Abstract
Acute myocarditis is one of the most challenging diagnosis in cardiology. At present, no diagnostic gold standard is generally accepted, due to the insensitivity of traditional diagnostic tests. This leads to the need for new diagnostic approaches, which resulted in the emergence of new molecular tests and a more detailed immunohistochemical analysis of endomyocardial biopsies. Recent findings using these new diagnostic tests resulted in increased interest in inflammatory cardiomyopathies and a better understanding of its pathophysiology, the recognition in overlap of virus-mediated damage, inflammation, and autoimmune dysregulation. Novel results also pointed towards a broader spectrum of viral genomes responsible for acute myocarditis, indicating a shift of enterovirus and adenovirus to parvovirus B19 and human herpes virus 6. The present review proposes a general diagnostic approach, focuses on the viral aetiology and associated autoimmune processes, and reviews treatment options for patients with acute viral myocarditis.
Keywords: Myocarditis, Virus, Heart failure, Inflammation
Introduction
The term ‘myocarditis’ was first introduced in the early 19th century by Corvisart. However, at the beginning of the 20th century with the recognition of coronary artery disease as an important cause for heart disease, the term was largely discarded.1 In attempts to standardize the diagnostic criteria for myocarditis, the Dallas classification2 was introduced in 1987. This classification, however, has several pitfalls, being susceptible to variation in pathological interpretation,3 sampling error,4 and not considering the exact cause of pathological findings. Therefore, it is not anymore used as the gold standard for the diagnosis of viral or autoimmune myocarditis, mainly due to its lack of additional immunostaining for inflammation and polymerase chain reaction (PCR) for viral diagnosis. Recent efforts to redefine viral and autoimmune heart disease may therefore result in the so-called ‘death of the Dallas Criteria’.5 The diagnostic gold standard is endomyocardial biopsy (EMB) with the histological Dallas criteria in conjunction with the new tools of immunohistochemistry and viral PCR, according to the 1995 WHO classification of cardiomyopathies6 (Table 1). The use of these new diagnostic tools has evolved in a better identification of the aetiology of myocarditis and a renewed interest in the mechanisms of the inflammatory process in the heart. These new insights of the disease are mandatory to allow the development of novel aetiology-directed treatment strategies.
Table 1.
Definition and classification of cardiomyopathies according to the 1995 WHO/International Society and Federation of Cardiology Task Force
| Dilated cardiomyopathy |
| Hypertrophic cardiomyopathy |
| Restrictive cardiomyopathy |
| Arrhythmogenic right ventricular cardiomyopathy |
| Unclassified cardiomyopathies |
Acute myocarditis is one of the most challenging diagnosis in cardiology. Its entity is rarely recognized and its pathophysiology defectively understood. The present review therefore proposes a general diagnostic approach, focuses on the viral aetiology and associated autoimmune process, and reviews treatment options for patients with acute viral myocarditis.
Pathogenesis
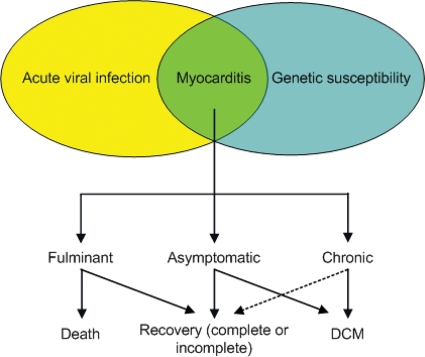
Myocarditis is a disease with variable clinical presentation and progression.7,8 In order to better understand its often unpredictable progression, one has to address its underlying pathophysiological process. Myocarditis is habitually viewed as a chronological sequence of three pathologically distinct phases.9 During the first phase, direct destruction of the cardiomyocytes occurs by virus-mediated lysis,10 causing degradation of cell structures, which in turn facilitates entry of the virus into the cells with consequential myocyte injury and cardiac dilatation.11 This initial phase frequently passes unnoticed since the initial damage is often prevented by the innate immune response.12 The second phase develops as a result of immune dysregulation triggered by the initial cardiomyocyte injury. The initial cellular and humoral immune responses may improve the outcome during phase 1; conversely, they are responsible for the harmful effect during phase 2. This is in part induced by molecular mimicry,13 which is caused by mimicked epitopes shared between the viral and cardiac antigens.14 Finally, in the third phase, a typical picture of dilated cardiomyopathy (DCM) develops as a result of extensive myocardial injury.9 Cross-reacting antibodies with auto-antigens have been found in patients with myocarditis15 and are indicative of progression to DCM.15 In relation to cardiac auto-antibodies as predictors of progression to DCM, the recent study by Caforio et al.16 demonstrated that cardiac auto-antibodies predict disease development in asymptomatic relatives. Another mechanism for the development of autoimmune responses is the virus-induced cardiomyocyte injury with consequent release of intracellular cardiac proteins. The latter may activate T-cells and associated cytokine mechanisms17 evolving in autoimmune myocarditis.18 Initial myocardial injury by the virus, and continuous low grade inflammation, induces enduring myocardial damage and reparative fibrosis. Inflammatory cells also produces matrix-degrading proteases,19,20 all together leading to left ventricular (LV) dilatation21 and cardiac dysfunction22 in stage 3 (Figure 1).
Figure 1.
Pathophysiological process of virus myocarditis.
Two important observations in the progression of virus infection to heart failure remain unclear. First, patients with initial fulminant myocarditis that require intense haemodynamic support rapidly recover within days and have a better long-term prognosis when compared with patients with (sub)acute myocarditis, which have a worse prognosis due to development of DCM.7,12 The precise reason for this difference is uncertain. One may postulate that a fulminant inflammatory reaction, which is able to clear the virus, may induce temporal stunning and dysfunction of the myocardium. These patients probably do not enter the second phase of viral-injury-mediated autoimmune responses, whereas patients with virus persistence due to a (sub)acute, but insufficient inflammatory reaction, are more prone to chronic autoimmune inflammation and injury of the heart.
Secondly, it remains unclear why some patients develop myocarditis. The cardiotropic viruses causing myocarditis, including adenovirus (ADV), enterovirus (EV), Epstein-Barr virus, human herpes virus 6 (HHV6), parvovirus B19 (PVB19) and cytomegalovirus are common cough viruses. Although up to 90% of people will catch one or more of these viruses in their life without getting their heart affected, only a selected few develop clinical symptoms. The exact incidence of myocarditis remains unclear, partly due to the insensitivity of diagnostic tools used in previous studies. This may cause an underestimation of its true incidence. The prevalence of myocarditis has been found to be up to 42% in cases of unexplained deaths in people aged 35 or younger.23–25 Therefore, a certain genetic background, either or not related to immune alterations, appears to be a requisite to develop clinical symptoms of myocarditis and/or progression to DCM following virus infection in the heart. A recent review, by Caforio and Iliceto,26 shows that in myocarditis/DCM, cardiac-specific and disease-specific antibodies of IgG class are potential biomarkers for identifying ‘at-risk’ relatives. As such, it has been shown that dystrophin mutations may facilitate myocarditis and cardiac failure during coxsackievirus B3 infection, whereas dystrophin and/or sarcoglycan disruption by viral proteases account for myocardial injury.14,27 Future research should therefore focus on the understanding of the underlying genetic susceptibility and related immune responses that explain why some persons are susceptible to develop clinical symptoms of myocarditis following viral infection, whereas others ‘spontaneously’ improve afterwards or progress to an ‘idiopathic’ DCM afterwards (Figure 2).
