Abstract
A new double-antigen sandwich-based enzyme-linked immunosorbent assay (ELISA) for the detection of total antibodies (immunoglobulin G [IgG] and IgM) specific for hepatitis E virus (HEV) was developed by utilizing well-characterized recombinant protein ET2.1 and its peroxidase-labeled counterpart. Our study showed that the ELISA detected all the positive patient samples (n = 265) regardless of whether they contained IgM or IgG antibodies, or both, while it maintained an excellent specificity of 98.8% with samples from various patient or healthy control groups (total number of samples, 424). The test had a detection limit for anti-HEV IgG antibodies that was equivalent to 62 mIU/ml of the international reference. Compared with the serological status of the specimens determined on the basis of tests performed at the individual collection sites, the testing outcome generated by the new ELISA had a good agreement of 99.3%, with a kappa value of 0.985. The positive predictive value and the negative predictive value for the new test reached 98.1% and 100%, respectively. This ELISA had a positive delta value of 4.836 and a negative delta value of 3.314 (where delta is a measure of the number of standard deviations by which the cutoff is separated from the mean of the sample groups) (N. Crofts, W. Maskill, and I. D. Gust, J. Virol. Methods 22:51-59, 1988), indicating that it had an excellent ability to differentiate the infected and noninfected cohorts. Furthermore, the new design enables the detection of antibodies not only in human samples but also in pig samples. Our preliminary data showed that the ELISA could detect seroconversion in samples from pigs at as early as 14 days postinoculation. The potential utility of detecting specific antibodies in pigs will be an added advantage for managing the disease, with suggested zoonotic implications.
Hepatitis E virus (HEV) is enterically transmitted and causes a self-limited disease with a mortality rate in the range of 1 to 3% in general adult populations and up to 20% in pregnant women (13). However, two very recent reports provide more disturbing statistics (2, 11). HEV was once again established as the cause of a large outbreak of acute hepatitis; this time it was among a displaced population in Darfur, Sudan (11). In a period of 6 months, 2,621 HEV cases were recorded, with an attack rate of 3.3% among 78,800 inhabitants in a camp in Mornay, Sudan (11). Concurrently, among the 253 recorded HEV cases hospitalized, the overall case fatality rate was reported to be 17.8%, with the corresponding figure for pregnant women being 24.1% (2). These data demonstrate once again the dramatic impact that HEV infection has on pregnant women and serve as a reminder of the need for timely intervention for the control of epidemics. Rapid and accurate diagnostic tools that enable the prompt identification of HEV-infected patients remain essential for such outbreak management.
Diagnostic tests, especially serological assays for the detection of HEV infection, have been available for more than a decade (10). A more recent development in the field includes a new immunochromatographic test that enables decision making at the point of care (5). In addition, an alternative approach that uses the simultaneous detection of anti-HEV immunoglobulin A (IgA) and IgM antibodies for the diagnosis of acute HEV infection has also been suggested (23). However, to date, few reports are available on double-antigen sandwich-based enzyme-linked immunosorbent assay (ELISA) for the detection of anti-HEV antibodies. The double-antigen sandwich format provides an advantage because it detects total rather than class-specific antibodies and has been utilized with success in third-generation ELISAs to improve their sensitivity for the detection of human immunodeficiency virus infection (6). Although there are fundamental differences between infections with the two viruses, the need for a more sensitive detection tool is believed to be common to both types of infections. For the detection of human immunodeficiency virus infection, the need is to detect low levels of antibody, such as those that occur during early infection (6). For the detection of HEV infection, on the other hand, the requirement is more apparent for outbreak investigations, in which it is necessary to identify infected persons in remote areas (22). It is understood that the detection of anti-HEV IgM antibodies is an established procedure for the diagnosis of acute HEV infection (22). Furthermore, an attempt to accommodate the need for a more sensitive detection method in outbreak settings was made by adjusting the cutoff point of an ELISA for anti-HEV IgM antibodies (22). However, in practice, epidemiological studies often required both ELISAs for the detection of anti-HEV IgM and IgG antibodies, in addition to a PCR test for HEV RNA, especially in outbreak investigations (2). Besides, the concerns during the management of an outbreak include the detection of individuals with asymptomatic infection for the identification of risk factors (11). Accordingly, an ELISA with the utility to address the concerns described above would be an ideal addition to the existing tools for combating the disease.
Recognizing the vital role that an antigen plays in an ELISA, we selected well-characterized recombinant protein ET2.1, whose origin is open reading frame 2 (ORF2), as the capture antigen as well as the labeled detector. The protein is the carboxyl-end portion of the ORF2 region previously known as ORF2.1 and has been found to contain a highly conserved conformational epitope (21) suitable for the specific and sensitive detection of anti-HEV IgG or IgM antibodies by the respective ELISAs (1, 5). With the full anticipation of the advantages inherent with the double-antigen sandwich design, the capability and the performance of the newly developed test were verified with not only human serum samples but also swine serum samples derived from animal studies or collected from the field. The aim of the present study was to establish an easily adaptable test with utilities not only in epidemic and serological studies with samples from humans but also in animal studies. To date, as very few kits dedicated for use for animal testing are commercially available, a new test with the desired utilities will be a useful addition for management of the disease.
MATERIALS AND METHODS
Recombinant protein and conjugation.
Recombinant protein pET2.1 was prepared as described in detail elsewhere (1, 5, 17, 21). Briefly, fusion protein ET2.1, which consists of the ORF2.1 fragment (amino acids 394 to 660, Chinese strain) and a six-histidine tag, was expressed in Escherichia coli. Subsequently, the protein was solubilized in 5 M urea and purified in the presence of 5 M urea with Talon resin (Clontech Laboratories, Palo Alto, CA) (1). The purified protein was refolded by serial twofold dilution in carbonate buffer (pH 9.6) at 4°C prior to application (1).
