Abstract
Escherichia coli O157:H7 causes hemorrhagic colitis and the life-threatening hemolytic-uremic syndrome in humans and transiently colonizes healthy cattle at the terminal rectal mucosa. To investigate the role of the O antigen in persistence and colonization in the animal host, we generated an E. coli O157:H7 mutant defective in the synthesis of the lipopolysaccharide side chain (O antigen) by deletion of a putative perosamine synthetase gene (per) in the rfb cluster. The lack of O antigen was confirmed by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and anti-O157 antibody. The growth rate and cell membrane permeability of the Δper mutant were similar to the growth rate and cell membrane permeability of the wild type. Changes in membrane and secreted proteins were observed, but the expression of intimin, EspA, and EspB, implicated in bacterial intestinal colonization, was not altered, as determined by immunoblotting and reverse transcription-PCR. Similar to other O-antigen deletion mutants, the Δper mutant was pleiotropic for autoaggregation and motility (it was FliC negative as determined by immunoblotting and flagellum negative as determined by electron microscopy). The abilities of the mutant and the wild type to persist in the murine intestine and to colonize the bovine terminal rectal mucosa were compared. Mice fed the Δper mutant shed lower numbers of bacteria (P < 0.05) over a shorter time than mice fed the wild-type or complemented strain. After rectal application in steers, lower numbers of the Δper mutant than of the wild type colonized the rectoanal junction mucosa, and the duration of the colonization was shorter (P < 0.05). Our previous work showed that flagella do not influence E. coli O157:H7 colonization at the bovine terminal rectal mucosa, so the current findings suggest that the O antigen contributes to efficient bovine colonization.
The enterohemorrhagic Escherichia coli (EHEC) strains are a subset of Shiga toxin-producing E. coli strains that have been associated with human illnesses, including self-limited watery diarrhea, hemorrhagic colitis, and the hemolytic-uremic syndrome (19, 27). Among the EHEC serotypes, O157:H7, which expresses somatic (O) antigen 157 and flagellar (H) antigen 7, causes serious morbidity and large disease outbreaks, making this bacterium one of the most important food-borne and waterborne pathogens worldwide (12, 27). Healthy cattle are the primary reservoirs for E. coli O157:H7 and non-O157 EHEC pathogens (2, 41) and the most common source of food-borne and direct-animal-contact infections (6, 14). Cattle frequently carry E. coli O157:H7 for either transient or long periods without suffering from pathological symptoms (4). Previous studies have demonstrated that the bovine terminal rectal mucosa is the primary site of E. coli O157:H7 colonization (28, 32).
The O-specific polysaccharide side chain or O antigen contains many repeats of an oligosaccharide unit and is part of the lipopolysaccharide (LPS) present in the outer membrane of gram-negative bacteria. The structure of the repeating units exhibits enormous antigenic variability and determines serological specificity. The O157 O side chain contains N-acetyl-d-perosamine, l-fucose, d-glucose, and N-acetyl-d-galactose (30). Among the four different sugars in the O157 repeated unit, GDP-d-perosamine and GDP-l-fucose are specific for the O side chain of LPS and are derived from d-mannose-6-phosphate (38, 40). The genes required for the synthetic steps occur in the rfb cluster. The O157 rfb region is comprised of 12 genes, including four GDP-l-fucose pathway genes (manB, manC, gmd, and fcl), a putative perosamine synthetase gene (per, also referred to as rfbE), an acetyltransferase gene (wbdR), three presumptive glycosyl transferase genes (wbdN, wbdO, and wbdP), the O-unit flippase gene (wzx), the O-antigen polymerase gene (wzy), and a remnant H-repeat unit (38, 40).
A recent study in our laboratory demonstrated that bacterial intimin, the translocated intimin receptor (Tir), and factors encoded by plasmid pO157 in E. coli O157:H7 are required for efficient bacterial colonization at the bovine terminal rectal mucosa (37). Other effector proteins associated with the type III secretion system (TTSS) may also play a role in bovine colonization (28). LPS and O antigen have been implicated in cell adherence and colonization in animals for various microorganisms, including Brucella melitensis 16M (13), Salmonella enterica serovar Typhi (26), Salmonella enterica serovar Typhimurium (8, 21), and Vibrio cholerae O139 (29, 39). The O antigen of E. coli O157:H7 has been suggested to be important for bacterial survival in an infant rabbit intestinal disease model (15). However, the role of the O antigen of E. coli O157:H7 in persistence and colonization in the healthy ruminant host is not known.
In this study, we (i) constructed a defined O-antigen-negative mutant by deleting the perosamine synthetase gene in the rfb locus (rfbE) and constructed a corresponding complemented strain; (ii) analyzed LPS profiles; (iii) characterized the O-antigen-negative mutant and wild type in terms of growth, membrane permeability, and expression of membrane and secreted proteins, including intimin, and representative effector proteins and in terms of pleiotropic effects; and (iv) compared the abilities of the Δper mutant and the wild type to persist in the murine intestine and to colonize the bovine terminal rectal mucosa.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and growth conditions.
Table 1 lists the bacterial strains and plasmids used in this study. Bacteria were grown in Luria-Bertani (LB) (10 g/liter Bacto tryptone, 5 g/liter Bacto yeast extract, 5 g/liter NaCl) broth or agar (1.5% [wt/vol] agar), Eagle's minimal essential medium (MEM), or sorbitol MacConkey agar (SMAC), as indicated below. Ampicillin (100 μg/ml), kanamycin (50 μg/ml), cefixime (50 ng/ml), potassium tellurite (2.5 μg/ml), 4-methylumbelliferyl-β-d-glucuronide (0.1 mg/ml), and/or novobiocin (200 μg/ml) was added when appropriate (Sigma Aldrich, St. Louis, MO). Oligonucleotide primers were purchased from Invitrogen (Carlsbad, CA).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| ATCC 43895 | Wild-type E. coli O157:H7 ATCC 43894, clinical isolate, stx1+/stx2+ | ATCCa |
| ATCC 43894 | Wild-type E. coli O157:H7 ATCC 43894, clinical isolate, stx1+/stx2+ | ATCC |
| 43895Δper | O-antigen-negative mutant of ATCC 43894 with perosamine synthetase deleted | This study |
| 43895ΔperComp | 43895Δper transformed with pCRII::per | This study |
| 43895Δeae | ATCC 43895 with intimin gene deleted | 37 |
| 43894ΔfliC | ATCC 43894 with flagellin gene deleted | 11 |
| 8624 | Wild-type E. coli O157:H7, clinical isolate, stx1/stx2+ | 3 |
| 8624 mutant | O-antigen-negative mutant of 8624 (F12) | 3 |
| Plasmids | ||
| pKD4 | Template plasmid for mutagenesis (Apr Knr) | 9 |
| pCRII | E. coli plasmid vector (Apr Knr) | Invitrogen |
| pCRII::per | per cloned into pCRII (Apr Knr) | This study |
ATCC, American Type Culture Collection, Manassas, VA.
