Abstract
High percentages of harmful microbes or their secreting toxins bind to specific carbohydrate sequences on human cells at the recognition and attachment sites. A number of studies also show that lectins react with specific structures of bacteria and fungi. In this report, we take advantage of the fact that a high percentage of microorganisms have both carbohydrate and lectin binding pockets at their surface. We demonstrate here for the first time that a carbohydrate non-labeled mass sensor in combination with lectin-bacterial O-antigen recognition can be used for detection of high molecular weight bacterial targets with remarkably high sensitivity and specificity. A functional mannose self-assembled monolayer (SAM) in combination with lectin Con A was used as molecular recognition elements for the detection of E. coli W1485 using Quartz Crytsal Microbalance (QCM) as a transducer. The multivalent binding of Concanavalin A (Con A) to the Escherichia coli (E. coli) surface O-antigen favors the strong adhesion of E. coli to mannose modified QCM surface by forming bridges between these two. As a result, the contact area between cell and QCM surface increases that leads to rigid and strong attachment. Therefore it enhances the binding between E. coli and the mannose. Our results show a significant improvement of the sensitivity and specificity of carbohydrate QCM biosensor with a experimental detection limit of a few hundred bacterial cells. The linear range is from 7.5 × 102 to 7.5 × 107 cells/mL that is four decade wider than the mannose alone QCM sensor. The change of damping resistances for E. coli adhesion experiments was no more than 1.4% suggesting that the bacterial attachment was rigid, rather than a viscoelastic behavior. Little non-specific binding was observed for Staphylococcus aureus and other proteins (Fetal Bovine serum, Erythrina cristagalli lectin). Our approach not only overcomes the challenges of applying QCM technology for bacterial detection but also increases the binding of bacteria to their carbohydrate receptor through bacterial surface binding lectins that significantly enhanced specificity and sensitivity of QCM biosensors. Combining carbohydrate and lectin recognition events with an appropriate QCM transducer can yield sensor devices highly suitable for the fast, reversible and straightforward on-line screening and detection of bacteria in food, water, clinical and biodefense areas.
Keywords: Non-labeled Quartz Crystal Microbalance, Carbohydrate, Self-Assembled Monolayers, Lectin, Escherichia coli
INTRODUCTION
Rapid Methods for bacterial detection are essential in food, industrial, environmental monitoring, clinical diagnostics and biodefense to allow faster decisions to be made with respect to food poisoning, water contamination, the presence of disease and, therefore, treatment options. Most conventional methods (e.g. plating and culturing, biochemical tests, microscopy, flow cytometry, luminescence) are time consuming, often requiring 1-2 days to obtain results. Although much faster detection methods such as immunosensors or DNA chips are becoming available, they have failed to gain wide acceptance due to the high user expertise required, high cost of labeling reagents, low stability of antibody and DNA recognition elements. As a result, a rapid, quantitative, sensitive and specific method for one step bacterial detection is highly sought after.
A high percentage of harmful bacteria and their secreted toxins bind specific carbohydrate sequences on the surface of human cells at the initial recognition and attachment site. Recently it has become clear that type 1 fimbriae present on the surface of Enterobacteriaceae such as E. coli, are responsible for mannose- and mannoside-binding activity.1,2 Knowing the nature of bacteria and their host cell invasion processes, where carbohydrate interactions play an important role in the stability and rigidity of saccharide assemblies, it seems logical to use carbohydrate structures as receptor elements in pathogen detection schemes. However, carbohydrate-protein interactions are often weaker than protein-protein interactions, by perhaps a factor of 102-103 based on typical antibody equilibrium dissociation constants (KD). Additionally, it is commonly accepted that apart from toxins and bacteria, many other endogenous and exogenous proteins could recognize the carbohydrate ligand, which could lead to high cross activity. Up to now, the optimal conditions for their application as receptor elements, particularly when a non-labeled transducer is selected as the transduction mechanism, have yet to be developed.
A non-labeled biosensor such as Quartz Crystal Microbalance offers significant advantages over current labeled techniques. Being labeled free, it dispenses with the time and cost demanding labeling step, and also eliminates any possible interference of the “true” binding process due to the presence of the labels. Among various non-label transducers, QCM, is a mass sensor, and is ideal for detecting analytes of high molecular weights. It gives a response that characterizes the binding event between the analyte to be detected and a sensing layer, which is immobilized on the surface of the QCM transducer. The resonant QCM frequency depends on the mass attached to the quartz crystal surface according to the Sauerbrey relationship,3 Δf = -2 Δmnf02 / [A(μqρq)1/2], where n is the overtone number, μq is the shear modulus of the quartz (2.947 × 1011 g/(cm·sec2)), and ρq is the density of the quartz (2.648 g/cm3), which assumes the foreign mass is strongly coupled to the resonator. Methods based on the use of piezoelectric crystal devices have been developed for immunoassays,4-6 bacterial detection7-22 and virus and toxin detection.23-26 Due to the non-rigid nature of bacterial cells, researchers are still skeptical about the potential of piezoelectric mass sensing devices for detection of bacteria. QCM measures only those materials that are acoustically coupled to the sensor surface and requires the surface layer to be rigid.27,28 Bacterial binding often involves energy dissipation due to internal friction or trapping of water by the cells, which cause damping of the oscillation of the crystals. As a result, surface chemistry needs to be developed to ensure that the bacteria are strongly attached on the QCM transducer surface, which is not a trivial task. For example, in many bacteria, the carbohydrate binding lectins are usually in the form of fimbriae (or pili). The pili typically have a diameter of 3-7 nm and can extend 100-200 nm in length.29 The bulk of the fimbrial filament is made up of polymers of the major subunit. Only one of the subunits, usually a minor component of the fimbriae, possesses a carbohydrate combining site and is responsible for the binding activity and sugar specificity of the fimbriae. For example, in type 1 fimbriae, which are made up of hundreds of subunits of four different kinds, the subunit (MW 29-31 KDa) is present in small numbers at intervals along the fimbrial filament and at the distal tip. However, only these subunits appear to be able to mediate mannose-sensitive adhesive interactions, whereas the subunits at the other positions are inaccessible to the ligand.29 Consequently, binding is generally of low affinity and not rigid. But since the adhesions and the receptors often cluster in the plane of the membrane, the resulting strength of the interaction can be quite strong.
Bacteria cells have rigid cell wall so they are more rigid than eukaryotic cells. The major bacterial cell wall component in all gram-negative bacterial families consists of a class of glycoconjugates called Lipopolysaccharides (LPS). LPS are present in the outer monolayer of the outer membrane along with phospholipids (inner leaflet) and proteins. The LPS fraction comprises about 10-15% of the total molecules in the outer membrane and is estimated to occupy about 75% of bacteria surface area.30 Bacteria express either smooth or rough LPS. Smooth LPS consists of a hydrophobic domain known as Lipid A anchored in the membrane, a polysaccharide (O-antigen) made of repetitive subunits of one to eight sugars and a “core” oligosaccharide at the junction between the two other regions. Rough LPS lacks O-antigen, but possesses lipid A and core oligosaccharides.31 The O-antigens, being at the utmost cell surface, are at the interface between the bacterium and its environment, and are important virulence factors and antigens for many pathogenic bacteria.32 Many bacteria are sub-classified by the O-antigen on their surface. Therefore, LPS O-antigens are unique to specific bacterial strains (i.e. sub-species) and provide the selective specificity needed for the lectin recognition.
