Abstract
AmyA, an α-amylase from the hyperthermophilic bacterium Thermotoga maritima, is able to hydrolyze internal α-1,4-glycosidic bonds in various α-glucans at 85°C as the optimal temperature. Like other glycoside hydrolases, AmyA also catalyzes transglycosylation reactions, particularly when oligosaccharides are used as substrates. It was found that when methanol or butanol was used as the nucleophile instead of water, AmyA was able to catalyze alcoholysis reactions. This capability has been evaluated in the past for some α-amylases, with the finding that only the saccharifying fungal amylases from Aspergillus niger and from Aspergillus oryzae present measurable alcoholysis activity (R. I. Santamaria, G. Del Rio, G. Saab, M. E. Rodriguez, X. Soberon, and A. Lopez, FEBS Lett. 452:346-350, 1999). In the present work, we found that AmyA generates larger quantities of alkyl glycosides than any amylase reported so far. In order to increase the alcoholytic activity observed in AmyA, several residues were identified and mutated based on previous analogous positions in amylases, defining the polarity and geometry of the active site. Replacement of residue His222 by glutamine generated an increase in the alkyl glucoside yield as a consequence of a higher alcoholysis/hydrolysis ratio. The same change in specificity was observed for the mutants H222E and H222D, but instability of these mutants toward alcohols decreased the yield of alkyl glucoside.
α-Amylases (EC 3.2.1.1) are retaining glycosidases that catalyze the hydrolysis of internal α-1,4-glycosidic bonds in starch through a double-displacement mechanism in which a covalent intermediate glycosyl enzyme is deglycosylated by water (43, 62). α-Amylases contain 5 to 11 subsites that bind glucose moieties (8, 51), with their numbers and relative affinities defining their product profiles (38). Like all retaining glycosidases, α-amylases can also catalyze transfer reactions, which are the result of employing molecules other than water as glucosyl acceptors, such as carbohydrates (transglycosylation reactions) or alcohols (alcoholysis reactions). When a high-molecular-weight alcohol is used as an acceptor, the product is an alkyl glycoside with surface tension activity properties that are important in several industrial applications. Therefore, the extremely laborious and inefficient chemical synthesis of alkyl glycosides presents an opportunity to develop enzymatic methods devoted to increasing the yields and specificities of these reactions.
The feasibility of alcoholysis reactions using various exoglycosidases has been extensively investigated (references 57 and 65 and references therein), and although efficient processes have been developed using activated substrates, such as p-nitrophenyl-glucoside or p-nitrophenyl-galactoside, with α- and β-glucosidases and galactosidases, the use of a readily available substrate, such as starch or amylodextrins, could prove attractive if efficient reactions employing α-amylases are developed.
For a given degree of starch depolymerization, endoamylases (such as α-amylase) generate lower-molecular-weight products than exoamylases (such as β-amylase or glucoamylase); while the former hydrolyze the polymer chain at random, the latter perform the hydrolysis from the nonreducing end of the molecule. However, in the case of maltogenic or saccharifying α-amylases, which are in principle endoenzymes, lower-molecular-weight products are obtained, presumably as a result of differences in subsite binding site affinities (3, 32) and the combination of hydrolytic and transfer mechanisms during the degradation process (42). Consequently, saccharifying α-amylases, such as Aspergillus oryzae and Aspergillus niger α-amylases, can transfer glucoside residues to alcohols (39), a limited property in liquefying α-amylases (55).
The direct use of fungal α-amylases for the production of alkyl glycosides from starch is precluded by the high temperature required for starch solubilization and the required stability of the protein toward alcohols. Currently, the industrial processing of starch requires initial hydrolysis by a liquefying (bacillar) thermostable α-amylase during the phase known as liquefaction, which is followed by a saccharifying step after pH and temperature adjustment due to the thermal stability of the saccharifying glucoamylase (63). In this context, the use of a thermophilic saccharifying α-amylase would be attractive, not only in the development of alcoholysis reactions, but also in the starch-processing industry, considering that thermophilic proteins are by their nature more stable at higher temperatures than their mesophilic equivalents and that thermostability has often been related to other important properties for a biocatalyst, such as resistance to substrate or product inhibition, as well as substrate and product solubility (66).
In 1997, Liebl et al. described an α-amylase (AmyA) found in the hyperthermophilic bacterium Thermotoga maritima MSB8 (DSM 3109) as a saccharifying amylase with an optimal temperature at 85°C that was able to hydrolyze internal α-1,4-glycosidic bonds in various α-glucans, such as starch, amylose, amylopectin, and glycogen, yielding mainly glucose and maltose as final products (37). As AmyA offers the advantage of a saccharifying profile in a very stable scaffold, we decided to explore its properties as a transferase, particularly in alcoholysis reactions, compared to other α-amylases and to improve this property by site-directed mutagenesis.
From the structural point of view, the direct comparison through multiple-sequence alignments of liquefying and saccharifying enzymes, as well as maltogenic amylases, neopullulanases, and CGTases, that have important transglycosylation activities has allowed the identification of residues potentially involved in transglycosylation activity (20, 30, 54). In spite of the fact that the α-amylase AmyA from T. maritima is already a saccharifying enzyme, after a sequence and structure comparison, we were able to identify various residues that are important in saccharifying amylases and are not conserved in the T. maritima α-amylase, suggesting that their mutation may generate an even more saccharifying enzyme; actually, as alcoholysis can be considered a specific type of transfer reaction, this would probably result in a more alcoholytic enzyme.
Several reports in the literature indicate that transglycosylation activity can be introduced or modified by promoting the effective concentration of less polar substrates in the active site, either by decreasing the affinity for water (7, 20, 34) or by modifying the geometry of the active site to disable the activation of the catalytic water molecule (39) and thereby favoring the activation of other, bulkier acceptor groups. The importance of aromatic residues in substrate binding at the active site of glycosidases through stacking interactions is well documented (7), and it has been proven that the introduction or substitution of these kinds of residues can modify the affinity of the protein at the different substrate subsites, affecting both the product profile and the activity of the enzyme (40, 45, 50, 54).
With this background, we focused our mutagenic strategy especially on those aromatic residues that, according to our model, might be involved in stacking interactions with the substrate, like Trp177, Tyr178, and Phe179 in domain B and His222 and Val259 in domain A of T. maritima α-amylase. Our expectations were that the modification of these residues to linear side chains might favor the entrance of other substrates to compete with water.
In the present work, we report some properties of AmyA and analyze the properties of several mutant proteins intended to improve the capacity of AmyA to carry out transglycosylation and alcoholysis reactions, in particular, its alcoholytic activity using starch as a substrate. The mutation positions were defined based on multiple-sequence alignments and a structural model constructed for AmyA.
MATERIALS AND METHODS
Homology modeling of AmyA.
There is no structural information on AmyA. However, it contains the four most highly conserved signatures present in members of family 13 of glycoside hydrolases (16, 26, 44, 52, 61) and 31% sequence identity to its closest homologous with known structure. In order to identify residues near the active site, a homology model for α-amylase AmyA was generated with the Swiss Model server (http://www.expasy.ch/swissmod/SWISS-MODEL.html) (19) using the coordinates of the α-amylase from A. oryzae (Protein Data Bank [PDB] entry 7TAA) (7) complexed with a modified acarbose inhibitor as a template. The side chain conformations in the model (root mean square score) were evaluated using the WHAT CHECK program (21). The model was minimized, and the substrate was introduced by homology to the TAA structure using InsightII molecular-modeling software (Accelrys Software, Inc.). Alternatively, using the program Deep View/Swiss PDB viewer version 3.7 (http://expasy.org/spdbv/) (19), the modified acarbose inhibitor crystallized in the 7TAA structure was superimposed on the generated model, guided by the four conserved regions characteristic of the amylase family (46).
Gene cloning and site-directed mutagenesis.