Gene manipulation.
To make a mutant deficient in expression of the O157 antigen, the per gene encoding a putative perosamine synthetase was selected for deletion based on its position in the rfb cluster and previous reports of the effect of disruption of this gene (3, 23, 40). The λRed recombinase system (9) was used for gene deletion, as previously described (37). The following PCR primers for per deletion were designed based on the sequence of the rfb cluster (accession number AF061251): Per-dF (5′-ATGAAATATATACCAGTTTACCAACCGTCATTGACAGGAAAAGGTGTAGGCTGGAGCTGCTTCG-3′) and Per-dR (5′-CTATTTATCACTATAAATTCGTTAATAGATTCACAAATATACATA TGAATATCCTCCTTA-3′). Recombinants were selected on LB agar plates containing kanamycin. The failure of the Δper mutant to express the O157 antigen was confirmed by anti-O157 latex agglutination (Pro-Lab Diagnostics, Toronto, Canada). The kanamycin resistance gene was eliminated, and the mutant was confirmed to be similar to the parental wild-type strain by a multiplex PCR to detect eae, ehxA, stx1, and stx2, as previously described (34, 37). The replacement of the per gene by a nonpolar scar structure (9) was confirmed by PCR and DNA sequencing with a Perkin-Elmer thermocycler using the ABI Big Dye sequencing mixture according to the manufacturer's instructions and an ABI 3730 genetic analyzer.
To generate the complemented strain, PCR primers PerF (5′-GACAGTTAAATATAAGAGGATGAAAATG-3′) and PerR (5′-CATGAATGACCTTTACAATATTTTAGG-3′) were used to amplify an internal 1,146-bp fragment of per in the rfb region. The amplicon was purified and cloned into the E. coli plasmid vector pCRII-TOPO using a TOPO-TA cloning kit (Invitrogen) to obtain pCRII::per. The pCRII::per plasmid was electroporated into the Δper mutant, and the recombinants were selected on LB agar containing kanamycin or ampicillin. The O157 antigen expression of the complemented clones was confirmed by anti-O157 latex agglutination.
LPS analysis.
To confirm the loss of O antigen in the Δper mutant and the restoration of the O antigen in the complemented strain, LPS was isolated and analyzed using the method described by Moller et al., with a slight modification (25). Briefly, cells were grown in 3 ml LB broth to an optical density at 600 nm (OD600) of 1.0, harvested by centrifugation, resuspended in 1.0 ml of phosphate-buffered saline, and incubated at 60°C for 30 min. The suspension was centrifuged at 11,750 × g for 30 min, and the supernatant was mixed with an equal volume of Tricine sample buffer (Bio-Rad Laboratories, Hercules, CA) and boiled for 10 min. Protease K was added to a final concentration of 0.4 mg/ml, and the sample was incubated at 60°C for 60 min and centrifuged at 16,000 × g for 30 min. LPS was analyzed by Tricine-sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) as described by Pradel and Schnaitman (31) and was visualized by silver staining.
Analysis of secreted and membrane proteins.
Secreted proteins were isolated from the wild type and the Δper mutant as previously described (17). Bacteria were grown in MEM with aeration at 37°C to an OD600 of 1.0. The cells were removed by centrifugation (10,000 × g, 10 min), and the supernatants containing secreted proteins were mixed with phenylmethylsulfonyl fluoride (50 μg/ml; Sigma-Aldrich), aprotinin (0.5 μg/ml; Sigma-Aldrich), and EDTA (0.5 μM; Sigma-Aldrich) and passed through 0.45-μm-pore-size filters. The proteins were concentrated to 1 ml using a stirred-cell ultrafiltration device (Millipore/Amicon) with 40 lb/in2 N2 gas. The proteins were separated by 10% SDS-PAGE and visualized by silver staining or blotted onto a polyvinylidene difluoride (PVDF) membrane.
Membrane protein fractions were prepared as follows. Each strain was grown overnight at 37°C with aeration in LB broth, and cells were harvested by centrifugation and lyophilized for 48 h. The outer membrane proteins were extracted using a ProteoPrep membrane extraction kit (Sigma-Aldrich) by following the manufacturer's protocol and were analyzed by 10% SDS-PAGE.
Membrane permeability.
To assess membrane permeability, twofold serial dilutions of novobiocin from 200 to 1.6 μg/ml were inoculated with 105 CFU/ml and incubated at 37°C for 8 h. Growth was scored positive if the OD600 was >0.2.
Immunoblotting.
Proteins derived from whole-cell lysates or secreted proteins from concentrated culture supernatants were separated by SDS-PAGE and transferred to PVDF membranes using a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad). Immunoblotting was performed using standard techniques with the appropriate primary antibody followed by secondary antibodies conjugated with horseradish peroxidase (HRP), and reaction mixtures were visualized with a supersignal West Femto trial kit (Pierce, Rockford, IL) by using autoradiography.