Figure 2.
Evolution of acute viral myocarditis. DCM, dilated cardiomyopathy.
Diagnosis
The initial evaluation of acute myocarditis includes a detailed history and a careful physical examination searching for any potential features that may provide clues to its aetiology. Additional technical examination should include an electrocardiogram (ECG), chest X-ray, blood studies, non-invasive imaging techniques, and EMB.
Acute myocarditis has been associated with various infections (Table 2). Viruses are the most important cause of acute myocarditis, especially in developed countries.28–31 Based on serological studies, ADV and EV have long been considered the most common cardiotropic viruses resulting in myocarditis,32,33 but recent studies using PCR for viral diagnosis in cardiac biopsies pointed towards PVB19 and HHV6 as the most frequent pathogens in patients with acute myocarditis.28,31,34,35
Table 2.
Infectious causes of myocarditis
| Viral |
| Adenovirus, Arbovirus, Arenavirus, Coxsackie virus, Epstein–Barr virus, Cytomegalovirus, Echovirus, Encephalomyocarditis virus, Hepatitis B, Human Herpes virus 6, Human immunodeficiency virus-1, Influenza virus B, Mumps virus, Parvovirus B19, Poliomyelitis virus, Rabies, Respiratory syncytial virus, Rubella virus, Rubeola virus, Vaccinia virus, Varicella virus, Variola virus |
| Bacterial |
| Brucellosis, Clostridia, Diphtheria, Francisella, Gonococcus, Haemophilus, Legionella, Meningococcus, Mycobacterium, Mycoplasma, Pneumococcus, Psittacosis, Salmonella, Staphylococcus, Streptococcus, Tropheryma whippleii |
| Fungal |
| Actinomyces, Aspergillus, Blastomyces, Candida, Coccidioides, Cryptococcus, Histoplasma, Nocardia, Sporothrix |
| Rickettsial |
| Rocky Mountain spotted fever, Q fever, Scrub typhus, Typhus |
| Spirochetal |
| Borrelia, Leptospira, Syphilis |
| Helminthic |
| Cysticercus, Echinococcus, Schistosoma, Toxocara, Trichinella |
| Protozoal |
| Entamoeba, Leishmania, Trypanosoma, Toxoplasmosis |
Clinical presentation
Symptoms of acute myocarditis vary, often starting with flu-like symptoms, either of the upper respiratory or gastrointestinal tract, before any cardiac symptoms appear. Cardiac symptoms may follow after a delay of days to weeks, including fatigue, dyspnoea, palpitations, malaise, and atypical chest discomfort.36 Further clinical signs may comprise sinus tachycardia, a diminished first heart sound, gallops, murmurs of mitral or tricuspid insufficiency, and, rarely but pathognomic, a pericardial friction rub. Still, the clinical cardiac signs and symptoms may be vague in many patients.35 Therefore, this disease should be considered in patients who present with rapidly progressive cardiomyopathy, idiopathic ventricular arrhythmias, cardiovascular collapse, and/or an ECG mimicking an acute myocardial infarction (MI) but with normal coronary arteries.37 Since the relation between clinical findings and presence of acute myocarditis remains obscure, a very specific and sensitive diagnostic procedure is warranted.38
The differential diagnosis for acute viral myocarditis includes other infectious causes for myocarditis (Table 2), acute MI, giant cell myocarditis, eosinophilic myocarditis, peripartum cardiomyopathy and cardiac sarcoidosis.
Viral serology
Traditional serological studies, peripheral viral cultures, have been used to identify the most frequent pathogens for viral myocarditis in the past. Unfortunately, these methods lack sensitivity and specificity.5 When seroconversion (low IgG, raised IgM, and IgA) occurs at the time of the cardiac symptoms, it may suggest viral cardiac manifestation. However, these assays do not prove direct presence of virus infection in the heart due to the high background prevalence, and hence they can be helpful to identify the aetiology in a selected patient group who do not undergo EMB for additional PCR and immunohistochemical analysis of inflammation.
Biopsies
The current indication for EMB is ‘a strong reason to believe that the results will have a meaningful effect on subsequent therapeutic decisions’ according to the American College of Cardiology/American Heart Association guidelines.39,40 This guideline supports the use of EMB for patients presenting with unexplained new-onset heart failure <2 weeks in duration associated with a normal-sized or dilated left ventricle in addition to haemodynamic compromise.
The timeframe in obtaining EMBs remains controversial. A conservative approach where clinicians wait for a maximum of 2 days before performing EMBs is justified. This is due to a high amount of patients who recover spontaneously, as seen in up to 57% of the cases.7,8 However, in the case of persistent or increasing cardiac dysfunction, urgent coronary angiography should be performed and EMB taken without delay in the presence of non-significant coronary artery disease.
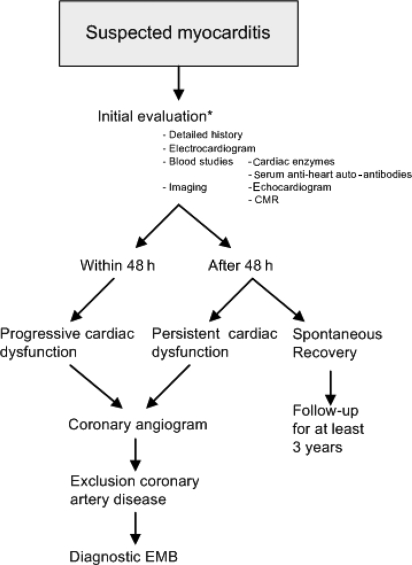
In contrast, a more invasive approach where EMBs are obtained in all patients with suspected myocarditis could be justified taking into account its high diagnostic sensitivity in the earliest stages of myocarditis. In addition, aetiology-based therapy is more effective when started early, before inflammation causes injury and the remodelling progress has become irreversible. Obtaining EMBs at presentation is valuable in understanding the potential role of specific aetiological factor, such as specific viral agents, double infections, high viral load and/or immunohistological inflammation. New studies in which informed written consent is obtained from the patient with his/her full understanding of performing EMBs at presentation are required (Figure 3).
Figure 3.
Proposal of diagnostic approach for patients with suspected myocarditis. Asterisk indicates that when informed consent is obtained to perform endomyocardial biopsy at presentation, the procedure is continued, if not proceed with the steps as suggested in the flowchart. CMR, cardiovascular magnetic imaging; EMB, endomyocardial biopsy.
The EMB analysis should include routine light microscopy, immunohistological techniques for inflammation, and PCR for virus detection.