The amyA gene from T. maritima (GenBank accession number CAA72194) was cloned into pET28a+ from a T. maritima library kindly provided by Enrique Morett, using the oligonucleotides 5′-CGAAGGCATATGAAGGTTGTGAAG-3′ and 5′-CGATCCAAGCTTCACTTTTTGAAAATGTACGC-3′ through the NdeI and HindIII restriction sites. The megaprimer method (56) was used to construct a DNA fragment carrying mutations at positions Trp177, Tyr178, and Phe179 to valine; His222 to aspartic acid, glutamic acid, and glutamine; and Val259 to tryptophan using the oligonucleotides 5′-CCGAAATACACGACTTTTTGACC-3′, 5′-CCGAAAACCCAGACTTTTTGACCC-3′, 5′-AGCCAAAAAGTCCGACATACCAGAC-3′, 5′-GACCAGCCATATATSTSCTTTGCAGC-3′ (where S = G or C), and 5′-CCGCTGAACCACTCTCCCAC-3′, respectively, by PCR, in combination with the oligonucleotide 5′-CGAAGGCATATGAAGGTTGTGAAG-3′, which anneals at the 5′ terminus of the gene. The megaprimers obtained were purified and further extended in a second round of PCR with the oligonucleotide 5′-CCGCAAGCTTTTTGAAAATGTACGCTTTC-3′, which anneals at the 3′ end to complete the genes. The resulting mutated genes were cloned through NdeI and HindIII restriction sites in the pET28a+ expression vector from Novagen, which adds a sequence that encodes a six-His tag at the 3′ termini of the genes. The ligated vectors were used to transform electrocompetent ER2566 E. coli cells (New England Biolabs) by electroporation and plated on Luria-Bertani medium supplemented with 25 μg/ml kanamycin. Plasmid DNA was purified from six independent colonies using the High Pure plasmid isolation kit (Roche) and sequenced completely to ensure that mutations other than the ones designed did not occur. In order to carry out a fine characterization of particular mutants, the genes were subcloned, eliminating the signal peptide-coding region. To do this, the already mutated genes were amplified with the oligonucleotides 5′-TTGCT GAGCCGAAGGCATATGTGCTTTCAAACGTCTATGAGTCAATCC-3′ and 5′-CGATCCAAGCTTCACTTTTTGAAAATGTACGC-3′ and subcloned through NdeI and HindIII restriction sites in vector pET28a+ as described above. Production of proteins with these vectors had a much higher yield, and contamination of unprocessed peptide was avoided.
Enzyme purification.
Transformants of wild-type AmyA and mutants were cultured in 3 liters of Luria-Bertani medium supplemented with kanamycin and induced with 0.25 mM dioxane-free IPTG (isopropyl-β-d-thiogalactopyranoside) at an optical density at 600 nm of 0.4 and grown further at 21°C for 12 h. The cells were spun down at 5,000 × g for 30 min at 4°C. The pellet was resuspended in 7 ml of 300 mM NaCl, 50 mM Na2HPO4 buffer, pH 7.7, and lysed by sonication on an ice bath (Branson Sonifier 450). The cell extracts were heated at 82°C for 20 min, centrifuged for 30 min at 5,000 × g at 4°C, filtered through a 0.2-μm membrane, and purified using a 5-ml affinity column (HiTrap Chelating HP; Amersham Biosciences) using Ni2+ as a ligand in an Ákta-Pharmacia fast protein liquid chromatography system. The column was washed with 20 volumes of 20 mM imidazole, and the protein was eluted with a 25-volume linear gradient from 20 to 300 mM imidazole in 300 mM NaCl, 50 mM Na2HPO4 buffer, pH 7.7, at a flow rate of 5 ml/min. Fractions with high amylase activity were pooled, concentrated, and dialyzed against 20 mM Tris buffer, pH 8, and purified on a MonoQ 4.6/100PE (Amersham Biosciences) anion-exchange column employing a linear gradient of 0 to 1,000 mM NaCl in a 20 mM Tris buffer, pH 8. Fractions with high amylase activity were dialyzed against 50 mM Tris, 150 mM NaCl, 2 mM CaCl2 buffer, pH 7. Protein purity was assured by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the concentration was quantified by the Bradford method (Bio-Rad) using bovine serum albumin as a standard. The proteins expressed without signal peptide were purified from 500-ml cultures treated as described above, except that as a second purification step, size exclusion chromatography instead of anion exchange was used. For this step, a Superose HR12 column (Amersham Bioscience) was used in a fast protein liquid chromatography system, using 50 mM Tris, 150 mM NaCl, and 2 mM CaCl2 buffer at pH 7 as an eluent.
Activity determination.
The enzyme activities of wild-type AmyA and the mutants were estimated by measuring the initial velocities of formation of reducing sugars released upon starch hydrolysis by the 3,5-dinitrosalicylic acid method as reported previously (50). The reaction was carried out in 1 ml of 10-mg/ml soluble starch (Sigma-Aldrich) dissolved in 50 mM Tris, 150 mM NaCl, 2 mM CaCl2 buffer, pH 7, at 85°C, employing approximately 4 U/ml of activity in each enzyme assay. Only in the case of the wild-type and mutants at position His222, the concentration of substrate (soluble starch from Sigma-Aldrich) was varied from 0.1 to 10 mg/ml to obtain Michaelis-Menten parameters. A unit of enzyme activity is defined as the amount of glucose equivalents (μmol) released per min and is reported in terms of mg of AmyA.
Product profile.
Starch hydrolysis reactions carried out with 10 U/ml of wild-type AmyA were conducted at 85°C in a Thermomixer Compact (Eppendorf) in 1 ml of 50 mM Tris-Bis-HCl, 150 mM NaCl, 2 mM CaCl2 buffer, pH 7, with 3% soluble starch as a substrate. Samples were taken after 30 min to determine the reaction progress and after 24 h to obtain the product concentration at equilibrium. Samples were analyzed by thin-layer chromatography (TLC) and quantified by high-pressure liquid chromatography (HPLC). For TLC analysis, 10-cm by 10-cm by 200-μm Whatman Silica Gel 60 plates were used, with a mixture of ethanol, butanol, and water as a solvent (50:30:20), and visualized as previously reported (54). Reverse-phase HPLC was carried out in a Waters-Millipore 510 HPLC system with a refractive-index detector (Waters model 410) equipped with an automatic sampler (model 717 Plus) and a C18 column (4.6 by 250 mm) using water as the mobile phase at a flow rate of 0.7 ml/min.
Alcoholysis reactions.
20 U/ml of each enzyme was incubated at 85°C in a Thermomixer Compact (Eppendorf) with a suspension of 6% starch and either 20% methanol in 50 mM Tris, 150 mM NaCl, 2 mM CaCl2 buffer, pH 7, or a solution of the same buffer containing 8% butanol saturated with the same buffer. The reaction products were analyzed after 48 h of reaction. Methyl and butyl glycosides were visualized by TLC as previously described (54). In order to quantify the total amount of alkyl glycosides after the reaction, all products were hydrolyzed for 3 h at 40°C with A. niger glucoamylase (43 U/ml) (Sigma-Aldrich) to transform all the alkyl glucosides into alkyl glucoside and glucose. Samples of the reaction mixture were diluted 1:2 and analyzed in an HPLC system equipped with a Nova-Pak aminated column (4.6 mm by 250 mm) using acetonitrile and water as the mobile phase (67:33) at a flow rate of 0.8 ml/min. The peak areas were measured and compared against those of a standard curve containing known amounts of oligosaccharides from glucose (G1) to maltoheptaose (G7) and the corresponding alkyl glucoside (Sigma-Aldrich). Alcoholysis and hydrolysis events were calculated taking into account the amounts of equivalents of alkyl glucoside quantified by HPLC and the number of equivalents of glucose released in the reaction.
Determination of alcoholysis yields.