LPS was detected with rabbit anti-O157 antisera (1:2,000; Remel, Lenexa, KS) and goat anti-rabbit HRP conjugate (1:50,000; Sigma-Aldrich). EspA and EspB were detected among secreted proteins with rabbit anti-EspA and anti-EspB, respectively (1:10,000; gifts from J. Kaper, University of Maryland), and goat anti-rabbit HRP conjugate (1:50,000; Sigma-Aldrich). Intimin was detected among whole-cell lysate proteins from cells grown in MEM with anti-intimin (1:300,000) and goat anti-rat HRP conjugate (1:50,000; Invitrogen). Antibody to intimin was made by immunization of Sprague-Dawley rats (Simonsen Labs, San Diego, CA) using a standard procedure. Intimin was purified as previously described (24) from an EHEC γ-intimin fusion with a six-His construct in E. coli M15 (a gift from R. Keller, University of Maryland). Rat anti-intimin serum was purified by absorption with whole E. coli M15 and an E. coli O157:H7 Δeae deletion mutant. Flagellin was detected by immunoblotting using rabbit anti-H7 antibody (1:3,000; Remel) and goat anti-rabbit HRP conjugate (1:50,000; Sigma-Aldrich).
Real-time PCR.
Bacterial cultures were grown in LB broth at 37°C with shaking to either mid- or late-exponential phase (OD600, 0.6 and 1.2, respectively). Cells were collected by centrifugation at 3,000 × g at 4°C, and RNA was isolated with a FASTRPREP RNA kit (Qbiogene, Irvine, CA) used according to the directions of the manufacturer. First-strand cDNA was generated from 1 μg of DNase I-treated total RNA using Superscript II reverse transcriptase and random hexamers (Life Technologies) according to the manufacturer's specifications. The primers used for the intimin gene (eaeA) were forward primer 5′-CCAGCTTCAGTCGCGATCTC-3′ and reverse primer 5′-GCCAGCATTTTTTCGGAATC-3′. The primers used for the 16S rRNA gene, which was used as an internal control for reverse transcription-PCRs, were forward primer 5′-AGCGGACCTCATAAAGTGCG-3′ and reverse primer 5′-CACAAAGTGGTAAGCGCCCT-3′. All primers were designed by using the Primer Express program (version 2.0; PE Applied Biosystems, Foster City, CA) and the GenBank accession number AF071034 and J01859 sequences. The reverse transcription-PCR assays included a positive control assay with genomic DNA and a negative control assay in which the mixture lacked reverse transcriptase. Reactions were performed in triplicate for each sample using the SYBR green I dye master mixture and the ABI Prism 7500 real-time PCR system (PE Applied Biosystems) according to the manufacturer's instructions. Data were analyzed using sequence detector system software (version 1.2.2; PE Applied Biosystems) and were normalized by calculating the change in the cycle threshold (CT) (CT of target − CT of the internal control [16S rRNA]).
Autoaggregation.
Autoaggregation was determined by visual examination of 4-ml overnight LB broth cultures aerated by shaking. Tubes were removed from the shaker, and the static cultures were examined for sedimentation for 1 to 3 h. Microscopic examination for autoaggregation was performed with cell wet mounts prepared directly from shake cultures using a phase-contrast microscope (Olympus) with a ×100 oil immersion objective lens.
Motility assays.
Tryptone swarm plates containing 1% Bacto tryptone, 0.5% NaCl, and 0.3% Bacto agar were used to assess the motility of the wild-type, Δper mutant, and complemented strains. Briefly, 2 μl of an overnight culture was point inoculated into swarm plates and incubated at 37°C for up to 40 h.
Electron microscopy.
Colonies of the wild type and the Δper mutant were grown overnight on LB agar plates. Formvar-coated copper grids were touched to isolated colonies, stained with 2% phosphotungstic acid in 0.1 M cacodylate buffer (pH 6.5) for 3 min, rinsed two times in 0.1 M cacodylate buffer, and air dried. The preparations were viewed to determine the presence of flagellated cells using a Zeiss 10A transmission electron microscope (Carl Zeiss, Thornwood, NY).
Experimental animals and bacterial challenge.
All animal procedures were approved by the Institutional Animal Care and Use and Biosafety Committees. Eighteen 8-week-old female Swiss Webster mice (Simonsen Laboratories, Inc., Gilroy, CA) were maintained in a biosafety level-2 isolation room. Mice were randomly allocated to cages and housed under standard day length, temperature, and humidity conditions. Water and pelleted food were provided ad libitum. The six mice in each of three groups were given oral doses containing approximately 108 CFU of wild-type E. coli O157:H7 strain ATCC 43895, the Δper mutant, or the complemented strain (43895ΔperComp) by using previously described procedures (36).
Two groups (n = 7) of 5-month-old Holstein steers were housed in a quarantined facility at the University of Idaho Agriculture Experiment Station as previously described (37); one group received a single rectal application of 1010 CFU of the E. coli O157:H7 wild-type strain, and the other group received a single rectal application of 1010 CFU of the Δper mutant.
Sample collection, bacterial culture, and enumeration.
Mouse fecal samples (three freshly voided pellets from each mouse) were collected daily, placed into 15-ml conical tubes containing 3 ml ice-cold Trypticase soy broth, suspended by vortexing, and cultured. Following rectal application of E. coli O157:H7 to cattle, recto-anal junction mucosa swab (RAMS) samples from each steer were obtained on days 1, 4, 7, 11, 18, 25, and 32 and cultured as previously described (32, 37). Briefly, the RAMS samples were vortexed with 3 ml ice-cold sterile Trypticase soy broth and cultured. Direct and enrichment bacterial culture procedures were used. Serial dilutions of the RAMS homogenates were plated on SMAC-CTVM (SMAC with cefixime [50 ng/ml], potassium tellurite [2.5 μg/ml], vancomycin [40 mg/liter], 4-methylumbelliferyl-β-d-glucuronide [0.1 mg/ml]). The identities of E. coli O157:H7 colonies on the plates were confirmed by the anti-O157 latex agglutination test. The presence of the eae, ehxA, stx1, and stx2 genes and the absence of the per gene in the colorless, nonfluorescent isolates recovered from animals treated with the mutant strain were confirmed by PCR.