For the conjugation of the ET2.1 antigen to horseradish peroxidase (HRP), a periodate oxidation method (16, 24) was used, with modifications. Briefly, to obtain an activated form, 5 mg of HRP in 0.5 ml of deionized water was mixed with 0.5 ml of 20 mM sodium periodate in 10 mM sodium phosphate buffer (pH 7.0), and the mixture was incubated for 2 h at room temperature (RT; 25 ± 3°C) in the dark. Subsequently, 0.1 ml of ethylene glycol was added to the mixture and the mixture was incubated for a further 20 min. The activated HRP was then dialyzed in 3 liters of 1 mM sodium acetate (pH 4.0) overnight at 4°C. Meanwhile, 2.5 mg ET2.1 antigen at a concentration of 2.2 mg/ml in 20 mM Tris containing 5 M urea was dialyzed in 1 liter of sodium carbonate buffer (pH 9.5) at 4°C overnight with one change of the buffer (2 liters). The dialyzed ET2.1 antigen was then mixed with the dialyzed HRP solution, and the mixture was incubated for 2 h at RT in the dark. The conjugate was stabilized by the addition of 50 μl of 5 M sodium cyanoborohydride to the reaction solution and further incubation for 15 min at RT. Subsequently, 100 μl of quench buffer (3.0 M ethanolamine, pH 9.0) was added to the mixture and was allowed to react for 15 min at RT. The resultant solution was dialyzed in 1 liters of 1× phosphate-buffered saline (PBS) at 4°C overnight with one time change of buffer (2 liters).
Human serum specimens.
Sera containing IgM or IgG antibodies to HEV were obtained from patients in areas where HEV is endemic or sites with sporadic HEV infections through collaborations with various institutions. These included specimens from patients in Nepal (n = 151) and China (n = 56) and from the archive (n = 58) of MP Biomedicals (formerly Genelabs Diagnostics, Singapore, Republic of Singapore). The 151 serum specimens from Nepal were obtained from patients with acute HEV infection and were found to contain ant-HEV IgM antibodies by the Walter Reed Army Institute of Research by an in-house ELISA (14). The 56 specimens from China were collected from two patient groups with sporadic infections in Shanghai, China. One group of samples was positive for anti-HEV IgM antibodies (n = 38) and the other (n = 18) was positive for anti-HEV IgG antibodies, as determined at each of the sites by commercial tests (HEV IgM ELISA and HEV ELISA; Genelabs Diagnostics). In addition, 58 serum samples positive for anti-HEV IgG antibodies but not for anti-HEV IgM antibodies from the serum samples archive of MP Biomedicals were also included in the study (archive sample, n = 58). For evaluation of the specificity of the new sandwich ELISA, serum samples negative for anti-HEV antibodies but positive for anti-hepatitis A virus antibodies (n = 41) or anti-hepatitis C virus antibodies (n = 44) were obtained from the archive of MP Biomedicals. Furthermore, 16 samples containing rheumatoid factor were included for additional verifications. These samples, together with 323 control serum samples from healthy donors of U.S. origin, were purchased from BioClinical Partner Inc. (Franklin, MA). All serum specimens were stored at −70°C until use. In addition, the WHO reference reagent for HEV antibody (human serum, NIBSC code 95/584) in lyophilized form was purchased from the National Institutes for Biological Standards and Control, United Kingdom. This reference reagent was reconstituted at 100 IU/ml in deionized water and was subsequently diluted with ELISA diluent (see below) in serial twofold dilutions starting from 1:100. Each dilution was tested in triplicate to obtain the titration curve. Furthermore, in view of the fact that few serum samples from the field would contain anti-HEV IgM antibodies without detectable anti-HEV IgG antibodies, 15 field samples (Nepal) were serially diluted twofold (with additional dilutions made when necessary) to a point at which their reactivity was just below the level detectable by the reference ELISA for anti-HEV IgG antibodies. These samples were used for additional verification of the ability of the new ELISA to detect anti-HEV IgM antibodies.
Swine serum specimens.
The swine sera were provided by C. Reusken of the National Institute of Public Health and Environment (RIVM) in The Netherlands. These sera included 50 samples collected from individual pigs from 10 different locations in an industrialized country where genotype 3 circulates and 74 longitudinal samples from inoculation studies with 10 pigs in a controlled environment. The details of the entire study have been published elsewhere (2a), but the samples relevant to the preliminary evaluation of the present study were collected at scheduled intervals after the pigs were inoculated intravenously with HEV genotype 3. Infection was confirmed by the detection of HEV RNA in stool samples collected 4 days postinoculation (dpi) from eight pigs and 7 days dpi from the other two. The two exceptions were pigs 8 and 10, but both became PCR positive at 7 dpi.
Reference HEV IgG- and IgM-specific ELISAs.
The two ELISAs used as references in the present study have been described separately elsewhere (1, 5). In particular, the IgG-specific ELISA was described previously and was performed with slight modifications, as detailed below (1), whereas the IgM-specific ELISA was performed on the basis of the version described in a recent update (5). Briefly, the materials and methods used to prepare the antigen-coated plates were identical for both reference HEV IgG- and IgM-specific ELISAs, according to the recent modification (5). The antigen-coated plates were prepared with the ET2.1 protein at a concentration of 0.6 μg/ml in carbonate buffer (pH 9.0). The 96-well polystyrene microtiter plates (MaxiSorp; Nunc, Roskilde, Denmark) were coated with the protein solution at a volume of 100 μl per well. The plates were incubated overnight (16 to 18 h) at 4°C prior to five washes with PBS-Tween 20 (PBST). Subsequently, nonspecific binding sites on the plates were blocked with 200 μl per well of a Tris-based diluent for 1 h at RT.
