Abstract
Human- and animal-pathogenic Bartonella species are fastidious and slow-growing bacteria difficult to isolate and cultivate. We describe a novel, easy-to-prepare liquid medium for the fast and reliable growth of several Bartonella spp. that does not affect bacterial protein expression patterns or interactions with host cells.
Bartonella spp. are important pathogens in human and veterinary medicine, of which B. henselae and B. quintana are considered to represent the most-relevant human-pathogenic Bartonella species (8). New members of the genus with unclear epidemiology (e.g., “B. rochalimae”) were recently described (10). Bartonella spp. are present in a broad spectrum of mammals (4, 14), which either suffer from these infections (11) or serve as asymptomatic reservoir hosts (8).
Usually, Bartonella spp. are cultivated using highly supplemented blood agars, which still results in long incubation periods. To date, the slow growth of Bartonella spp. on solid media has limited diagnostic (e.g., primary isolation) and experimental (e.g., analyses of B. henselae pathogenicity) approaches. In light of these problems, a laboratory diagnosis of a Bartonella infection is generally made by serology (5) or molecular approaches (7).
Here, we describe the discovery, characterization, and optimization of a liquid growth medium for Bartonella spp. This inexpensive and easy-to-prepare medium allows for relatively fast planktonic growth of the fastidious bacteria. It facilitates approaches for analyzing, e.g., B. henselae pathogenicity and might also be evaluated for its use in the primary cultivation of Bartonella spp. from human or veterinary samples in the future.
Development of a liquid growth medium for Bartonella henselae.
While analyzing the interaction of B. henselae Marseille (9) with Schneider cells from Drosophila melanogaster (19), we noticed the growth of B. henselae in the insect cell culture medium (Schneider's drosophila powder medium, revised [Serva, Heidelberg, Germany], supplemented with 10% fetal calf serum [FCS] and 2 mM glutamine [data not shown]). As no easy-to-prepare liquid growth medium for Bartonella spp. has been described before, we further evaluated Schneider's medium for its use as a growth medium. For all of the following experiments, Bartonella spp. were grown either in liquid media in cell culture flasks with constant, slow shaking (60 rpm/min) or on Columbia blood agar (CBA) in a humidified atmosphere at 37°C with 5% CO2.
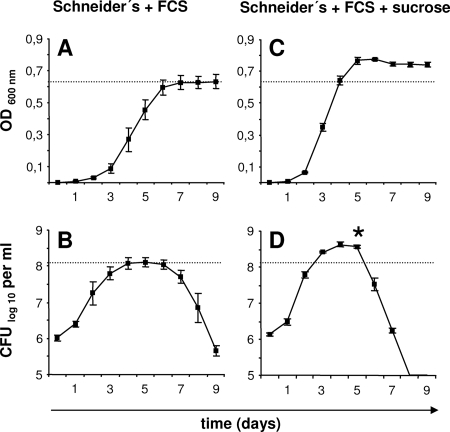
First, Schneider's medium supplemented with 10% FCS was inoculated with B. henselae (1.0 × 106 CFU/ml) and incubated for 9 days. Bacterial growth was determined by measuring the optical densities at 600 nm (OD600) at 24-h intervals, revealing a typical growth curve with a lag phase, a logarithmic growth phase, and a stationary phase (Fig. 1A). At the same time points, viable bacteria were quantified by plating serial dilutions on CBA (Fig. 1B). As described previously (6, 13), the number of viable bacteria decreased rapidly after the exponential growth phase (death phase), with no detectable stationary phase. We speculate that this early death phase might be caused by a lack of nutrients in the culture medium and not by lytic phage induction as described earlier (6), as the OD does not decrease during the death phase. Our observation has direct consequences when, e.g., infection experiments are performed with such liquid-grown Bartonella spp.; here, for each bacterial strain, the time point of entering the death phase needs to be evaluated to avoid an excess of dead bacteria.
FIG. 1.
Growth curves of B. henselae in Schneider's medium containing 10% FCS depending on the addition of 5% sucrose. Media were inoculated with B. henselae Marseille (1.0 × 106 CFU/ml), and bacterial growth was determined in triplicates by measuring the OD600 (A, C) and by quantifying the number of viable bacteria (CFU/ml) at 24-h intervals (B, D). Note that the growth rates and the maximum number of viable bacteria are higher in the presence of sucrose. *, significant difference from the control value (P < 0.01).
