Abstract
Water is arguably the most important constituent of microbial microhabitats due to its control of physical and physiological processes critical to microbial activity. In natural environments, bacteria often live on unsaturated surfaces, in thin (micrometric) liquid films. Nevertheless, no experimental systems are available that allow real-time observation of bacterial processes in liquid films of controlled thickness. We propose a novel, inexpensive, easily operated experimental platform, termed the porous surface model (PSM) that enables quantitative real-time microscopic observations of bacterial growth and activity under controlled unsaturated conditions. Bacteria are inoculated on a porous ceramic plate, wetted by a liquid medium. The thickness of the liquid film at the surface of the plate is set by imposing suction, corresponding to soil matric potential, to the liquid medium. The utility of the PSM was demonstrated using Pseudomonas putida KT2440 tagged with gfp as a model bacterium. Single cells were inoculated at the surface of the PSM, and the rate at which colonies expanded laterally was measured for three matric potentials (−0.5, −1.2, and −3.6 kPa). The matric potential exerted significant influence on colony expansion rates, with a faster rate of spreading at −0.5 than at −1.2 or −3.6 kPa (diameter increase rate, ca. 1,000, 200, and 17 μm h−1, respectively). These differences can be attributed to cell motility, strongly limited under the most negative matric potential. The PSM constitutes a tool uniquely adapted to study the influence of liquid film geometry on microbial processes. It should therefore contribute to uncovering mechanisms of microbial adaptation to unsaturated environments.
Bacteria are often found in environments where they are not fully immersed in aqueous solution but are surrounded by thin (micrometric) water films. This would, for example, be the case in non-water-saturated terrestrial habitats (surface soil and vadose zone), which harbor most of the Earth's prokaryotes (29), but also in other environmentally and economically important habitats, such as the surface of leaves (2, 20) and food products (14).
In porous media, the energy state of water is conveniently expressed as water potential (unit, Pa) (24). In unsaturated soils, the most important components of water potential are the matric potential and, in saline soils, the osmotic potential. The matric potential results from the interactions between water molecules and solids, the water being held on solid surfaces by capillary and adsorptive forces (13). If the matric component of the water potential of a system is 0, the system is saturated; unsaturated conditions are associated with negative matric potentials.
The water potential measures the thermodynamic availability of water for bacteria. Low water potentials cause cell desiccation, which, in severe cases, can result in the cessation of metabolic activity and in cell death (25). Bacteria have evolved mechanisms to maintain their homeostasis when challenged by variation in water potential (8, 28), which have often been studied using bacteria grown in liquid culture (4, 10). Indeed, osmotic stress is readily created by adding salts to liquid medium (24). Lower water potentials can also be created by adding a nonpermeating solute, such as polyethylene glycol (PEG), to a solution. While the resulting decrease in water potential is of osmotic rather than of matric nature (i.e., not caused by solid-water interactions), it is considered that bacteria react in the way they would under a thermodynamically equivalent matric stress because nonpermeating molecules are too large to enter bacterial cells (11).
The consequences of low matric potential in porous media are not limited to low water availability. Unsaturated conditions are associated with the existence of thin liquid films at the surface of pore walls, which impose specific physical constraints on microbes, as recently reviewed in reference 21. Thin and discontinuous aqueous films limit bacterial motility because swimming requires pathways that are sufficiently hydrated (9). Liquid film geometry also affects diffusive processes to and from cells, with potential effects on competitive interactions and, thus, consequences at the community scale (6).