For the testing, 5 μl of serum in 100 μl of Tris-based diluent (containing 1% each bovine serum albumin and skim milk powder) was added to each of the wells of the antigen-coated plates. The plates were then incubated for 30 min at 37°C, followed by six washes with PBST. For the detection of IgG antibodies, HRP-conjugated goat anti-human IgG antibodies at a 1:700 dilution were added at 100 μl per well, whereas for the detection of IgM antibodies, HRP-conjugated mouse anti-human IgM antibodies at a 1:200 dilution were added at 100 μl per well. After the addition of the respective conjugate, the plates were incubated for 30 min at 37°C and subsequently washed with PBST six times. The color was developed by the addition of 100 μl per well of a substrate solution containing 3,3′,5,5′-tetramethylbenzidine (TMB). After a 15-min incubation in the dark at RT, the reaction was stopped by the addition of 100 μl of 1 N HCl into each well. The optical densities (ODs) were measured at 450 nm with a 620-nm reference filter. The results were considered positive for anti-HEV IgG antibodies if the ODs for individual samples were greater than or equal to the cutoff value (COV) of 0.3 plus the mean OD for the negative controls (OD/COV ≥ 1). The COV set for a positive result for anti-HEV IgM antibodies was not changed and remained 0.4 plus the mean OD for the negative controls (5). For quality control purposes, a positive control and a negative control were included in each assay run in triplicate. An assay run was considered valid only when the OD readings were less than 0.1 for both the blank and the negative control and greater than 0.6 for the positive control. All control samples, including samples negative and positive for anti-HEV IgM or IgG antibodies, were from the in-house preparations used for the respective commercial kits (MP Biomedicals).
Double-antigen sandwich ELISA.
For the double-antigen sandwich ELISA, the plates were prepared as described above for the reference plates, but the double-antigen sandwich ELISA relied on the HRP-labeled antigen (HRP-ET2.1) for anti-HEV antibody detection.
For the testing, the HRP-labeled pET2.1 (conjugate) was diluted 1:800 in the Tris-based diluent before it was dispensed into the wells of the antigen-coated microplate; it was used at 50 μl per well for human serum samples and 80 μl per well for pig serum samples. Subsequently, 50 μl of human serum sample or 20 μl of pig serum sample was added to the well containing the diluted conjugate. The plate was then incubated for 1 h at 37°C, followed by aspiration and six washes with PBST. Again, the substrate solution containing TMB was used at 100 μl per well for development of the color, and the plates were incubated for 30 min at 37°C in the dark. The reaction was stopped by adding 50 μl of 2 M H2SO4 to each well, and the ODs at 450 nm were obtained with a 620-nm reference filter.
Again, for quality control purposes, a positive control and a negative control were included in each assay run in triplicate. An assay run was considered valid only when the OD readings were less than 0.1 for both the blank and the negative control and greater than 0.6 for the positive control. The negative and positive control samples for anti-HEV IgG and IgM antibodies were from the in-house preparations used for the commercial kit (MP Biomedicals).
Statistical analysis.
The kappa statistic was used to measure the strength of agreement between the results of the new ELISA and those of the reference ELISAs. Kappa statistic values of >0.75, 0.40 to 0.75, and <0.40 represent excellent agreement, good to fair agreement and poor agreement, respectively (20). For data analysis for the new ELISA, an arbitrary COV of 0.3 plus the mean OD for the negative controls was used for human specimens but was verified with the delta values (7), which could provide an indication of an optimal means of differentiation between the positive and the negative populations. For the data generated with swine serum samples, the arbitrary COV was set equal to 0.2 plus the mean OD for the negative controls but was again verified with the delta values (measuring the number of standard deviations by which the cutoff is separated from the mean of the sample groups) (7). Because the ELISA plate reader used in the study was set to yield the maximum OD value of 3.000, the maximum OD readings obtained with various positive samples thus consisted of values of 3.000 or greater. To obtain the average OD value or OD/COV for the positive samples, all data with the maximum values were given a value of 3.000.
RESULTS
Detection of HEV-specific antibodies in human serum samples.
As shown in Table 1, field samples identified as containing either anti-HEV IgM or IgG antibodies were tested by the reference ELISAs as well as by the new test. Although anti-HEV IgG antibody-positive samples (76 of 76) remained reactive exclusively by the reference ELISA specific for IgG, many samples (187 of 189) originally identified as anti-HEV IgM antibody positive in the field by the Walter Reed Army Institute of Research ELISA (14) or a commercial test were also found to contain detectable amounts of anti-HEV IgG antibodies. The overall detection rates for the group of patient samples by the reference IgM-specific ELISA, the IgG-specific ELISA, and the new test were 59.5% (189 of 265), 99.0% (263 of 265), and 100% (265 of 265), respectively. Because in this initial test only two serum samples among the patient samples contained anti-HEV IgM antibodies and had no detectable anti-HEV IgG antibodies, we decided to expand the number of samples of this nature in order to illustrate further the advantage of the new design, which is antibody class independent. For this purpose, 15 field serum samples (from Nepal) were diluted to a point at which their reactivity was just below the detectable level by the reference ELISA for anti-HEV IgG antibodies (Table 2). These diluted samples were then verified to contain detectable anti-HEV IgM antibodies by the reference ELISA for anti-HEV IgM antibodies (Table 2). When these samples were further tested by the new ELISA, all produced higher ODs and had an average OD/COV five times greater than that generated by the reference ELISA for anti-HEV IgM antibodies (Table 2). Similar high OD values were also obtained for undiluted patient samples (n = 265), with a mean ± standard deviation value of 2.885 ± 0.470, in contrast to a value of 0.054 ± 0.114 for the healthy controls (Fig. 1). When these OD data were analyzed, they gave a positive delta value of 4.836 and a negative delta value of 3.314, which provided a good differentiation of the two populations.
TABLE 1.
Performance of sandwich ELISA and reference ELISAs with sera from hepatitis E patients, other patient controls, and healthy donors
| Serum group | No. of samples | No. of samples positive by the following method:
|
||
|---|---|---|---|---|
| Reference ELISA
|
New ELISA | |||
| IgM | IgG | |||
| Samples known to contain anti-HEV antibodies | ||||
| Anti-HEV IgM antibody positive | ||||
| Nepal | 151 | 151 | 149 | 151 |
| China | 38 | 38 | 38 | 38 |
| Anti-HEV IgG antibody positive | ||||
| Archived | 58 | 0 | 58 | 58 |
| China | 18 | 0 | 18 | 18 |
| Total | 265 | 189 | 263 | 265 |
| Controls | ||||
| Samples known to contain antibodies to other hepatitis viruses | ||||
| HAVa antibody positive | 41 | 1 | 2 | 0 |
| HCV antibody positive | 44 | 0 | 3 | 2 |
| Other patient samples known to contain rheumatoid factor | 16 | 0 | 0 | 0 |
| Samples from healthy blood donors (United States) | 323 | 3 | NDb | 3 |
| Total | 424 | 4 | ND | 5 |
HAV, hepatitis A virus.