In this report, we developed an innovative approach so that the characteristics of multivalency of lectin and bacterial surface LPS are fully exploited for their benefits of non-labeled sensing. Shown in Scheme 1, both the selective lectin-O-antigen recognition and lectin-carbohydrate recognition can be used synergetically for bacterial detection that provides enhanced specificity and needed rigidity for non-labeled QCM biosensors. Specifically, we use a mannose receptor immobilized on the gold QCM sensor surface and use it to detect E. coli W1485 as a model system. As discussed earlier, type 1 fimbriae present on the surface of most E. coli strains are responsible for mannose- and mannoside-binding activity.1 According to the studies conducted by Otto and coworkers,20,33 the direct adhesion of the fimbriated E. coli onto the mannose immobilized QCM surface, is quiet flexible, and there might be water layers trapped between the bacteria and QCM surface. This non-rigid binding may cause damping of the oscillation. E coil W1485 is the wild type of E Coli K-12.34 LPS analysis (Supplementary S1) showed that E coil W1485 is a “semi-rough” bacterial strain in which the intact LPS core is capped by a single O-antigen subunit. Structural study of E coli K-12 O-antigen showed that the repetitive subunit of O-antigen consists of glucose, N-acetylglucosamine, galactose and rhamnose in the ratio 1.8:1.0:0.7:0.6.35 Given the fact that gram-negative bacteria such as E. coli, have chemically distinct surface LPS that could be recognized by specific lectins,36 we first bind the Concanavalin A (Con A) to the E. coli W1485. Con A, isolated from Jack bean (Canavalian ensiformis) is the most widely used and well-characterized mannose and glucose binding lectin. Con A can aggregate on specific terminal carbohydrates of bacterial surface LPS with different binding ability.37 The multivalent binding of Con A to the E. coli W1485 surface O-antigen glucose receptor favors the strong adhesion of E. coli W1485 to mannose immobilized on the QCM surface. As a result, Con A increases the contact area between the E. coli W1485 cell and the mannose ligands immobilized on the gold QCM surface. This leads to a relatively rigid and strong attachment that enhances and magnifies the binding between E. coli and the mannose receptor (Scheme 1). Our approach not only overcomes the challenges of applying QCM technology for bacterial detection but also increases the binding of bacteria to their carbohydrate receptor through bacterial surface binding lectins, thus significantly enhanced specificity and sensitivity of QCM biosensors.
Scheme 1.
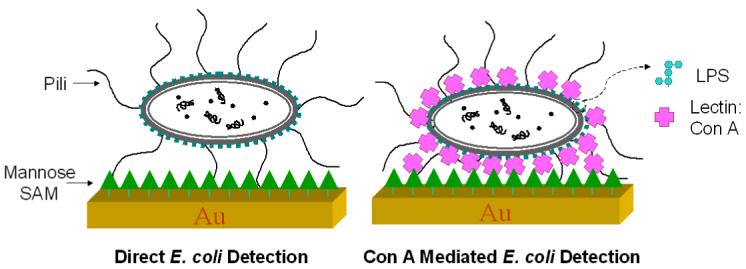
Schematic presentations of direct E. coli detection and Con A mediated E. coli detection.
Additionally, we combined the advantage of SAM and the synthetic strength of molecular design by building a functional mannose coating to prevent non-specific adsorption. The linker for the functional mannose coating consists of two parts: the polyethylene glycol ([OCH2CH2]nOH, n=4) portion and the saturated alkyl portion (R= (CH2)11) (Scheme 1). The polyethylene glycol part is linked with mannose, while the alkyl portion is terminated with -SH group, which will anchor the molecule on the Au surface of the QCM sensor. mPEG-thiol was used as a blocking reagent to reduce the nonspecific adsorption. Previous research shows that monolayers terminated in short oligomers of the ethylene glycol group ([OCH2CH2]nOH, n=3-6) prevent the adsorption of proteins under a wide range of conditions.38 This alternative approach has several advantages over current methods including well-defined surface chemistry, relative stability and facile formation into defined supramicron to nanometer-scale two-dimensional patterns.
MATERIALS AND METHODS
Chemicals and Materials
1H and 13C NMR spectra were recorded on a VXR400 NMR and a Varian Unity 500 MHz spectrometers. Mass spectra were run on Kratos MS-80 and Kratos MS-50 instruments. Thin-layer chromatography was conducted on precoated Whatman K6F silica gel 60 Å TLC plates with a fluorescent indicator. EM Science silica gel 60 Geduran (230-400 Mesh) was used for column chromatography. Concanavalin A (Con A), and lectin from Erythrina cristagelli (ECL) were purchased from Sigma. Escherichia coli, a lambda-derivative of E. coli strain W1485 (ATCC® 12435™) was obtained from ATCC. mPEG-thiol was purchased from NEKTAR. Phosphate buffered saline and fetal bovine serum were obtained from Gibco.
Bacterial strain, culture and LPS analysis
The pure culture of Escherichia coli, a lambda-derivative of E. coli strain W1485 (ATCC® 12435™) was grown in ATCC®294 broth at 37 °C for 24 h in a shaking incubator. The viable cell number was determined by conventional agar plate counting. The crude cultured cell sample was directly diluted with PBS buffer to the desired concentrations and frozen for further use without any washing steps. The E coli LPS was analyzed by silver-stained SDS-PAGE (See supplementary file Figure. S1).
Quartz Crystal Microbalance Set-up
The QCM measurement set-up is shown in scheme 2. A non-polished gold quartz crystal (International Crystal Manufacturing Co. Inc.) was mounted in a custom-made Kel-F cell. It was cleaned three times using a mixture of concentrated nitric acid and sulfuric acid (1:1 v/v), biograde water and ethanol in series, and then the cell was dried using a nitrogen stream. The frequency of the electrode was measured in PBS (pH 7.2). One side of the gold quartz crystal was incubated in a solution of 4 mg/mL mannose thiol linker conjugate in anhydrous ethanol at 4 °C overnight. After incubation, the gold surface was washed with ethanol and biograde water and then dried under nitrogen to give mannose SAMs. Any remaining sites on the mannose modified QCM surface were blocked using 3 mg/mL mPEG-thiol (PI-03D-18, NEKTAR) for 6 h. A magnetic stir bar was used to increase the mass transfer through convection. The changes in frequency and damping resistance of the QCM were monitored simultaneously using a network/spectrum/impedance analyzer (Agilent 4395A) controlled by a PC via an Intel card.
Scheme 2.
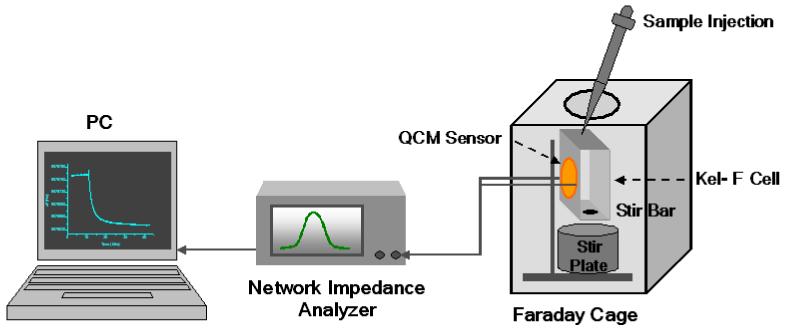
QCM sensor set-up.