Even if the addition of PEG in stirred liquid media has proven to be a successful approach to study specifically the “water deprivation role” of low matric potentials (16), it does not recreate the thin liquid films characteristic of unsaturated habitats nor the sessile mode of life commonly adopted by bacteria in these habitats. Therefore, other methods, reviewed by Holden (15), have been proposed to grow biofilms under controlled matric potential. In these methods, the matric potential is controlled either by adding the appropriate quantity of water to a porous medium, by controlling the relative humidity of the headspace, or by supplementing a supporting medium with a nonpermeating substrate. The merits of these methods relative to various research objectives are discussed in reference 15. In spite of the development of these methods, our knowledge of microbial ecology under unsaturated conditions has lagged behind our knowledge of its “saturation” counterpart. In the last decade, great insights in the structure, dynamics, physiology, and genetics of saturated biofilms have been gained by online microscopic observation of organisms expressing fluorescent proteins growing in flow cells (23). To date, no experimental system exists that offers the same opportunities to explore unsaturated biofilms. A number of detailed microscopic observations of unsaturated biofilms have been obtained using, for example, atomic force microscopy (1) or confocal microscopy (5), but these were offline and destructive observations.
We propose a novel experimental platform for direct microscopic observation of microbial growth and activity under controlled unsaturated conditions, with a specific focus on the role of thin aqueous films. Bacteria are grown on the surface of a porous ceramic plate connected to a solution reservoir containing a growth medium. The thickness of the surface liquid film is defined by the topography (or roughness) of the plate and the matric potential, set by imposing suction on the solution wetting the ceramic plate. This way to control matric potential in porous media is well known in soil physics (13), and its application to soil microbiology has been proposed (18); however, it is without the direct observability possible with our experimental platform. This novel experimental system, termed the porous surface model (PSM), was used to directly observe and quantify the surface colonization of a gfp-tagged model bacterium, Pseudomonas putida KT2440, at three different matric potentials.
MATERIALS AND METHODS
Bacterial strain.
The soil bacterium Pseudomonas putida KT2440 was chosen as a model strain for inoculation on the PSM. Two derivatives of this strain, constitutively expressing either the green fluorescent protein (GFP) or its red variant (DsRed), were created by inserting the appropriate mini Tn7 (17). To illustrate the role of motility, a spontaneous nonmotile mutant of the same parental strain was obtained and tagged with gfp, using the same tagging procedure. The strains were routinely grown on ABT minimal medium supplemented with 5 mM benzoate (ABTB) as the sole carbon source (6).
Experimental system.
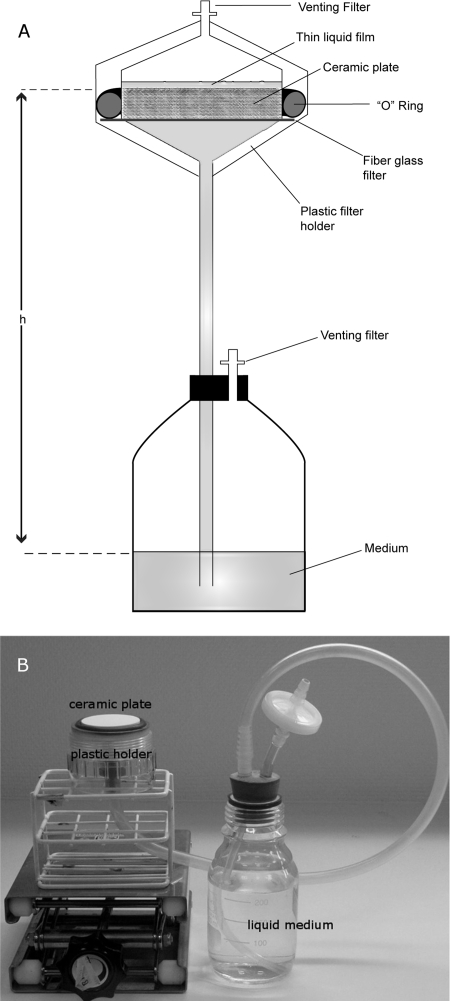
The PSM is made up of a ceramic plate placed in a plastic holder, the bottom of which is connected to a medium reservoir by a plastic tube (Fig. 1). The matric potential at the surface of the plate is controlled by the difference in height between the ceramic plate and the surface of the solution in the reservoir. The ceramic plate, which is 7.1 mm thick and 41.3 mm in diameter, was chosen because its pores are small enough to exclude bacterial growth inside the plate (specified maximum pore radius, <1.5 μm; 1-bar bubbling pressure plate; Soilmoisture, Santa Barbara, CA). This type of plate has been recently used to expose bacteria to chemical gradients in saturated conditions (30). Prior to use, the ceramic plates were grounded with a 25-μm diamond abrasive to minimize surface irregularities. The ceramic plate is housed in a polycarbonate filter holder