ND, not done; as healthy donor samples tested negative by a commercial ELISA for anti-HEV IgG antibodies prior to use in the study, no additional tests were done by the reference ELISA for IgG.
TABLE 2.
Performance of sandwich ELISA with diluted serum samples containing detectable amounts of anti-HEV IgM antibodies only
| Serum sample no. (dilution) | Reference ELISA
|
New ELISA
|
||||
|---|---|---|---|---|---|---|
| IgG
|
IgM
|
|||||
| OD | OD/COV | OD | OD/COV | OD | OD/COVa | |
| 20 (1:800) | 0.236 | 0.7 | 0.430 | 1.0 | ≥3.000 | ≥8.9 |
| 87 (1:256) | 0.209 | 0.6 | 0.444 | 1.1 | 1.901 | 5.6 |
| 49 (1:256) | 0.269 | 0.8 | 0.446 | 1.1 | 1.252 | 3.7 |
| 200 (1:10) | 0.310 | 0.9 | 0.481 | 1.1 | ≥3.000 | ≥8.9 |
| 65 (1:256) | 0.258 | 0.8 | 0.506 | 1.2 | 1.012 | 3.0 |
| 275 (1:80) | 0.218 | 0.6 | 0.517 | 1.2 | 1.078 | 3.2 |
| 62 (1:160) | 0.291 | 0.8 | 0.525 | 1.2 | ≥3.000 | ≥8.9 |
| 22 (1:160) | 0.275 | 0.8 | 0.622 | 1.5 | 1.366 | 4.0 |
| 198 (1:40) | 0.216 | 0.6 | 0.630 | 1.5 | 1.816 | 5.4 |
| 67 (1:128) | 0.287 | 0.8 | 0.723 | 1.7 | 1.625 | 4.8 |
| 201 (1:10) | 0.200 | 0.6 | 0.731 | 1.7 | ≥3.000 | ≥8.9 |
| 245 (1:4) | 0.326 | 0.9 | 1.043 | 2.4 | ≥3.000 | ≥8.9 |
| 194 (1:40) | 0.242 | 0.7 | 1.277 | 3.0 | ≥3.000 | ≥8.9 |
| 257 (1:70) | 0.187 | 0.5 | 1.370 | 3.2 | ≥3.000 | ≥8.9 |
| 244 (1:40) | 0.151 | 0.4 | 1.625 | 3.9 | ≥3.000 | ≥8.9 |
For the reason indicated in Materials and Methods, all maximum ODs were treated as 3.000 for the calculation of OD/COV.
FIG. 1.
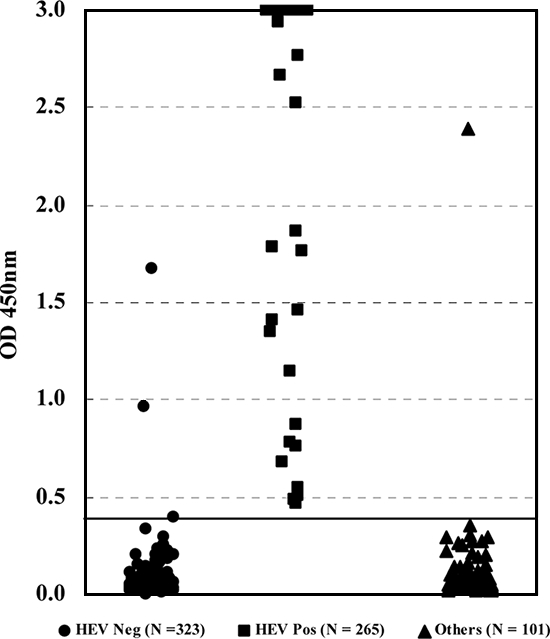
Scatter chart of OD values obtained by the new sandwich ELISA for sera known to contain anti-HEV IgM or IgG antibodies, or both, and sera from other patients or healthy controls. The solid horizontal line represents the COV. Neg, negative; Pos, positive.
When the ELISA was further subjected to testing with samples from patients infected with other hepatitis viruses (n = 85) or samples known to contain rheumatoid factor (n = 16), a good specificity of 98.0% (99 of 101) was obtained (Table 1). In addition, the assay presented an excellent specificity of 99.1% (320 of 323) with samples from healthy individuals from areas where HEV is not endemic (Table 1). The ELISA was therefore shown to have an overall specificity of 98.8% (419 of 424). The positive predictive value and the negative predictive value of the ELISA were 98.1% and 100%, respectively, with samples from the test populations described in Table 1.
Since no universal standard for IgM antibody to HEV is currently available, the detection limit of the ELISA was established only for IgG antibody by using the WHO reference reagent for HEV antibody (NIBSC code 95/584). As shown in Fig. 2, when the titration curve was obtained with the reference sample, the reactivity end point was found at the 1:1,600 dilution for the new test. This reactivity end point equated to a detection limit equivalent to 62 mIU/ml of the international reference standard. In comparison, the corresponding detection limit for the reference ELISA for anti-HEV IgG antibody detection was 124 mIU/ml (Fig. 2).
FIG. 2.
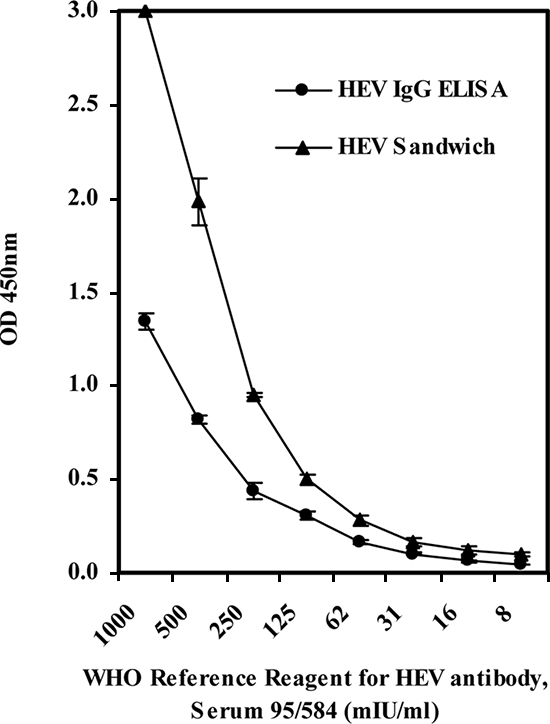
Titration curves of the WHO reference reagent for HEV antibody (NIBSC code 95/584) obtained by the new ELISA and the reference HEV ELISA for IgG. Each data point is the average OD reading for triplicate tests, and the error bars represent the standard deviations.
