (*P < 0.028; C vs. HS; n = 4). Activation of FAK continued to be higher after 10 min of chemical inhibition (CI) in HS compared with control cells (**P < 0.034; C + 10'CI vs. HS +10'CI; n = 4). y-Axis data are plotted as fraction of control myocyte levels. There was no difference in the total amount of FAK protein in any of the samples.
We previously demonstrated (39) that the myocardial stress associated with HS causes increased interaction between integrin and FAK, increased paxillin in the membrane fraction of cell lysates, and protection against lethal ischemic injury. Therefore, we propose that myocardial stress activates a protective signaling pathway in myocytes that is initiated by activation of FAK. The purpose of the present study was to characterize the subcellular signaling pathways activated by myocardial stress and to determine whether activation of the pathway results in protection from lethal cell injury through activation of AKT.
MATERIALS AND METHODS
All experiments reported here conformed to the standards in the Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, revised 1996). The animal protocol used for these studies was approved by the Wayne State University Animal Investigation Committee (protocol no. A03-07-08). Wayne State University is approved by the American Association for Accreditation of Laboratory Animal Care.
Isolation of neonatal myocytes.
For each isolate, the ventricular portions of six or seven hearts from 1- to 2-day-old rats were pooled and gently agitated overnight at 4°C with trypsin (0.1 g in 100 ml) in Hanks' balanced salt solution (HBSS). The next day, the myocytes were digested further with serial incubations in collagenase (0.07 g in 100 ml HBSS). The final cell isolate was centrifuged for 3 min at 1,000 rpm and 4°C. The resulting supernatant was discarded, and the cells were resuspended in ice-cold DMEM, transferred to a 50-ml conical tube, and centrifuged again for 4 min at 1,000 rpm and 4°C. The resulting supernatant was discarded, and the cell yield was determined with a hemocytometer. Each isolate yielded enough cells for three six-well plates, with each well containing 2–3 × 106 cells plated at a density of 2 × 103/mm2.
Cell culture.
After isolation and purification, the myocytes were resuspended in DMEM supplemented with antibiotics (penicillin-streptomycin and gentamycin to inhibit bacterial growth) and cultured on standard six-well plates or 35-mm dishes (Corning, Corning, NY). Cells were placed in each well and allowed to attach for 1 h to reduce the number of fibroblasts in the final preparation. After 1 h, the cells were removed and transferred to a fresh plate with fresh medium before initiation of the experimental protocol. Previous studies (40) have shown that this procedure results in >95% myocytes.
Experimental design/protocol.
Myocytes were divided into two main groups: control and heat stressed (myocardial stress). Myocytes subjected to HS were subdivided into two additional groups, each of which was subjected to simulated ischemia. For studies of signaling pathways, myocytes were treated with ≤30 min of simulated ischemia. For studies of cell injury/death, the duration of simulated ischemia was extended to 150 min or the myocytes were subjected to simulated reperfusion by removal of the chemical inhibitors (CI) after 30 min of simulated ischemia. For all studies, the number of separate replicates (i.e., isolates) is indicated in resultsand in Figs. 1–9. Data from at least three separate cell isolations were averaged for all cell injury data [i.e., Trypan blue (TB)/terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) counts]. Western blot data were generated in parallel from the same isolates as the cell injury data.
Fig. 9.
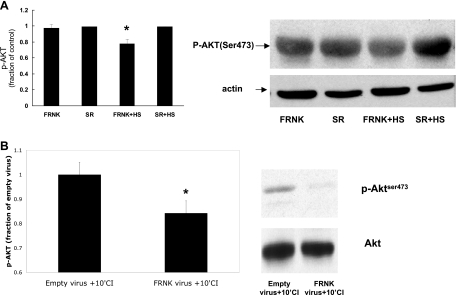
Effect of FRNK virus on AKT activation. A: NRVM were infected with empty virus (SR) or adenovirus designed to increase expression of FRNK (inhibitor of FAK). Increased FRNK expression had no effect on AKT activation as measured by phosphorylation of AKT at Ser473 compared with myocytes infected with the empty virus (P = NS, FRNK vs. control; n = 4) but reduced the amount of HS-induced activation of AKT compared with myocytes infected with the empty virus (*P < 0.04, FRNK+HS vs. SR+HS; n = 4). y-Axis data are plotted as fraction of empty virus (SR)-infected myocyte levels. The total amount of AKT loaded was not different between the groups. B: NRVM were infected with empty virus (SR) or FRNK virus and subsequently exposed to 10 min of simulated ischemia. Myocytes infected with FRNK exhibited significantly less activated AKT than myocytes infected with empty virus after 10 min of simulated ischemia (*P < 0.01; n = 4). y-Axis data are plotted as fraction of empty virus (SR)-infected myocyte level. The total amount of AKT loaded was not different between the groups.
Induction of HS in NRVM.
HS was used as a myocardial stress. Briefly, myocytes were subjected to HS by rapidly increasing the temperature of the culture plates to 42°C for 20 min, followed by 18–20 h of recovery at 37°C. Control myocytes were cultured in parallel at 37°C but not subjected to HS.
Simulated ischemia and/or reperfusion.