for in-line filtration (50-mm diameter; Sartorius, Goettingen, Germany). The holder's inside walls were machined to accommodate the ceramic, placed on a 50-mm diameter glass fiber prefilter (GF92; Whatman), and circled with the silicone O-ring (40 by 5 mm) provided with the holder. Once the holder's lid is closed, the system is air- and watertight (i.e., gaseous and liquid exchanges only happen through the pores of the ceramic plate). A venting filter is connected to the opening of the holder's lid in order to prevent airborne contamination of the ceramic surface. A 50-cm-long silicone tube (inner diameter, 7 mm) connects the holder's bottom part to a 250-ml bottle filled with 200 ml of liquid ABTB medium. The bottle is closed with a two-hole cap. The tube connecting the solution in the bottle to the ceramic-plate holder goes through one of the holes; the other, equipped with a venting filter, serves to equilibrate the gas phase inside the bottle with the atmosphere.
FIG. 1.
(A) Sketch of the PSM. The liquid film thickness at the surface of the ceramic plate is determined by h, the height of the fluid column connecting the medium reservoir to the ceramic plate. (B) Photo of a PSM with its lid removed to expose the ceramic surface.
PSM sterilization and operation.
After the PSM was assembled, the ceramic plate was saturated from its bottom by elevating the medium container above the surface of the ceramic plate in its holder. The system was then autoclaved for 25 min at 121°C. After the system was autoclaved, the plate was brought to the desired matric potential by adjusting the height of the medium bottle relative to that of the ceramic plate. It has been verified using a pressure transducer (PX170; Omega, Stamford, CT) connected to the base of the plate holder that this allows a reliable, stable, and temperature-independent way to set the matric potential at the surface of the plate. The holder's lid prevents evaporation, which would decrease the matric potential. When suction is imposed on the ceramic plate, the plastic-holder lid can be open without compromising the airtightness of the system, permitting the inoculation of the plate.
PSM inoculation.
Colonies from a 24-h-old ABTB plate were scraped and resuspended in sterile saline solution (9 g l−1 NaCl). The optical density at 600 nm of the suspension was adjusted to obtain a cell density of ca. 1 cell μl−1. Four 1-μl drops of this suspension were directly pipetted onto the surface of at least three replicate ceramic plates brought to a matric potential of −1.7 kPa by placing the reservoir 17 cm below the surface of the ceramic plate. The drops, ca. 3 to 5 mm in diameter, were rapidly absorbed by the plate. After 30 min, the plates were brought to a matric potential of −0.5, −1.2, or −3.6 kPa (−5, −12, or −35 cm of water, respectively) for long-term incubation at room temperature (ca. 22°C). The inoculum density was verified by drop-plating 20-μl aliquots of the cell suspension onto LB plates (12).
Washing of the ceramic plates.
The ceramic plates can be reused after a cycle of bacterial growth. Nevertheless, removing the residual fluorescent signal due to bacterial remnants was challenging. To effectively restore their initial low background fluorescence, the ceramic plates were immersed in boiling HCl (1 M) for 1 hour. To normalize the pH of the plates, 60 ml of phosphate buffer, equivalent to the one present in the ABT medium, was forced through the plates (pore volume, ca. 3.5 ml) by using a vacuum pump. The plates were then placed in a boiling solution of 3% agar (Fluka, Switzerland) for 0.5 h before being rinsed in boiling deionized water. The treatment in agar was shown to improve the culturability of inoculated cells, but its role was not elucidated. After the last rinsing in boiling water, no visible trace of agar remained on the ceramic plates. We hypothesize that the agar may help remove toxic aluminum that could have been mobilized by the acid wash. This cleaning procedure was also applied prior to the first use of the ceramic plates.