Biopsies: viral diagnosis
With the introduction of new molecular techniques, such as PCR and in situ hybridization, many different viruses have been found to be associated with myocarditis: especially a high prevalence of PVB19 and HHV6 emerged in the last years28,31,34,35,41 (reviewed in Table 3). Some but not all studies reveal a high prevalence of PVB19 ranging from 15 to 60% and HHV6 ranging from 8 to 30% using PCR analyses in EMBs from patients with immunohistological myocarditis. They show similar virus prevalence in patients with DCM compared with acute myocarditis, underscoring the pathogenic process where viruses are the triggers for long-term cardiac injury and dysfunction. The presence of PVB19 and especially a dual infection of PVB19 and HHV6 are associated with a worse prognosis in patients with acute myocarditis.28,31
Table 3.
Prevalence of viruses using PCR in endomyocardial biopsies
| Type of patients | Number of patients | Reference | PVB19 | HHV6 | EV | ADV | EBV | CMV | No virus | Comment |
|---|---|---|---|---|---|---|---|---|---|---|
| Dallas MC criteria | 624 | Bowles et al.29 | ND | ND | 85 (14%) | 142 (23%) | 3 (0.5%) | 18 (3%) | 385 (62%) | No analyses for PVB19 was done |
| DCM | 149 | ND | ND | 18 (8%) | 12 (12%) | 0 (0%) | 0 (0%) | 119 (80%) | Parvovirus 6 in MC was <1% | |
| Immunohistochemical MC criteria | 36 | Pankuweit et al.91 | 7 (19%) | ND | ND | ND | ND | ND | 19 (81%) | Only analyses for PVB19 |
| DCMi | 13 | 3 (23%) | ND | ND | ND | ND | ND | 10 (77%) | ||
| Clinical MC | 32 | Mahrholdt et al.34 | 12 (38%) | 6 (19%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 14 (43%) | |
| With Dallas MC criteria | 20 out of 32 | 12 (60%) | 6 (30%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (10%) | ||
| PCR-proven viral MC | 172 | Kuhl et al.31 | 63 (37%) | 18 (11%) | 56 (33%) | 14 (8%) | ND | ND | NA | Dual infections in 21 patients |
| PPCM | 26 | Bultmann et al.92 | 4 (15%) | 2 (8%) | 0 (0%) | 0 (0%) | 1 (4%) | 1 (4%) | 18 (69%) | |
| With immunohistochemical inflammation | 19 out of 26 | 4 (21%) | 2 (11%) | 0 (0%) | 0 (0%) | 1 (5%) | 1 (5%) | 11 (58%) | ||
| Idiopathic DCM | 245 | Kuhl et al.28 | 126 (51%) | 53 (22%) | 23 (9%) | 4 (2%) | 5 (2%) | 2 (<1%) | 80 (33%) | Dual infections in 45 patients |
| Dallas MC criteria | 87 | Mahrholdt et al.35 | 49 (56%) | 16 (18%) | 1 (1%) | 0 (0%) | 1 (1%) | 0 (0%) | 5 (6%) | |
| Dallas MC criteria | 120 | Caforio et al.61 | 3 (2.5%) | ND | 15 (12.5%) | 6 (5%) | 5 (4%) | 3 (2.5%) | 89 (74%) | Mumps in three patients (2.5%), HHV1 in one (1%), and hepatitis C in two (2%). Dual infections in five patients |
DCM, dilated cardiomyopathy; DCMi, dilated cardiomyopathy with inflammation; PPCM, peripartum cardiomyopathy; PVB19, parvovirus B19; EV, enterovirus; HHV6, human herpes virus 6; ADV, andenovirus; EBV, Ebstein–Barr virus; CBV Coxacksie B virus; CMV, cytomegalovirus; MC, myocarditis; HHV1, human herpes virus 1; ND, not determined; NA, not applicable.
In conclusion, recent findings using these new diagnostic tests point towards a broader spectrum of viral genomes responsible for acute myocarditis, indicating a shift of EV and ADV to PVB19 and HHV6 as the most frequent viruses causing acute myocarditis.
Biopsies: histological evaluation
Histological evaluation of biopsies was initially done according to the Dallas criteria.2 The presence of an inflammatory infiltrate with or without necrosis under light microscopy was required for the histological diagnosis of myocarditis. The absence of myocyte necrosis and a sparser inflammatory infiltrate suggests borderline myocarditis. However, several investigations have raised questions whether the Dallas criteria are sensitive enough to identify viral cardiomyopathy. In addition, various studies have shown a rather large variability of 39% among pathologists examining the same cardiac tissue samples.7 Biopsies obtained from different parts of the right ventricle may or may not show focal infiltrates (spatial variability), and biopsies obtained at different time points document intermittent inflammatory infiltrates (temporal variability).4 In EMBs containing PCR-proven viral pathogens, the Dallas criteria were absent in 50% of the specimens.41 For this reason, the solitary use of the Dallas criteria to diagnose myocarditis is poor, since it is susceptible to sampling error and variation in observer expertise, and it does not consider viral persistence and auto-immune regulation.5
To address the shortcomings, additional immunohistological and quantitative evaluation of the EMB is required. Immunohistochemical techniques allow quantification, identification, and differentiation of inflammatory cells, focusing on T-lymphocytes.42 Criteria for immunohistological diagnosis in the endomyocardial biopsy of inflammatory cardiomyopathy is specified quantitatively as ≥14 infiltrating leukocytes/mm2, preferably T-lymphocytes or activated T-cells. If foci of T-lymphocytes are present, active myocarditis can be diagnosed due to the nature of the infiltrate even when the critical number of 14 leukocytes/mm2 is not reached, as has been defined by the task force of the World Heart Federation's Council on Cardiomyopathies.43
Blood studies
Cardiac enzymes
Specific markers for acute myocarditis in routine blood studies are lacking. In general, cardiac enzymes are elevated, but its reliability to detect myocarditis has repeatedly been questioned.44 Serum levels of cardiac troponins are more sensitive in patients with clinically suspected myocarditis than conventional determination of creatine kinase levels and indicate myocyte injury.45 Cardiac troponin was mainly elevated in patients with acute, early-onset myocarditis, whereas the absence of increased levels suggests long-term presence of myocarditis. Unfortunately, the prognostic value of cardiac troponin remains unclear.
Cardiac auto-antibodies
The pathophysiological role of auto-antibodies in cardiac injury after acute viral infection is still under discussion.13,46,47 Auto-antibodies against contractile structures,48–50 proteins involved in energy metabolism/transfer,51,52 ion channels and transporters,53 and sarcolemmal receptors54 have been identified in myocarditis. Circulating cardiac auto-antibodies, in particular antibodies against cardiac myosin heavy chain, may play a key role as a clinical and pathogenetic marker and may also have some prognostic value.15,55 Indications for their role in the pathophysiology of myocarditis are that: (i) various auto-antibodies can produce inadequate inflammation in the heart and directly injure cardiomyocytes56; (ii) transfer of human lymphocytes from patients with proven heart-reactive auto-antibodies into severe combined immunodeficiency mice leads to autoantibody production, myocardial inflammation, and impaired cardiac function57; (iii) active immunization with cardiac auto-antigens leads to anto-antibody production, heart-specific inflammation, and cardiac dysfunction48,58; and (iv) direct transfer of auto-antibodies against cTnI and β-adrenoreceptor into rodents results in cardiac dilatation and dysfunction.59 Antibodies of the IgG class are cardiac and disease specific for myocarditis, can be used to determine an autoimmune pathogenesis,15,60 and are present in up to 50% of patients with active to borderline biopsy-proven myocarditis.61 Further studies are required to study whether this subgroup of patients might benefit from immunosuppressive and/or immunomodulation therapy.59,61
Electrocardiogram
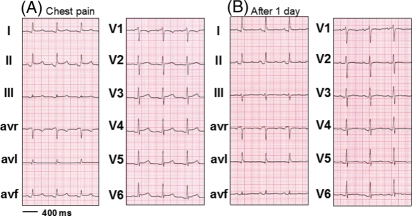
Patients with acute myocarditis frequently have abnormal ECGs ranging from conduction delays to ECGs mimicking acute MI or acute pericarditis.37,62 Its sensitivity is estimated at 47%, but its specificity remains unknown.63 Acute myocarditis may also result in ‘idiopathic’ atrial or ventricular arrhythmias, PQ-segment depression, and diffusely elevated ST-T-segments (Figure 4) which evolve in ST-T-segment depression.64
Figure 4.