To induce ischemia, the culture medium was removed and replaced with PBS containing 1.0 mM iodoacetic acid to inhibit glycolysis and 1.0 mM amobarbital to inhibit mitochondrial respiration as described previously (40). In studies of apoptotic cell death, myocytes were returned to fresh culture medium without inhibitors (“reperfused”) after the indicated period of incubation with medium plus inhibitors.
Western blot procedures.
Myocytes were harvested for protein analysis by standard Western blot techniques as described previously (40). For analysis of subcellular fractions, myocytes were separated into three fractions according to our previous methodology (40). Western blot analysis of the membrane fraction showed the absence of positive staining for myosin heavy chain compared with cytoskeletal and cytosolic fractions and an enrichment in Na+-K+-ATPase compared with the other two fractions, confirming the nature of the membrane fraction (data not shown). Each lane was loaded with an equal amount of protein [as determined by bicinchoninic acid (BCA) protein assay] and subjected to SDS-protein electrophoresis. After electrophoresis, the proteins were transferred to nitrocellulose membranes and then incubated with one of the following primary antibodies: 1) rabbit anti-FAK (Upstate Cell Signaling Solutions; catalog no. 06-543); 2) rabbit anti-FAKpy397 (Biosource; catalog no. 44-624G); 3) rabbit anti-PI3K p85 (Upstate Cell Signaling Solutions catalog no. 06-195); 4) anti-AKT/phospho (p)AKT (Cell Signaling catalog nos. 9271, 9172). The membranes were incubated with donkey anti-rabbit IgG (PI3K/pPI3K and AKT/pAKT) (Santa Cruz Biotechnology; catalog no. SC-2005) or goat anti-rabbit IgG (FAK), and final protein expression was detected with a standard horseradish peroxidase (HRP) chemiluminescence system (Amersham, Arlington, IL). For quantification, films were scanned and the data are reported in arbitrary units and/or percent elevation over control cells as indicated.
Coimmunoprecipitation.
For these experiments myocytes were cultured on standard six-well or 35-mm culture plates. After infection with virus, induction of HS, or induction of metabolic inhibition, cells from two 35-mm dishes were rinsed with PBS at room temperature and immunoprecipitation lysis buffer was added to the cultured myocytes. The immunoprecipitation lysis buffer contained 150 mM NaCl, 1% Triton X-100, 50 mM Tris·HCl, pH 7.5, a cocktail of protease inhibitors [in mM: 10.4 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBST), 0.2 leupeptin, 0.4 bestatin, 0.15 pepstatin A, and 0.14 E-64, with 8 μM aprotinin], and phosphatase inhibitors (1 mM Na3VO4 and 10 nM okadaic acid). The myocytes were scraped from the culture dish, passed through a 26.5-gauge needle, and placed in a microcentrifuge tube. The lysates were incubated on ice for 45 min and centrifuged at 18,000 g for 15 min at 4°C. Approximately 0.4 ml of the supernatant containing equal amounts of protein (confirmed by BCA protein assay) was incubated with 5 μl of mouse anti-FAK antibody (Upstate Cell Signaling no. 06-543) or anti-phosphotyrosine (PY20) for 3 h at 4°C. Twenty-five microliters of protein A/G agarose was then added, and the lysate was rocked gently overnight at 4°C. The cell pellet was collected by centrifugation at 18,000 g for 30 s at 4°C and washed four times with immunoprecipitation lysis buffer. After the final wash, the supernatant was discarded and the pellet was resuspended in sample buffer and subjected to SDS-PAGE. The separated proteins were transferred to nitrocellulose membranes and probed for PI3K-p85 with anti-PI3K-p85 (1:1,000 dilution) or for pPYK2 with anti-PYK2 (1:1,000 dilution; BD Transduction Laboratories) followed by secondary antibody conjugated to peroxidase (1:1,000 dilution; Roche Diagnostics). Membranes were probed with the same chemiluminescence system described for routine Western blots. For quantitative Western blot analysis, films were scanned and data are reported in arbitrary units and/or percent elevation over control cells.
Integrin linked-kinase assay.
Initial cell lysates were generated as described above. After the whole cell lysate was precleared with normal rabbit IgG, ∼0.4 ml of the supernatant containing equal amounts of protein (confirmed by BCA protein assay) was incubated with 5 μl of rabbit anti-integrin-linked kinase (ILK) antibody (Cell Signaling no. 3862) for 3 h at 4°C. Twenty-five microliters of protein A agarose was added, and the lysate was rocked gently overnight at 4°C. The cell pellet was collected by centrifugation at 18,000 g for 30 s at 4°C and washed twice with immunoprecipitation lysis buffer and twice with kinase buffer (Cell Signaling no. 9802). After the final wash, the pellet was resuspended in kinase buffer supplemented with 1 μl of 10 mM ATP and 1 μg of GSK-3 fusion protein (Cell Signaling no. 9278). After 30 min of incubation at 30°C, the reaction was terminated with sample buffer and subjected to SDS-PAGE. After transfer, the membrane was probed for pGSK-3 with antibody (GSK-3α/β ser 21/9) followed by secondary antibody conjugated to peroxidase as above. Quantification was carried out as described above.
Cell injury assay.