Microscopy.
Colonies developing at the surface of the ceramic plates were observed during the course of the incubations by using a Leica MZ16 FA epifluorescent stereomicroscope, maintaining the height of the medium reservoir relative to that of the ceramic plate (i.e., the imposed matric potential). The plastic holder housing the ceramic plate was opened in proximity to a Bunsen burner flame, and its screw lid was replaced by a lid which had been machined to remove its central part to create an “observation window” made with the lid of a plastic petri dish. The objectives and magnifications used depended on the colony size (from a 10× objective with magnification set at 40 to a 1× objective with magnification at 7.1, which correspond to fields of view of 0.7 mm and 29.6 mm in diameter, respectively). Filter sets appropriate for GFP (excitation wavelength, 480 nm; emission, 525 nm) or DsRed (excitation, 565 nm; emission, 620 nm) detection were used, and the images were acquired with a charge-coupled-device camera and analyzed with the Image Pro Plus software (version 5; Media Cybernetics, Silver Spring, MD).
The colonization pattern over the complete plate surface can also be imaged at the end of the incubation period. This was done by removing the plate from its holder and by sequentially capturing several fields of view by using a motorized stage piloted by Image Pro Plus before reconstructing a tiled image with the same software.
Effective liquid film thickness.
Since the amount of liquid retained on an unsaturated surface depends on both the matric potential and the surface geometry (e.g., see reference 21), a description of the microtopography of the ceramic plates was obtained by combining two approaches. To describe the smaller details of the topography with a submicrometric resolution, 12 profiles were acquired at four different locations (10 by 10 μm) on a plate, using an atomic force microscope (Dimension 3100 SPM with a metrology AFM head; Veeco, Woodbury, NY) equipped with capacitive distance sensors working in the dynamic resonant mode. These analyses were carried out at Danish Fundamental Metrology (Kongens Lyngby, Denmark). Larger-scale topography was quantified over 10 400- by 400-μm zones by using scanning laser confocal microscopy (Leica TCS SP5). A large number (approximately 2 × 109) of GFP-producing P. putida KT2440 cells were filtered onto the surface of a ceramic plate so that they formed a fluorescing layer revealing the plate topography. Parameters describing an “average roughness element” (angle, depth, and frequency) were derived from the profiles. Following the approach by Or and Tuller (22), these parameters allowed a calculation of the thickness of the effective liquid film present at the surface of the PSM as function of the matric potential.
Using PEG-amended medium in the reservoir of the PSM gives the opportunity to decouple the effects of the total matric potential experienced by the cell (imposed by the cumulated contributions of PEG concentration and of the suction created by the PSM) from the effects of the effective liquid film thickness (controlled by the suction created by the PSM). Two situations with the same total water potential of −3.6 kPa (i.e., same thermodynamic availability of water) but different liquid film thicknesses were created. We compared a situation where this potential was obtained through suction by the hanging water column to a situation where −0.5 kPa was obtained by suction and the remaining −3.1 kPa was obtained by adding 37.8 g PEG 8000 per liter of ABTB medium in the reservoir (19).
RESULTS AND DISCUSSION
Early stages of surface colonization.
The low autofluorescence of the ceramic surface allowed for detection of colonized zones as small as 100 μm in diameter, using epifluorescence stereomicroscopy. Early stages of microcolony development (between 0 and 17 h after inoculation) were not documented due to the difficulty of detecting small cell clusters on the relatively large surface of the plate (4.13-cm diameter).