Statistical analysis.
The numbers of E. coli O157:H7 bacteria recovered from individual samples were determined by calculating the averages of duplicate plate counts. Values from experiments were transformed to log10 CFU/fecal pellet for the mouse experiments and to log10 CFU/swab for the cattle experiments, and the average was calculated for each group. Samples that were positive only when the enrichment procedure was used were assigned a value of 5 CFU/fecal pellet for the mice or 10 CFU/swab for the cattle. The differences in the number of bacteria in fecal samples between the E. coli O157:H7 wild-type strain, the rough mutant, and the complemented strain fed individually to mice were compared by repeated-measures analysis of variance of the logarithm of the group geometric mean of CFU/fecal pellet for all mice in each group. Graphpad Prism software 4.0 (San Diego, CA) was used for this analysis. The difference in the number of bacteria in RAMS samples between the wild type and the rough mutant in the cattle experiment was determined using the Statistical Analysis t test procedure (33).
RESULTS AND DISCUSSION
Deletion of per generated an O-antigen-deficient mutant.
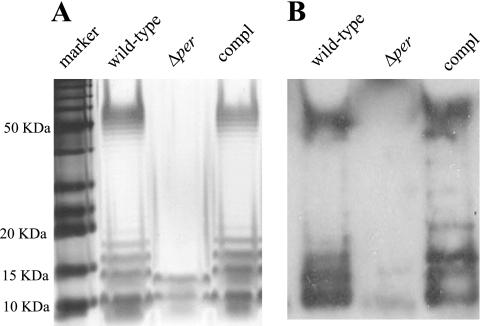
Deletion of per (the putative perosamine synthetase gene) resulted in a strain lacking the O157 antigen as determined by an LPS Tricine-SDS-PAGE assay, immunoblotting, and an anti-O157 latex bead agglutination assay. As a control, a per-complemented strain was constructed by cloning per in the E. coli vector pCRII and transforming pCRII::per into the mutant. Importantly, throughout all subculturing, O-antigen expression was not lost in the trans-complemented strain, indicating that the pCRII::per plasmid was stable. The per-complemented strain and the wild-type E. coli O157:H7 ATCC 43895 parental strain produced normal O157 profiles in a gel assay (Fig. 1A). The high-molecular-weight laddered bands represented the O-polysaccharide side chains and reacted with O157-specific antiserum (Fig. 1B). As Fig. 1 shows, these bands were missing in the Δper mutant. This LPS profile is in agreement with that of an O-antigen-negative mutant of E. coli O157:H7 8624 (F12) created by Bilge et al., who inactivated the per gene by TnphoA insertion (3).
FIG. 1.
LPS analysis of an E. coli O157:H7 strain and derivatives of this strain. (A) LPS from E. coli O157:H7 strain ATCC 43895 (wild type), the O-antigen-negative mutant (Δper), and the complemented mutant (compl). LPS samples from equal numbers of bacterial cells were loaded in the lanes, separated by Tricine-SDS-PAGE on a 12% acrylamide gel, and visualized by silver staining. (B) Immunoblot of bacterial LPS. Components separated by Tricine-SDS-PAGE were transferred to Immobilon-P and probed with antibody to the O157 antigen.
The growth rates of the wild-type, Δper mutant, and complemented strains did not differ in LB or SMAC-CTVM medium at 37°C when growth was monitored by using optical density (data not shown). However, the concentration of the O-antigen-negative mutant in stationary-phase cultures was higher than that of the wild type (2.5 × 109 CFU/ml versus 1.7 × 109 CFU/ml) when growth in LB broth at 37°C with aeration for 18 h was assessed by plate counting.
Cell permeability was not altered in the O-antigen-deficient Δper mutant.
To determine whether the overall permeability of the outer membrane was compromised by loss of the O antigen on the cell surface, the susceptibility of the O-antigen-deficient mutant to the hydrophobic antibiotic novobiocin was compared to the susceptibility of the wild-type and complemented strains. All three strains grew well in LB medium containing 200 μg/ml novobiocin, indicating that they were not sensitive to this antibiotic and therefore had similarly impermeable membranes (data not shown).
Some secreted and membrane proteins were altered by the Δper mutation, but intimin, EspA, or EspB was not altered.
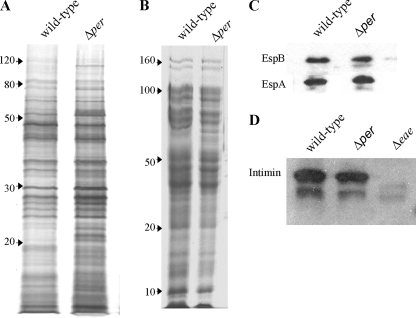
To determine if the lack of O antigen affected membrane proteins or secretion of proteins, including proteins exported by the TTSS, the Δper mutant and the wild type were compared. There were several differences in the secreted protein bands and/or the staining intensity of individual protein bands between the Δper mutant and the wild-type parental strain (Fig. 2A). The mutant produced several bands with low molecular masses (near 20 kDa) and contained a ∼100-kDa protein, and these proteins were not present in wild-type strain concentrates (Fig. 2A). Similarly, purified membrane proteins of the Δper mutant were slightly altered; two bands (∼70 kDa) were present in the Δper mutant and not in the wild type (Fig. 2B). However, there was no difference in the number or intensity of staining of the other protein bands. Thus, the loss of the O antigen correlated with minor alterations of secreted and membrane proteins.
FIG. 2.
Secreted and membrane proteins of E. coli O157:H7 strain ATCC 43895 (wild type) and the O-antigen-negative mutant (Δper). Proteins were separated by 10% SDS-PAGE. (A) Secreted proteins from culture supernatants visualized by silver staining. (B) Membrane proteins visualized by Coomassie blue staining. The positions of molecular mass markers (in kilodaltons) are indicated on the left. The proteins in similar gels were transferred to PVDF membranes for immunoblotting and probed with (C) anti-EspA and anti-EspB or (D) anti-intimin, reacted with secondary antibodies conjugated with HRP, and visualized by autoradiography. Δeae, E. coli O157:H7 with the intimin gene deleted.