Either TB or TUNEL staining was used as an indication of cell death. TB is a vital dye excluded by viable cells with intact cell membranes and is well documented as an indicator of oncotic cell death. Briefly, cells were gently trypsinized from the culture surface and neutralized with serum, and a small volume of 1% TB was added to cells from each dish. TUNEL staining was performed as indicated in the manufacturer's instruction kit (ApopTag; Chemicon International). For TB staining, cells were counted immediately after addition of the dye to prevent counting of nonspecific stained cells, which occur over time. A cell was considered TB positive if the entire cytoplasm was diffusely stained with any shade of blue and TUNEL positive when unequivocal bright nuclear staining was identified. The total numbers of viable cells and positive (dead) cells were counted with a Nikon TE300 inverted immunofluorescent microscope, and the resulting data are presented as the percentage of cells either TB positive or TUNEL positive. All cell counts were performed in a blinded fashion by two different observers to ensure objectivity.
Statistics.
Each myocyte isolate generates control and experimental groups, and therefore each isolate served as its own control. All Western blot data are expressed as means ± SE (in arbitrary units). Statistically significant differences between groups were tested with a paired t-test analysis. A P value <0.05 was considered statistically significant.
RESULTS
Effect of HS on FAK activity.
In our model system, HS caused a significant activation of FAK as measured by phosphorylation of FAK tyrosine residue 397 (Fig. 1). Furthermore, myocytes subjected to prior HS contained more activated FAK than control (nonstressed) myocytes subjected to the same duration of sustained ischemia even though the total amount of FAK protein present in each sample was the same (Fig. 1).
Effect of prior HS on association of FAK and phosphatidylinositol 3-kinase.
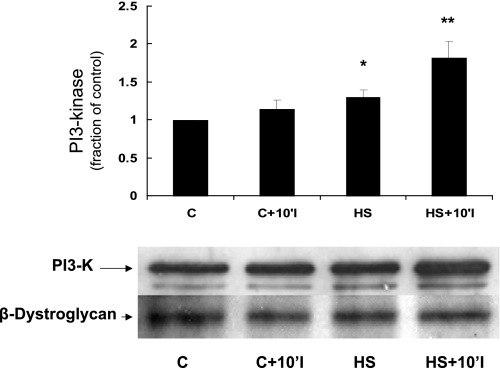
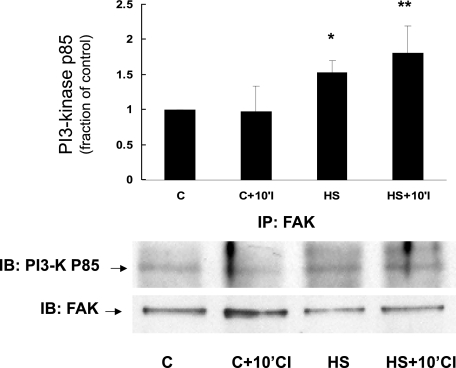
Phosphatidylinositol 3-kinase (PI3K) is a signaling protein that is known to link FAK with downstream protective proteins such as AKT (5, 6). Figure 2 shows that prior HS increases the amount of PI3K present in the membrane fraction both immediately after HS as well as 10 min after the onset of simulated ischemia. Figure 3 depicts immunoprecipitation data showing that prior HS increases the association between FAK and PI3K both immediately after HS and after 10 min of simulated ischemia. Importantly, the total amount of FAK was not different between the two groups in Fig. 3, indicating a HS-specific effect on FAK-PI3K interaction.
Fig. 2.
Effect of HS on phosphatidylinositol 3-kinase (PI3K) content in the membrane fraction of NRVM. A representative immunoblot is shown (bottom). Compared with control myocytes, myocytes subjected to HS contained significantly more PI3K in the membrane fraction (*P < 0.05, C vs. HS, n = 4; **P < 0.05, C+10'CI vs. HS+10'CI, n = 4). y-Axis data are plotted as fraction of control myocyte levels. β-Dystroglycan (a membrane protein) blotting confirmed equal loading between the samples.
Fig. 3.
Effect of HS on interaction between FAK and PI3K in NRVM. Cell lysates from control myocytes or myocytes subjected to HS were prepared and immunoprecipitated (IP) with anti-FAK antibody and then subjected to electrophoresis. The resulting Western blot was stained with anti-PI3K-p85 antibody (IB; bottom). Compared with control myocytes, there was increased interaction between FAK and PI3K in myocytes subjected to HS, consistent with assembly and/or increased interaction between 2 key members of the proposed signaling complex (*P < 0.029, C vs. HS, n = 4; **P < 0.06, C+10'CI vs. HS+10'CI, n = 4). y-Axis data are plotted as fraction of control myocyte levels. The total amount of FAK protein was not significantly different between the various groups.
Effect of HS on PKB/AKT activity.
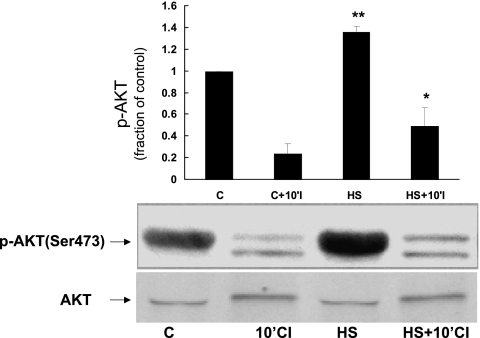
Activation of AKT, a well-known protective signal, has been linked to FAK through PI3K (7, 22). To measure AKT activity, we measured phosphorylation of AKT at serine 473, a site associated with increased AKT activity. Figure 4 demonstrates that HS causes a significant increase in AKT Ser473 phosphorylation before ischemia compared with control NRVM. HS also caused an increase in phosphorylation of AKT at threonine 308, another residue important in activation of AKT activity (data not shown).