Interestingly, no growth of B. henselae was observed in Schneider's medium without FCS or in Luria-Bertani broth, in brain heart medium, or in endothelial cell growth medium, all supplemented with 10% FCS (data not shown). Cultivation of B. henselae in Bartonella Alphaproteobacteria growth medium (BAPGM [13]; provided by Ricardo Maggi, Chapel Hill, NC) supplemented with 10% FCS (instead of 5% sheep blood) revealed growth curves similar to those of the cultures in Schneider's medium with 10% FCS. Remarkably, the maximum bacterial numbers were higher in Schneider's medium (1.9 × 108 CFU/ml) than in BAPGM (1.2 × 108 CFU/ml). In contrast to BAPGM, our medium is not hampered by turbidity due to the addition of blood, thus allowing the detection of bacterial growth by visual inspection or by measurement of OD. Furthermore, it does not contain hemin (a necessary component of other liquid Bartonella media [17, 20, 21]), of which various concentrations were shown to be essential for the growth of different Bartonella species (20).
While evaluating different methods for the genetic manipulation of B. henselae, we realized that the addition of 5% (wt/vol) sucrose to Schneider's medium with 10% FCS resulted in even faster bacterial growth. Schneider's medium with 10% FCS and 5% sucrose was inoculated with B. henselae (1.0 × 106 CFU/ml), and the OD and numbers of CFU/ml were determined at 24-h intervals. In accordance with the OD, the maximum bacterial number was ∼3.3-fold higher when sucrose was added, and again, we observed an early death phase after the exponential growth phase (Fig. 1C and D). The maximum growth rate between days 1 and 2 allowed us to calculate a generation time of ∼5.6 h [to calculate the generation time, we used the equation 1 × lg2(t − t0)/lgN − lgN0, where t is time, t0 is time zero, N is the bacterial number, and N0 is the bacterial number at time zero].
To analyze the influence of further carbohydrates on the growth of B. henselae, Schneider's medium containing 10% FCS was supplemented with 5% (wt/vol) fructose, glucose, mannose, or galactose. While the addition of fructose showed no significant effect, the addition of glucose lowered bacterial growth rates, and remarkably, the addition of mannose or galactose suppressed the growth of B. henselae completely (data not shown). Right now, it is unclear why sucrose improves the growth of B. henselae but other carbohydrates do not influence, reduce, or even inhibit bacterial growth. Several ABC transporters (including a sugar ABC transporter) are present in the genome of B. henselae (1) and might facilitate the use of sucrose as a carbon source, whereas mannose and galactose could be toxic for B. henselae. For example, mannose might be taken up in an energy-consuming process by a phosphoenolpyruvate-dependent sugar phosphotransferase system (2), causing phosphoenolpyruvate starvation and killing of B. henselae.
Growth of different Bartonella spp. in Schneider's medium containing 10% FCS.
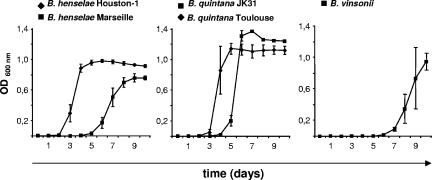
Next, the growth of medically and veterinarily important Bartonella spp. (B. henselae Marseille, B. henselae Houston-1 [ATCC 49882], B. quintana Toulouse [Collection de l'Institut Pasteur, Paris, France], B. quintana JK31 [22], and B. vinsonii subsp. berkhoffii [ATCC 51672]) was analyzed (inoculum, 1.0 × 105 bacteria/ml; OD determination at 24-h intervals [Fig. 2]). All five strains grew, but the amount of time needed to reach the respective exponential phase and the maximum OD differed from strain to strain. The best-growing strain was B. quintana JK31 (OD600, 1.37 at day 7), and the slowest-growing strains were B. henselae Marseille (OD600, 0.76 at day 9) and B. vinsonii (OD600, 0.95 at day 10), demonstrating that even within a certain species, generation times and maximum growth rates deviate significantly (e.g., for B. henselae strains Houston-1 and Marseille).
FIG. 2.
Growth curves of different Bartonella strains in Schneider's medium. Schneider's medium with 10% FCS was inoculated with B. henselae Marseille, B. henselae Houston-1, B. quintana Toulouse, B. quintana JK31, and B. vinsonii (1.0 × 105 bacteria/ml), and bacterial growth was determined in triplicates by measuring the OD600 at 24-h intervals. Note that all strains exhibited growth in this medium, although the intervals before the start of the logarithmic growth phase (lag phase) and the maximum OD differ.