When the data generated by the new ELISA were compared with those produced by the two reference ELISAs combined, the two approaches gave agreements of 97.9% with the control samples (from other patients who were HEV negative) and 97.8% with the samples from patients with HEV infection (Table 3). Overall, the results of the new ELISA were very consistent with the combined outcomes generated by the two reference ELISAs (determined by using the data obtained by the tests for IgM and IgG combined), with an excellent agreement of 97.8% (kappa statistic, 0.944) (Table 3). Furthermore, when the detection outcome of the new ELISA was compared with the serological status of the specimens obtained externally, the overall agreement was 99.3% and the kappa statistic was 0.985 (Table 4).
TABLE 3.
Agreement between the new ELISA and the reference ELISAs with sera from hepatitis E patients or other patient control groups
| New ELISA result | No. of samples with the following reference ELISA resulta:
|
% Agreement | Kappa statisticb | |
|---|---|---|---|---|
| Positive for IgM or IgG antibodies | Negative for IgM and IgG antibodies | |||
| Positive | 265 | 2 | 97.8 | 0.944 |
| Negative | 6 | 93 | 97.9 | |
Data generated by the two reference ELISAs were combined and compared with those generated by the new ELISA for the calculation of agreement. The negative results used for the calculation of agreement were from the disease controls only.
Calculated for the total agreement of 97.8%. A kappa statistic of ≥0.75 represents excellent agreement, one of 0.40 to 0.75 represents good to fair agreement, and one of <0.40 represents poor agreement (20).
TABLE 4.
Agreement between the detection outcome by the new ELISA and the serological status of specimens from hepatitis E patients or healthy or patient control groups
| New ELISA result | No. of patients with the following serological statusa:
|
% Agreement | Kappa statisticb | |
|---|---|---|---|---|
| Positive | Negative | |||
| Positive | 265 | 5 | 100 | 0.985 |
| Negative | 0 | 419 | 98.8 | |
The serological status was based on the test results for the respective sample providers with either the commercial ELISA kits (Genelabs Diagnostics, Singapore) or the respective institutes' well-established in-house ELISAs.
Calculated for the total agreement of 99.3%.
Detection of HEV antibodies in swine serum samples.
When the new ELISA was used to test serum samples obtained at scheduled intervals from 10 individual pigs inoculated with HEV, anti-HEV antibodies were detected at as early as 14 dpi in 3 pigs and at 18 dpi in 6 other pigs (Fig. 3). These included pigs 3, 4, and 8, in which anti-HEV antibodies were detected at 14 dpi, and pigs 1, 2, 5, 6, 9, and 10, in which anti-HEV antibodies were detected at 18 dpi. The only exception was pig 7, in which seroconversion to anti-HEV antibodies was detected at 25 dpi (Fig. 3). All samples found to contain anti-HEV antibodies were unequivocally positive by the sandwich ELISA, with sample OD/COV values ranging from 3 to 12. This new test was further used to test a group of 50 samples collected from individual pigs from 10 different locations. A total of 36 samples were found to be positive for anti-HEV antibodies. The mean OD values ± standard deviations for the positive and the negative samples were 2.06 ± 1.01 and 0.07 ± 0.03, respectively (Fig. 4). The positive and the negative delta values were 2.75 and 3.56, respectively, again indicating an excellent differentiation of the two groups.
FIG. 3.
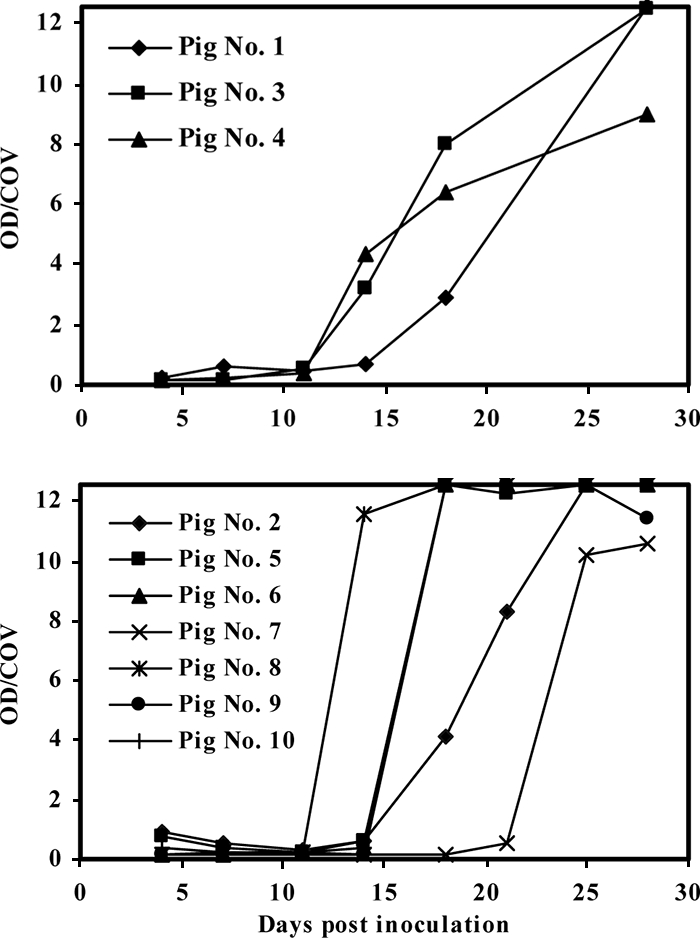
Performance of the new ELISA with seroconversion samples from 10 individual pigs collected at scheduled intervals. Due to the different schedules used for sample collection, the data obtained for the two groups of samples are shown in the upper and lower panels, respectively.