Fig. 4.
Effect of HS on activation of AKT in NRVM. NRVM were subjected to HS or control conditions. Cell lysates were probed for phosphorylated (p-)AKTser473 to detect activated AKT. Compared with control myocytes, myocytes subjected to HS showed increased phosphorylation/activation of AKT (*P < 0.007, C vs. HS; **P < 0.05, 10'CI vs. HS+10'CI; n = 3). y-Axis data are plotted as fraction of control myocyte levels. The amount of total AKT present in each sample was not significantly different.
Evidence that AKT is protective in NRVM.
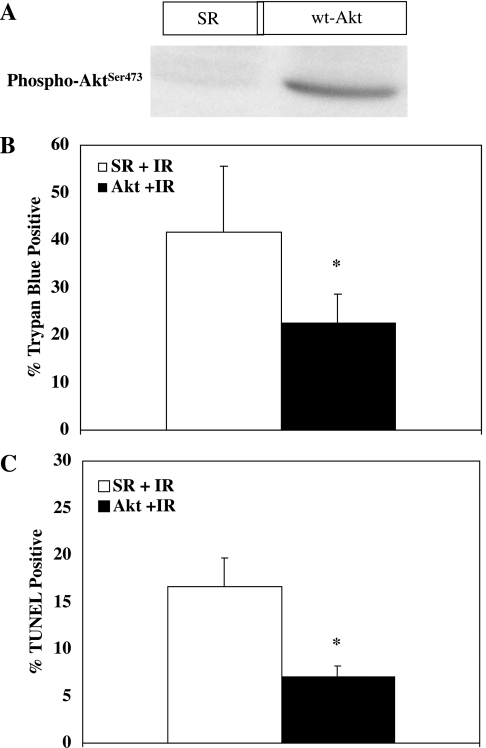
Previous studies have shown that HS is protective in our model system against both oncosis and apoptosis (39). Activation of AKT has been linked to FAK through PI3K (6). Figure 4 shows that HS increases phosphorylation of Ser473 on AKT (associated with increased AKT activity), and therefore we sought to determine whether direct activation of AKT was protective in our model system. To stimulate AKT directly, we increased expression of wild-type AKT within NRVM with an adenoviral vector (kindly supplied by Dr. Ken Walsh; Boston University; Boston, MA). Figure 5A shows that the adenovirus causes a significant increase in AKT activity at time 0 (as indicated by phosphorylated AKT) compared with NRVM infected with an empty adenovirus (SR virus). More importantly, Fig. 5B shows that when activation of AKT is increased in NRVM lethal oncotic injury (as measured by TB permeability) is significantly reduced compared with NRVM infected with the empty adenovirus. Figure 5C shows that when activation of AKT is increased apoptotic injury (as measured by TUNEL staining) is also significantly reduced compared with myocytes infected with the empty adenovirus.
Fig. 5.
Effect of AKT on lethal cell injury in cultured myocytes. A: NRVM were infected with an adenovirus (adv) designed to increase expression of AKT as described in materials and methods. A Western blot demonstrating the effect of adv-AKT on AKT phosphorylation is shown. Compared with myocytes infected with an empty adenovirus (SR), myocytes infected with wild-type (wt) adv-AKT contained significantly more activated (phosphorylated) AKT. B: NRVM were infected with either empty virus or virus designed to increase expression of AKT and then subjected to 30 min of simulated ischemia followed by 30 min of reperfusion (IR). y-Axis values indicate % of cells staining positive with Trypan blue (dead cells). Increased expression of AKT resulted in significant protection against oncotic cell death. *Significant difference from control cells (P ≤ 0.02; n = 5). C: NRVM were infected with either empty virus or virus designed to increase expression of wild-type AKT and then subjected to 30 min of simulated ischemia followed by 30 min of reperfusion. y-Axis values indicate % of TUNEL-positive cells (apoptotic cells). Increased expression of AKT resulted in significant protection against apoptosis. *Significant difference from control cells (P < 0.005; n = 5).
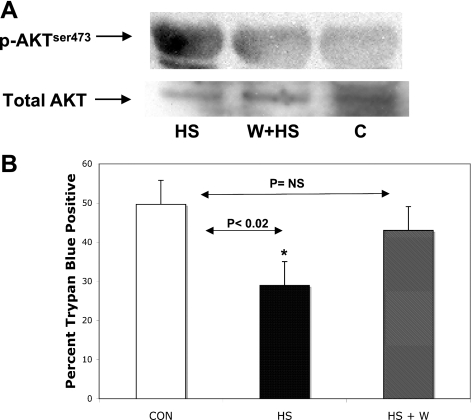
To further support the role of the proposed pathway in cardioprotection, we interrupted the pathway by pretreating one group with the PI3K inhibitor wortmannin (100 nM) before HS and subsequent CI. The control group was incubated in parallel with normal medium before HS and subsequent CI. Importantly, inhibition of PI3K with wortmannin inhibited HS-induced AKT phosphorylation at Ser473 (Fig. 6A). Figure 6B shows that inhibition of PI3K with wortmannin almost completely blocked HS-induced protection. Wortmannin alone did not significantly affect cell death compared with control myocytes (data not shown).