Electrocardiogram showing PQ-segment depression and diffusely elevated ST-T-segments at presentation (A) and evolution after 1 day (B).
Natural history
The natural history of acute myocarditis varies from early death to multisystem failure (fulminant myocarditis), ventricular arrhythmias, complete recovery, and long-term evolution to DCM.8 There are no large population-based, epidemiological studies defining the presenting symptoms of acute myocarditis, which is partly due to the absence of sensitive non-invasive diagnostic tests that are able to confirm its diagnosis. Keeping in mind its difficult diagnosis and often its asymptomatic course, D'Ambrosio et al.8 reviewed all current reports to describe the natural history of histology-proven acute myocarditis. They report spontaneous improvement in 50–57% of histology-confirmed cases7 and a variable incidence to develop DCM in 14–52%,65,66 with a higher incidence of DCM in a subset of patients presenting with congestive heart failure compared with patients with other types of clinical presentation (arrhythmias and/or chest pain).67 Long-term prognosis was usually good with a 3–5-year survival ranging from 56 to 83%, respectively.68,69 Patients with acute fulminant myocarditis, once they survive the acute illness, had an excellent long-term prognosis of 93% at 11 years, compared with 45% of the patients presenting with acute non-fulminant myocarditis.12 In patients with idiopathic CMP or a previous history of myocarditis, Kuhl et al.28 showed that viral persistence in the myocardium is associated with progressive LV deterioration, whereas clearance of the viral genomes resulted in a significant improvement of LV function. In patients with acute myocarditis, an initial decline with a 1-year mortality rate of 20%7,69 which gradually stabilizes after 3 years has been reported.12 Therefore, we advise a follow-up of at least 3 years after presentation or longer since myocarditis may recur even many years after the initial episode (Figure 3). Patients with cardiac deterioration or recurrent episodes of myocarditis during this time should receive adequate therapeutic and/or diagnostic measures, whereas clinically stable patients should be discharged from follow-up.
Imaging tools
Echocardiogram
The echocardiographic findings of (sub)acute myocarditis may largely vary, showing marked LV dilation, normal LV thickness, and decreased function. Systolic dysfunction, regional wall motion abnormalities, and diastolic dysfunction all have been described.70 However, many cases of myocarditis do not show abnormal LV function,71–73 thus limiting its utility for the diagnosis of myocarditis. It can be useful in distinguishing fulminant myocarditis from subacute myocarditis, as the former often presents with a non-dilated, thickened and hypocontractile left ventricle. This is likely due to the greater inflammatory response in fulminant myocarditis resulting in interstitial oedema, ventricular wall thickening and loss of ventricular contractility.70 New echocardiographic modalities (such as TDI strain) are emerging and hold promise in relation to the detection of dysfunction in patients with standard transthoracic echocardiography. Until these methods are validated in detecting myocarditis, we advise to always perform cardiovascular magnetic resonance (CMR) imaging in addition to echocardiography.
Nuclear imaging
Gallium scanning has been shown to be a useful diagnostic tool for detecting chronic inflammation; nevertheless, due to its lack of sensitivity (8%), its use has diminished over the course of time.74 Antimyosin antibody imaging has been described as a screening method for myocarditis with a high sensitivity (91–100%) and negative predictive value (93–100%) by detecting localized or diffuse myocardial necrosis.75 Unfortunately, due to its limited availability, pronounced radiation exposure, and 48 h delay in obtaining images, its use remained restricted.
Cardiovascular magnetic resonance imaging
CMR imaging has emerged as the most important imaging tool in the diagnostic procedure. Especially, gadolinium late enhancement (LE) and T2-weighted image sequences (Figure 5) distinguish between ischaemic and non-ischaemic cardiomyopathy. Patients with ischaemic cardiomyopathy have subendocardial or transmural enhancement compared with the non-ischaemic DCM group that may have three distinct patterns, including the absence of enhancement (59%), patchy or longitudinal striae of mid-wall enhancement (28%), with a minority having myocardial enhancement indistinguishable from patients with ischaemic cardiomyopathy (13%).76 LE distributions in MI patients show a smaller number of segmental vascular distributions in contrast to a more diffuse, nodular or patchy, non-segmental vascular distribution in myocarditis patients.77 Whereas increased LE indicates myocardial injury, T2-weighted images mark interstitial oedema, which is an integral part of the inflammatory response.78 Thus, visual assessment of T2 images relating the signal intensity of the myocardium to that of skeletal muscle (if not affected) allows estimating global oedema and offers a high accuracy to identify myocarditis, with a sensitivity of 100% and specificity of 90%.71
Figure 5.
Cardiovascular magnetic resonance image. Short-axis cardiac magnetic resonance imaging of a patient with acute myocarditis (A) T2-weighted image, showing regional oedema of the lateral left ventricle predominantly subepicardial involvement (arrow). (B) Late enhancement image, demonstrating high signal intensity in the epicardial region of the lateral wall of the left ventricle (arrow).
Therefore, combined CMR approach of T2-weighted imaging and early and late gadolinium enhancement provides a high diagnostic accuracy and should become standard imaging in patients with a suspicion of myocarditis.71,79
Treatment
Patients with acute myocarditis should limit physical activity, as exercise during active viral infection may increase viral replication and shorten survival.80 They should also receive standard heart failure treatment including diuretics, β-blockers, angiotensin-converting enzyme-inhibitors or angiotensin II receptor blockers. Arrhythmias should be monitored and treated. Mechanical support with intra-aortic balloon pump or LV assist device as a bridge to recovery or heart transplantation may be necessary in severe cases.81 These measures are, however, intended for symptomatic relief. To halt disease progression and possible evolution to DCM, the underlying pathogenic mechanisms, e.g. virus infection or persistence and autoimmune-mediated myocardial damage should be addressed. The challenge of treating these primary mechanisms requires detailed diagnosis of the pathogen and sufficient knowledge of the pathophysiology leading to heart failure (Figure 6).
Figure 6.
Proposal of treatment algorithm based on endomyocardial biopsy results. Immuno. infl., immunohistological inflammation; AHA, anti-heart auto-antibodies; pos, positive; neg, negative; HF, heart failure.