FIG. 4.
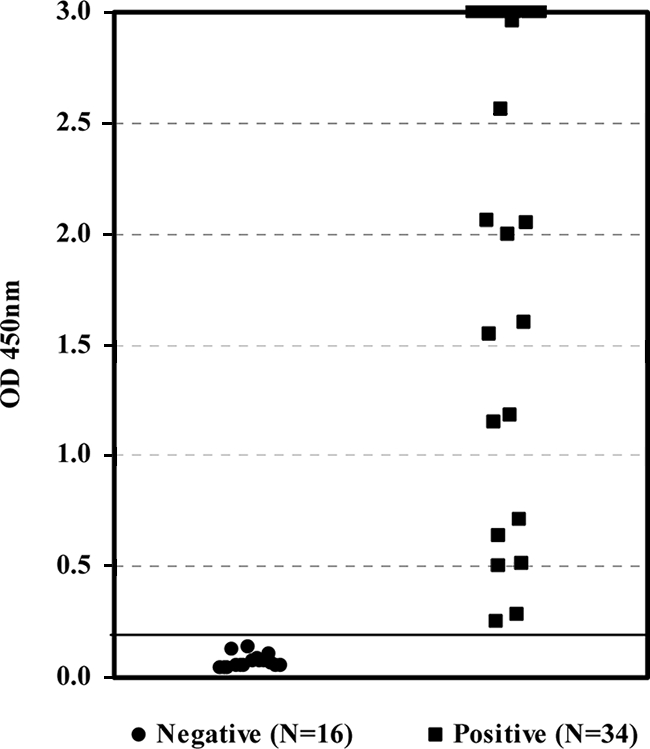
Scatter chart of OD values obtained by the new ELISA for 50 serum samples collected from individual pigs from 10 different locations. The solid horizontal line represents the COV.
DISCUSSION
As shown in Table 1, the new ELISA detected HEV antibodies in all 265 serum samples from HEV-infected patients, regardless of whether the specimens were originally found to be positive for anti-HEV IgM or anti-IgG antibodies at the collection sites, demonstrating an overall excellent sensitivity. However, when the results were verified by the reference tests, only 2 of the 265 samples were found to contain exclusively anti-HEV IgM antibodies. This finding thus suggested to the authors to a certain extent a limitation with the overall data in demonstrating the ability of the new test to detect anti-HEV IgM antibodies in addition to anti-HEV IgG antibodies. It is not unusual for samples from patients with acute infection who are positive for anti-HEV IgM antibodies to also contain anti-HEV IgG antibodies (2, 19). Hence, in practice, the number of cases whose specimens exclusively contain anti-HEV IgM antibodies may well be limited. Nevertheless, it was necessary for us to establish if the sandwich assay could indeed detect this class of antibodies adequately. The experiment with diluted samples was specifically designed to answer this question (Table 2). All 15 diluted samples were verified by the reference ELISAs of the indirect sandwich design to contain anti-HEV IgM antibodies exclusively, and the amount of anti-HEV IgG antibodies was diluted to an undetectable level. The high OD/COV ratios generated by the new ELISA for all 15 samples were a full supplement to the data obtained with the 265 patient samples, in that they demonstrated the capabilities of the new test. For example, samples 20, 200, and 62 produced the maximum ODs of 3.000 by the new ELISA but produced OD/COV ratios of only 1 to 1.2 by the reference IgM ELISA (Table 2). While these findings highlight the excellent sensitivity of the new assay, they cannot be taken as evidence of the more sensitive detection of anti-HEV IgM antibodies alone. In light of the significance of IgA antibodies in acute HEV infection (23), it is conceivable that the increases in reactivity obtained by the new assay could very well be due to the contribution of the detection of IgA. Although the contribution of this portion warrants further verification, it is still possible to conclude here that the new ELISA can detect anti-HEV antibodies independently of their class.
More importantly, our study also verified that the new ELISA is species independent and could readily detect HEV-specific antibody in swine samples with minimum modification. By adjusting only the amount of the sample used in the assay and adopting a different cutoff point, the new ELISA originally designed for the testing of human samples detected HEV-specific antibody in serum samples from pigs in inoculation studies at as early as 14 dpi. In fact, high titers of antibodies to HEV were detected in all except one of the samples from the 10 pigs by 18 dpi. Indeed, antibodies to HEV were detected even in the exceptional case no later than 25 dpi. In contrast, the study of Kasorndorkbua et al. (15) similarly showed that the detection of anti-HEV antibodies by an ELISA specific for IgG was possible only at 56 dpi for two of three intravenously inoculated pigs. Many other factors, such as the inoculum size and the inoculation scheme, in addition to the test kit used (which was specific for IgG antibodies only) in the study of Kasorndorkbua et al., probably resulted in the delay in the detection of seroconversion (15). However, the difference in the time to detection in the present study and that of Kasorndorkbua et al. was remarkable.
The new ELISA not only appeared to detect positive samples earlier but also generated higher reactivity, with the mean OD ± standard deviation being 2.885 ± 0.470 for serum samples from patients with HEV infection. Because the maximum OD value was set equal to 3.000 when the data were obtained, the average OD value of 2.885 for the positive samples was almost equal to the maximum OD value. In fact, more than 90% of the samples (246 of 265) from patients with HEV infections had reactivities at an OD value of 3.000 or greater (Fig. 1). In contrast, because the corresponding parameter for the healthy controls was 0.054 ± 0.114, the ability to differentiate the two cohorts was remarkable (Fig. 1). A similar ability to differentiate between positive and negative samples was also obtained with samples of swine origin (Fig. 4). In this regard, the finding by Engle et al. (9) in a study of the detection of anti-HEV antibodies in inoculated rhesus monkeys is noteworthy. The two ELISAs used in that study generated a maximum OD/COV value of 7 for seroconversion samples, which was very much lower than the OD/COV values of 12 to 13 produced by the new ELISA for a group of positive swine samples collected at an earlier time of infection (Fig. 3). Again, it may not be fair to compare the two sets of data directly because many other factors were involved, but the absolute difference between the two corresponding parameters does provide us to a certain extent an indication about the performance of the new ELISA.