Fig. 6.
Effect of PI3K inhibition (wortmannin) on AKT phosphorylation and cardioprotection. A: NRVM were treated for 1 h in culture medium containing 100 nM wortmannin (W) followed by return to normal culture medium or culture medium alone before HS. Cell lysates were probed for anti-p-AKTser473. A representative Western blot shows that 100 nM wortmannin prevents HS-induced phosphorylation of AKTser473. Total AKT protein was not different between conditions. B: in separate experiments, NRVM were incubated for 60 min with 100 nM wortmannin or with control medium (Con) before a standard HS protocol and then subjected to 60 min of simulated ischemia. HS significantly reduced lethal cell injury, but HS-induced protection was abolished by wortmannin pretreatment. *P < 0.02 Con vs. HS; P = not significant (NS), Con vs. HS+W; n = 5.
Effect of HS on proline-rich tyrosine kinase 2 and ILK activity.
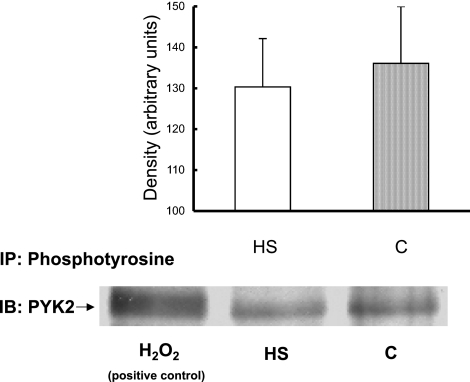
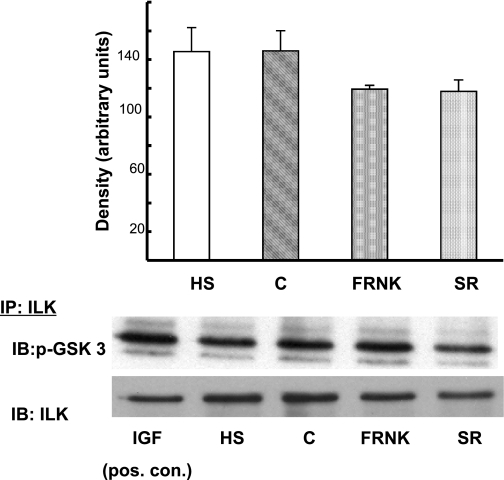
Proline-rich tyrosine kinase 2 (PYK2) has been shown to serve as a scaffolding protein for phosphoinositol-dependent kinase 1 (PDK1) in myocytes (10). PDK1 can phosphorylate/activate AKT at Thr308 and perhaps Ser473 and therefore could be responsible for the AKT activation we measured in response to HS. It is also possible that HS activates ILK that may in turn activate/phosphorylate AKT. Figure 7 shows that HS did not cause significant activation/tyrosine phosphorylation of PYK2. Similarly, to investigate the role of ILK in the activation of AKT, ILK activity was estimated by measuring GSK3-α/β phosphorylation on Western blot. Figure 8 shows that ILK activity was not significantly increased by HS.
Fig. 7.
Effect of HS on proline-rich tyrosine kinase 2 (PYK2) activity. NRVM were subjected to HS or treated for 10 min with PBS containing 5 μM H2O2 (positive control). Extracts were immunoprecipitated with anti-phosphotyrosine (PY20) antibody, followed by probing for PYK2. Hydrogen peroxide was used as a positive control for PYK2 tyrosine phosphorylation. In contrast to hydrogen peroxide, HS had no effect on PYK2 phosphorylation. y-Axis data are plotted as arbitrary units of density. Representative blot is shown. n = 4; P = NS.
Fig. 8.
Effect of HS on integrin-linked kinase (ILK) activity. NRVM were infected with FRNK virus (inhibitor of FAK) or empty virus (SR) for 48 h. Another group of myocytes were subjected to HS. In a separate control experiment (n = 1), control myocytes were treated with 100 ng/ml insulin-like growth factor for 15 min as a positive control for ILK activity. Myocyte extracts were immunoprecipitated with anti-ILK antibodies, followed by an in vitro kinase assay using GSK-3 fusion protein as a substrate (see materials and methodsfor details). Activation of ILK was measured by probing for anti-phospho-GSK-3α/β (Ser 21/9). Total ILK protein present in each lane was detected with anti-ILK antibody after stripping the blot. The graph shows that neither HS nor FRNK infection resulted in increased ILK activity. y-Axis data are plotted as arbitrary units of density. Total ILK present was not different between groups. Representative blot is shown. P = NS, HS vs. C and FRNK vs. SR; n = 3.
Effect of FRNK expression on AKT activation.