Earlier trials for myocarditis lacked specific diagnosis of viral presence and/or autoimmune diseases. The Myocarditis Treatment Trial by Mason et al.7 failed to show support for immunosuppressive therapy in 111 patients with biopsy-proven myocarditis and a depressed LV function. Immunosuppressive therapy on top of conventional therapy did not significantly improve LV systolic function or overall survival. This trial, however, had several pitfalls: treatment was not blinded and the diagnosis of myocarditis was made solely on the histological Dallas criteria. Immune suppression may be beneficial in patients with systemic disease-related or autoimmune myocarditis, whereas it may increase virus replication and worsen myocardial injury in viral myocarditis. In another trial by McNamara et al.82 including 62 patients with myocarditis or recent onset idiopathic DCM, intravenous immune globulin did not significantly improve LV function or overall mortality when compared with the placebo group. LV function improved significantly in both groups during a 12-month follow-up, indicating a favourable short-term prognosis in this subset of patients. This trial also based its diagnosis of myocarditis solely on the Dallas criteria. Both these trials failed to show any evidence to support immunomodulatory therapy for patients with myocarditis or recent-onset DCM. These findings emphasize the importance of identifying the underlying aetiological pathogenesis to find a specific subgroup of patients who might benefit from immunomodulatory therapy. In a study of 102 patients with idiopathic cardiomyopathy treated with immunosuppressive therapy, improvement of LV function was only seen in patients with evidence of inflammation, the so-called ‘reactive’ patients.83 ‘Reactive’ patients were those with fibroblastic or lymphocytic infiltration or immunoglobulin deposition on endomyocardial biopsy, a positive gallium scan, or an elevated erythrocyte sedimentation rate. The non-reactive patient group did not improve in LV function despite treatment with immunomodulatory therapy. A randomized, placebo-controlled study84 further confirmed this concept, revealing a long-term improvement in LVEF of 19% compared with 7% (P < 0.001) with the short-term use of prednisone and azathioprine compared with placebo in 84 patients with immunohistologically proven chronic myocarditis. Post hoc analysis revealed a high prevalence of virus persistence but absent anti-cardiac antibodies in the non-responders, in contrast to a high prevalence of anti-cardiac antibodies in the responders to immunosuppressive therapy.85 The importance of identifying a virological profile has been further elaborated86 in a small phase II study including 22 patients with myocarditis or DCM with virus persistence in the heart. Patients were treated with interferon-β (Beneferon) 18×106 IU/week for 24 weeks, which resulted in complete elimination of both enteroviral and adenoviral genomes and haemodynamic improvement as shown by improvement in LVEF. A prospective placebo-controlled randomized multicentre study, the Betaferon® in Chronic Viral Cardiomyopathy (BICC) trial, has been initiated since 2002.87
Preventing direct viral damage using antiviral therapy is one possible approach. Inhibition of viral proliferation by preventing interaction of viruses with their cellular receptor and their consequent signalling amplification systems, such as the tyrosine kinase p56lck, phosphatase CD45 and downstream ERK1/2, and the family of cytokines,88,89 is another approach. Therefore, additional research is required to further understand the mechanisms of viral heart disease and consequently identify new potential targets for therapy.
In summary, most trials suggest that immunomodulatory and/or antiviral treatment may be beneficial in cohorts of patients with a very specific diagnosis and related aetiology. Thus, classification of the underlying mechanisms should lead to different therapeutic approaches aimed to: (i) adequately target the specific aetiology and (ii) to prevent treatment of an autoimmune disease by global immunosuppressive treatment in patients with an infectious disease.90 Currently, evidence-based guidelines in favour of these specific treatment options are lacking. Future clinical studies, including immunosuppressive, immunomodulatory, and/or antiviral treatment, according to the algorithm proposed in Figure 6 should only be performed after obtaining informed consent from the patient in specific reference centres.
Conclusion
Patients suspected of acute myocarditis should undergo a complete cardiological work-up, including standard cardiological examination, ECG, blood analyses with standard cardiac enzymes, and serum tests for anti-heart auto-antibodies, imaging techniques (echocardiogram and especially CMR), and EMBs for both PCR and immunohistochemical analysis as suggested (Figure 3).
Myocarditis is a disease with a variable natural history. However, due to the insensitivity of traditional diagnostic tests, its diagnosis was difficult in the past. The emergence of CMR as a valuable diagnostic tool combined with new, more sensitive and specific, molecular diagnostic tests in blood and EMB has led to renewed interest in inflammatory cardiomyopathies and to the better understanding of their pathophysiology. The recognition in overlap of virus-mediated damage, inflammation, and autoimmune dysregulation in these patients, together with the combined effort of clinicians, pathologists, and immunologists resulted in a better classification of the disease. Future randomized placebo-controlled trials should be based upon aetiological diagnosis (viral vs. autoimmune patients) and may provide novel treatment options and possibly a better prognosis for these selected patients.
Funding
Funding to pay Open Access publication charges for this article was provided by Stephane Heymans, Centre for Heart Failure Research, CARIM, University of Maastricht.
Acknowledgements
The authors gratefully thank Prof. Dr A. Gorgels for providing the ECG and Dr. S. Schalla for providing the CMR images.
Conflict of interest: none declared.