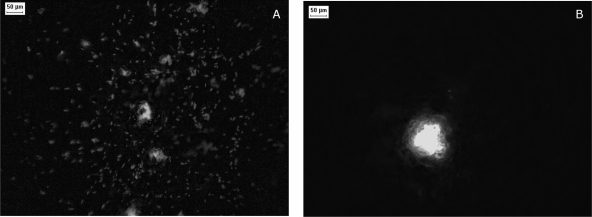
The number of colonies that arose from the 1-μl inoculation drops was very close to the number expected based on the counts on regular ABTB agar plates, suggesting that the inoculation procedure did not cause a stress that jeopardized cell culturability. After 17 h of incubation at −0.5 kPa or −1.2 kPa, the colonization zones that developed from an inoculated cell had diameters in the 300- to 800-μm range. These colonized zones did not consist of tight colonies but were made up of a diffuse accumulation of isolated cells and cell clusters typically smaller than 40 μm in diameter (Fig. 2A). A fraction of the isolated cells were mobile, as visible on a time-lapse video (see the supplemental material). The situation was different when the ceramic plates were incubated at the lowest matric potential (−3.6 kPa); each inoculated cell developed into a tight microcolony (Fig. 2B), with a mean diameter at 17 h estimated to be 176 μm (standard error, 26 μm). Neither isolated cells nor cell mobility was detected.
FIG. 2.
Epifluorescence micrographs of the early steps of surface colonization by P. putida KT2440. The cells visible on the images derive from the inoculation of one cell at the surface of the PSM incubated for 17 h at a matric potential of −0.5 kPa (A) or −3.6 kPa (B).
Rates of surface colonization.
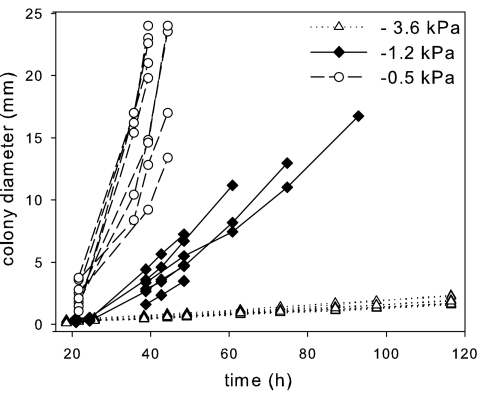
The evolution of colony diameter with time was recorded for replicate colonies subjected to one of the three matric potentials. Colony development was followed until the cells reached the edge of the plate or until two neighboring colonies overlapped. Over the course of the incubation period, the microcolonies grown at −0.5 and −1.2 kPa displayed fuzzy edges, comprised of isolated cells, some being mobile, and small cell clusters. This sparsely colonized fringe around the colonies later developed into a continuous biofilm. In contrast, the colonies grown at −3.6 kPa had sharp limits throughout their development, and cell mobility was rarely detected. While the diameters of these tight colonies were easily determined, measuring the size of diffuse colonies was more challenging. We chose to measure it using moderate or low magnifications (fields of view, >6 mm2), which excluded the detection of single cells or smaller clusters separated from the main colony. Colony diameter was found to increase linearly with time but at rates that varied greatly with the imposed matric potential (Fig. 3). The colony growth rate, as measured by the slope of the curves (Fig. 3), decreased with decreasing matric potentials (1,029.8 ± 373.5, 195.4 ± 61.7, and 16.6 ± 0.8 μm h−1 for −0.5, −1.2, and −3.6 kPa, respectively).
FIG. 3.
Kinetics of surface colonization by P. putida KT2440 at three matric potentials at the surface of the PSM. The kinetics obtained for several replicate colonies are shown.
Liquid film thickness controls surface colonization rates.