When a hydrolysis reaction takes place, for each molecule of a reducing substrate broken, one additional reducing end is formed, increasing its contribution to the solution reducing power, while when an alcoholysis reaction occurs, there is no real gain in reducing power. Therefore, a measure of the increasing equivalent dextrose concentration measured by the 3,5-dinitrosalicylic acid method (59) reflects the number of hydrolysis events occurred during the reaction, while the number of alcoholysis events can be independently determined by quantification of the increase in the alkyl glucoside concentration in the same period of time. From these data, it is possible to determine the numbers of hydrolysis and alcoholysis events in the reaction, as well as an alcoholysis/hydrolysis ratio and an alcoholysis efficiency: It is important to point out that this is an approximate calculation, as it assumes that the reducing power is independent of the oligosaccharide chain length. Nevertheless, it is useful as a comparative tool for modified amylases.
Transglycosylation reactions.
Aliquots with 4 U/ml of native and mutant enzymes were incubated at 85°C in a Thermomixer Compact (Eppendorf) with 2% maltotriose and maltotetraose (Sigma-Aldrich), each diluted in 50 mM Tris-Bis-HCl buffer, pH 7, containing 150 mM NaCl, 2 mM CaCl2. Aliquots were taken at 0 and 10 min and 1 and 12 h, and the reaction was stopped by transfer of the sample to ice; the products were visualized by TLC as previously reported (54), employing a mixture of oligosaccharides containing G1 to G7 as standards.
Thermostability.
Aliquots of the wild-type and mutant enzymes were incubated at 85°C in sealed Eppendorf tubes in a Thermomixer Compact (Eppendorf). Residual enzyme activity was measured at 85°C with 10 mg/ml of starch after different incubation times, starting from an activity of 3 U/ml. The value of stability is reported as a percentage of residual activity, taking the activity prior to incubation as 100% activity.
RESULTS
Product profile formation with wild-type AmyA.
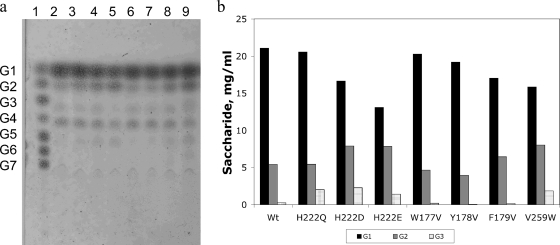
The starch hydrolysis product profile is shown in Fig. 1a, where it may be observed that glucose (G1) and maltose (G2) are the main products, as already demonstrated (37). In contrast to other bacillar α-amylases, such as those of Bacillus stearothermophilus or Bacillus licheniformis, which produce maltopentaose (G5) and maltohexaose (G6) as the main products after extensive starch hydrolysis, the product profile obtained with AmyA is similar to the profile observed with A. niger α-amylase (15, 55).
FIG. 1.
(a) TLC product profile of the hydrolysis reaction of a 30-mg/ml starch solution with 10 U/ml of the wild-type and mutant α-amylases from T. maritima after 24 h of reaction at 85°C. G1, glucose; G2, maltose; G3, maltotriose; G4, maltotetraose; G5, maltopentaose; G6, maltohexaose; G7, maltoheptaose. Lane 1, malto-oligosaccharides; lane 2, wild type; mutants, lanes 3, H222Q; 4, H222E; 5, H222D; 6, W177V; 7, Y178V; 8, F179V; and 9, V259W. (b) Quantification of G1, G2, and G3 for the different variants.
Alcoholysis reactions with wild-type AmyA.
The higher degree of starch depolymerization observed with AmyA in comparison to other bacterial α-amylases can be explained in terms of a combination of hydrolase and transferase reactions, as already demonstrated by del Río et al., (14). Therefore, we decided to explore if AmyA was also capable of catalyzing alcoholysis reactions. Figure 2a shows the alcoholysis profile of wild-type AmyA. Besides the spots that comigrate with maltodextrins, corresponding to hydrolysis products, we can observe spots that do not correspond to maltodextrins, among which methyl glucoside can be identified from a standard. In order to quantify the alcoholysis yield, given the wide diversity of methyl glycoside products, we treated the resulting reaction products with glucoamylase, an exoglycosidase that excises α-1,4-saccharides at the nonreducing end. The quantification of the resulting methyl glucoside was carried out by HPLC. After 48 h of incubation, the concentration of methyl glucoside generated by AmyA was 8 mg/ml.
FIG. 2.
(a) Alcoholysis reaction products obtained from 6% starch-20% methanol reaction medium with 20 U/ml of the respective enzymes after 48 h of incubation at 85°C. Lane 1, standards: MG, methyl glucoside; G1, glucose. Lanes 2 to 9, methanolysis reaction with the wild type and H222Q, H222D, H222E, W177V, Y178V, F179V, and V259W mutants, respectively; lanes 10 to 17, subsequent treatment of methanolysis reaction mixtures with A. niger glucoamylases of the wild type and H222Q, H222D, H222E, W177V, Y178V, F179V, and V259W mutants, respectively. (b) Yields of methyl glucoside after 48 h of incubation at 85°C and their subsequent treatment with A. niger glucoamylase of the wild type and variants at position 222. (c) Alcoholysis/hydrolysis ratios for the wild type and variants at position 222. The error bars indicate standard deviations.
Structural model of AmyA.
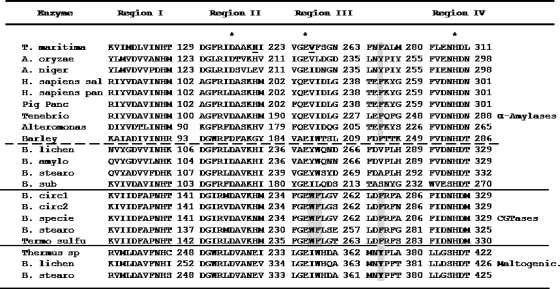
The structural model generated on the basis of homology with A. oryzae α-amylase (see Materials and Methods) shows that the catalytic (β/α)8 barrel domain, or A domain, is the more conserved domain. Domain B varies in size and structure from one amylase to another (25, 26, 47). Although some sequence similarities allow the classification of glycoside hydrolases into subgroups that presumably share the same supersecondary structure in domain B (23), AmyA shows a very low sequence identity to most amylases in this region. In effect, when a PSI-BLASTP (4, 5) search of its sequence was run against all nonredundant GenBank coding sequence databases, only a few high-score hits were obtained in the B domain sequence. This was the case for α-amylases from Thermosipho melanesiensis BI429 and from Fervidobacterium nodosum, while a lower score (under 60%) was obtained for oligo-1,6-glucosidases from some Lactobacillus species. As there is no crystal structure for any of these proteins, the domain B generated model is inaccurate and has to be taken with caution. As far as the C domain is concerned, it has a more conserved Greek key motif; however, it is less relevant for the reaction itself, since it is far from the active site (Fig. 3A). The modified acarbose inhibitor can be observed inside a crevice between domains A and B. Selection of residues to mutagenize was focused on residues located 4 Å around the modified acarbose (Fig. 3B). Based on the reports in the literature (30, 36, 64), special attention was devoted to aromatic residues and residues that could form hydrogen bonds near the acceptor binding sites (subsites +1, +2, and +3).
FIG. 3.
(A) Homology model obtained for AmyA in which the three characteristic amylase domains are depicted in different colors. The catalytic domain A is shown in gray; domain B and domain C are shown in green and blue, respectively. (B) The acarbose inhibitor (orange) is located between domains A and B and is surrounded by various residues located within 4 Å, among them the catalytic residue Glu258 (red). Possible residues involved in substrate binding are indicated in stick format; some of the amino acids subjected to site-directed mutagenesis were His222 and Phe179, shown in blue, and Val259, shown in yellow.