Since the outer membrane protein intimin, its receptor Tir, and potentially several effector proteins secreted by the TTSS are required for efficient E. coli O157:H7 colonization of the bovine terminal rectal mucosa (24, 33), the expression of several of these factors was examined in more detail. Intimin and the effector proteins EspA and EspB were examined by immunoblotting. There was no difference in the secretion levels of these three proteins (Fig. 2C and D). Anti-Tir antibody was not available, but no difference in the protein profile corresponding to the molecular weight of Tir was observed for the secreted proteins (Fig. 2A).
To further confirm these observations, quantitative real-time PCR was conducted to compare the relative amounts of intimin (eaeA)-specific transcripts of the Δper mutant, the wild-type parental strain, and the Δper complemented strain. CT values from triplicate assays were normalized to the levels of 16S rRNA to correct for variations in bacterial numbers and were calculated using the relative comparison method. The corrected values showed that the relative levels of expression of the eaeA gene for the wild-type, Δper mutant, and complemented strains were 9.52 ± 0.28, 8.6 ± 0.29, and 9.13.± 0.34, respectively, at an OD600 of 0.6 and 8.98 ± 0.39, 8.99 ± 0.49, and 9.07 ± 0.25, respectively, at an OD600 of 1.2. The differences were not statistically significant. Therefore, we concluded that expression of intimin and expression of the secreted effector proteins EspA and EspB were not affected by the Δper mutation. Therefore, the difference in the secreted proteins of the Δper mutant did not appear to be due to differences in TTSS substrates.
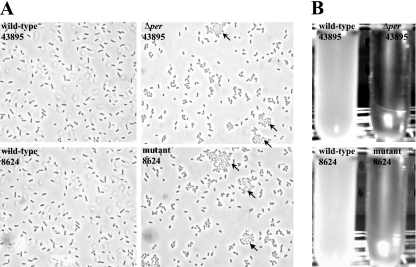
The Δper mutant displayed autoaggregation.
A prominent phenotype of the Δper mutant was autoaggregation of cells in broth culture (Fig. 3). To determine if this was a phenotype unique to this construct, we examined the per::TnphoA mutant of E. coli O157:H7 strain 8624 isolated by Bilge et al. (3). Microscopic examination of the 8624 per mutant revealed autoaggregation comparable to that seen for the ATCC 43895 Δper mutant; >80% of cells lacking the O antigen (43895Δper and 8624 mutant) clumped together. Most (>90%) of the clumping was reversed by brief vortexing, so plate enumeration in the experiments described below would not have been affected if such clumping occurred in vivo. This phenotype is in contrast to the phenotype of both parental wild-type strains (Fig. 3A). Liquid cultures of both strains showed pronounced rapid sedimentation of cells after removal from a shaker incubator. This phenotype is not displayed by either isogenic parent (Fig. 3B). Similar autoaggregation after the loss of O antigen has been observed with other strains of E. coli and S. enterica serovar Typhimurium (5, 10).
FIG. 3.
Autoaggregation of O-antigen-negative mutants. Wild-type E. coli O157:H7 strain ATCC 43895 (43895) and strain 8624 (8624) and the corresponding O-antigen mutants (43895Δper and 8624 mutant) were grown overnight with shaking in LB broth. Cells taken directly from the shaker were observed microscopically by phase-contrast microscopy (magnification, ×1,000) to examine clumping (A), or cultures were monitored visually for sedimentation after they stood undisturbed for 3 h at room temperature (B). Cell aggregation is indicated by arrows.
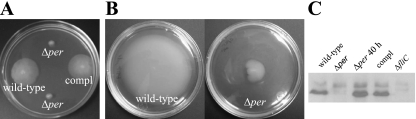
The Δper mutation resulted in a nonmotile phenotype.
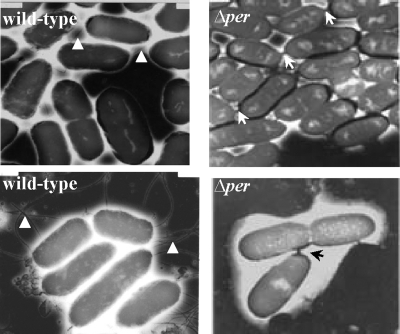
Bacterial autoaggregation can correlate with a loss of motility. To examine this possibility, we inoculated motility soft agar plates with the wild-type, Δper mutant, and complemented strains. As shown in Fig. 4A, the Δper mutant was nonmotile compared to the two control strains. Because both the motility and nonaggregative phenotypes resulting from the per mutation were restored by complementation with a wild-type copy of per in trans, these phenotypes are directly or indirectly linked to LPS structure and not due to unlinked mutations generated by the site-directed mutagenesis procedure. To further examine the motility phenotype, immunoblots prepared from whole-cell protein extracts of the wild-type, Δper mutant, and Δper complemented strains were probed with specific anti-H7 antibody. As shown in Fig. 4C, H7 flagellin was not present in the Δper mutant. The lack of motility observed for the Δper mutant was further confirmed by electron microscopy. Flagella were readily seen on wild-type cells but not on Δper mutant cells (Fig. 5). Interestingly, the Δper mutant cells in the images appeared to be attached to each other through a bridge of electron-dense material absent in the wild-type cells (Fig. 5). To our knowledge, this structure between cells has not been reported previously. Although we did not identify the composition of the apparent attachments, we assumed that they played a role in the autoaggregation. In summary, soft agar motility assays, immunoblots probed with specific anti-H7 (flagellin) antibody, and electron microscopy showed that the Δper mutation is pleiotropic for autoaggregation and nonmotile phenotypes.
FIG. 4.
Motility and flagellin expression in E. coli O157:H7 strain ATCC 43895 (wild type), the O-antigen-negative mutant (Δper), and the complemented strain (compl). Bacteria were stabbed into 0.3% motility agar and incubated at 37°C for 18 h (A) or 40 h (B). White areas represent bacterial growth (motility halo). Flagellin expression was examined using cells from the agar plates by immunoblotting (C) after 18 h of growth or 40 h of growth (Δper 40 h). ΔfliC, E. coli O157:H7 strain ATCC 43894 with the flagellin gene deleted.