Because there was no universal reference for HEV IgM antibody, the detection limit of the new ELISA was correlated only to the WHO anti-HEV human serum reference reagent (NIBSC code 95/584). With a detection limit correlated to a level equivalent to 62 mIU/ml (for IgG), the performance of the new test for the specific detection of IgG antibodies was highly comparable to that of the reference ELISA dedicated to the detection of IgG antibodies. In fact, this detection limit was also fairly consistent with, although not identical to, that found in a previous study (1). While this detection limit provided an indication of the performance of the test, the ultimate demonstration of its performance was obtained with data for field samples. Apart from the results mentioned above of the earlier detection of seroconversion in samples from pigs in controlled studies, data from the 50 samples collected from individual pigs from 10 different locations were particularly interesting. The overall prevalence of HEV in swine in the country where the samples originated will be established in a separate study. Nevertheless, the early detection of seroconversion and the high detection rate for the field samples with unequivocal OD/COV values provided at least a preliminary indication of the utility of the new test for the testing of animals, in addition to its utility established for the testing of humans.
ET2.1 is a well-defined recombinant antigen containing a major conformational epitope (17, 18) and has been used to detect human anti-HEV antibodies in a different platform, in addition to ELISA (5). Furthermore, this recombinant antigen was used in an indirect sandwich ELISA for the detection of anti-HEV antibodies in swine samples (4). Therefore, it is not surprising that in the current double-antigen sandwich ELISA, the recombinant protein produced optimal reactivity with serum samples from pigs from inoculation studies as well as from the field. In this aspect, the finding of Engle et al. (9) is again noteworthy. The researchers used two antigens of either human (Sar-55) or swine (strain Meng) origin for the development of ELISAs and found that the two antigens were in fact interchangeable with respect to their ability to detect anti-HEV antibodies (9). This was also in agreement with the understanding that all four recognized genotypes of HEV appeared to fall into a single serotype (8). In a sense, our findings suggest the same, because the genotype 1-derived antigen detected antibodies not only in human samples from regions where genotype 1 was the most prevalent but also in swine samples obtained after inoculation with a genotype 3-derived strain. The finding of the utility of ET2.1 in the new test is consistent with what had been demonstrated in the ELISA or Western immunoblot format with related ORF2.1 recombinant proteins (1, 17, 18).
The specificity of the ELISA was verified with serum samples from patients infected with other hepatitis viruses as well as healthy controls. The results obtained with serum samples known to contain antibodies to hepatitis A or C virus showed an excellent specificity of 97.6% (Table 1), indicating the likely utility of the new test for the detection of HEV infection among patients infected with other hepatitis viruses. This capability of differentiation is critical, particularly between hepatitis A virus and HEV, as the two share similarities not only in their clinical manifestations but also in their routes of transmission. Because the new ELISA was based on the double-antigen sandwich format for the detection of antibodies specific for HEV, it was, by design, free of the cross-reactivity otherwise associated with an indirect sandwich assay or a capture-based ELISA (3, 12). Nevertheless, verification with sera containing rheumatoid factors, which are generally known to cause cross-reactivity in ELISAs for IgM antibodies, was also carried out. As expected, this interference factor did not elicit any cross-reactivity in the assay with the double-antigen design, and the new test thus appeared to have adequate specificity, in addition to adequate sensitivity, established as described above. In fact, the results of the new test were found to be in good agreement with those of existing tests for the detection (but not the differentiation) of anti-HEV IgM or IgG antibodies. Compared with the serological status of the specimens determined on the basis of tests performed at the individual collection sites, the corresponding finding generated by the new test had an agreement of 99.3% (delta value, 0.985) (Table 4).
As mentioned above, the new ELISA was capable of detecting anti-HEV antibodies in samples of both human and swine origin. The minimum modification needed to test samples of human and swine origin, which consists of adjustment of the sample volume used in the assay and the cutoff point, is simple and involves no change to any components used in the assay. This is practical and allows the kit to be applied to samples from outbreak investigations, in which the detection of both IgM and IgG antibodies is needed (2), or to the testing of specimens of animal origin. The strong agreement established between the new test and the reference ELISAs or existing approaches with the wide range of human samples tested indicates the overall performance of the new test. The overall findings thus suggest that the new test not only is compatible with currently established methods but also is a valuable addition to the tools available for the detection and management of the disease.
Acknowledgments
We thank Chantal Reusken for providing the serum samples collected from the pigs.
Footnotes
Published ahead of print on 21 May 2008.