Among the potential residues to mutagenize, we identified residue His222, which points toward the sugar moiety at subsite +1 (Fig. 3B). In fact, in α-amylase from A. oryzae, the equivalent His222 hydrogen bonds the substrate sugar situated at subsite +1 (9). This residue has also been implicated in Ca2+ ion coordination and is conserved in α-amylases (Fig. 4). Therefore, we decided to investigate its role in AmyA transglycosylation and alcoholysis activities by replacing it with polar linear residues, like aspartic acid, glutamic acid, and glutamine. Based on the model (Fig. 3B) and on the multiple-sequence alignment shown in Fig. 4, we also decided to mutagenize Val259 to tryptophan. This position, located in proximity to subsite +2, shows a consensus tryptophan residue among CGTases that are natural trasferases. Therefore, we decided to investigate the effect of the mutation V259W in AmyA on transglycosylation and alcoholysis reactions. Finally, we identified one aromatic residue in domain B, Phe179, which points toward subsite −1. This residue is part of a cluster of contiguous aromatic residues. We figured out that even with the lack of accuracy of our model, any of these residues could have a role in placing and orienting the entrant acceptor for the second step of the reaction, so we decided to study the effects of the mutations W177V, Y178V, and F179V on the transglycosidic and alcoholytic activities of the enzyme.
FIG. 4.
Multiple structural alignment around the four characteristic regions observed in members of glycoside hydrolase family 13. The catalytic residues conserved in all the sequences are marked with asterisks. Aromatic residues involved in transglycosylation activity and the residues that structurally interact with them are highlighted in gray. Two of the residues mutagenized are shown in boldface and underlined. From the top: T. maritima, T. maritima α-amylase (GenBank accession number CAA72194) (37); A. oryzae, A. oryzae α-amylase (PDB accession number 2TAA) (41); A. niger, A. niger α-amylase (PDB accession number 2AAA) (6); H. sapiens sal, Homo sapiens salivary α-amylase (PDB accession number 1JXK) (49); H. sapiens pan, H. sapiens pancreatic α-amylase (PDB accession number 1HNY) (53); Pig panc, Sus scrofa pancreatic α-amylase (PDB accession number 1HX0) (48); Tenebrio, Tenebrio molitor α-amylase (PDB accession number 1JAE) (58); Alteromonas, Pseudoalteromonas haloplanktis α-amylase (PDB accession number 1G94) (1, 2); Barley, Hordeun vulgare α-amylase (PDB accession number 1AMY) (27); B. lichen, B. licheniformis α-amylase (PDB accession number 1VJS) (22); B. amylo, Bacillus amyloliquefaciens chimera α-amylase (PDB accession number 1E43) (8); B. stearo, B. stearothermophilus α-amylase (PDB accession number 1HVX) (60); B. sub, Bacillus subtilis 2633 α-amylase (PDB accession number 1BAG) (17), B. circ1, Bacillus circulans 251 cyclodextrin glycosyltransferase (CGTase) (PDB accession number 1CDG) (35); B. circ2, B. circulans 8 CGTase (PDB accession number 1CGT) (31); B. specie, Bacillus sp. strain 1011 CGTase (PDB accession number 1D7F) (23); B. stearo, B. stearothermophilus CGTase (PDB accession number 1CYG); Termo sulfu, Thermoanaerobacter thermosulfurogenes CGTase (PDB accession number 1A46) (68); Thermus sp., Thermus sp. maltogenic amylase (PDB accession number 1SMA) (29); B. lichen, B. licheniformis maltogenic amylase (GenBank accession number CAA47612) (28); B. stearo, B. stearothermopilus maltogenic amylase (PDB accession number 1QHO) (13).
Enzyme activity.
All the specific activities for starch, measured for the wild-type enzyme and the mutants (except for V259W), were in the same order of magnitude, around 1,000 U/mg. After purification of the proteins by Ni2+ affinity and anion-exchange chromatography, a certain amount of the unprocessed amylase was still present, as evidenced by the sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (data not shown). Nevertheless, in order to have uniform conditions for comparison, the protein concentrations were adjusted to have 4 U of activity per ml of reaction mixture.
In order to biochemically characterize mutants showing a higher alcoholysis/hydrolysis ratio, they were expressed in the cytoplasm by removing the signal peptide. Thus, the yields and purities of H222Q, H222E, and H222D mutants were improved, eliminating contamination with unprocessed peptide.
Product profile after starch hydrolysis with Amy mutants.
Although the wild-type AmyA and its mutants showed similar patterns of starch hydrolysis with G1 and G2 as the main products, their relative concentrations varied among the mutants, as shown in Fig. 1b. The mutants in domain B, W177V and Y178V, as well as the mutant H222Q, showed a G1/G2 ratio similar to that of the wild-type enzyme (Fig. 1). On the other hand, mutations of His222 to acidic residues showed a decrease in the G1/G2 ratio, especially mutant H222E, whose G1/G2 ratio decreased twofold. The V259W and F179V mutants also showed lower G1/G2 ratios and the presence of a larger product, G6, not detectable in the rest of the mutants (Fig. 1a). All the mutant and wild-type enzymes showed products that could be attributed to transglycosylation reactions different from α-1,4. During the first minutes of the reaction, accumulation of oligosaccharides took place (data not shown). However, once a pseudoequilibrium was reached, the band corresponding to G3 seemed to disappear, giving rise to a product band with lower mobility present in all variants but H222E. This band had a different mobility than G3, as can be appreciated in Fig. 1a, lane 9, where a double band can be observed for the mutant V259W, showing that in reactions performed with this mutant, G3 was still present, in addition to the other transglycosylation reaction products observed with the other enzymes. Besides this product, all the variants showed the presence of a second, unexpected band that ran between G4 and G5. Mutants at His222 and mutants F179V and V259W also showed a product that seemed to have a lower mobility than G5. The presence of these bands suggests the production of transglycosylation products in addition to those usually found in α-amylases. Further support for this hypothesis is shown in Fig. 2a, lanes 10 to 17, where glucoamylase was used to hydrolyze the final alcoholysis reaction products to methyl glucoside and glucose. As can be observed in the figure, besides the expected bands corresponding to the hydrolysis products, bands corresponding to the additional transglycosylation products were still present, indicating that they were not glucoamylase substrates. These species were produced more slowly, but since they were not substrates for α-amylase, they accumulated at equilibrium.
Alcoholysis reactions with Amy mutants.
With the exception of the mutants at position 222, the variants yielded a product profile similar to that of the wild-type enzyme (Fig. 2a). Therefore, the W177V, Y178V, and F179V (which was particularly unstable in the presence of alcohols) mutants were not further investigated. The alcoholysis products that can be unequivocally assigned are methyl glucoside and methyl maltoside, which have higher mobility in TLC than any hydrolysis product. However, the lack of methyl-saccharide standards of higher molecular weight and the possibility of overlapping of some alcoholysis and hydrolysis products precluded a direct evaluation of alcoholysis reaction yields. To overcome this limitation, the final products of the reaction were completely hydrolyzed with glucoamylase to generate glucose and methyl glucoside (a direct indicator of alcoholysis reactions). Figure 2a shows the product profiles of alcoholysis reactions of the wild-type enzyme and mutants before and after treatment with glucoamylase. His222 mutants showed a greater amount of methyl glucoside than the wild-type enzyme and the other mutants, as shown in Fig. 2a. The amounts of methyl glucoside generated for His222 mutants were quantified by HPLC (Table 1). From these results, it may be concluded that H222Q showed the highest yield of methyl glucoside. Mutant H222Q presented an increase in the alcoholysis events as a consequence of an increase in alcoholysis and a reduction in hydrolysis activity of almost 30%. The twofold increase in the alcoholysis/hydrolysis ratio resulted in approximately 80% increase in the amount of methyl glucoside produced by this mutant (Fig. 2c). Although mutant H222D showed the highest alcoholysis/hydrolysis ratio, its production of methyl glycosides was lower than that of the H222Q mutant. This can be attributed to loss of activity in the presence of methanol, as suggested by the presence of residual starch in the reaction, even though equal numbers of activity units were used for all assays (Fig. 2a, lane 4). The same seems to be true for the H222E mutant, although it had the lowest alcoholysis/hydrolysis ratio among the mutants.
TABLE 1.