Surface Plasmon Resonance
A homemade surface plasmon resonance (SPR) biosensor was used to detect bacterial attachment. The light source was a 06-DAL-103 model semiconductor diode laser (Melles Griot Inc., California) with 4 mW power output. The wavelength of the polarized laser was 650 nm. The refractive index of the glass prism (ZF7) used is 1.79. A series-600 angular sensor (Trans -Tek Inc., Ellington, CT) is used to measure laser incident angles. With a fifteen volt direct current (15V DC) input, the capacitance angle sensor can give an angle signal output of 100 mV/Degree. Also a solar cell is used to convert reflected light intensity into electric voltage. The two analog signals from the angular sensor and the solar cell are converted into 16-bit digital signals by an USB-1608FS data acquisition module (Measurement Computing Corporation, MA), which is connected to a PC through a USB cable. The hardware of the homemade SPR biosensor is controlled by a program written in LabviewR 8.0.
Detection of Con A by mannose-QCM
Con A has identical subunits of 237 amino acid residues (M.W. 26,000). At neutral pH, Con A is predominantly tetrameric with optimal activity.39 At pH 4.5-5.6, Con A exists as a single dimer.40 Two metal ions (Mn2+ and Ca2+) can bind to Con A; both must be present for carbohydrate binding. Therefore, Con A has been used to examine the mannose-QCM sensor performance.
To examine our mannose sensor’s specificity, Erythrina cristagalli lectin (ECL), a galactose-specific legume lectin,41 was used as a negative control. Fetal bovine serum (FBS), the most widely used serum in the culturing of cells, tissues and organs, was also selected as a negative control. As shown in Figure 1 insertion, there were negligible frequency changes for the addition of FBS and ECL. This result shows that the mannose-QCM sensor is antigen specific. After exposure to FBS and ECL, Con A at different concentrations was consecutively added to the sensor (Figure 1). This study demonstrates that the mannose-QCM sensor has high sensitivity and specificity for binding with the Con A even after exposure of the sensor surface in a complex matrix (i.e. FBS and ECL).
Figure 1.
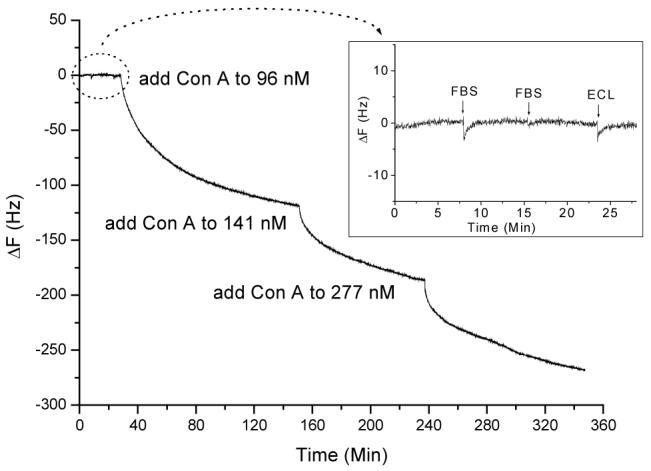
Frequency change vs. time curve when FBS (5.2 μg/mL), ECL (142 nM) and different concentrations of Con A solutions were added sequentially to the mannose-QCM in 1.0 mL PBS buffer (pH = 7.2) with 1 mM Mn2+ and 1 mM Ca2+. (The final concentrations of Con A were 96 nM, 141 nM, and 277 nM.)
To obtain the affinity constant of ConA binding with mannose, we used experiment conditions in which mass transfer is very fast and is not the rate-limiting step by thoroughly stirring the solution using a magnetic stir bar. Under this condition, the binding will be the rate limiting step and the apparent binding affinity for Con A binding to the mannose QCM surface could be estimated by using the Langmuir adsorption model.42 According to the following equation, the mass change at equilibrium was related to the original concentration of Con A.
| (1) |
In this equation, ΔMmax is the maximum binding amount, ΔM is the measured binding amount at equilibrium, and [Con A] is the original concentration of Con A.
The value of KA for the binding between Con A and mannose was estimated to be (5.6±1.4) × 106 M-1 (n=5). This result is in good agreement with reported literature values [(5.6±1.7) × 106 M-1] 43 and our previous work [(8.7±2.8) × 105 M-1 (QCM), (3.9±0.2) × 106 M-1 (SPR)].44
Detection of E. coli W1485 by E. coli pili-Mannose binding and by Con A mediated E. coli LPS and Mannose binding
One of the motivations in this work is to validate our hypothesis that QCM mass sensor senses surface binding phenomena and requires rigid and tight binding. This is why the measured mass was often smaller than expected when QCM is used to detect big targets such as bacteria. Therefore, we compared two recognition events; the first one is the fimbriae mediated E. coli detection based on less rigid pili and mannose adhesion (Scheme 1 Left), the second one is based on the tight and rigid binding via the lectin mediated sandwich assay (Scheme 1 Right).
One major issue which must be considered in bacterial detection is the antigenic or phase variation.45,46,47. Phase variation is the adaptive process by which bacteria undergo frequent and reversible phenotypic changes as a result of genetic alterations in specific loci of their genomes.45 This process is crucial for the survival of pathogens in hostile and ever-changing host environments.46,47 As a result, type 1 E. coli bacteria might shift from a fimbriated phase to a nonfimbriated phase and back spontaneously,48 which might affect the fimbriated E. coli attachment. Various environmental factors, such as centrifuge can change the phase of the Type 1 E. coli bacteria from a fimbriated phase to a non-fimbriated phase48. To eliminate this possible variation, we used crude culture supernatant to omit the centrifuge and washing steps so that we can compare these two methods using consistent bacterial samples. Using crude culture samples also allow us to observe the amount of non-specific binding from the crude cell matrix.
1) E. coli pili-Mannose binding
E. coli W1485 (ATCC® 12435™) carries type 1 fimbriae, which is specific for mannose binding. The E. coli cell is about a million times heavier than Con A and typical antibodies; theoretically the binding between E. coli and mannose receptor on the QCM surface should lead to a very large response. However, when the mannose modified QCM electrode was exposed to the different concentrations of E. coli W1485 (2.9 × 107, 9.8 × 107, 1.6 × 108, and 2.7 × 108 cells/mL), only small signals were observed (Figure 2). The linear range is very narrow (i.e. 2.9 × 107 - 2.7 × 108 cells/mL). This result confirmed our hypothesis that the fimbriae mediated adhesion is relatively weak and flexible. Along with the high mobility of the bacteria, it created large freedom of movement of the bacteria on the QCM surface. This non-acoustic attachment cannot be easily measured by QCM technique. QCM will give a signal only if the above interaction results in a net change of mass. The weak and flexible binding of the fimbriae to the mannose may result in a displacement of one species with another.49 Consequently, the surface is only a temporary host to the E. coli and the net change of mass is very small. Phase variation in which type 1 E. coli bacteria might shift from a fimbriated phase to a nonfimbriated phase and back spontaneously,48 might also contribute to the weak fimbriated E. coli attachment.