References
- 1.Mattingly TW. Changing concepts of myocardial diseases. JAMA. 1965;191:33–37. doi: 10.1001/jama.1965.03080010039011. [DOI] [PubMed] [Google Scholar]
- 2.Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18:619–624. doi: 10.1016/s0046-8177(87)80363-5. [DOI] [PubMed] [Google Scholar]
- 3.Shanes JG, Ghali J, Billingham ME, Ferrans VJ, Fenoglio JJ, Edwards WD, Tsai CC, Saffitz JE, Isner J, Furner S. Interobserver variability in the pathologic interpretation of endomyocardial biopsy results. Circulation. 1987;75:401–405. doi: 10.1161/01.cir.75.2.401. [DOI] [PubMed] [Google Scholar]
- 4.Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64:1235–1245. doi: 10.1016/s0025-6196(12)61286-5. [DOI] [PubMed] [Google Scholar]
- 5.Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113:593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 6.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 7.Mason JW, O'Connell JB, Herskowitz A, Rose NR, McManus BM, Billingham ME, Moon TE. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med. 1995;333:269–275. doi: 10.1056/NEJM199508033330501. [DOI] [PubMed] [Google Scholar]
- 8.D'Ambrosio A, Patti G, Manzoli A, Sinagra G, Di Lenarda A, Silvestri F, Di Sciascio G. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart. 2001;85:499–504. doi: 10.1136/heart.85.5.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mason JW. Myocarditis and dilated cardiomyopathy: an inflammatory link. Cardiovasc Res. 2003;60:5–10. doi: 10.1016/s0008-6363(03)00437-1. [DOI] [PubMed] [Google Scholar]
- 10.McManus BM, Chow LH, Wilson JE, Anderson DR, Gulizia JM, Gauntt CJ, Klingel KE, Beisel KW, Kandolf R. Direct myocardial injury by enterovirus: a central role in the evolution of murine myocarditis. Clin Immunol Immunopathol. 1993;68:159–169. doi: 10.1006/clin.1993.1113. [DOI] [PubMed] [Google Scholar]
- 11.Maekawa Y, Ouzounian M, Opavsky MA, Liu PP. Connecting the missing link between dilated cardiomyopathy and viral myocarditis: virus, cytoskeleton, and innate immunity. Circulation. 2007;115:5–8. doi: 10.1161/CIRCULATIONAHA.106.670554. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy RE, 3rd, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Hare JM, Baughman KL. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N Engl J Med. 2000;342:690–695. doi: 10.1056/NEJM200003093421003. [DOI] [PubMed] [Google Scholar]
- 13.Lawson CM. Evidence for mimicry by viral antigens in animal models of autoimmune disease including myocarditis. Cell Mol Life Sci. 2000;57:552–560. doi: 10.1007/PL00000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badorff C, Knowlton KU. Dystrophin disruption in enterovirus-induced myocarditis and dilated cardiomyopathy: from bench to bedside. Med Microbiol Immunol (Berl) 2004;193:121–126. doi: 10.1007/s00430-003-0189-7. [DOI] [PubMed] [Google Scholar]
- 15.Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–417. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 16.Caforio AL, Mahon NG, Baig MK, Tona F, Murphy RT, Elliott PM, McKenna WJ. Prospective familial assessment in dilated cardiomyopathy: cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation. 2007;115:76–83. doi: 10.1161/CIRCULATIONAHA.106.641472. [DOI] [PubMed] [Google Scholar]
- 17.Shioi T, Matsumori A, Sasayama S. Persistent expression of cytokine in the chronic stage of viral myocarditis in mice. Circulation. 1996;94:2930–2937. doi: 10.1161/01.cir.94.11.2930. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz MS, La Cava A, Fine C, Rodriguez E, Ilic A, Sarvetnick N. Pancreatic expression of interferon-gamma protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat Med. 2000;6:693–697. doi: 10.1038/76277. [DOI] [PubMed] [Google Scholar]
- 19.Pauschinger M, Chandrasekharan K, Schultheiss HP. Myocardial remodeling in viral heart disease: possible interactions between inflammatory mediators and MMP-TIMP system. Heart Fail Rev. 2004;9:21–31. doi: 10.1023/B:HREV.0000011391.81676.3c. [DOI] [PubMed] [Google Scholar]
- 20.Heymans S, Pauschinger M, De Palma A, Kallwellis-Opara A, Rutschow S, Swinnen M, Vanhoutte D, Gao F, Torpai R, Baker AH, Padalko E, Neyts J, Schultheiss HP, Van de Werf F, Carmeliet P, Pinto YM. Inhibition of urokinase-type plasminogen activator or matrix metalloproteinases prevents cardiac injury and dysfunction during viral myocarditis. Circulation. 2006;114:565–573. doi: 10.1161/CIRCULATIONAHA.105.591032. [DOI] [PubMed] [Google Scholar]
- 21.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, DeMayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 22.Heymans S. Inflammation and cardiac remodeling during viral myocarditis. Ernst Schering Res Found Workshop. 2006:197–218. doi: 10.1007/3-540-30822-9_12. [DOI] [PubMed] [Google Scholar]
- 23.Doolan A, Langlois N, Semsarian C. Causes of sudden cardiac death in young Australians. Med J Aust. 2004;180:110–112. doi: 10.5694/j.1326-5377.2004.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 24.Basso C, Calabrese F, Corrado D, Thiene G. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc Res. 2001;50:290–300. doi: 10.1016/S0008-6363(01)00261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- 26.Caforio AL, Iliceto S. Genetically determined myocarditis: clinical presentation and immunological characteristics. Curr Opin Cardiol. 2008;23:219–226. doi: 10.1097/HCO.0b013e3282fbf572. [DOI] [PubMed] [Google Scholar]
- 27.Lee GH, Badorff C, Knowlton KU. Dissociation of sarcoglycans and the dystrophin carboxyl terminus from the sarcolemma in enteroviral cardiomyopathy. Circ Res. 2000;87:489–495. doi: 10.1161/01.res.87.6.489. [DOI] [PubMed] [Google Scholar]
- 28.Kuhl U, Pauschinger M, Noutsias M, Seeberg B, Bock T, Lassner D, Poller W, Kandolf R, Schultheiss HP. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with ‘idiopathic’ left ventricular dysfunction. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 29.Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction. Evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- 30.Fujioka S, Kitaura Y, Ukimura A, Deguchi H, Kawamura K, Isomura T, Suma H, Shimizu A. Evaluation of viral infection in the myocardium of patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2000;36:1920–1926. doi: 10.1016/s0735-1097(00)00955-4. [DOI] [PubMed] [Google Scholar]
- 31.Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss HP. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- 32.Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000;343:1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 33.Pauschinger M, Doerner A, Kuehl U, Schwimmbeck PL, Poller W, Kandolf R, Schultheiss HP. Enteroviral RNA replication in the myocardium of patients with left ventricular dysfunction and clinically suspected myocarditis. Circulation. 1999;99:889–895. doi: 10.1161/01.cir.99.7.889. [DOI] [PubMed] [Google Scholar]
- 34.Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Multimedia article. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 35.Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581–1590. doi: 10.1161/CIRCULATIONAHA.105.606509. [DOI] [PubMed] [Google Scholar]