FIG. 5.
Transmission electron microscopy. Bacterial cells grown overnight on LB agar were visualized by electron microscopy. Typical E. coli O157:H7 strain ATCC 43895 (wild-type) cells with flagella (indicated by arrowheads) are shown. Among the O-antigen-negative mutant (Δper) cells, cells without flagella were observed, and the cells were closer to each other than wild-type cells and electron-dense material apparently linked some cells together (indicated by arrows). Total magnification, ×20,000.
Pleiotropic mutations can restore partial phenotypes by secondary or compensatory mutations (18, 22). To test whether this phenomenon occurred, motility agar plates were point inoculated with the Δper mutant and incubated for an extended time. After prolonged incubation (40 h), motility flares were observed (Fig. 4B). The motile revertants showed restored expression of H7 flagellin (Fig. 4C) and loss of autoaggregation (data not shown), and they were nonreactive with anti-O157 antibody as determined by latex agglutination. Similar nonmotile phenotypes have been reported for E. coli and Salmonella LPS-deficient strains in which mutations in certain genes involved in the organization of the bacterial membrane impaired flagellum formation (1, 16, 20, 22).
The Δper mutation decreased the persistence of E. coli O157:H7 in the mouse intestine.
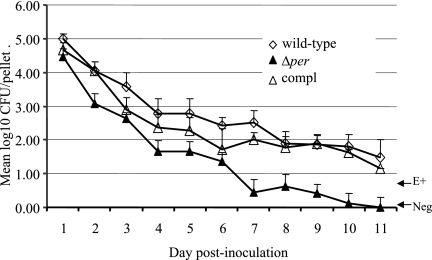
Although mice are not susceptible to EHEC disease unless they are pretreated with streptomycin, they have been used as a model for E. coli O157:H7 survival and persistence in the intestinal tract (36). To determine if O-antigen modification affected intestinal persistence, three groups of six Swiss Webster mice were given a single oral dose of the E. coli O157:H7 ATCC 43895 wild-type, Δper mutant, or complemented strain. The bacteria shed by the mice were monitored daily for 11 days after the dose was given. The logarithm of the group geometric mean for the number of bacteria shed by each mouse is shown in Fig. 6. Repeated-measures analysis of variance of these data accounting for bacterial strain, day, and amount of shedding indicated that significantly lower numbers of the Δper mutant than of the wild-type and complemented strains were shed on days 4, 7, and 11 postinoculation (P < 0.05). There was not a significant difference in the number of bacteria shed by animals on any day when the wild-type and complemented strains were compared. The Δper mutant was eliminated from three of six mice by day 7 after the dose was given, while all mice in the wild-type and Δper complemented groups remained culture positive on day 7. By day 11 after the dose was given, all six animals that received the Δper mutant were culture negative. In contrast, five of six mice that received either the wild-type or complemented strain remained culture positive. Thus, the persistence of the Δper mutant in the murine intestine was reduced.
FIG. 6.
Fecal shedding of E. coli O157:H7 by mice. Three groups of six mice received a single oral dose of 108 CFU E. coli O157:H7 strain ATCC 43895 (wild type), the O-antigen-negative mutant (Δper), or the complemented strain (compl). The geometric means of log-transformed viable counts (CFU/fecal pellet) are shown for each strain cultured from fecal samples on the indicated days. E+, fecal cultures positive for E. coli O157:H7 only as determined by using enrichment cultures and containing <10 E. coli O157 CFU/pellet; Neg, feces cultures that were negative as determined by both direct and enrichment culture procedures. The error bars indicate standard errors of the means.
The Δper mutation reduced colonization of E. coli O157:H7 at the bovine rectal terminal mucosa.
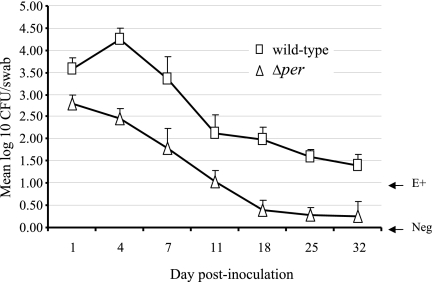
Rectal application of bacteria in cattle is a suitable model for investigating the interaction of E. coli O157:H7 with this intestinal mucosal colonization site (35, 37). Given that the persistence in the mouse intestine and the shedding patterns of the Δper mutant and wild type were significantly different, we examined the ability of the Δper mutant to persist in cattle. Our previous work showed that efficient colonization of rectal-anal junction mucosa by E. coli O157 can occur after direct application of bacteria to this site. Two groups of seven 6-month-old Holstein steers received a single rectal dose of 1 × 1010 CFU of wild-type E. coli O157:H7 or the Δper mutant. Colonization at the terminal rectal mucosa was monitored by culture of E. coli O157:H7 in RAMS samples from each steer on days 1, 4, 7, 11, 18, 25, and 32 (Fig. 7). Consistent with the mouse results, significantly lower numbers of the Δper mutant than of the wild type were shed, and the duration of shedding was shorter (P < 0.05). The Δper mutant was not detectable in five of seven steers by day 18 postchallenge, whereas the wild type was recovered from all seven steers on day 18 postchallenge (Fig. 7). By day 32 postinoculation, six steers in the Δper group were culture negative, while only one of the steers treated with wild-type E. coli O157:H7 was culture negative. These results indicate that the O side chain of E. coli O157:H7 LPS is involved in the persistence and colonization in bovine rectal terminal mucosa.
FIG. 7.