Our previous study (39) showed that increased expression of the FAK inhibitor FRNK (focal adhesion kinase-related nonphosphorylatable kinase) resulted in increased ischemic injury compared with control NRVM expressing empty adenovirus. Since FRNK inhibited the activation of a key pathway member (FAK), HS-induced activation of AKT should also be reduced or inhibited. Figure 9 shows that at baseline (i.e., before HS or CI) increased FRNK expression has no effect on AKT activity as measured by Ser473 phosphorylation. However, FRNK expression prevented the HS-induced increase in AKT activation/phosphorylation. Moreover, after 10 min of CI, the amount of AKT activation was less in FRNK-expressing myocytes than control myocytes expressing empty virus. These results support the conclusion that interruption/inhibition of key members of the signaling pathway results in a reduced stimulation of the survival pathway and therefore reduced protection (Fig. 6) or increased cell injury (39).
DISCUSSION
The present results extend the conclusions of our previous study regarding heat shock-induced cardioprotection (39) in several important ways. First, the present study demonstrates that, in addition to oncosis, HS also protects against apoptotic cell death that becomes prevalent when NRVM are subjected to CI followed by reoxygenation. Similar to oncosis, the extent of apoptotic cell death increased as the duration of the simulated ischemic insult increased before reperfusion. Second, the present study shows that additional cytoskeleton-linked proteins are activated by HS, including FAK and PI3K, and that activation of the pathway results in activation of the known cardioprotective protein AKT. Third, activation of FAK/PI3K/AKT is selective since other proteins linked to AKT activation, PYK2 and ILK, are not activated by HS. Fourth, inhibition of PI3K abolished the protective effect of HS, indicating that inhibition of key pathway members abolishes protection. Finally, expression of the FAK inhibitor protein FRNK inhibited HS-induced phosphorylation of AKT at time 0 as well as AKT phosphorylation 10 min after the onset of simulated ischemia, results consistent with our previous study (39) that showed that FRNK expression in nonstressed neonatal myocytes (i.e., interruption of the survival role of the proposed pathway) caused increased cell death.
HS and lethal injury.
Although it is has been reported that HS protects myocardium against subsequent ischemia-reperfusion injury, most if not all prior studies have focused on the role of heat shock proteins (HSPs) per se in mediating the cardioprotective effect. Many mechanisms have been hypothesized to explain the protective role of HSPs in the cardiovascular system, including their role as chaperone proteins, their ability to inhibit caspase-dependent and caspase-independent apoptotic stimuli and/or stabilize cytoskeletal proteins, and the ability of some family members to inhibit cytochrome c-dependent activation of procaspase-9 (1). However, the precise mechanism of cardioprotection is not known. Recently, it has been suggested that induction of a general cell stress response may be all that is necessary for cardioprotection (1).
The results of the present study show for the first time that HS activates a series of linked subcellular signaling proteins that significantly reduces cell death in ventricular myocytes and support the notion that generalized myocardial stress activates a generalized cell survival/protection pathway in myocardium.
Role of HS in assembling a cell survival signaling complex.
Our previous study (39) suggested a critical role for FAK in cell survival by showing that HS caused activation of FAK and decreased cell death while inhibition of FAK (through increased expression of FRNK) increased cell death. Therefore, one of the goals of the present study was to investigate further the role of HS in the assembly of an integrated signaling complex.
If cytoskeleton-based signaling is important in cell survival, then myocardial stress should result in the assembly/activation of the docking protein p130cas into a membrane/cytoskeleton-based signaling complex. HS does indeed result in increased p130cas in the membrane fraction (data not shown). This result, coupled with the data from our previous study (39) showing that HS both enhanced interaction between integrin and paxillin and increased localization of paxillin in the membrane fraction, suggests that stress results in the assembly of a signaling complex localized to the membrane fraction consisting of at least integrin, FAK, p130cas, and paxillin. Although not specifically interrogated in this study, it is likely that talin and vinculin are also localized in the membrane-based signaling complex (9).
Role of other signaling molecules.
Although FRNK expression was used to specifically inhibit FAK activation in these studies, FRNK also inhibits the related protein PYK2. PYK2 has been shown to serve as a scaffolding protein for PDK1 in myocytes, and PDK1 can phosphorylate AKT at Thr308 and perhaps Ser473 (10). Furthermore, it is possible that HS activates the serine-threonine protein kinase ILK, which may in turn directly phosphorylate AKT and therefore account for the measured cardioprotective effect. Therefore, we investigated whether HS protection may in part be mediated through PYK2 and/or ILK activation. However, the results in Figs. 7 and 8 show that HS did not significantly increase either ILK or PYK2 activity, making a role for these important proteins unlikely in the cardioprotective effect of HS in our model system.
Role of AKT in survival pathway.
Our data confirmed that HS reduced lethal ischemic injury in NRVM. However, if the cytoskeleton-based cell survival pathway is important, then the final mediator of protection should be linked with members of the proposed signaling complex. One known survival protein linked with FAK through PI3K is AKT. Although much of the original work describing the protective effect of AKT activation was carried out in cultured myocyte systems, subsequent studies showed a marked reduction in infarct size with in vivo models of ischemia-reperfusion injury (8, 20).