Characterization of wild-type and mutant α-amylase alcoholysis reactionsa
| Enzyme | No. of alcoholysis events | No. of hydrolysis events | Alcoholysis/hydrolysis ratio | Efficiency of alcoholysis reaction (%) | Amt of methyl glucoside (mg/ml) |
|---|---|---|---|---|---|
| Wild type | 80 | 239 | 0.33 | 25 | 7.5 ± 0.6 |
| H222D | 103 | 132 | 0.78 | 44 | 10.6 ± 4.0 |
| H222E | 85 | 169 | 0.51 | 34 | 9.7 ± 1.8 |
| H222Q | 121 | 177 | 0.68 | 41 | 12.5 ± 2.0 |
Starch, 6%; methanol, 20%.
Alcoholysis reactions with butanol.
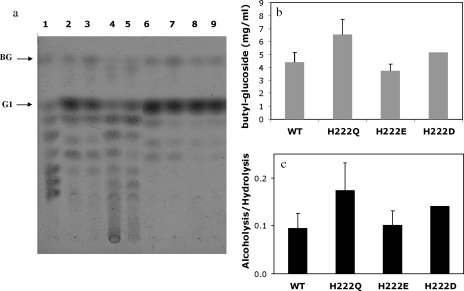
The ultimate goal is to generate alkyl glucosides with good surfactant properties, for which the use of higher-molecular-weight alcohols as acceptors is imperative. We decided to test the abilities of the wild type and mutants H222Q and H222E to carry out butanolysis. From previous experience (55), the yield of butanolysis was higher when the reaction was carried out in a single phase (buffer saturated with butanol) than when larger amounts of alcohol were used, probably due to an inefficient homogenization of the two phases. We therefore used a butanol-saturated buffer solution to carry out these reactions. Figure 5a shows the product profiles of the wild type and the different His222 mutants. As can be observed in Fig. 5c, mutant H222Q maintained a higher alcoholysis/hydrolysis ratio than the wild-type enzyme (twofold) and yielded 50% more butyl glucoside (Fig. 5b). However, the amount of butyl glucoside produced might have been limited by the availability of butanol in the reaction mixture relative to the methanolysis reactions. The instability of mutants H222E and H222D toward butanol was more prominent, as can be observed in Fig. 5a, lanes 4 and 5, yielding an amount of butyl glucoside similar to that of the wild-type enzyme.
FIG. 5.
(a) Alcoholysis reaction products obtained from 6% starch-8% butanol saturated with buffer (50 mM Tris, 150 mM NaCl, 2 mM CaCl2 buffer, pH 7) with 20 U/ml of the respective enzymes after 48 h of incubation at 85°C. Lane 1, standards: BG (butyl glucoside), G1, glucose. Lanes 2 to 5, butanolysis reaction with the wild type and H222Q, H222D, and H222E mutants, respectively; lanes 6 to 9, subsequent treatment of butanolysis reaction mixtures with A. niger glucoamylase of the wild type and H222Q, H222D, and H222E mutants, respectively. (b) Yields of butyl glucoside after 48 h of incubation at 85°C and their subsequent treatment with A. niger glucoamylase from the different variants. (c) Alcoholysis/hydrolysis ratios with the different variants. The error bars indicate standard deviations.
Transglycosylation activity.
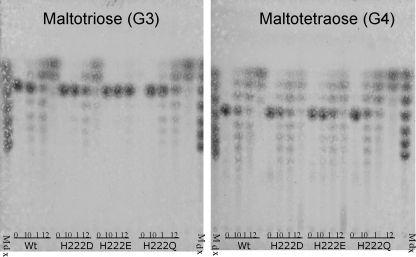
In order to establish if reduction in hydrolysis events in mutants H222D and H222E was the result of a change in substrate specificity or a decrease in transglycosylation activity, we compared the hydrolysis/transglycosylation patterns in the enzymes using small oligosaccharides as substrates. When G3 was used as a substrate (Fig. 6a), the wild-type enzyme, H222D, and H222Q, after producing some transglycosylation products, generated G1 and G2 as the predominant end species; however, H222E did not recognize G3 as a substrate, accepting G4 as the smallest substrate for activity (Fig. 6b), resulting in a product pattern very similar to the one obtained with H222D, accumulating different amounts of maltodextrins (data not shown). These results suggest that there is a loss of affinity binding to small oligosaccharides in H222E, rather than a loss in transglycosylation activity.
FIG. 6.
Transglycosylation products obtained from maltotriose (a) and maltotetraose (b) using 4 U/ml of the wild type and mutants after 0 and 10 min and 1 and 12 h of incubation at 85°C. Lane 1 is the molecular marker, a mixture of oligosaccharides from glucose to maltoheptaose.
Thermostability.
One of the main reasons to select T. maritima α-amylase for alcoholysis was its high temperature stability, a condition required for starch processing that may also be extended to stability in organic solvents. It was therefore important for industrial applications to demonstrate that the thermostability of AmyA was preserved in the alcoholytic mutants. We measured the residual activity of the wild-type enzyme, as well as that in the H222D, H222E, and H222Q mutants, after incubation at 85°C. The wild-type AmyA was found to be stable for more than 30 h at 85°C, a property that was unaltered in the mutants. Although no activity loss existed after 30 h of incubation, extending the incubation time beyond this period was difficult due to solvent evaporation (Fig. 7); however for practical purposes, the half-lives of the enzyme and its mutants fulfilled the stability requirements of the industrial process.
FIG. 7.
Thermal inactivation of the wild type and mutants at His222 of α-amylase from T. maritima after incubation at 85°C.
DISCUSSION
In contrast to bacillar α-amylases from B. licheniformis and B. stearothermophilus, the saccharifying α-amylase AmyA from T. maritima is capable of efficiently carrying out alcoholysis reactions by transferring starch hydrolysis products to methanol. These results are consistent with the previous conclusion that saccharifying α-amylases are better transferases than liquefactant enzymes (54). Moreover, AmyA generates 3.7 and 11 times more alkyl glucosides than those observed with the saccharifying α-amylases from A. niger and A. oryzae, respectively, under selected reaction conditions (55).
Among the family 13 glycoside hydrolases, CGTases, maltogenic amylases, and neopullulanases have an important transglycosylation activity. Even though they share a common fold and general mechanism (33), there are some differences among these enzymes. For example, CGTases and maltogenic amylases contain extra domains besides the canonical A, B, and C domains. Some differences can also be identified in the catalytic domain (24). Identification of these differences led us in the past to introduce transglycosylation activity into the liquefying α-amylase from B. stearothermophilus through the replacement of Ala289 by phenylalanine or tyrosine (54). This replacement conformed to the observation of aromatic residues at that position in natural glycoside transferases (like CGTases), as well as in a conserved region among maltogenic glycoside hydrolases (10) and in saccharifying amylases of fungal origin (54). It has been widely accepted that an aromatic residue at position 286 (B. licheniformis number) is a key condition for transglycosylation (34, 40, 45, 50, 54). An interesting observation is the conservation of a phenylalanine residue at position 277 in AmyA (equivalent to position 286 in B. licheniformis and 289 in B. stearothermophilus α-amylases), in agreement with the importance of an aromatic residue at this position for transglycosylation activity. In fact, we have previously proposed that the effect of transglycosylation of aromatic residue 289 in B. stearothermophilus could not necessarily be due only to a change to a more hydrophobic environment in the active site, but also to a change in the constellation of the active site (54). When the mutant A289F of B. stearothermophilus amylase was modeled, a steric clash between this residue and Trp266 produced a twist in the tryptophan side chain. This residue, which is just two positions after the acid-base Glu264 residue, bridges the incoming nucleophilic water in the structure of barley α-amylase (27), so that a change in its orientation could displace the water molecule, preventing it from being activated by Glu264, acting as a base in this step of the reaction. In fact, in B. licheniformis α-amylase, we found that the V286Y W263L double mutant counteracts the effect produced by the single mutation V286Y (50). From a multiple-sequence alignment, we found that aromatic residues already occupy the positions equivalent to Val286 and Trp263 in AmyA (Fig. 4); however, other transglycosidic enzymes (specifically, CGTases) contain the signature tryptophan-phenylalanine invariably after the acid-base glutamic acid residue (24). The importance of this aromatic dipeptide in CGTases for cyclizing reactions has been demonstrated by Fujiwara and coworkers (18). When these residues were replaced by the aliphatic valine-isoleucine pair, the formation of α-cyclodextrins was completely abolished and liquefying activity decreased drastically. The phenylalanine is one of the two aromatic residues in subsite +2 that sandwich the incoming nonreducing end of the saccharide for the cyclization reaction and seems to also be important for the structural integrity of the active site through its hydrophobic interaction with the lysine residue three positions after the nucleophile aspartic residue at beta 4 (64). AmyA has a valine-phenylalanine dipeptide at these positions. The replacement of the valine residue by tryptophan decreased the specific activity and changed the product profile of the enzyme, leaving some longer products, like G5 and G6, in addition to the G1 and G2 main products. Interestingly, in the context of AmyA, this substitution, as well as the F179V mutation in domain B, abolished the formation of a product with mobility between G4 and G5 observed by TLC upon digestion with the wild type and mutants that was not a substrate for glucoamylase (this is shown in Fig. 2, where the alcoholysis reactions were treated with glucoamylase). Thus, mutations at Phe179 and Val259 seem to have removed at least one transglycosylation activity different from α-1,4.