Figure 2.
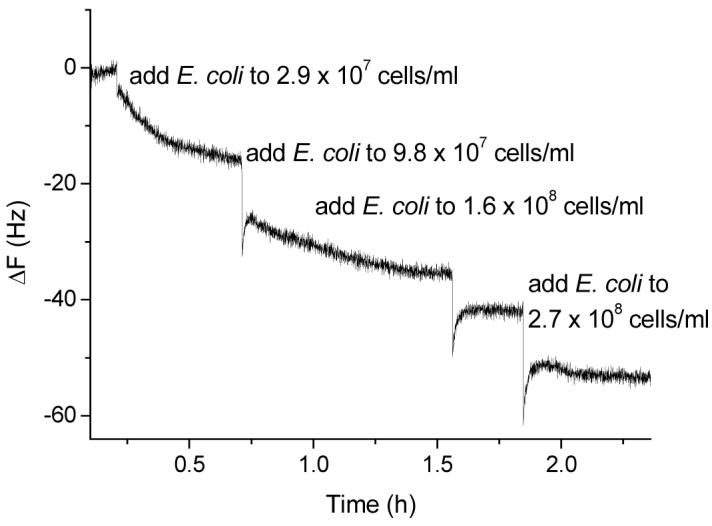
Frequency change vs. time curve when the mannose-QCM electrode was exposed to different concentrations of E. coli W1485 in 1.0 mL stirred PBS buffer (pH = 7.2) with 1mM Ca2+ and 1 mM Mn2+.. (The final concentrations of E. coli were: 2.9 × 107, 9.8 × 107, 1.6 × 108, and 2.7 × 106 cells/mL.)
2) Con A mediated E. coli LPS and Mannose binding
As shown above and also pointed out by E. V. Olsen, et. al.,49 a piezoelectric mass sensor will not be able to provide quantitative information for bacterial detection if binding of bacteria on the sensor surface is neither predominantly rigid nor predominantly flexible. The key is to ensure adequate bacterial binding by using recognition molecules with high affinity and multiple binding valences. Taking advantage of the fact that gram negative bacteria have chemically distinct surface LPS structures that can be recognized by lectins in agglutination studies.37 We used lectin Con A as an E. coli adhesion promoter to strongly attach E. coli to the mannose so a rigid binding layer would be formed on the QCM surface (Scheme 1 Right).
Experimental conditions were first studied so that we can unquestionably demonstrate that the Con A adsorbed on the E. coli cell surface facilitates the binding of E. coli to the mannose receptor rather than free Con A in the mixture of bacteria that binds to the mannose receptor. A low concentration of Con A was first added to the mannose sensor test chamber. The concentration of Con A added is relatively low so that the mannose surface is not saturated based on the Con A/mannose binding study (Figure 1) and the surface still has a plethora of available mannose binding sites. After the Con A-mannose binding reached equilibrium, E. coli W1485 was added. At this time, small amounts of free Con A in the bulk solution will facilitate the binding of E. coli to the mannose receptor as described in Scheme 1.
As shown in Figure 3, the addition of 100 nM Con A to the mannose-QCM generated ∼100 Hz frequency change at equilibrium. The subsequent addition of E. coli provided a large binding signal (∼230 Hz). This result shows that the presence of Con A in the binding solution leads to the signal enhancement. Since the interaction between Con A and E. coli has already been proved by several studies,50 we suggest that Con A in the binding solution aggregates on the E. coli cell walls through binding to the glucose unit in their O-antigen structures that promotes the formation of rigid adhesion onto mannose-QCM.
Figure 3.
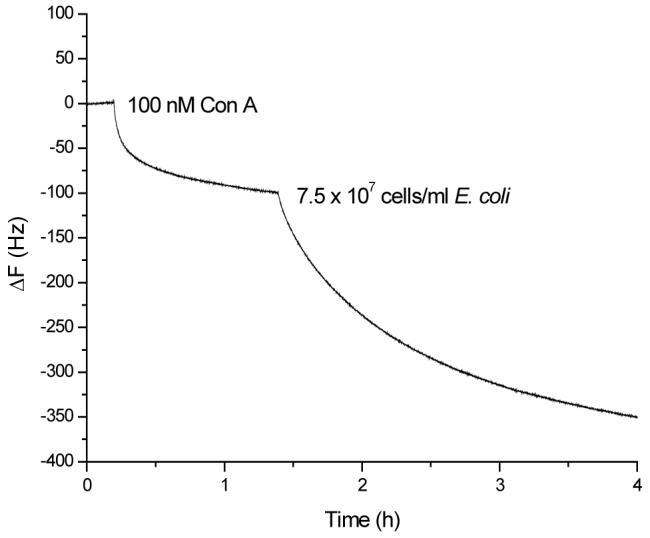
Frequency change vs. time curve when the mannose-QCM sensor was first exposed to 100 nM Con A, followed by the addition of E.coli W1485 (7.5 × 107 cells/mL) in 1.0 mL stirred PBS buffer (pH = 7.2) with 1mM Ca2+ and 1 mM Mn2+.
(a) Sensor sensitivity
The mannose modified QCM surface was first exposed to the 100 nM Con A solution for 2 h to reach the binding equilibrium to ensure the consistency between each experiment, then E. coli W1485 samples ranging from 7.5 × 102 to 7.5 × 107 cells/mL were injected onto the Con A pretreated mannose-QCM sensor chambers which contain 1 mL PBS with 1 mM Mn2+, 1 mM Ca2+ and 0.1 mM Con A. Fast and large signal responses were observed. A linear relationship between the frequency shift and logarithm of cell concentration was found from 7.5 × 102 to 7.5 × 107 cells/mL (Figure 4), which is four decade wider than the early mannose alone sensor. The damping resistance in the Butterworth-Van Dyke-equivalent circuit was also determined simultaneously with the frequency shift for the bacterial binding study. The change of damping resistances for E. coli adhesion experiments in Figure 3 and Figure 4 were no more than 1.4%. This suggests that the bacterial attachment was rigid, rather than a viscoelastic behavior.
Figure 4.