Four results from the present study support the notion that AKT plays an important protective role in our model system. First, we showed that HS caused activation of AKT (Fig. 4). Second, we increased expression of wild-type AKT with an adenoviral expression vector and showed that increased expression of AKT resulted in reduction of both oncotic and apoptotic cell death (Fig. 5). Third, we showed that inhibition of PI3K blocked both the increased phosphorylation of AKT and the protection resulting from HS (Fig. 6). Finally, increased expression of FRNK inhibited HS-induced activation of AKT as well as reducing the amount of activated AKT present 10 min after the onset of simulated ischemia (Fig. 9). The present results (Figs. 4, 5, 6, 9), coupled with the results of our previous study showing that FRNK expression increases cell injury in nonstressed cells, strongly support the hypothesis that HS results in assembly and activation of a cytoskeleton-based cell survival pathway that results in activation of AKT, which is responsible for reducing cell death resulting from simulated ischemia-reperfusion injury.
Mechanism of AKT protection.
The mechanism of AKT protection has been best described in apoptotic cell death, where it has been hypothesized to inhibit apoptotic cell death (caspase mediated) through three potential mechanisms: 1) phosphorylation of Bcl/Bcl-associated death promoter (BAD), which releases Bcl-2 family members (antiapoptotic); 2) direct phosphorylation of caspase-9; and 3) blocking Fas ligand expression. In mammalian cells, the apoptotic cascade is initiated by loss of integrity of the outer mitochondrial membrane and the associated release of cytochrome c (32). Cytochrome c in turn results in the cleavage and activation of caspase-9 and the rest of the caspase cascade. Therefore, some studies have hypothesized that AKT protection is secondary to inhibition of cytochrome c release from mitochondria (16). However, AKT has been shown to increase glucose uptake via increased sarcolemmal Glut-4 expression in rodent and human skeletal muscle (31, 38). Since it is well known that increased glucose uptake can mitigate many ischemia-induced derogatory effects on myocardial function and viability, it is feasible that AKT protection could result through modulation of glucose metabolism/bioenergetics. At the present time, it is not clear which potential mechanism is most likely to provide protection in our model system.
Summary and conclusions.
Data from our previous study showed that heat shock in cultured NRVM is associated with changes in paxillin localization, assembly of an integrin-paxillin-FAK signaling complex, and a reduction in lethal injury in response to simulated ischemic injury. The data from the present study confirm and extend our previous studies by showing that HS causes activation of a series of linked signaling molecules starting at the focal adhesion complex (costamere equivalent in cultured myocytes) and resulting in activation of the well-known cardioprotective protein AKT. Activation of AKT, whether through HS or directly through increased expression of the wild-type AKT protein, protected myocytes from oncotic and apoptotic death. Inhibition of PI3K with wortmannin inhibited activation of AKT and reduced HS-induced protection. These results suggest that myocardial stress activates a cytoskeleton-based survival pathway that may play an important role in protection against acute ischemia-reperfusion injury in ventricular myocardium.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants RO1-HL59563-A2 and RO1-HL-84405-A1 to R. S. Vander Heide.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alireza-Tousi A, Halcox JP, Henderson B. Stressing the obvious? Cell stress and cell stress proteins in cardiovascular disease. Cardiovasc Res 74: 19–28, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Angell WW, Rikkers L, Dong E Jr, Shumway NE. Organ viability with hypothermia. J Thorac Cardiovasc Surg 58: 619–624, 1969. [PubMed] [Google Scholar]
- 3.Bogoyevitch MA, Parker PJ, Sugden PH. Characterization of protein kinase C isotype expression in adult rat heart. Protein kinase C-epsilon is a major isotype present, and it is activated by phorbol esters, epinephrine and endothelin. Circ Res 72: 757–767, 1993. [DOI] [PubMed] [Google Scholar]
- 4.Chan PC, Lai JF, Cheng CH, Tang MJ, Chiu CC, Chen HC. Suppression of ultraviolet irradiation-induced apoptosis by overexpression of focal adhesion kinase in Madin-Darby canine kidney cells. J Biol Chem 274: 26901–26906, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Chen HC, Appeddu PA, Isoda H, Guan JL. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem 271: 26329–26334, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Chen HC, Guan JL. Association of focal adhesion kinase with its potential substrate phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA 91: 10148–10152, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franke TF, Yang BC, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell 81: 727–736, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Fujio Y, Nguyen T, Wencker D, Kitsis R, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giancotti FG, Ruoslahti E. Integrin signaling. Science 285: 1028–1032, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Guo J, Sabri A, Elouardighi H, Rybin V, Steinberg S. Alpha-1 adrenergic receptors activate AKT via a Pyk2/PDK-1 pathway that is tonically inhibited by novel protein kinase C isoforms in cardiomyocytes. Circ Res 99: 1367–1375, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Hauck CR, Hsia DA, Schlaepfer DD. The focal adhesion kinase—a regulator of cell migration and invasion. IUBMB Life 53: 115–119, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Hauck CR, Hsia DA, Schlapfer DD. Focal adhesion kinase facilitates platelet-derived growth factor-BB-stimulated ERK2 activation required for chemotaxis, migration of vascular smooth muscle cells. J Biol Chem 275: 41092–41093, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res 90: 1282–1289, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Jones RN, Hill ML, Reimer KA, Wechsler AS, Jennings RB. Effect of hypothermia on the rate of myocardial ATP and adenine nucleotide degradation in total ischemia. Fed Proc 39: 1111, 1980. [Google Scholar]