His222 is part of the second highly conserved region among glycoside hydrolases after the catalytic nucleophile (aspartate at beta 4) (Fig. 4). It is worth noting that a histidine residue is highly conserved among α-amylases and CGTases, but maltogenic amylases, neopullulanases, and cyclodextrinases contain a glutamic acid at the equivalent position (33). The importance of this position in the catalytic process has been demonstrated in various enzymes. Mutagenesis of the equivalent residue His269 to leucine in the Bacillus sp. strain TS-23 α-amylase completely inactivated the enzyme (11). In the case of B. stearothermophilus α-amylase, the replacement of the equivalent His238 by aspartic acid generated an enzyme with a reduced hydrolysis rate and a modified final product profile (67) that could be the consequence of an increase in its transferase activity. In contrast, maltogenic Thermus amylase, an enzyme with an important transferase activity, contains a glutamic acid residue at the position equivalent to His222; when this residue was replaced by an uncharged or positively charged amino acid, like glutamine or histidine, its transferase activity was considerably reduced (10).
In AmyA, the change of His222 to linear residues, like aspartic acid, glutamine, and glutamic acid, increased its alcoholitic activity; however, with the H222Q mutant, the increase in the amount of methyl glucoside obtained represents, to our knowledge, the best alcoholytic α-amylase reported so far. The lower yields observed for the H222D and H222E mutants suggest that the presence of the carboxylic group has a destabilizing effect in the presence of alcohols. A destabilizing effect should not be surprising when a potentially positively charged residue is replaced by a negatively charged residue. Based on the structure of the α-amylase from A. oryzae (PDB entry 7TAA) (7) in the model generated by AmyA, the glucose located in subsite +1 interacts with His222 (His210 in A. oryzae) by means of hydrogen bonds. The common effect of increased alcoholysis and decreased hydrolysis observed upon mutation to the isosteric glutamic acid or glutamine residue could be attributed to the enhanced flexibility of these side chains compared to the imidazol ring, leaving more space to accommodate larger acceptor groups, favoring the entrance of alcohol over water. The presence of a charge can also alter the electrostatic potential of the active site. As previously suggested by Kim et al. (30), it is possible that the carboxylate group of the glutamic acid side chain helps to position the acceptor molecule in the proper orientation for the transfer reaction. However, the presence of the charge in the glutamic acid mutant was destabilizing in the presence of methanol, giving a lower yield in the alcoholysis reaction.
Exploration of new and more interesting activities from an industrial point of view requires robust enzymes capable of withstanding harsh conditions and mutagenesis. In this sense, thermophilic organisms are a good source of potential scaffolds for protein engineering. In this case, we relied on the correlation of thermal stability observed in thermophilic or hyperthermophilic enzymes with their stability in the presence of denaturing agents and organic solvents (12) to tolerate reaction conditions involving a high content of alcohol concentrations. The α-amylase AmyA from T. maritima not only provided this key requirement, it turned out to have an important transferase activity that was further increased by site-directed mutagenesis.
Acknowledgments
This work was supported by grants IN214803-3 from PAPIIT and P49590-Q from CONACyT to G.S.-R.
We thank Leticia Olvera and Mary Olvera for providing a T. maritima library and Ricardo Ciria and the computer group under his supervision for their technical support.
Footnotes
Published ahead of print on 13 June 2008.
REFERENCES
- 1.Aghajari, N., G. Feller, C. Gerday, and R. Haser. 2002. Structural basis of alpha-amylase activation by chloride. Protein Sci. 11:1435-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aghajari, N., M. Roth, and R. Haser. 2002. Crystallographic evidence of a transglycosylation reaction: ternary complexes of a psychrophilic alpha-amylase. Biochemistry 41:4273-4280. [DOI] [PubMed] [Google Scholar]
- 3.Allen, J. D., and J. A. Thoma. 1976. Subsite mapping of enzymes. Application of the depolymerase computer model to two alpha-amylases. Biochem. J. 159:121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altschul, S. F., J. C. Wootton, E. M. Gertz, R. Agarwala, A. Morgulis, A. A. Schaffer, and Y. K. Yu. 2005. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 272:5101-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady, R. L., A. M. Brzozowski, Z. S. Derewenda, E. J. Dodson, and G. G. Dodson. 1991. Solution of the structure of Aspergillus niger acid alpha-amylase by combined molecular replacement and multiple isomorphous replacement methods. Acta Crystallogr. B 47:527-535. [DOI] [PubMed] [Google Scholar]
- 7.Brzozowski, A. M., and G. J. Davies. 1997. Structure of the Aspergillus oryzae alpha-amylase complexed with the inhibitor acarbose at 2.0 Å resolution. Biochemistry 36:10837-10845. [DOI] [PubMed] [Google Scholar]
- 8.Brzozowski, A. M., D. M. Lawson, J. P. Turkenburg, H. Bisgaard-Frantzen, A. Svendsen, T. V. Borchert, Z. Dauter, K. S. Wilson, and G. J. Davies. 2000. Structural analysis of a chimeric bacterial alpha-amylase. High-resolution analysis of native and ligand complexes. Biochemistry 39:9099-9107. [DOI] [PubMed] [Google Scholar]
- 9.Brzozowski, A. M., A. C. Pike, Z. Dauter, R. E. Hubbard, T. Bonn, O. Engstrom, L. Ohman, G. L. Greene, J. A. Gustafsson, and M. Carlquist. 1997. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753-758. [DOI] [PubMed] [Google Scholar]
- 10.Cha, H. J., H. G. Yoon, Y. W. Kim, H. S. Lee, J. W. Kim, K. S. Kweon, B. H. Oh, and K. H. Park. 1998. Molecular and enzymatic characterization of a maltogenic amylase that hydrolyzes and transglycosylates acarbose. Eur. J. Biochem. 253:251-262. [DOI] [PubMed] [Google Scholar]
- 11.Chang, C. T., H. F. Lo, M. C. Chi, C. Y. Yao, W. H. Hsu, and L. L. Lin. 2003. Identification of essential histidine residues in a recombinant alpha-amylase of thermophilic and alkaliphilic Bacillus sp. strain TS-23. Extremophiles 7:505-509. [DOI] [PubMed] [Google Scholar]
- 12.Cowan, D. A. 1997. Thermophilic proteins: stability and function in aqueous and organic solvents. Comp. Biochem. Physiol. A 118:429-438. [DOI] [PubMed] [Google Scholar]
- 13.Dauter, Z., M. Dauter, A. M. Brzozowski, S. Christensen, T. V. Borchert, L. Beier, K. S. Wilson, and G. J. Davies. 1999. X-ray structure of Novamyl, the five-domain “maltogenic” alpha-amylase from Bacillus stearothermophilus: maltose and acarbose complexes at 1.7Å resolution. Biochemistry 38:8385-8392. [DOI] [PubMed] [Google Scholar]
- 14.del Río, G., E. Morett, and X. Soberon. 1997. Did cyclodextrin glycosyltransferases evolve from alpha-amylases? FEBS Lett. 416:221-224. [DOI] [PubMed] [Google Scholar]
- 15.Fogarty, W. M. 1983. Microbial amylases, p. 1-92. In W. M. Fogarty (ed.), Microbial enzymes and biotechnology. Applied Science Publishers Ltd., New York, NY.