The three matric potentials tested in this work, although spanning a relatively narrow range, resulted in very different rates of surface colonization. To evaluate the specific role of liquid film thickness in these differences, P. putida KT2440 was grown under a total water potential of −3.6 kPa, of which −0.5 kPa was obtained by the suction imposed by the PSM and the remaining −3.1 kPa by PEG amendment to the liquid medium. The high colony expansion rate observed (data not shown) was similar to the one measured under a suction of −0.5 kPa in the absence of PEG and was much higher than the one observed under a suction of −3.6 kPa. Therefore, the liquid film thickness (controlled by the difference in height between the ceramic surface and solution reservoir), rather than the water potential per se, was the primary factor controlling the surface colonization rate. This was expected because even the lowest water potential used here (−3.6 kPa) corresponds to a very mild potential for soil bacteria, commonly facing matric potentials exceeding −1 MPa (11). Specifically, Holden et al. showed, by simulating matric potentials in saturated conditions by PEG amendment, that the growth rate of P. putida is not adversely affected by water stress at water potentials above −0.75 MPa (16).
Effective liquid film thickness and bacterial motility.
P. putida is a flagellated bacterium, and we observed ubiquitous cell motion when the matric potential was at its highest (−0.5 kPa) but almost none when it was at −3.6 kPa. This suggests that at high matric potentials, the liquid film is sufficiently thick for bacteria to rapidly disperse, while more negative matric potentials reduce the thickness of the liquid film, limiting bacterial motion. Surface colonization would, in the latter case, rely on slower processes such as cell shoving. To illustrate this, a coinoculation experiment was performed on a PSM set at −0.9 kPa; it consisted of a mixture of the wild-type strain KT2440 tagged with DsRed and a gfp-tagged spontaneous nonswimming mutant of the same strain. The result clearly suggests a role of bacterial locomotion in the rapid surface colonization by KT2440; the nonmotile mutant formed small, well-defined microcolonies, while the wild type formed larger and more diffuse colonies (Fig. 4). These findings are in line with indirect observations made in soils, where reduction of bacterial dispersal rate with decreasing matric potential is attributed to the reduction of hydrated pathways (7, 26).
FIG. 4.
Differences in surface colonization of the wild type, P. putida KT2440 (red), and a nonmotile spontaneous mutant (green). The ceramic plate (diameter, 4.17 cm) was coinoculated with approximately 50 mutant cells and 25 wild-type cells and incubated for 48 h at a matric potential of −0.9 kPa.
Our result indicates that at −3.6 kPa, KT2440 was largely limited in its swimming behavior while the other tested suctions allowed for a faster dispersal. If the ceramic surface were perfectly flat, the expected theoretical water film thicknesses on the PSM would be 18.4, 13.7, and 9.6 nm at −0.5, −1.2, and −3.6 kPa, respectively (27). In reality, imperfections were present at the surface of the plates. Atomic force microscopy revealed the existence of microroughness. Owing to their geometry, these roughness elements (typical depth, 2.5 μm; typical opening angle, 50°) remained water-saturated for the entire matric potential range we used, significant draining being expected only at matric potentials lower than −51 kPa (see reference 22 for the equations). Therefore, this type of microroughness does not explain the differences in bacterial dispersal rate observed in the −0.5- to −3.6-kPa range. Coarser and larger roughness elements, with depths of 22 μm on average and opening angles of 92° on average, were observed using the approach based on confocal microscopy. Since these elements would begin to drain around −1.0 kPa, they are consistent with the differences in bacterial motion we observed at the different matric potentials. From these observations, a plate-wide effective liquid film thickness of approximately 1.4 μm is calculated at matric potentials larger than −1.0 kPa, decreasing markedly after this point, reaching a value of approximately 0.4 μm at −3.6 kPa.
Conclusions.
This report presents a novel, directly microscopically observable, experimental platform to grow microorganisms and to study and quantify microbial processes under controlled unsaturated conditions, from the cell scale (μm) to the centimeter scale. The PSM allows an easy, precise, and constant (including the phases of microscopic observations) control of the matric potential. It enables exploring the effects of slightly negative matric potentials, which have rarely been studied in laboratories, and simulating matric potential cycles to mimic soil-wetting and -drying events. The PSM in its current form is nevertheless not well adapted to matric potentials lower than −10 kPa because this would require that the water column connecting the ceramic surface to the medium reservoir exceed 1 m. Such long water columns can be unpractical to handle, especially considering bubble formation from medium degassing, occurring relatively rapidly under those suctions. At a matric potential in the 0- to −10-kPa range, the PSM is very suitable for experiments lasting several days or weeks, thanks to its large medium reservoir.