Bacterial colonization of the bovine rectal-anal junction mucosa. The numbers of E. coli O157:H7 strain ATCC 43895 (wild-type) or O-antigen-negative mutant (Δper) bacteria in RAMS samples from experimentally inoculated steers were determined. Two groups of seven steers received a single rectal application of 1010 CFU E. coli O157:H7 strain ATCC 43895 or the Δper mutant. The geometric means of log-transformed bacterial numbers (CFU/swab) are shown for each strain isolated from RAMS cultures on the indicated days. E+, RAMS cultures positive for E. coli O157:H7 as determined only by an enrichment procedure and containing <30 E. coli O157 CFU/swab; Neg, RAMS cultures that were negative as determined by both direct and enrichment culture procedures.
Involvement of O antigen in bacterial adherence to epithelial cells in vivo and/or in vitro has been reported for other gram-negative bacteria. In S. enterica serovar Typhi, the O side chain is required for adhesion to HeLa cells (26). In S. enterica serovar Typhimurium, O-antigen mutations significantly reduced colonization of the avian intestinal tract (8), and the O side chain was found to be required for adherence to the epithelium of the colonic lumen in infected mice (21). The V. cholerae O139 antigen has also been reported to be important for colonization of the mouse intestine (29, 39). Likewise, a rough (O-antigen-deficient) strain of B. melitensis 16M did not survive in the murine intestine (13). The results of this study are also consistent with a similar infant rabbit colonization model using an O-antigen-defective E. coli O157:H7 galETKM deletion mutant. Loss of O antigen in this deletion strain reduced the colonization of rabbits (15).
The results of our in vivo studies with the strain ATCC 43895 Δper mutant differ from conclusions drawn from in vitro cell adhesion assays performed with the strain 8624 per::TnphoA mutant. The latter O-antigen-negative strain adhered better in HeLa and HEp-2 cells than the wild type (3, 7). Demonstration here that both per mutant strains autoaggregated, a phenotype not mentioned for the 8624 mutant, suggests that the increased adherence to eukaryotic cells may be attributed in part to more rapid settling of the O-antigen-negative bacterial cells onto the eukaryotic monolayers compared to the settling expected for planktonic suspensions of wild-type bacteria.
Because the Δper mutant was pleiotropic, we cannot definitively say that decreased colonization in cattle was a direct consequence of O-antigen display. However, it is likely not due to loss of flagella. In a previous study, a nonmotile filament mutant (fliC deletion) of E. coli O157:H7 strain ATCC 43894 colonized the bovine terminal rectal mucosa as well as the wild type (11). Furthermore, intimin, EspA, and EspB protein levels were not affected by the Δper mutation. Therefore, the Δper mutation does not appear to have affected the expression and secretion of TTSS substrates or the outer membrane protein intimin, known factors that contribute to E. coli O157:H7 colonization of cattle.
In summary, we report that deletion of per resulted in a mutant lacking the O antigen with a concomitant nonmotile, autoaggregative phenotype. The O-antigen-defective mutant was cleared faster that the wild type in the mouse intestine and did not colonize at the bovine rectal terminal mucosa as effectively as the wild type. These data show that the O polysaccharide side chain of E. coli O157:H7 LPS is a required factor for efficient intestinal colonization.
Acknowledgments
We gratefully acknowledge Lonie Austin and Marilyn Austin for animal care and handling. We thank Ann Norton and Franklin Bailey for assistance with transmission electron microscopy.
This work was supported in part by the Idaho Agriculture Experiment Station, by grant 04-04562 from the National Research Initiative of the USDA Cooperative State Research, Education and Extension Service, by Public Health Service grants U54-AI-57141, P20-RR16454, and P20-RR15587 from the National Institutes of Health, and by grants from the Idaho Beef Council.
Footnotes
Published ahead of print on 13 June 2008.
REFERENCES
- 1.Bertin, P., E. Terao, E. H. Lee, P. Lejeune, C. Colson, A. Danchin, and E. Collatz. 1994. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J. Bacteriol. 176:5537-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettelheim, K. A. 2000. Role of non-O157 VTEC. Symp. Ser. Soc. Appl. Microbiol. 29:38S-50S. [DOI] [PubMed] [Google Scholar]
- 3.Bilge, S. S., J. C. Vary, Jr., S. F. Dowell, and P. I. Tarr. 1996. Role of the Escherichia coli O157:H7 O side chain in adherence and analysis of an rfb locus. Infect. Immun. 64:4795-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco, M., J. E. Blanco, J. Blanco, E. A. Gonzalez, A. Mora, C. Prado, L. Fernandez, M. Rio, J. Ramos, and M. P. Alonso. 1996. Prevalence and characteristics of Escherichia coli serotype O157:H7 and other verotoxin-producing E. coli in healthy cattle. Epidemiol. Infect. 117:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan, R. E., and N. E. Gibbons (ed.). 1974. Bergey's manual of determinative bacteriology, 8th ed. The Williams and Wilkins Company, Baltimore, MD.
- 6.Chapman, P. A., A. T. Cerdan Malo, M. Ellin, and R. Ashton. 2001. Escherichia coli O157 in cattle and sheep at slaughter, on beef and lamb carcasses and in raw beef and lamb products in South Yorkshire, UK. Int. J. Food Microbiol. 64:139-150. [DOI] [PubMed] [Google Scholar]
- 7.Cockerill, F., III, G. Beebakhee, R. Soni, and P. Sherman. 1996. Polysaccharide side chains are not required for attaching and effacing adhesion of Escherichia coli O157:H7. Infect. Immun. 64:3196-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craven, S. E. 1994. Altered colonizing ability for the ceca of broiler chicks by lipopolysaccharide-deficient mutants of Salmonella typhimurium. Avian Dis. 38:401-408. [PubMed] [Google Scholar]
- 9.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diderichsen, B. 1980. flu, a metastable gene controlling surface properties of Escherichia coli. J. Bacteriol. 141:858-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobbin, H. S., C. J. Hovde, C. J. Williams, and S. A. Minnich. 2006. The Escherichia coli O157 flagellar regulatory gene flhC and not the flagellin gene fliC impacts colonization of cattle. Infect. Immun. 74:2894-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle, M. P., L. R. Beuchat, and T. J. Montville (ed.). 2001. Food microbiology: fundamentals and frontiers, 2nd ed., p. 193-213. ASM Press, Washington, DC.