Functional analysis of B. henselae grown in Schneider's medium-based Bartonella growth medium.
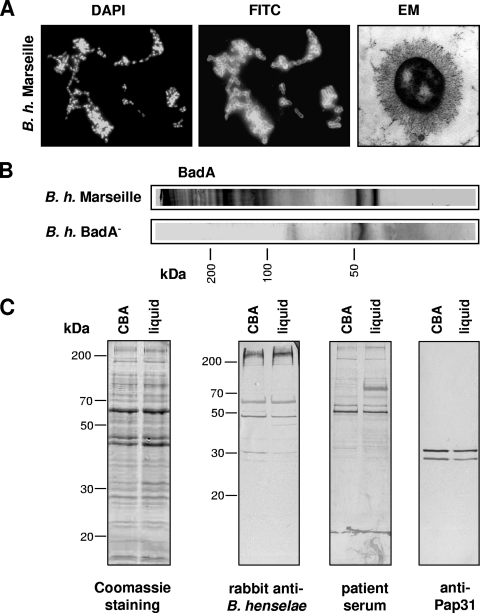
Expression of Bartonella adhesin A (BadA) depends on the cultivation procedures used (3, 16). Therefore, we analyzed B. henselae Marseille grown in our liquid medium for BadA expression. Immunofluorescence using specific antibodies and electron microscopy (done as described previously [15]) demonstrated a strong surface expression of BadA (Fig. 3A). BadA was also present in trichloroacetic acid-precipitated culture supernatants (inoculum, B. henselae [1.0 × 107 CFU/ml]; incubation period, 10 days) and detectable by the appearance of the typical ladder-like pattern of BadA bands (15, 16) in immunoblotting (Fig. 3B). No eminent differences were detected in the total protein compositions and immunoreactivities of B. henselae Marseille grown on CBA and in liquid medium (exponential growth phase), compared by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotting using the sera of a rabbit infected with B. henselae Marseille (15) and of a patient suspected of having a B. henselae infection (indirect immunofluorescence assay titer, 1:400). Finally, no difference was observed in the levels of expression of Pap31 (detected by immunoblotting using the monoclonal antibody VKS29 [23]) (Fig. 3C).
FIG. 3.
Protein expression pattern and antigenic profile of B. henselae (B. h.) grown on CBA and in liquid medium. (A) BadA expression by B. henselae Marseille grown in Schneider's medium containing 10% FCS and 5% (wt/vol) sucrose is shown by BadA immunostaining (fluorescein isothiocyanate [FITC] represents specific fluorescence, and DAPI [4′,6-diamidino-2-phenylindole] is used as an internal control) and electron microscopy (EM) (long, “hairy” structures). (B) Detection of BadA in liquid culture supernatants of B. henselae Marseille by BadA immunoblotting. A B. henselae BadA− strain (not expressing BadA [15]) was used as a negative control. (C) Protein composition of B. henselae grown on CBA or in liquid medium (Coomassie blue-stained sodium dodecyl sulfate gel) and immunoblots incubated with sera from a B. henselae-infected rabbit and from a patient suspected of having a B. henselae infection (indirect immunofluorescence assay titer, 1:400). The expression of Pap31 was detected with a specific monoclonal antibody (VKS29).
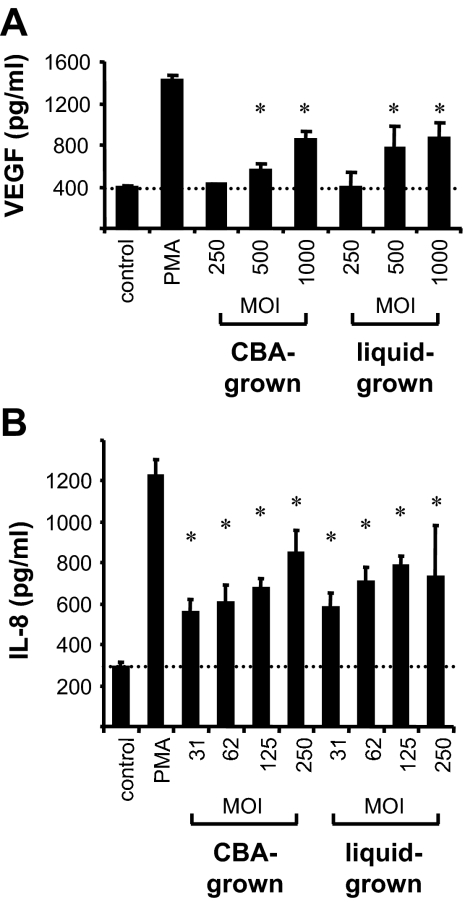
B. henselae adheres to and invades endothelial cells (12). We next analyzed the interaction of liquid-grown B. henselae with human umbilical vein endothelial cells (HUVEC) which were infected with B. henselae Marseille grown either on CBA or in Schneider's medium containing 10% FCS and 5% sucrose for 6 days. Adhesion and invasion were analyzed 30 min, 2 h, and 24 h after infection by confocal laser scanning microscopy as described previously (12), revealing no differences in the amounts of adherent and intracellular B. henselae (data not shown). Moreover, experiments investigating the secretion of vascular endothelial growth factor (VEGF) from HeLa cells (15) or of interleukin-8 (IL-8) from HUVEC (18) demonstrated that using our liquid medium to grow B. henselae (exponential growth phase) does not affect bacterium-triggered cytokine secretion (Fig. 4).