- 15.Jones RN, Reimer KA, Hill ML, Jennings RB. Effect of hypothermia on changes in high-energy phosphate production and utilization in total ischemia. J Mol Cell Cardiol 14: 123–130, 1982. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol 19: 5800–5810, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloner RA, Shook T, Przyklenk K, Davis VG, Junio L, Matthews RV, Burstein S, Gibson M, Poole WK, Cannon CP, McCabe CH, Braunwald E. Previous angina alters in-hospital outcome in TIMI 4: a clinical correlate to preconditioning. Circulation 91: 37–47, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Lamping KA, Christensen CW, Pelc LR, Warltier DC, Gross GJ. Effects of nicorandil and nifedipine on protection of ischemic myocardium. J Cardiovasc Pharmacol 6: 536–542, 1984. [DOI] [PubMed] [Google Scholar]
- 19.Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillmann WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation 96: 4343–4348, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Matsui T, Tao J, del Monte F, Lee K, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 104: 330–335, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Murry CE, Reimer KA, Long JB, Jennings RB. Preconditioning with ischemia protects ischemic myocardium (Abstract). Circulation 72, Suppl III: 119, 1985.4006123 [Google Scholar]
- 22.Oh H, Fujio Y, Kunisada K, Hirota H, Matsui H, Kishimoto T, Yamauchi-Takihara K. Activation of phosphatidylinositol 3-kinase through glycoprotein 130 induces protein kinase B and p70 S6 kinase phosphorylation in cardiac myocytes. J Biol Chem 273: 9703–9710, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Prasad MR, Jones RM. Enhanced membrane protein kinase C activity in myocardial ischemia. Basic Res Cardiol 87: 19–26, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Reimer KA, Jennings RB. Verapamil in two reperfusion models of myocardial infarction. Temporary protection of severely ischemic myocardium without limitation of ultimate infarct size. Lab Invest 51: 655–666, 1984. [PubMed] [Google Scholar]
- 25.Richardson A, Parsons T. A mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK. Nature 380: 538–540, 1996. [DOI] [PubMed] [Google Scholar]
- 26.Samarel AM Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol 289: H2291–H2301, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Schaller MD Paxillin: a focal adhesion-associated adaptor protein. Oncogene 20: 6459–6472, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Schaller MD, Hildebrand JD, Parsons JT. Complex formation with focal adhesion kinase: a mechanism to regulate activity and subcellular localization of Src kinases. Mol Biol Cell 10: 3489–3505, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schott RJ, Rohmann S, Braun ER, Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res 66: 1133–1142, 1990. [DOI] [PubMed] [Google Scholar]
- 30.Stabel S, Parker P. Protein kinase C. Pharmacol Ther 51: 71–95, 1991. [DOI] [PubMed] [Google Scholar]
- 31.Thorell A, Hirshman MF, Nygren J, Jorfeldt L, Wojtaszewski JF, Dufresne SD, Horton ES, Ljungqvist O, Goodyear LJ. Exercise and insulin cause GLUT-4 translocation in human skeletal muscle. Am J Physiol Endocrinol Metab 277: E733–E741, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science 281: 312–316, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Thornton JD, Striplin S, Liu GS, Swafford A, Stanley AWH, Van Winkle DM, Downey JM. Inhibition of protein synthesis does not block myocardial protection afforded by preconditioning. Am J Physiol Heart Circ Physiol 259: H1822–H1825, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Turner CE Paxillin and focal adhesion signaling. Nat Cell Biol 2: E231–E235, 2000. [DOI] [PubMed] [Google Scholar]
- 35.van de Water B, Houtepen F, Huigsloot M, Tijdens IB. Suppression of chemically induced apoptosis but not necrosis of renal proximal tubular epithelial (LLC-PK1) cells by focal adhesion kinase (FAK). J Biol Chem 276: 36183–36193, 2001. [DOI] [PubMed] [Google Scholar]
- 36.van de Water B, Nagelkerke JF, Stevens JL. Dephosphorylation of focal adhesion kinase (FAK) and loss of focal contacts precede caspase-mediated cleavage of FAK during apoptosis in renal epithelial cells. J Biol Chem 274: 13328–13337, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Vander Heide RS, Schwartz L, Reimer KA. The novel calcium channel antagonist Ro 40-S967 limits infarct size in the dog. Cardiovasc Res 28: 1526–1532, 1994. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol 19: 4008–4018, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei H, Campbell W, Vander Heide RS. Heat shock-induced cardioprotection activates cytoskeletal-based cell survival pathways. Am J Physiol Heart Circ Physiol 291: H638–H647, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Wei H, L'Ecuyer T, Vander Heide RS. Effect of increased expression of cytoskeletal protein vinculin on ischemia-reperfusion injury in ventricular myocytes. Am J Physiol Heart Circ Physiol 284: H911–H918, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Ytrehus K, Liu Y, Downey JM. Preconditioning protects ischemic rabbit heart by protein kinase C activation. Am J Physiol Heart Circ Physiol 266: H1145–H1152, 1994. [DOI] [PubMed] [Google Scholar]