- 13.Godfroid, F., B. Taminiau, I. Danese, P. Denoel, A. Tibor, V. Weynants, A. Cloeckaert, J. Godfroid, and J. J. Letesson. 1998. Identification of the perosamine synthetase gene of Brucella melitensis 16M and involvement of lipopolysaccharide O side chain in Brucella survival in mice and in macrophages. Infect. Immun. 66:5485-5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hancock, D. D., T. E. Besser, M. L. Kinsel, P. I. Tarr, D. H. Rice, and M. G. Paros. 1994. The prevalence of Escherichia coli O157.H7 in dairy and beef cattle in Washington State. Epidemiol. Infect. 113:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho, T. D., and M. K. Waldor. 2007. Enterohemorrhagic Escherichia coli O157:H7 gal mutants are sensitive to bacteriophage P1 and defective in intestinal colonization. Infect. Immun. 75:1661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoare, A., M. Bittner, J. Carter, S. Alvarez, M. Zaldivar, D. Bravo, M. A. Valvano, and I. Contreras. 2006. The outer core lipopolysaccharide of Salmonella enterica serovar Typhi is required for bacterial entry into epithelial cells. Infect. Immun. 74:1555-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvis, K. G., and J. B. Kaper. 1996. Secretion of extracellular proteins by enterohemorrhagic Escherichia coli via a putative type III secretion system. Infect. Immun. 64:4826-4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, C. J., and S. Aizawa. 1991. The bacterial flagellum and flagellar motor: structure, assembly and function. Adv. Microb. Physiol. 32:109-172. [DOI] [PubMed] [Google Scholar]
- 19.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 20.Komeda, Y., T. Icho, and T. Iino. 1977. Effects of galU mutation on flagellar formation in Escherichia coli. J. Bacteriol. 129:908-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Licht, T. R., K. A. Krogfelt, P. S. Cohen, L. K. Poulsen, J. Urbance, and S. Molin. 1996. Role of lipopolysaccharide in colonization of the mouse intestine by Salmonella typhimurium studied by in situ hybridization. Infect. Immun. 64:3811-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macnab, R. M. 1992. Genetics and biogenesis of bacterial flagella. Annu. Rev. Genet. 26:131-158. [DOI] [PubMed] [Google Scholar]
- 23.Marolda, C. L., J. Vicarioli, and M. A. Valvano. 2004. Wzx proteins involved in biosynthesis of O antigen function in association with the first sugar of the O-specific lipopolysaccharide subunit. Microbiology 150:4095-4105. [DOI] [PubMed] [Google Scholar]
- 24.McKee, M. L., and A. D. O'Brien. 1996. Truncated enterohemorrhagic Escherichia coli (EHEC) O157:H7 intimin (EaeA) fusion proteins promote adherence of EHEC strains to HEp-2 cells. Infect. Immun. 64:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moller, A. K., M. P. Leatham, T. Conway, P. J. M. Nuijten, L. A. M. de Haan, K. A. Krogfelt, and P. S. Cohen. 2003. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect. Immun. 71:2142-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mroczenski-Wildey, M. J., J. L. Di Fabio, and F. C. Cabello. 1989. Invasion and lysis of HeLa cell monolayers by Salmonella typhi: the role of lipopolysaccharide. Microb. Patholog. 6:143-152. [DOI] [PubMed] [Google Scholar]
- 27.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesper, J., S. Schild, C. M. Lauriano, A. Kraiss, K. E. Klose, and J. Reidl. 2002. Role of Vibrio cholerae O139 surface polysaccharides in intestinal colonization. Infect. Immun. 70:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry, M. B., L. MacLean, and D. W. Griffith. 1986. Structure of the O-chain polysaccharide of the phenol-phase soluble lipopolysaccharide of Escherichia coli O157:H7. Biochem. Cell Biol. 64:21-28. [DOI] [PubMed] [Google Scholar]
- 31.Pradel, E., and C. A. Schnaitman. 1991. Effect of rfaH (sfrB) and temperature on expression of rfa genes of Escherichia coli K-12. J. Bacteriol. 173:6428-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice, D. H., H. Q. Sheng, S. A. Wynia, and C. J. Hovde. 2003. Rectoanal mucosal swab culture is more sensitive than fecal culture and distinguishes Escherichia coli O157:H7-colonized cattle and those transiently shedding the same organism. J. Clin. Microbiol. 41:4924-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SAS Institute. 1982. Statistical analysis system. SAS Institute, Inc., Cary, NC.
- 34.Sheng, H., M. A. Davis, H. J. Knecht, D. D. Hancock, J. Van Donkersgoed, and C. J. Hovde. 2005. Characterization of a Shiga toxin-, intimin-, and enterotoxin hemolysin-producing Escherichia coli ONT:H25 strain commonly isolated from healthy cattle. J. Clin. Microbiol. 43:3213-3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng, H., M. A. Davis, H. J. Knecht, and C. J. Hovde. 2004. Rectal administration of Escherichia coli O157:H7: novel model for colonization of ruminants. Appl. Environ. Microbiol. 70:4588-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheng, H., H. J. Knecht, I. T. Kudva, and C. J. Hovde. 2006. Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Appl. Environ. Microbiol. 72:5359-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheng, H., J. Y. Lim, H. J. Knecht, J. Li, and C. J. Hovde. 2006. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect. Immun. 74:4685-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu, T., S. Yamasaki, T. Tsukamoto, and Y. Takeda. 1999. Analysis of the genes responsible for the O-antigen synthesis in enterohaemorrhagic Escherichia coli O157. Microb. Pathog. 26:235-247. [DOI] [PubMed] [Google Scholar]
- 39.Waldor, M. K., R. Colwell, and J. J. Mekalanos. 1994. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc. Natl. Acad. Sci. USA 91:11388-11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, L., and P. R. Reeves. 1998. Organization of Escherichia coli O157 O antigen gene cluster and identification of its specific genes. Infect. Immun. 66:3545-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]