FIG. 4.
Induction of VEGF and IL-8 secretion by B. henselae grown in Schneider's medium. Bacteria were grown either on CBA or in Schneider's medium containing 10% FCS and 5% (wt/vol) sucrose. (A) HeLa 229 cells (VEGF secretion) were infected with B. henselae Marseille (multiplicity of infection [MOI], 250, 500, and 1,000. (B) HUVEC (IL-8 secretion) were infected with B. henselae ATCC 49882 (MOI, 31, 62, 125, and 250). Phorbol myristate acetate (PMA; 25 ng/ml) was used as a positive control. Cytokines were quantified by enzyme-linked immunosorbent assay from cell culture supernatants 24 h after infection. Note that no significant differences in their abilities to trigger VEGF or IL-8 secretion were observed between bacteria grown on agar and bacteria grown in liquid culture. Uninfected cells were used as a control. *, significant difference from the control value (VEGF, P < 0.05; IL-8, P < 0.01).
Taken together, we describe the development of an inexpensive and easy-to-prepare liquid medium for the cultivation of Bartonella spp. that uses only three widely used ingredients (Schneider's medium, FCS, and sucrose). This medium allows relatively fast planktonic growth and does not affect host-cell interaction in vitro. The use of Schneider's medium-based growth medium for microbiological diagnostics of Bartonella infections in veterinary or human medicine and for the cultivation of other fastidious bacteria will be evaluated in the future.
Acknowledgments
We thank Ricardo Maggi (Chapel Hill, NC) for providing the BAPGM and Silke Dorner (Max Planck Institute for Developmental Biology, Tübingen, Germany) for providing the Schneider cells.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (grant DFG-SFB 766) and from the University of Tübingen (Center for Interdisciplinary Clinical Research, junior group program) to V.A.J.K.
Footnotes
Published ahead of print on 20 June 2008.
REFERENCES
- 1.Alsmark, C. M., A. C. Frank, E. O. Karlberg, B. A. Legault, D. H. Ardell, B. Canback, A. S. Eriksson, A. K. Naslund, S. A. Handley, M. Huvet, B. LaScola, M. Holmberg, and S. G. Andersson. 2004. The louse-borne human pathogen Bartonella quintana is a genomic derivative of the zoonotic agent Bartonella henselae. Proc. Natl. Acad. Sci. USA 101:9716-9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barabote, R. D., and M. H. Saier, Jr. 2005. Comparative genomic analyses of the bacterial phosphotransferase system. Microbiol. Mol. Biol. Rev. 69:608-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batterman, H. J., J. A. Peek, J. S. Loutit, S. Falkow, and L. S. Tompkins. 1995. Bartonella henselae and Bartonella quintana adherence to and entry into cultured human epithelial cells. Infect. Immun. 63:4553-4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breitschwerdt, E. B., and D. L. Kordick. 2000. Bartonella infection in animals: carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin. Microbiol. Rev. 13:428-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1999. Serodiagnosis of emerging infectious diseases: Bartonella and Ehrlichia infections. Centers for Disease Control and Prevention, Atlanta, GA.