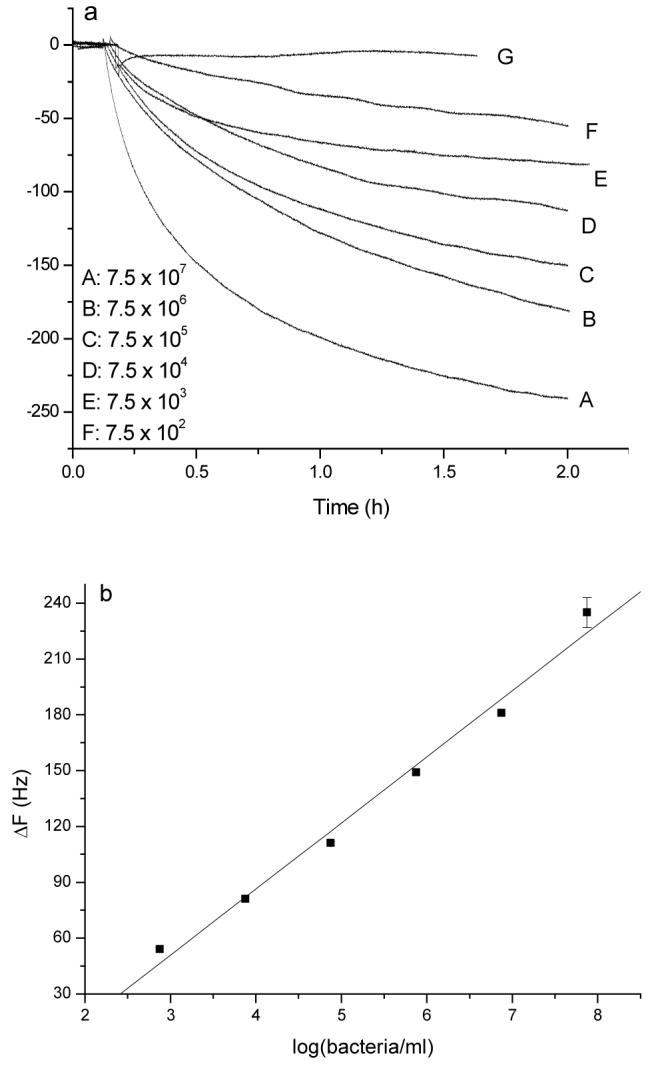
(a) Frequency change vs. time curve when mannose-QCM electrodes were exposed to different concentrations of E. coli W1485 from 7.5 × 102 to 7.5 × 107 cells/mL in 1 mL stirred PBS with 1 mM Mn2+ and 1 mM Ca2+ and 100 nM Con A.Curve G: the addition of blank solution (50 μL culture medium without E. coli) (The mannose-QCM was first exposed to 100 nM Con A solution for about 2 h); (b) Calibration curve: frequency shift vs. log of E. coli concentration.
Table 1 lists the limits of detections (LOD) obtained by the QCM technique using antibody or DNA recognition elements as reported in the literature and the reliable quantitative detection via the two methods we used (carbohydrate or carbohydrate/lectin recognition elements). Comparing to the use of carbohydrate alone, ∼104 fold improvement in reliable quantitative detection was achieved using lectin amplification. Currently, the combined carbohydrate/lectin detection methodsgave 54 Hz signal after adding 7.5 × 102 cells/mL for 2 hours. Therefore, the detection limit in our case could be much smaller if calculated from the statistical methods than those reported in the literature. Since enrichment could give various detection limits, Table 1 showed only the reliable quantitative detection concentration for our study. Additionally, as evidence from the Sauerbrey equation above, the sensitivity of the QCM sensor increases with the square of f0 and linearly with n; thus by working with crystals of higher f0 or at higher harmonics, even higher sensitivity and lower detection limits can be obtained.
Table 1.
Comparison of QCM biosensors for the detection of E. coli
| References | E. coli strains | Assay principle | LOD (cells/mL) | Linear ranges (cells/mL) |
|---|---|---|---|---|
| 8 | K-12 | Immunosensor: Anti-ECA antibody cross-linked to PEI precoated surface | 106 | 106-109 |
| 17 | O157:H7 | Immunosensor: Antibody linked to MHDA SAM | 103 | 103-108 |
| 51 | O157:H7 | DNA sensor: Nanoparticle amplification | 2.67 × 102 | 2.67 × 102-2.67 × 106 |
| Our results | E. coli strains | Assay principle | Reliable quantitative detection (cells/mL) | Linear ranges (cells/mL) |
|---|---|---|---|---|
| W1485 | Carbohydrate sensor | 3.0 × 107 | 2.9 × 107-2.7 × 108 | |
| W1485 | Carbohydrate/lectin sensor | 7.5 × 102 | 7.5 × 102-7.5 × 107 |
ECA: Enterobacterial common antigen; PEI: polyetheneimine; MHDA: 16-Mercaptohexadecanoic acid; SAM: self-assembled monolayer
(b) Sensor specificity
Several control experiments were performed to validate the conclusions and to test the sensor specificity. The mannose modified QCM sensor surface was first exposed to 100 nM Con A solution for about 2 h, which allowed Con A to partially occupy the mannose surface activity sites leaving free Con A in the bulk solution. When the Con A/mannose binding reaction reached to the equilibrium, the final concentration of 7.5 × 107 cells/mL E. coli W1485 were added and generated a large signal response (∼230 Hz) (Figure 5, Curve A), which was about 8 times larger than the direct adhesion of E. coli W1485 onto the mannose-QCM alone (∼30 Hz signal, Curve C). To determine whether the surface bound Con A or the Con A in the binding solution was the major factor for the enhanced E. coli W1485 adhesion, the following control experiment was performed. First, the mannose modified QCM surface was immersed in a 100 nM Con A solution for 2 h, then the electrode was rinsed with PBS buffer to remove the unbounded Con A and the cell was refilled with fresh PBS buffer containing 1 mM Ca2+ and Mn2+. When the similar concentration of E. coli W1485 was added to the test chamber in which the mannose modified QCM surface has preadsorbed Con A but no Con A is in the binding solution, only ∼40 Hz frequency shift was observed (Curve B). This result confirms that the Con A in the binding solution rather than the Con A on the QCM surface played the key role in enhancing E. coli cell adhesion onto the mannose modified QCM surface.
Figure 5.
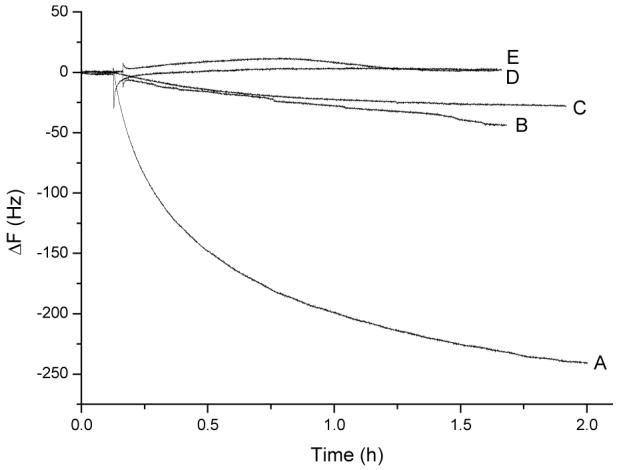
Comparison of sensor specificity Different electrodes were exposed to 7.5 × 107 cells/mL E. coli W1485: A) Con A pretreated mannose electrode with Con A in binding solution; B) Con A pretreated mannose electrode without Con A in binding solution; C) mannose surface with no Con A present on surface and in binding solution; and D) Con A pretreated 210E scFv-cys electrode, Control antigen test (curve E): 1.4 × 109 cells/mL Staphylococcus aureus were added to Con A pretreated mannose electrode with Con A in binding solution. All the test chambers contain 1.0 mL stirred PBS buffer (pH = 7.2) with 1 mM Mn2+ and 1 mM Ca2+.
A recombinant antibody 210E scFv-cys modified QCM surface was used additionally to exam the specificity of the above system. Recombinant antibody 210E scFv-cys binds specifically to rabbit IgG antigen.52 With the same experimental condition as Figure 5 Curve A, negligible frequency change was observed for the addition of E. coli W1485 (Curve D).
Staphylococcus aureus serotype 1, a gram-positive bacterium, was further used as a negative control. When Staphylococcus aureus was added to the Con A pretreated mannose-QCM electrode, only a very small signal was detected (Curve E).