- 36.Dec GW, Jr, Palacios IF, Fallon JT, Aretz HT, Mills J, Lee DC, Johnson RA. Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N Engl J Med. 1985;312:885–890. doi: 10.1056/NEJM198504043121404. [DOI] [PubMed] [Google Scholar]
- 37.Dec GW, Jr, Waldman H, Southern J, Fallon JT, Hutter AM, Jr, Palacios I. Viral myocarditis mimicking acute myocardial infarction. J Am Coll Cardiol. 1992;20:85–89. doi: 10.1016/0735-1097(92)90141-9. [DOI] [PubMed] [Google Scholar]
- 38.Heymans S. Myocarditis and heart failure: need for better diagnostic, predictive, and therapeutic tools. Eur Heart J. 2007;28:1279–1280. doi: 10.1093/eurheartj/ehm111. [DOI] [PubMed] [Google Scholar]
- 39.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC., Jr ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996–3007. doi: 10.1161/hc4901.102568. [DOI] [PubMed] [Google Scholar]
- 40.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J, Virmani R. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. Eur Heart J. 2007;28:3076–3093. doi: 10.1093/eurheartj/ehm456. [DOI] [PubMed] [Google Scholar]
- 41.Martin AB, Webber S, Fricker FJ, Jaffe R, Demmler G, Kearney D, Zhang YH, Bodurtha J, Gelb B, Ni J, et al. Acute myocarditis. Rapid diagnosis by PCR in children. Circulation. 1994;90:330–339. doi: 10.1161/01.cir.90.1.330. [DOI] [PubMed] [Google Scholar]
- 42.Noutsias M, Pauschinger M, Schultheiss H, Kh U. Phenotypic characterization of infiltrates in dilated cardiomyopathy—diagnostic significance of T-lymphocytes and macrophages in inflammatory cardiomyopathy. Med Sci Monit. 2002;8:CR478–487. [PubMed] [Google Scholar]
- 43.Maisch B, Portig I, Ristic A, Hufnagel G, Pankuweit S. Definition of inflammatory cardiomyopathy (myocarditis): on the way to consensus. A status report. Herz. 2000;25:200–209. doi: 10.1007/s000590050007. [DOI] [PubMed] [Google Scholar]
- 44.Karjalainen J, Heikkila J. ‘Acute pericarditis’: myocardial enzyme release as evidence for myocarditis. Am Heart J. 1986;111:546–552. doi: 10.1016/0002-8703(86)90062-1. [DOI] [PubMed] [Google Scholar]
- 45.Lauer B, Niederau C, Kuhl U, Schannwell M, Pauschinger M, Strauer BE, Schultheiss HP. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997;30:1354–1359. doi: 10.1016/s0735-1097(97)00317-3. [DOI] [PubMed] [Google Scholar]
- 46.Rose NR, Mackay IR. Molecular mimicry: a critical look at exemplary instances in human diseases. Cell Mol Life Sci. 2000;57:542–551. doi: 10.1007/PL00000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawson CM, O'Donoghue HL, Reed WD. Mouse cytomegalovirus infection induces antibodies which cross-react with virus and cardiac myosin: a model for the study of molecular mimicry in the pathogenesis of viral myocarditis. Immunology. 1992;75:513–519. [PMC free article] [PubMed] [Google Scholar]
- 48.O'Donoghue HL, Lawson CM, Reed WD. Autoantibodies to cardiac myosin in mouse cytomegalovirus myocarditis. Immunology. 1990;71:20–28. [PMC free article] [PubMed] [Google Scholar]
- 49.Wolff PG, Kuhl U, Schultheiss HP. Laminin distribution and autoantibodies to laminin in dilated cardiomyopathy and myocarditis. Am Heart J. 1989;117:1303–1309. doi: 10.1016/0002-8703(89)90410-9. [DOI] [PubMed] [Google Scholar]
- 50.Sato Y, Matsumori A, Sasayama S. Autoantibodies against vimentin in a murine model of myocarditis. Autoimmunity. 1994;18:145–148. doi: 10.3109/08916939409007988. [DOI] [PubMed] [Google Scholar]
- 51.Pankuweit S, Portig I, Lottspeich F, Maisch B. Autoantibodies in sera of patients with myocarditis: characterization of the corresponding proteins by isoelectric focusing and N-terminal sequence analysis. J Mol Cell Cardiol. 1997;29:77–84. doi: 10.1006/jmcc.1996.0253. [DOI] [PubMed] [Google Scholar]
- 52.Schulze K, Becker BF, Schultheiss HP. Antibodies to the ADP/ATP carrier, an autoantigen in myocarditis and dilated cardiomyopathy, penetrate into myocardial cells and disturb energy metabolism in vivo. Circ Res. 1989;64:179–192. doi: 10.1161/01.res.64.2.179. [DOI] [PubMed] [Google Scholar]
- 53.Khaw BA, Narula J, Sharaf AR, Nicol PD, Southern JF, Carles M. SR-Ca2+ ATPase as an autoimmunogen in experimental myocarditis. Eur Heart J. 1995;16(Suppl. O):92–96. doi: 10.1093/eurheartj/16.suppl_o.92. [DOI] [PubMed] [Google Scholar]
- 54.Perez Leiros C, Goren N, Sterin-Borda L, Lustig L, Borda E. Alterations in cardiac muscarinic acetylcholine receptors in mice with autoimmune myocarditis and association with circulating muscarinic receptor-related autoantibodies. Clin Auton Res. 1994;4:249–255. doi: 10.1007/BF01827430. [DOI] [PubMed] [Google Scholar]
- 55.Takeda N. Cardiomyopathy: molecular and immunological aspects (review) Int J Mol Med. 2003;11:13–16. [PubMed] [Google Scholar]
- 56.Afanasyeva M, Georgakopoulos D, Rose NR. Autoimmune myocarditis: cellular mediators of cardiac dysfunction. Autoimmun Rev. 2004;3:476–486. doi: 10.1016/j.autrev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 57.Schwimmbeck PL, Badorff C, Schultheiss HP, Strauer BE. Transfer of human myocarditis into severe combined immunodeficiency mice. Circ Res. 1994;75:156–164. doi: 10.1161/01.res.75.1.156. [DOI] [PubMed] [Google Scholar]
- 58.Alvarez FL, Neu N, Rose NR, Craig SW, Beisel KW. Heart-specific autoantibodies induced by coxsackievirus B3: identification of heart autoantigens. Clin Immunol Immunopathol. 1987;43:129–139. doi: 10.1016/0090-1229(87)90164-4. [DOI] [PubMed] [Google Scholar]
- 59.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, Ishida M, Hiai H, Matsumori A, Minato N, Honjo T. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 60.Caforio AL, Goldman JH, Haven AJ, Baig KM, Libera LD, McKenna WJ. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. The Myocarditis Treatment Trial Investigators. Eur Heart J. 1997;18:270–275. doi: 10.1093/oxfordjournals.eurheartj.a015230. [DOI] [PubMed] [Google Scholar]
- 61.Caforio AL, Calabrese F, Angelini A, Tona F, Vinci A, Bottaro S, Ramondo A, Carturan E, Iliceto S, Thiene G, Daliento L. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J. 2007;28:1326–1333. doi: 10.1093/eurheartj/ehm076. [DOI] [PubMed] [Google Scholar]
- 62.Sarda L, Colin P, Boccara F, Daou D, Lebtahi R, Faraggi M, Nguyen C, Cohen A, Slama MS, Steg PG, Le Guludec D. Myocarditis in patients with clinical presentation of myocardial infarction and normal coronary angiograms. J Am Coll Cardiol. 2001;37:786–792. doi: 10.1016/s0735-1097(00)01201-8. [DOI] [PubMed] [Google Scholar]
- 63.Liu PP, Yan AT. Cardiovascular magnetic resonance for the diagnosis of acute myocarditis: prospects for detecting myocardial inflammation. J Am Coll Cardiol. 2005;45:1823–1825. doi: 10.1016/j.jacc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 64.Wang K, Asinger RW, Marriott HJ. ST-segment elevation in conditions other than acute myocardial infarction. N Engl J Med. 2003;349:2128–2135. doi: 10.1056/NEJMra022580. [DOI] [PubMed] [Google Scholar]