- 16.Friedberg, F. 1983. On the primary structure of amylases. FEBS Lett. 152:139-140. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto, Z., K. Takase, N. Doui, M. Momma, T. Matsumoto, and H. Mizuno. 1998. Crystal structure of a catalytic-site mutant alpha-amylase from Bacillus subtilis complexed with maltopentaose. J. Mol. Biol. 277:393-407. [DOI] [PubMed] [Google Scholar]
- 18.Fujiwara, S., H. Kakihara, K. Sakaguchi, and T. Imanaka. 1992. Analysis of mutations in cyclodextrin glucanotransferase from Bacillus stearothermophilus which affect cyclization characteristics and thermostability. J. Bacteriol. 174:7478-7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 20.Hinz, S. W., C. H. Doeswijk-Voragen, R. Schipperus, L. A. van den Broek, J. P. Vincken, and A. G. Voragen. 2006. Increasing the transglycosylation activity of alpha-galactosidase from Bifidobacterium adolescentis DSM 20083 by site-directed mutagenesis. Biotechnol. Bioeng. 93:122-131. [DOI] [PubMed] [Google Scholar]
- 21.Hooft, R. W., G. Vriend, C. Sander, and E. E. Abola. 1996. Errors in protein structures. Nature 381:272. [DOI] [PubMed] [Google Scholar]
- 22.Hwang, K. Y., H. K. Song, C. Chang, J. Lee, S. Y. Lee, K. K. Kim, S. Choe, R. M. Sweet, and S. W. Suh. 1997. Crystal structure of thermostable alpha-amylase from Bacillus licheniformis refined at 1.7 Å resolution. Mol. Cells 7:251-258. [PubMed] [Google Scholar]
- 23.Ishii, N., K. Haga, K. Yamane, and K. Harata. 2000. Crystal structure of alkalophilic asparagine 233-replaced cyclodextrin glucanotransferase complexed with an inhibitor, acarbose, at 2.0 Å resolution. J. Biochem. 127:383-391. [DOI] [PubMed] [Google Scholar]
- 24.Janecek, S., E. A. MacGregor, and B. Svensson. 1995. Characteristic differences in the primary structure allow discrimination of cyclodextrin glucanotransferases from alpha-amylases. Biochem. J. 305:685-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janecek, S., B. Svensson, and B. Henrissat. 1997. Domain evolution in the alpha-amylase family. J. Mol. Evol. 45:322-331. [DOI] [PubMed] [Google Scholar]
- 26.Jespersen, H. M., E. A. MacGregor, B. Henrissat, M. R. Sierks, and B. Svensson. 1993. Starch- and glycogen-debranching and branching enzymes: prediction of structural features of the catalytic (beta/alpha)8-barrel domain and evolutionary relationship to other amylolytic enzymes. J. Protein Chem. 12:791-805. [DOI] [PubMed] [Google Scholar]
- 27.Kadziola, A., J. Abe, B. Svensson, and R. Haser. 1994. Crystal and molecular structure of barley alpha-amylase. J. Mol. Biol. 239:104-121. [DOI] [PubMed] [Google Scholar]
- 28.Kim, I. C., J. H. Cha, J. R. Kim, S. Y. Jang, B. C. Seo, T. K. Cheong, D. S. Lee, Y. D. Choi, and K. H. Park. 1992. Catalytic properties of the cloned amylase from Bacillus licheniformis. J. Biol. Chem. 267:22108-22114. [PubMed] [Google Scholar]
- 29.Kim, J. S., S. S. Cha, H. J. Kim, T. J. Kim, N. C. Ha, S. T. Oh, H. S. Cho, M. J. Cho, M. J. Kim, H. S. Lee, J. W. Kim, K. Y. Choi, K. H. Park, and B. H. Oh. 1999. Crystal structure of a maltogenic amylase provides insights into a catalytic versatility. J. Biol. Chem. 274:26279-26286. [DOI] [PubMed] [Google Scholar]
- 30.Kim, T. J., C. S. Park, H. Y. Cho, S. S. Cha, J. S. Kim, S. B. Lee, T. W. Moon, J. W. Kim, B. H. Oh, and K. H. Park. 2000. Role of the glutamate 332 residue in the transglycosylation activity of Thermus maltogenic amylase. Biochemistry 39:6773-6780. [DOI] [PubMed] [Google Scholar]
- 31.Klein, C., and G. E. Schulz. 1991. Structure of cyclodextrin glycosyltransferase refined at 2.0 Å resolution. J. Mol. Biol. 217:737-750. [DOI] [PubMed] [Google Scholar]
- 32.Kondo, H., H. Nakatani, R. Matsuno, and K. Hiromi. 1980. Product distribution in amylase-catalyzed hydrolysis of amylose. Comparison of experimental results with theoretical predictions. J. Biochem. 87:1053-1070. [PubMed] [Google Scholar]
- 33.Kuriki, T., and T. Imanaka. 1999. The concept of the alpha-amylase family: structural similarity and common catalytic mechanism. J. Biosci. Bioeng. 87:557-565. [DOI] [PubMed] [Google Scholar]
- 34.Kuriki, T., H. Kaneko, M. Yanase, H. Takata, J. Shimada, S. Handa, T. Takada, H. Umeyama, and S. Okada. 1996. Controlling substrate preference and transglycosylation activity of neopullulanase by manipulating steric constraint and hydrophobicity in active center. J. Biol. Chem. 271:17321-17329. [DOI] [PubMed] [Google Scholar]
- 35.Lawson, D. M., A. M. Brzozowski, S. Rety, C. Verma, and G. G. Dodson. 1994. Probing the nature of substrate binding in Humicola lanuginosa lipase through X-ray crystallography and intuitive modelling. Protein Eng. 7:543-550. [DOI] [PubMed] [Google Scholar]
- 36.Leemhuis, H., H. J. Rozeboom, M. Wilbrink, G. J. Euverink, B. W. Dijkstra, and L. Dijkhuizen. 2003. Conversion of cyclodextrin glycosyltransferase into a starch hydrolase by directed evolution: the role of alanine 230 in acceptor subsite +1. Biochemistry 42:7518-7526. [DOI] [PubMed] [Google Scholar]
- 37.Liebl, W., I. Stemplinger, and P. Ruile. 1997. Properties and gene structure of the Thermotoga maritima alpha-amylase AmyA, a putative lipoprotein of a hyperthermophilic bacterium. J. Bacteriol. 179:941-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacGregor, E. A., S. Janecek, and B. Svensson. 2001. Relationship of sequence and structure to specificity in the alpha-amylase family of enzymes. Biochim. Biophys. Acta 1546:1-20. [DOI] [PubMed] [Google Scholar]
- 39.Matsubara, S. 1961. Studies on Taka-amylase A. VII. Transmaltosidation by Taka-amylase A. J. Biochem. 49:226-231. [PubMed] [Google Scholar]
- 40.Matsui, I., S. Yoneda, K. Ishikawa, S. Miyairi, S. Fukui, H. Umeyama, and K. Honda. 1994. Roles of the aromatic residues conserved in the active center of Saccharomycopsis alpha-amylase for transglycosylation and hydrolysis activity. Biochemistry 33:451-458. [DOI] [PubMed] [Google Scholar]
- 41.Matsuura, Y., M. Kusunoki, W. Harada, and M. Kakudo. 1984. Structure and possible catalytic residues of Taka-amylase A. J. Biochem. 95:697-702. [DOI] [PubMed] [Google Scholar]