Our control experiments in Figure 5 indicated that the extracellular matrix didn’t interfere with the E. coli detection. They also verified that Con A in the binding solution aggregated onto the E. coli W1485 cell wall thus enhanced the sensitivity and specificity for mannose binding with E. coli W1485 by promoting the formation of rigid attachment to the mannose modified QCM surface.
(c) Validating Con A mediated E. coli LPS and Mannose binding by a SPR biosensor
Surface plasmon affinity sensors are based on monitoring the changes in the effective refractive index of the guided waves caused by the interactions of their evanescent field with analyte molecules binding specifically to their reaction partners immobilized on the sensor surfaces. Even though SPR spectroscopy and QCM are based on different physical phenomena, it is possible to use SPR to validate our surface chemistry since both techniques are non-labeled mass sensors and can theoretically provide information for the binding events occurring at solution metal interfaces. Figure 6 shows the SPR spectra of the stepwise binding of surface receptor to the target analyte. The surface was thoroughly washed after each step and then recorded the spectrum. Little angle shift was observed when E. coli W1485 was directly bound to the mannose SAM (Curve B). Con A binds to the mannose surface and leads to 0.41 ° angle shift (Curve C). However, when E. coli W1485 was added to mannose-Con A surface, negligible angle shift was observed (Curve D) confirming our early rational that the agglutination of Con A to the E. coli in solution phase is the key reason for the signal amplification. Finally, as shown in the Figure 6 insertion, when the incident angle of SPR was fixed at 54.32 °, and the mixture of E. coli W1485 and Con A were injected into the Con A pretreated the mannose SAM chamber in which the concentration of Con A was fixed to be the same as that in curve C, the reflected light intensity change vs. time shows real time binding events. After about 70 min, the chamber was rinsed with PBS and SPR spectrum shows a significant angle shift 0.48 ° (Curve E). In summary, the amplification of binding between the mannose recognition element on the surface and E. coli W1485 by lectin Con A was observed by the SPR biosensor using a similar surface chemistry approach as in the QCM experiments.
Figure 6.
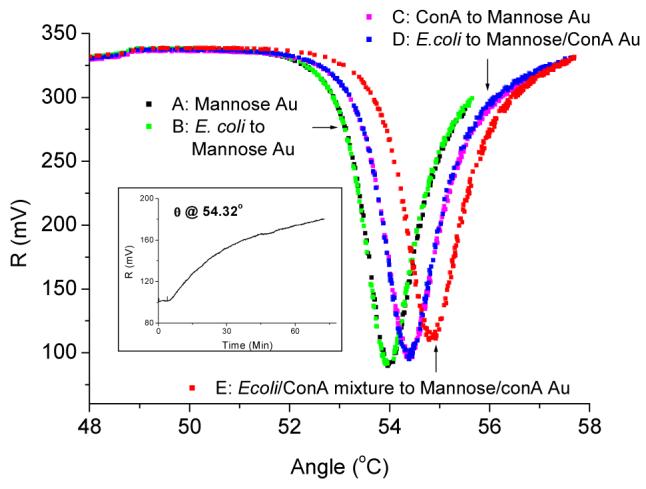
SPR spectra A) mannose SAM, B) 3.7 × 108 cells/mL E. coli W1485 was injected into mannose SAM for 60 min, C) 1.25 μM Con injected into mannose SAM for 40 min, D) 3.7 × 108 cells/mL E. coli W1485 was injected to the Mannose/Con A surface for 60 min, E) the mixture of 1.9 × 108 cells/mL E. coli W1485 and 1.25 μM Con A injected to the Con A pretreated Mannose SAM for 70 min. Insertion: light intensity change with time after the injection of 1.9 × 108 cells/mL E. coli W1485 and 1.25 μM Con A mixture onto the Con A pretreated Mannose SAM. The wavelength of the SPR biosensor light source was 650 nm and the refractive index of its prism was 1.79. The sample chamber of the SPR biosensor was thoroughly washed before recording the spectrum, and filled with 200 μl PBS with 1mM Ca2+, 1mM Mn2+ for each of the experiments.
CONCLUSION
Routine identification and characterization of toxins and microorganisms are commonly performed by biosensors containing either antibodies or nucleic acid probes as the detection element. However, neither antibodies nor nucleic acids are necessarily the best or even most valuable means of identification. In order to have a realistic chance of detecting and identifying an unknown agent, a vast array of antibodies would be required. For DNA or RNA biosensors, often the DNA/RNA probe must be known and amplification of the DNA/RNA probe is needed before immobilization. Taking advantage of the fact that a high percentage of microorganisms have both carbohydrates and carbohydrate binding pockets at their surfaces, we demonstrate here that a carbohydrate epitope in combination with a lectin-bacterial O-antigen recognition event represents a real possibility for developing a highly sensitive and specific non-labeled biosensor for bacteria detection. Our mannose QCM biosensor show a significant improvement of the sensitivity and specificity for E. coli W1485 detection with a detection limit of a few hundred bacterial cells and a linear range from 7.5 × 102 to 7.5 × 107 cells/mL that is four decade wider than the mannose alone sensor. The change of damping resistances for E. coli adhesion experiments were no more than 1.4% suggesting that the bacterial attachment was rigid, rather than a viscoelastic behavior. Little non-specific binding was observed for Staphylococcus aureus and other proteins (Fetal Bovine serum, Erythrina cristagalli lectin). Carbohydrate epitopes offer several advantages over antibody/nucleic acid detection of antigens. Carbohydrates possess broad interaction specificity; carbohydrate recognition could enable identification of unexpected or even novel agents. Carbohydrates do not denature or lose activity upon changes of temperature or pH; they are stable and could have long lifetime. Oligosaccharides are smaller than antibodies; consequently, higher densities of carbohydrate sensing elements could lead to higher sensitivity and less non-specific adsorption. A few carefully chosen carbohydrate epitopes in combination with additional specificity of lectin-bacterial O-antigen recognition could provide the desired fingerprinting of a high number of biological agents and have a propensity to be extremely specific for one particular biological agent, possessing minimal cross-reactivity for other agents. In addition, combining lectin and carbohydrate SAM recognition allows rigid binding of bacteria to the QCM sensor surface, which significantly enhances specificity and sensitivity of detection. We believe that the approach used in this work is potentially universal and could provide a versatile platform for the high-throughput analysis of bacteria at very low concentrations in complex samples.
Supplementary Material
ACKNOWLEDGEMENT
X. Zeng thanks the support of Oakland University and the NIH (4R33 EB00067202). P. G. Wang acknowledges support from the Ohio State University Ohio Eminent Scholar adornments. We thank Mr. Hironobu Eguchi contribution for E coli W1485 lipopolysaccaride analysis. Dr. Zeng likes to thank Dr. Dagmar Cronn for her help of proofreading, Dr. Art Bull for his kindness allowing us to use his biochemistry laboratory and Dr. Heping Yan and Ray L. Mernaugh at Biochemistry Department at Vanderbilt University for his help regarding bacterial biology.