The PSM was validated by quantifying the impact of matric potential on surface colonization of a model soil bacterium. Even moderately negative matric potentials significantly decreased the surface colonization rate of P. putida KT2440, probably through a limitation of bacterial motility caused by the shallowness of the liquid films at the surface of the PSM. The use of PEG to decrease the water potential did not restrict the surface colonization rate. This clearly demonstrates that the PSM allows the detection of constraints to bacterial growth and activity, which would have been overlooked with other experimental approaches. More thorough tests of the role of flagellar motility in unsaturated conditions using isogenic mutants are currently under way. At a time when application of landscape ecology framework to microbial systems is being advocated (3), it is of critical importance to improve our understanding of microbe-water interactions on rough mineral surfaces, water arguably being the most essential constituent of microbial landscapes.
Supplementary Material
Acknowledgments
This work was funded by the National Science Foundation (Hydrologic Sciences) grant EAR-0409364 and by the Villum Kann Rasmussen Foundation Center of Excellence, Center for Environmental and Agricultural Microbiology (CREAM). D.O. gratefully acknowledges funding by the Swiss National Science Foundation project 200021-113442.
We are grateful to Hector Diaz for technical assistance and to Carole Dauby and Karim El Azouzi for helping with the development of the PSM.
Footnotes
Published ahead of print on 27 June 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Auerbach, I. D., C. Sorensen, H. G. Hansma, and P. A. Holden. 2000. Physical morphology and surface properties of unsaturated Pseudomonas putida biofilms. J. Bacteriol. 182:3809-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axtell, C. A., and G. A. Beattie. 2002. Construction and characterization of a proU-gfp transcriptional fusion that measures water availability in a microbial habitat. Appl. Environ. Microbiol. 68:4604-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battin, T. J., W. T. Sloan, S. Kjelleberg, H. Daims, I. M. Head, T. P. Curtis, and L. Eberl. 2007. Microbial landscapes: new paths to biofilm research. Nat. Rev. Microbiol. 5:76. [DOI] [PubMed] [Google Scholar]
- 4.Busse, M. D., and P. J. Bottomley. 1989. Growth and nodulation responses of Rhizobium meliloti to water stress induced by permeating and nonpermeating solutes. Appl. Environ. Microbiol. 55:2431-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, W. S., and L. J. Halverson. 2003. Reduced water availability influences the dynamics, development, and ultrastructural properties of Pseudomonas putida biofilms. J. Bacteriol. 185:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dechesne, A., D. Or, and B. F. Smets. 2008. Limited substrate diffusive fluxes facilitate coexistence of two competing bacterial strains. FEMS Microbiol. Ecol. 64:1-8. [DOI] [PubMed] [Google Scholar]
- 7.Gammack, S. M., E. Paterson, J. S. Kemp, M. S. Cresser, and K. Killham. 1992. Factors affecting the movement of microorganisms in soils, p. 263-305. In G. Stotzky and J. Bollag (ed.), Soil biochemistry, vol. 7. Marcel Dekker, New York, NY. [Google Scholar]
- 8.Griffin, D. M. 1981. Water and microbial stress. Adv. Microb. Ecol. 5:91-136. [Google Scholar]
- 9.Griffin, D. M., and G. Quail. 1968. Movement of bacteria in moist, particulate systems. Aust. J. Biol. Sci. 21:579-582. [DOI] [PubMed] [Google Scholar]
- 10.Halverson, L. J., and M. K. Firestone. 2000. Differential effects of permeating and nonpermeating solutes on the fatty acid composition of Pseudomonas putida. Appl. Environ. Microbiol. 66:2414-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris, R. F. 1981. Effect of water potential on microbial growth and activity, p. 23-95. In J. F. Parr, W. R. Gardner, and L. F. Elliot (ed.), Water potential relations in soil microbiology. Soil Science Society of America, Madison, WI.