- 6.Chenoweth, M. R., G. A. Somerville, D. C. Krause, K. L. O'Reilly, and F. C. Gherardini. 2004. Growth characteristics of Bartonella henselae in a novel liquid medium: primary isolation, growth-phase-dependent phage induction, and metabolic studies. Appl. Environ. Microbiol. 70:656-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dauga, C., I. Miras, and P. A. Grimont. 1996. Identification of Bartonella henselae and B. quintana 16s rDNA sequences by branch-, genus- and species-specific amplification. J. Med. Microbiol. 45:192-199. [DOI] [PubMed] [Google Scholar]
- 8.Dehio, C. 2005. Bartonella-host-cell interactions and vascular tumour formation. Nat. Rev. Microbiol. 3:621-631. [DOI] [PubMed] [Google Scholar]
- 9.Drancourt, M., R. Birtles, G. Chaumentin, F. Vandenesch, J. Etienne, and D. Raoult. 1996. New serotype of Bartonella henselae in endocarditis and cat-scratch disease. Lancet 347:441-443. [DOI] [PubMed] [Google Scholar]
- 10.Eremeeva, M. E., H. L. Gerns, S. L. Lydy, J. S. Goo, E. T. Ryan, S. S. Mathew, M. J. Ferraro, J. M. Holden, W. L. Nicholson, G. A. Dasch, and J. E. Koehler. 2007. Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N. Engl. J. Med. 356:2381-2387. [DOI] [PubMed] [Google Scholar]
- 11.Kelly, P., J. M. Rolain, R. Maggi, S. Sontakke, B. Keene, S. Hunter, H. Lepidi, K. T. Breitschwerdt, and E. B. Breitschwerdt. 2006. Bartonella quintana endocarditis in dogs. Emerg. Infect. Dis. 12:1869-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kempf, V. A., M. Schaller, S. Behrendt, B. Volkmann, M. Aepfelbacher, I. Cakman, and I. B. Autenrieth. 2000. Interaction of Bartonella henselae with endothelial cells results in rapid bacterial rRNA synthesis and replication. Cell. Microbiol. 2:431-441. [DOI] [PubMed] [Google Scholar]
- 13.Maggi, R. G., A. W. Duncan, and E. B. Breitschwerdt. 2005. Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J. Clin. Microbiol. 43:2651-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maggi, R. G., C. A. Harms, A. A. Hohn, D. A. Pabst, W. A. McLellan, W. J. Walton, D. S. Rotstein, and E. B. Breitschwerdt. 2005. Bartonella henselae in porpoise blood. Emerg. Infect. Dis. 11:1894-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riess, T., S. G. Andersson, A. Lupas, M. Schaller, A. Schäfer, P. Kyme, J. Martin, J. H. Wälzlein, U. Ehehalt, H. Lindroos, M. Schirle, A. Nordheim, I. B. Autenrieth, and V. A. Kempf. 2004. Bartonella adhesin A mediates a proangiogenic host cell response. J. Exp. Med. 200:1267-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riess, T., G. Raddatz, D. Linke, A. Schäfer, and V. A. Kempf. 2007. Analysis of Bartonella adhesin A expression reveals differences between various B. henselae strains. Infect. Immun. 75:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sander, A., S. Kretzer, W. Bredt, K. Oberle, and S. Bereswill. 2000. Hemin-dependent growth and hemin binding of Bartonella henselae. FEMS Microbiol. Lett. 189:55-59. [DOI] [PubMed] [Google Scholar]
- 18.Schmid, M. C., R. Schülein, M. Dehio, G. Denecker, I. Carena, and C. Dehio. 2004. The VirB type IV secretion system of Bartonella henselae mediates invasion, proinflammatory activation and antiapoptotic protection of endothelial cells. Mol. Microbiol. 52:81-92. [DOI] [PubMed] [Google Scholar]
- 19.Schneider, I. 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J. Embryol. Exp. Morphol. 27:353-365. [PubMed] [Google Scholar]
- 20.Schwartzman, W. A., C. A. Nesbit, and E. J. Baron. 1993. Development and evaluation of a blood-free medium for determining growth curves and optimizing growth of Rochalimaea henselae. J. Clin. Microbiol. 31:1882-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong, M. T., D. C. Thornton, R. C. Kennedy, and M. J. Dolan. 1995. A chemically defined liquid medium that supports primary isolation of Rochalimaea (Bartonella) henselae from blood and tissue specimens. J. Clin. Microbiol. 33:742-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, P., B. B. Chomel, M. K. Schau, J. S. Goo, S. Droz, K. L. Kelminson, S. S. George, N. W. Lerche, and J. E. Koehler. 2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc. Natl. Acad. Sci. USA 101:13630-13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmermann, R., V. A. J. Kempf, E. Schiltz, K. Oberle, and A. Sander. 2003. Hemin binding, functional expression, and complementation analysis of Pap 31 from Bartonella henselae. J. Bacteriol. 185:1739-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]