REFERENCE
- (1).Lis H, Sharon N. Lectins as Molecules and as Tools. Annual Review of Biochemistry. 1986;55:35–67. doi: 10.1146/annurev.bi.55.070186.000343. [DOI] [PubMed] [Google Scholar]
- (2).Buchanan RL, Doyle MP. Foodborne disease significance of Escherichia coli O157:H7 and other enterohemorrhagic E-coli. Food Technology. 1997;51:69–76. [Google Scholar]
- (3).Sauerbrey G. Z. Phys. 1959;155:206–222. [Google Scholar]
- (4).Ngeh-Ngwainbi J, Suleiman AA, Guilbault GG. Piezoelectric crystal biosensors. Biosensors and Bioelectronics. 1990;5:13–26. doi: 10.1016/0956-5663(90)80023-7. [DOI] [PubMed] [Google Scholar]
- (5).Schmitt N, Tessier L, Watier H, Patat F. A new method based on acoustic impedance measurements for quartz immunosensors. Sensors and Actuators B-Chemical. 1997;43:217–223. [Google Scholar]
- (6).Su XD, Chew FT, Li SFY. Self-assembled monolayer-based piezoelectric crystal immunosensor for the quantification of total human immunoglobulin E. Analytical Biochemistry. 1999;273:66–72. doi: 10.1006/abio.1999.4186. [DOI] [PubMed] [Google Scholar]
- (7).Muramatsu H, Kajiwara K, Tamiya E, Karube I. Piezoelectric immuno sensor for the detection of candida albicans microbes. Analytica Chimica Acta. 1986;188:257–261. [Google Scholar]
- (8).Plomer M, Guilbault GG, Hock B. Development of a piezoelectric immunosensor for the detection of enterobacteria. Enzyme and Microbial Technology. 1992;14:230–235. doi: 10.1016/0141-0229(92)90071-u. [DOI] [PubMed] [Google Scholar]
- (9).Ivnitski D, Abdel-Hamid I, Atanasov P, Wilkins E. Biosensors for detection of pathogenic bacteria. Biosensors and Bioelectronics. 1999;14:599–624. doi: 10.1016/s0956-5663(99)00004-4. [DOI] [PubMed] [Google Scholar]
- (10).Pathirana ST, Barbaree J, Chin BA, Hartell MG, Neely WC, Vodyanoy V. Rapid and sensitive biosensor for Salmonella. Biosensors and Bioelectronics. 2000;15:135–141. doi: 10.1016/s0956-5663(00)00067-1. [DOI] [PubMed] [Google Scholar]
- (11).Fung YS, Wong YY. Self-Assembled Monolayers as the Coating in a Quartz Piezoelectric Crystal Immunosensor To Detect Salmonella in Aqueous Solution. Analytical Chemistry. 2001;73:5302–5309. doi: 10.1021/ac010655y. [DOI] [PubMed] [Google Scholar]
- (12).Vaughan RD, Sullivan CK, Guilbault GG. Development of a quartz crystal microbalance (QCM) immunosensor for the detection of Listeria monocytogenes. Enzyme and Microbial Technology. 2001;29:635–638. [Google Scholar]
- (13).Kim N, Park I-S. Application of a flow-type antibody sensor to the detection of Escherichia coli in various foods. Biosensors and Bioelectronics. 2003;18:1101–1107. doi: 10.1016/s0956-5663(02)00240-3. [DOI] [PubMed] [Google Scholar]
- (14).Su X-L, Li Y. A self-assembled monolayer-based piezoelectric immunosensor for rapid detection of Escherichia coli O157:H7. Biosensors and Bioelectronics. 2004;19:563–574. doi: 10.1016/s0956-5663(03)00254-9. [DOI] [PubMed] [Google Scholar]
- (15).Wong YY, Ng SP, Ng MH, Si SH, Yao SZ, Fung YS. Immunosensor for the differentiation and detection of Salmonella species based on a quartz crystal microbalance. Biosensors and Bioelectronics. 2002;17:676–684. doi: 10.1016/s0956-5663(02)00030-1. [DOI] [PubMed] [Google Scholar]
- (16).Minunni M, Mascini M, Carter RM, Jacobs MB, Lubrano GJ, Guilbault GG. A quartz crystal microbalance displacement assay for Listeria monocytogenes. Analytica Chimica Acta. 1996;325:169–174. [Google Scholar]
- (17).Bao LL, Deng L, Nie LH, Yao SZ, Wei WZ. Determination of microorganisms with a quartz crystal microbalance sensor. Analytica Chimica Acta. 1996;319:97–101. [Google Scholar]
- (18).Carter RM, Mekalanos JJ, Jacobs MB, Lubrano GJ, Guilbault GG. Quartz-Crystal Microbalance Detection of Vibrio-Cholerae O139 Serotype. Journal of Immunological Methods. 1995;187:121–125. doi: 10.1016/0022-1759(95)00176-b. [DOI] [PubMed] [Google Scholar]
- (19).Pavey KD, Ali Z, Olliff CJ, Paul F. Application of the quartz crystal microbalance to the monitoring of Staphylococcus epidermidis antigen-antibody agglutination. Journal of Pharmaceutical and Biomedical Analysis. 1999;20:241–245. doi: 10.1016/s0731-7085(99)00026-6. [DOI] [PubMed] [Google Scholar]
- (20).Otto K, Elwing H, Hermansson M. Effect of ionic strength on initial interactions of Escherichia coli with surfaces, studied on-line by a novel quartz crystal microbalance technique. Journal of Bacteriology. 1999;181:5210–5218. doi: 10.1128/jb.181.17.5210-5218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Konig B, Gratzel M. Detection of Viruses and Bacteria with Piezoelectric Immunosensors. Analytical Letters. 1993;26:1567–1585. [Google Scholar]
- (22).BenDov I, Willner I, Zisman E. Piezoelectric immunosensors for urine specimens of Chlamydia trachomatis employing quartz crystal microbalance microgravimetric analyses. Analytical Chemistry. 1997;69:3506–3512. doi: 10.1021/ac970216s. [DOI] [PubMed] [Google Scholar]
- (23).Susmel S, O’Sullivan CK, Guilbault GG. Human cytomegalovirus detection by a quartz crystal microbalance immunosensor. Enzyme and Microbial Technology. 2000;27:639–645. doi: 10.1016/s0141-0229(00)00196-4. [DOI] [PubMed] [Google Scholar]
- (24).Eun AJC, Huang LQ, Chew FT, Li SFY, Wong SM. Detection of two orchid viruses using quartz crystal microbalance-based DNA biosensors. Phytopathology. 2002;92:654–658. doi: 10.1094/PHYTO.2002.92.6.654. [DOI] [PubMed] [Google Scholar]
- (25).Abad JM, Pariente F, Hernandez L, Lorenzo E. A quartz crystal microbalance assay for detection of antibodies against the recombinant African swine fever virus attachment protein p12 in swine serum. Analytica Chimica Acta. 1998;368:183–189. [Google Scholar]
- (26).Homola J, Dostalek J, Chen SF, Rasooly A, Jiang SY, Yee SS. Spectral surface plasmon resonance biosensor for detection of staphylococcal enterotoxin B in milk. International Journal of Food Microbiology. 2002;75:61–69. doi: 10.1016/s0168-1605(02)00010-7. [DOI] [PubMed] [Google Scholar]
- (27).Marx KA. Quartz Crystal Microbalance: A Useful Tool for Studying Thin Polymer Films and Complex Biomolecular Systems at the Solution-Surface Interface. Biomacromolecules. 2003;4:1099–1120. doi: 10.1021/bm020116i. [DOI] [PubMed] [Google Scholar]