- 65.Take M, Sekiguchi M, Hiroe M, Hirosawa K. Long-term follow-up of electrocardiographic findings in patients with acute myocarditis proven by endomyocardial biopsy. Jpn Circ J. 1982;46:1227–1234. doi: 10.1253/jcj.46.1227. [DOI] [PubMed] [Google Scholar]
- 66.Billingham ME, Tazelaar HD. The morphological progression of viral myocarditis. Postgrad Med J. 1986;62:581–584. doi: 10.1136/pgmj.62.728.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinagra G, Maras P, D'Ambrosio A, Gregori D, Bussani R, Silvestri F, Morgera T, Pinamonti B, Salvi A, Alberti E, Di Lenarda A, Lardieri G, Klugmann S, Camerini F. Clinical polymorphic presentation and natural history of active myocarditis: experience in 60 cases. G Ital Cardiol. 1997;27:758–774. [PubMed] [Google Scholar]
- 68.Dec GW, Jr, Fallon JT, Southern JF, Palacios IF. Relation between histological findings on early repeat right ventricular biopsy and ventricular function in patients with myocarditis. Br Heart J. 1988;60:332–337. doi: 10.1136/hrt.60.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grogan M, Redfield MM, Bailey KR, Reeder GS, Gersh BJ, Edwards WD, Rodeheffer RJ. Long-term outcome of patients with biopsy-proved myocarditis: comparison with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1995;26:80–84. doi: 10.1016/0735-1097(95)00148-s. [DOI] [PubMed] [Google Scholar]
- 70.Felker GM, Boehmer JP, Hruban RH, Hutchins GM, Kasper EK, Baughman KL, Hare JM. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000;36:227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 71.Abdel-Aty H, Boye P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 72.Yilmaz A, Mahrholdt H, Athanasiadis A, Vogelsberg H, Meinhardt G, Voehringer M, Kispert EM, Deluigi C, Baccouche H, Spodarev E, Klingel K, Kandolf R, Sechtem U. Coronary vasospasm as the underlying cause for chest pain in patients with PVB19-myocarditis. Heart. 2008 doi: 10.1136/hrt.2007.131383. [DOI] [PubMed] [Google Scholar]
- 73.Gutberlet M, Spors B, Thoma T, Bertram H, Denecke T, Felix R, Noutsias M, Schultheiss HP, Kuhl U. Suspected chronic myocarditis at cardiac MR: diagnostic accuracy and association with immunohistologically detected inflammation and viral persistence. Radiology. 2008;246:401–409. doi: 10.1148/radiol.2461062179. [DOI] [PubMed] [Google Scholar]
- 74.O'Connell JB, Henkin RE, Robinson JA, Subramanian R, Scanlon PJ, Gunnar RM. Gallium-67 imaging in patients with dilated cardiomyopathy and biopsy-proven myocarditis. Circulation. 1984;70:58–62. doi: 10.1161/01.cir.70.1.58. [DOI] [PubMed] [Google Scholar]
- 75.Dec GW, Palacios I, Yasuda T, Fallon JT, Khaw BA, Strauss HW, Haber E. Antimyosin antibody cardiac imaging: its role in the diagnosis of myocarditis. J Am Coll Cardiol. 1990;16:97–104. doi: 10.1016/0735-1097(90)90463-y. [DOI] [PubMed] [Google Scholar]
- 76.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 77.Laissy JP, Hyafil F, Feldman LJ, Juliard JM, Schouman-Claeys E, Steg PG, Faraggi M. Differentiating acute myocardial infarction from myocarditis: diagnostic value of early- and delayed-perfusion cardiac MR imaging. Radiology. 2005;237:75–82. doi: 10.1148/radiol.2371041322. [DOI] [PubMed] [Google Scholar]
- 78.Gagliardi MG, Bevilacqua M, Di Renzi P, Picardo S, Passariello R, Marcelletti C. Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am J Cardiol. 1991;68:1089–1091. doi: 10.1016/0002-9149(91)90501-b. [DOI] [PubMed] [Google Scholar]
- 79.Skouri HN, Dec GW, Friedrich MG, Cooper LT. Noninvasive imaging in myocarditis. J Am Coll Cardiol. 2006;48:2085–2093. doi: 10.1016/j.jacc.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 80.Woodruff JF. Viral myocarditis. A review. Am J Pathol. 1980;101:425–484. [PMC free article] [PubMed] [Google Scholar]
- 81.Grinda JM, Chevalier P, D'Attellis N, Bricourt MO, Berrebi A, Guibourt P, Fabiani JN, Deloche A. Fulminant myocarditis in adults and children: bi-ventricular assist device for recovery. Eur J Cardiothorac Surg. 2004;26:1169–1173. doi: 10.1016/j.ejcts.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 82.McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre-Amione G, Gass A, Janosko K, Tokarczyk T, Kessler P, Mann DL, Feldman AM. Controlled trial of intravenous immune globulin in recent-onset dilated cardiomyopathy. Circulation. 2001;103:2254–2259. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 83.Parrillo JE, Cunnion RE, Epstein SE, Parker MM, Suffredini AF, Brenner M, Schaer GL, Palmeri ST, Cannon RO, 3rd, Alling D, et al. A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. N Engl J Med. 1989;321:1061–1068. doi: 10.1056/NEJM198910193211601. [DOI] [PubMed] [Google Scholar]
- 84.Wojnicz R, Nowalany-Kozielska E, Wojciechowska C, Glanowska G, Wilczewski P, Niklewski T, Zembala M, Polonski L, Rozek MM, Wodniecki J. Randomized, placebo-controlled study for immunosuppressive treatment of inflammatory dilated cardiomyopathy: two-year follow-up results. Circulation. 2001;104:39–45. doi: 10.1161/01.cir.104.1.39. [DOI] [PubMed] [Google Scholar]
- 85.Frustaci A, Chimenti C, Calabrese F, Pieroni M, Thiene G, Maseri A. Immunosuppressive therapy for active lymphocytic myocarditis: virological and immunologic profile of responders versus nonresponders. Circulation. 2003;107:857–863. doi: 10.1161/01.cir.0000048147.15962.31. [DOI] [PubMed] [Google Scholar]
- 86.Kuhl U, Pauschinger M, Schwimmbeck PL, Seeberg B, Lober C, Noutsias M, Poller W, Schultheiss HP. Interferon-beta treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107:2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 87.Betaferon®/Betaseron® (interferon beta-1b) in patients with chronic viral cardiomyopathy. Bayer Schering Pharma AG, Germany (2002) [Google Scholar]
- 88.Liu P, Aitken K, Kong YY, Opavsky MA, Martino T, Dawood F, Wen WH, Kozieradzki I, Bachmaier K, Straus D, Mak TW, Penninger JM. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nat Med. 2000;6:429–434. doi: 10.1038/74689. [DOI] [PubMed] [Google Scholar]
- 89.Liu P, Fuse K, Chu G, Liu Y, Opavsky A. Recent insights into the role of host innate and acquired immunity responses. Ernst Schering Res Found Workshop. 2006:123–139. doi: 10.1007/3-540-30822-9_8. [DOI] [PubMed] [Google Scholar]
- 90.Rose NR. Viral damage or ‘molecular mimicry’-placing the blame in myocarditis. Nat Med. 2000;6:631–632. doi: 10.1038/76199. [DOI] [PubMed] [Google Scholar]
- 91.Pankuweit S, Moll R, Baandrup U, Portig I, Hufnagel G, Maisch B. Prevalence of the parvovirus B19 genome in endomyocardial biopsy specimens. Hum Pathol. 2003;34:497–503. doi: 10.1016/s0046-8177(03)00078-9. [DOI] [PubMed] [Google Scholar]
- 92.Bultmann BD, Klingel K, Nabauer M, Wallwiener D, Kandolf R. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. Am J Obstet Gynecol. 2005;193:363–365. doi: 10.1016/j.ajog.2005.01.022. [DOI] [PubMed] [Google Scholar]
References
The above article uses a new reference style being piloted by the EHJ that shall soon be used for all articles.