- 12.Herigstad, B., M. Hamilton, and J. Heersink. 2001. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 44:121-129. [DOI] [PubMed] [Google Scholar]
- 13.Hillel, D. 1998. Environmental soil physics. Academic Press, San Diego, CA.
- 14.Hills, B. P., C. E. Manning, Y. Ridge, and T. Brocklehurst. 1997. Water availability and the survival of Salmonella typhimurium in porous systems. Int. J. Food Microbiol. 36:187-198. [DOI] [PubMed] [Google Scholar]
- 15.Holden, P. A. 2001. Biofilms in unsaturated environments. Methods Enzymol. 337:125-143. [DOI] [PubMed] [Google Scholar]
- 16.Holden, P. A., L. J. Halverson, and M. K. Firestone. 1997. Water stress effects on toluene biodegradation by Pseudomonas putida. Biodegradation 8:143-151. [DOI] [PubMed] [Google Scholar]
- 17.Lambertsen, L., C. Sternberg, and S. Molin. 2004. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ. Microbiol. 6:726-732. [DOI] [PubMed] [Google Scholar]
- 18.McGovern, H. J., L. K. Deeks, P. D. Hallett, K. Ritz, and I. M. Young. 2001. A sterile environment for growing, and monitoring, micro-organisms under a range of soil matric potentials. Soil Biol. Biochem. 33:689-691. [Google Scholar]
- 19.Michel, B. E. 1983. Evaluation of the water potentials of solutions of polyethylene glycol 8000 both in the absence and presence of other solutes. Plant Physiol. 72:66-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monier, J. M., and S. E. Lindow. 2003. Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc. Natl. Acad. Sci. USA 100:15977-15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Or, D., B. F. Smets, J. M. Wraith, A. Dechesne, and S. P. Friedman. 2007. Physical constraints affecting microbial habitats and activity in unsaturated porous media—a review. Adv. Water Resour. 30:1505-1527. [Google Scholar]
- 22.Or, D., and M. Tuller. 2000. Flow in unsaturated fractured porous media: hydraulic conductivity of rough surfaces. Water Resour. Res. 36:1165-1177. [Google Scholar]
- 23.Palmer, R. J., Jr., J. A. J. Haagensen, T. Neu, and C. Sternberg. 2006. Confocal microscopy of biofilms—spatiotemporal approaches. In J. Pawley (ed.), Confocal microscopy handbook, 3rd ed. Plenum Press, New York, NY.
- 24.Papendick, R. I., and G. S. Campbell. 1981. Theory and measurement of water potential, p. 1-23. In J. F. Parr, W. R. Gardner, and L. F. Elliot (ed.), Water potential relations in soil microbiology. Soil Science Society of America, Madison, WI.
- 25.Potts, M. 1994. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58:755-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soby, S., and K. Bergman. 1983. Motility and chemotaxis of Rhizobium meliloti in soil. Appl. Environ. Microbiol. 46:995-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tuller, M., D. Or, and L. M. Dudley. 1999. Adsorption and capillary condensation in porous media: liquid retention and interfacial configurations in angular pores. Water Resour. Res. 35:1949-1964. [Google Scholar]
- 28.Vriezen, J. A. C., F. J. de Bruijn, and K. Nusslein. 2007. Responses of rhizobia to desiccation in relation to osmotic stress, oxygen, and temperature. Appl. Environ. Microbiol. 73:3451-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitman, W. B., D. C. Coleman, and W. J. Wiebe. 1998. Prokaryotes: the unseen majority. Proc. Natl. Acad. Sci. USA 95:6578-6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolfaardt, G. M., M. J. Hendry, T. Birkham, A. Bressel, M. N. Gardner, A. J. Sousa, D. R. Korber, and M. Pilaski. 2008. Microbial response to environmental gradients in a ceramic-based diffusion system. Biotechnol. Bioeng. 100:141-149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.