- (28).Zhou T, Marx KA, Warren M, Schulze H, Braunhut SJ. The Quartz Crystal Microbalance as a Continuous Monitoring Tool for the Study of Endothelial Cell Surface Attachment and Growth. Biotechnology Progress. 2000;16:268–277. doi: 10.1021/bp000003f. [DOI] [PubMed] [Google Scholar]
- (29).Lis H, Sharon N. Lectins: Carbohydrate-Specific Proteins That Mediate Cellular Recognition. Chemical Reviews. 1998;98:637–674. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- (30).Hsu KL, Pilobello KT, Mahal LK. Analyzing the dynamic bacterial glycome with a lectin microarray approach. Nature Chemical Biology. 2006;2:153–157. doi: 10.1038/nchembio767. [DOI] [PubMed] [Google Scholar]
- (31).Rietschel ET, Kirikae T, Schade FU, Ulmer AJ, Holst O, Brade H, Schmidt G, Mamat U, Grimmecke HD, Kusumoto S, Zahringer U. The Chemical-Structure of Bacterial-Endotoxin in Relation to Bioactivity. Immunobiology. 1993;187:169–190. doi: 10.1016/S0171-2985(11)80338-4. [DOI] [PubMed] [Google Scholar]
- (32).Andersson M, Carlin N, Leontein K, Lindquist U, Slettengren K. Structural studies of the O-antigenic polysaccharide of Escherichia coli O86, which possesses blood-group B activity. Carbohydrate Research. 1989;185:211–223. doi: 10.1016/0008-6215(89)80036-9. [DOI] [PubMed] [Google Scholar]
- (33).Otto K, Hermansson M. Inactivation of ompX causes increased interactions of type 1 fimbriated Escherichia coli with abiotic surfaces. Journal of Bacteriology. 2004;186:226–234. doi: 10.1128/JB.186.1.226-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Porco A, Peekhaus N, Bausch C, Tong SX, Isturiz T, Conway T. Molecular genetic characterization of the Escherichia coli gntT gene of GntI, the main system for gluconate metabolism. Journal of Bacteriology. 1997;179:1584–1590. doi: 10.1128/jb.179.5.1584-1590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Stevenson G, Neal B, Liu D, Hobbs M, Packer NH, Batley M, Redmond JW, Lindquist L, Reeves P. Structure of the O-Antigen of Escherichia-Coli-K-12 and the Sequence of Its Rfb Gene-Cluster. Journal of Bacteriology. 1994;176:4144–4156. doi: 10.1128/jb.176.13.4144-4156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pistole TG. Interaction of Bacteria and Fungi with Lectins and Lectin-Like Substances. Annual Review of Microbiology. 1981;35:85–112. doi: 10.1146/annurev.mi.35.100181.000505. [DOI] [PubMed] [Google Scholar]
- (37).Ertl P, Mikkelsen SR. Electrochemical Biosensor Array for the Identification of Microorganisms Based on Lectin-Lipopolysaccharide Recognition. Analytical Chemistry. 2001;73:4241–4248. doi: 10.1021/ac010324l. [DOI] [PubMed] [Google Scholar]
- (38).Mrksich M. A surface chemistry approach to studying cell adhesion. Chemical Society Reviews. 2000;29:267–273. [Google Scholar]
- (39).Wang JL, Cunningh BA, Edelman GM. Proceedings of the National Academy of Sciences of the United States of America. 1971;68:1130. doi: 10.1073/pnas.68.6.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Mckenzie GH, Sawyer WH, Nichol LW. Biochimica et Biophysica Acta. 1972;263:283. doi: 10.1016/0005-2795(72)90081-5. [DOI] [PubMed] [Google Scholar]
- (41).Turton K, Natesh R, Thiyagarajan N, Chaddock JA, Acharya KR. Crystal structures of Erythrina cristagalli lectin with bound N-linked oligosaccharide and lactose 10.1093/glycob/cwh114. Glycobiology. 2004;14:923–929. doi: 10.1093/glycob/cwh114. [DOI] [PubMed] [Google Scholar]
- (42).Ebara Y, Itakura K, Okahata Y. Kinetic Studies of Molecular Recognition Based on Hydrogen Bonding at the Air-Water Interface by Using a Highly Sensitive Quartz-Crystal Microbalance. Langmuir. 1996;12:5165–5170. [Google Scholar]
- (43).Smith EA, Thomas WD, Kiessling LL, Corn RM. Journal of the American Chemical Society. 2003;125:6140. doi: 10.1021/ja034165u. [DOI] [PubMed] [Google Scholar]
- (44).Zhang Y, Luo S, Tang Y, Yu L, Hou K-Y, Cheng J-P, Zeng X, Wang PG. Carbohydrate-Protein Interactions by ”Clicked“ Carbohydrate Self-Assembled Monolayers. Anal. Chem. 2006;78:2001–2008. doi: 10.1021/ac051919+. [DOI] [PubMed] [Google Scholar]
- (45).Hallet B. Playing Dr Jekyll and Mr Hyde: combined mechanisms of phase variation in bacteria. Current Opinion in Microbiology. 2001;4:570–581. doi: 10.1016/s1369-5274(00)00253-8. [DOI] [PubMed] [Google Scholar]
- (46).Kyes S, Horrocks P, Newbold C. Antigenic variation at the infected red cell surface in malaria. Annual Review of Microbiology. 2001;55:673–707. doi: 10.1146/annurev.micro.55.1.673. [DOI] [PubMed] [Google Scholar]
- (47).Barbour AG, Restrepo BI. Antigenic variation in vector-borne pathogens. Emerging Infectious Diseases. 2000;6:449–457. doi: 10.3201/eid0605.000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Eisenstein BI. Phase Variation of Type-1 Fimbriae in Escherichia-Coli Is under Transcriptional Control. Science. 1981;214:337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- (49).Olsen EV, Pathirana ST, Samoylov AM, Barbaree JM, Chin BA, Neely WC, Vodyanoy V. Specific and selective biosensor for Salmonella and its detection in the environment. Journal of Microbiological Methods Detection of Microbial Pathogens using Molecular Methods. 2003;53:273–285. doi: 10.1016/s0167-7012(03)00031-9. [DOI] [PubMed] [Google Scholar]
- (50).Picken R, Beacham IR. Interaction of Concanavalin a with Mutant and Wild-Type Strains of Escherichia-Coli-K12. Biochemical Society Transactions. 1975;3:387–388. doi: 10.1042/bst0030387. [DOI] [PubMed] [Google Scholar]
- (51).Mao X, Yang L, Su X-L, Li Y. A nanoparticle amplification based quartz crystal microbalance DNA sensor for detection of Escherichia coli O157:H7. Biosensors and Bioelectronics. 2006;21:1178–1185. doi: 10.1016/j.bios.2005.04.021. [DOI] [PubMed] [Google Scholar]
- (52).Shen Z, Stryker GA, Mernaugh RL, Yu L, Yan H, Zeng X. Single-Chain Fragment Variable Antibody Piezoimmunosensors. Anal. Chem. 2005;77:797–805. doi: 10.1021/ac048655w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