REFERENCES
- 1.Anderson, D. A., F. Li, M. Riddell, T. Howard, H.-F. Seow, J. Torresi, G. Perry, D. Sumarisidi, S. M. Shrestha, and I. L. Shrestha. 1999. ELISA for IgG-class antibody to hepatitis E virus based on a highly conserved, conformational epitope expressed in Escherichia coli. J. Virol. Methods 81:131-142. [DOI] [PubMed] [Google Scholar]
- 2.Boccia, D., J. P. Guthmann, H. Klovstad, N. Hamid, M. Tatay, I. Ciglenecki, J. Y. Nizou, E. Nicand, and P. J. Guerin. 2006. High mortality associated with an outbreak of hepatitis E among displaced persons in Darfur, Sudan. Clin. Infect. Dis. 42:1679-1684. [DOI] [PubMed] [Google Scholar]
- 2a.Bouwknegt, M., K. Frankena, S. A. Rutjes, G. J. Wellenberg, A. M. de Roda Husman, W. H. van der Poel, and M. C. de Jong. 2008. Estimation of hepatitis E virus transmission among pigs due to contact-exposure. Vet. Res. 39:40. [DOI] [PubMed] [Google Scholar]
- 3.Briantais, M. J., L. Grangeot-Keros, and J. Pillot. 1984. Specificity and sensitivity of the IgM capture immunoassay: studies of possible factors inducing false positive or false negative results. J. Virol. Methods 9:15-26. [DOI] [PubMed] [Google Scholar]
- 4.Chandler, J. D., M. A. Riddell, F. Li, R. J. Love, and D. A. Anderson. 1999. Serological evidence for swine hepatitis E virus infection in Australian pig herds. Vet. Microbiol. 68:95-105. [DOI] [PubMed] [Google Scholar]
- 5.Chen, H. Y., Y. Lu, T. Howard, D. Anderson, P. Y. Fong, W. P. Hu, C. P. Chia, and M. Guan. 2005. Comparison of a new immunochromatographic test to enzyme-linked immunosorbent assay for rapid detection of immunoglobulin m antibodies to hepatitis E virus in human sera. Clin. Diagn. Lab. Immunol. 12:593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantine, N. T., and H. Zink. 2005. HIV testing technologies after two decades of evolution. Indian J. Med. Res. 121:519-538. [PubMed] [Google Scholar]
- 7.Crofts, N., W. Maskill, and I. D. Gust. 1988. Evaluation of enzyme-linked immunosorbent assays: a method of data analysis. J. Virol. Methods 22:51-59. [DOI] [PubMed] [Google Scholar]
- 8.Emerson, S. U., and R. H. Purcell. 2003. Hepatitis E virus. Rev. Med. Virol. 13:145-154. [DOI] [PubMed] [Google Scholar]
- 9.Engle, R. E., C. Yu, S. U. Emerson, X. J. Meng, and R. H. Purcell. 2002. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J. Clin. Microbiol. 40:4576-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldsmith, R., P. O. Yarbough, G. R. Reyes, K. E. Fry, K. A. Gabor, M. Kamel, S. Zakaria, S. Amer, and Y. Gaffar. 1992. Enzyme-linked immunosorbent assay for diagnosis of acute sporadic hepatitis E in Egyptian children. Lancet 339:328-331. [DOI] [PubMed] [Google Scholar]
- 11.Guthmann, J. P., H. Klovstad, D. Boccia, N. Hamid, L. Pinoges, J. Y. Nizou, M. Tatay, F. Diaz, A. Moren, R. F. Grais, I. Ciglenecki, E. Nicand, and P. J. Guerin. 2006. A large outbreak of hepatitis E among a displaced population in Darfur, Sudan, 2004: the role of water treatment methods. Clin. Infect. Dis. 42:1685-1691. [DOI] [PubMed] [Google Scholar]
- 12.Ho, D. W. T., P. R. Field, and A. L. Cunningham. 1989. Rapid diagnosis of acute Epstein-Bar virus infection by an indirect enzyme-linked immunosorbent assay for specific immunoglobulin M (IgM) antibody without rheumatoid factor and specific IgG interference. J. Clin. Microbiol. 27:952-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussani, S. H., S. J. Skidmore, P. Richardson, L. M. Sherratt, B. T. Cooper, and J. G. O'Grady. 1997. Severe hepatitis E infection during pregnancy. J. Viral Hepat. 4:51-54. [DOI] [PubMed] [Google Scholar]
- 14.Innis, B. L., J. Seriwatana, R. A. Robinson, M. P. Shrestha, P. O. Yarbough, C. F. Longer, R. M. Scott, D. W. Vaughn, and K. S. A. Myint. 2002. Quantitation of immunoglobulin to hepatitis E virus by enzyme immunoassay. Clin. Diagn. Lab. Immunol. 9:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasorndorkbua, C., D. K. Guenette, F. F. Huang, P. J. Thomas, X. J. Meng, and P. G. Halbur. 2004. Routes of transmission of swine hepatitis E virus in pigs. J. Clin. Microbiol. 42:5047-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laman, J. D., A. J. van den Eertwegh, C. Deen, N. Vermeulen, W. J. Boersma, and E. Claassen. 1991. Synthetic peptide conjugates with horseradish peroxidase and beta-galactosidase for use in epitope-specific immunocytochemistry and ELISA. J. Immunol. Methods 145:1-10. [DOI] [PubMed] [Google Scholar]
- 17.Li, F., H. Zhuang, S. Kolivas, S. A. Locarnini, and D. A. Anderson. 1994. Persistent and transient antibody responses to hepatitis E virus detected by Western immunoblot using open reading frame 2 and 3 and glutathione S-transferase fusion proteins. J. Clin. Microbiol. 32:2060-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, F., J. Torresi, S. A. Locarnini, H. Zhuang, W. F. Zhu, X. X. Guo, and D. A. Anderson. 1997. Amino-terminal epitopes are exposed when full-length open reading frame 2 of hepatitis E virus is expressed in Escherichia coli, but carboxy-terminal epitopes are masked. J. Med. Virol. 52:289-300. [DOI] [PubMed] [Google Scholar]
- 19.Myint, K. S., T. P. Endy, R. V. Gibbons, K. Laras, M. P. Mammen, Jr., E. R. Sedyaningsih, J. Seriwatana, J. S. Glass, S. Narupiti, and A. L. Corwin. 2006. Evaluation of diagnostic assays for hepatitis E virus in outbreak settings. J. Clin. Microbiol. 44:1581-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pottumarthy, S., A. J. Morris, A. C. Harrison, and V. C. Wells. 1999. Evaluation of the tuberculin gamma interferon assay: potential to replace the Mantoux skin test. J. Clin. Microbiol. 37:3229-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riddell, M. A., F. Li, and D. A. Anderson. 2000. Identification of immunodominant and conformational epitopes in the capsid protein of hepatitis E virus by using monoclonal antibodies. J. Virol. 74:8011-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seriwatana, J., M. P. Shrestha, R. M. Scott, S. A. Tsarev, D. W. Vaughn, K. S. Myint, and B. L. Innis. 2002. Clinical and epidemiological relevance of quantitating hepatitis E virus-specific immunoglobulin M. Clin. Diagn. Lab. Immunol. 9:1072-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi, M., S. Kusakai, H. Mizuo, K. Suzuki, K. Fujimura, K. Masuko, Y. Sugai, T. Aikawa, T. Nishizawa, and H. Okamoto. 2005. Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies against hepatitis E virus (HEV) is highly specific for diagnosis of acute HEV infection. J. Clin. Microbiol. 43:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tijssen, P., and E. Kurstak. 1984. Highly efficient and simple methods for the preparation of peroxidase and active peroxidase-antibody conjugates for enzyme immunoassays. Anal. Biochem. 136:451-457. [DOI] [PubMed] [Google Scholar]