- 42.Moreno, A., G. Saab-Rincon, R. I. Santamaia, X. Soberon, and A. Lopez-Munguia. 2004. A more efficient starch degradation by the combination of hydrolase and transferase activities of alpha-amylase and cyclomaltodextrin glucanotransferase. Starch 56:63-68. [Google Scholar]
- 43.Mosi, R., S. He, J. Uitdehaag, B. W. Dijkstra, and S. G. Withers. 1997. Trapping and characterization of the reaction intermediate in cyclodextrin glycosyltransferase by use of activated substrates and a mutant enzyme. Biochemistry 36:9927-9934. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima, R., T. Imanaka, and S. Aiba. 1986. Comparison of amino acid sequences of eleven different α-amylases. Appl. Microbiol. Biotechnol, 23:355-360. [Google Scholar]
- 45.Nakamura, A., K. Haga, and K. Yamane. 1994. The transglycosylation reaction of cyclodextrin glucanotransferase is operated by a ping-pong mechanism. FEBS Lett. 337:66-70. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen, J. E., and T. V. Borchert. 2000. Protein engineering of bacterial alpha-amylases. Biochim. Biophys. Acta 1543:253-274. [DOI] [PubMed] [Google Scholar]
- 47.Pujadas, G., and J. Palau. 2001. Evolution of α-amylases: architectural features and key residues in the stabilization of the (β/α)8 scaffold. Mol. Biol. Evol. 18:38-54. [DOI] [PubMed] [Google Scholar]
- 48.Qian, M., V. Nahoum, J. Bonicel, H. Bischoff, B. Henrissat, and F. Payan. 2001. Enzyme-catalyzed condensation reaction in a mammalian alpha-amylase. High-resolution structural analysis of an enzyme-inhibitor complex. Biochemistry 40:7700-7709. [DOI] [PubMed] [Google Scholar]
- 49.Ramasubbu, N., C. Ragunath, and P. J. Mishra. 2003. Probing the role of a mobile loop in substrate binding and enzyme activity of human salivary amylase. J. Mol. Biol. 325:1061-1076. [DOI] [PubMed] [Google Scholar]
- 50.Rivera, M. H., A. Lopez-Munguia, X. Soberon, and G. Saab-Rincon. 2003. Alpha-amylase from Bacillus licheniformis mutants near to the catalytic site: effects on hydrolytic and transglycosylation activity. Protein Eng. 16:505-514. [DOI] [PubMed] [Google Scholar]
- 51.Robyt, J. F., and D. French. 1967. Multiple attach hypothesis of alpha-amylase action: action of porcine pancreatic, human salivary, and Aspergillus oryzae alpha-amylases. Arch. Biochem. Biophys. 122:8-16. [DOI] [PubMed] [Google Scholar]
- 52.Rogers, J. C. 1985. Conserved amino acid sequence domains in alpha-amylases from plants, mammals, and bacteria. Biochem. Biophys. Res. Commun. 128:470-476. [DOI] [PubMed] [Google Scholar]
- 53.Rydberg, E. H., G. Sidhu, H. C. Vo, J. Hewitt, H. C. Cote, Y. Wang, S. Numao, R. T. MacGillivray, C. M. Overall, G. D. Brayer, and S. G. Withers. 1999. Cloning, mutagenesis, and structural analysis of human pancreatic alpha-amylase expressed in Pichia pastoris. Protein Sci. 8:635-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saab-Rincon, G., G. del-Rio, R. I. Santamaria, A. Lopez-Munguia, and X. Soberon. 1999. Introducing transglycosylation activity in a liquefying alpha-amylase. FEBS Lett. 453:100-106. [DOI] [PubMed] [Google Scholar]
- 55.Santamaria, R. I., G. Del Rio, G. Saab, M. E. Rodriguez, X. Soberon, and A. Lopez. 1999. Alcoholysis reactions from starch with alpha-amylases. FEBS Lett. 452:346-350. [DOI] [PubMed] [Google Scholar]
- 56.Sarkar, G., and S. S. Sommer. 1990. The “megaprimer” method of site-directed mutagenesis. BioTechniques 8:404-407. [PubMed] [Google Scholar]
- 57.Sarney, D. B., and E. N. Vulfson. 1995. Application of enzymes to the synthesis of surfactants. Trends Biotechnol. 13:164-172. [DOI] [PubMed] [Google Scholar]
- 58.Strobl, S., K. Maskos, M. Betz, G. Wiegand, R. Huber, F. X. Gomis-Ruth, and R. Glockshuber. 1998. Crystal structure of yellow meal worm alpha-amylase at 1.64 Å resolution. J. Mol. Biol. 278:617-628. [DOI] [PubMed] [Google Scholar]
- 59.Summer, J. B., and S. F. Howell. 1935. A method for determination of saccharase activity. J. Biol. Chem. 108:51-54. [Google Scholar]
- 60.Suvd, D., Z. Fujimoto, K. Takase, M. Matsumura, and H. Mizuno. 2001. Crystal structure of Bacillus stearothermophilus alpha-amylase: possible factors determining the thermostability. J. Biochem. 129:461-468. [DOI] [PubMed] [Google Scholar]
- 61.Svensson, B. 1988. Regional distant sequence homology between amylases, α-glucosidases and transglucanosylases. FEBS Lett. 230:72-76. [DOI] [PubMed] [Google Scholar]
- 62.Uitdehaag, J. C. M., R. Mosi, K. H. Kalk, B. A. van der Veen, L. Dijkhuizen, S. G. Withers, and B. W. Dijkstra. 1999. X-ray structures along the reaction pathway of cyclodextrin glycosyltransferase elucidate catalysis in the α-amylase family. Nat. Struct. Mol. Biol. 6:432-436. [DOI] [PubMed] [Google Scholar]
- 63.van der Maarel, M. J., B. van der Veen, J. C. Uitdehaag, H. Leemhuis, and L. Dijkhuizen. 2002. Properties and applications of starch-converting enzymes of the alpha-amylase family. J. Biotechnol. 94:137-155. [DOI] [PubMed] [Google Scholar]
- 64.van der Veen, B. A., H. Leemhuis, S. Kralj, J. C. M. Uitdehaag, B. W. Dijkstra, and L. Dijkhuizen. 2001. Hydrophobic amino acid residues in the acceptor binding site are main determinants for reaction mechanism and specificity of cyclodextrin-glycosyltransferase. J. Biol. Chem. 276:44557-44562. [DOI] [PubMed] [Google Scholar]
- 65.van Rantwijk, F., M. Woudenberg-van Oosterom, and R. A. Sheldon. 1999. Glycosidase-catalysed synthesis of alkyl glycosides. J. Mol. Catal. B 6:511-532. [Google Scholar]
- 66.Vieille, C., and G. J. Zeikus. 2001. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol. Mol. Biol. Rev. 65:1-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vihinen, M., P. Ollikka, J. Niskanen, P. Meyer, I. Suominen, M. Karp, L. Holm, J. Knowles, and P. Mantsala. 1990. Site-directed mutagenesis of a thermostable alpha-amylase from Bacillus stearothermophilus: putative role of three conserved residues. J. Biochem. 107:267-272. [DOI] [PubMed] [Google Scholar]
- 68.Wind, R. D., R. M. Buitelaar, and L. Dijkhuizen. 1998. Engineering of factors determining alpha-amylase and cyclodextrin glycosyltransferase specificity in the cyclodextrin glycosyltransferase from Thermoanaerobacterium thermosulfurigenes EM1. Eur. J. Biochem. 253:598-605. [DOI] [PubMed] [Google Scholar]