Abstract
We previously reported that protein antigens of serodiagnostic potential were more abundant in culture filtrates than cellular extracts from liquid cultures of Mycobacterium avium subsp. paratuberculosis (D. Cho and M. T. Collins, Clin. Vaccine Immunol. 13:1155-1161, 2006). Based on this observation, a novel enzyme-linked immunosorbent assay (ELISA) using antigens secreted by young (early- to mid-log-phase) cultures of M. avium subsp. paratuberculosis JTC303 (a low-passage isolate originating from the ileum of a Holstein bull) in mycobactin-supplemented Watson-Reid medium (pH 6.0) was developed and evaluated using a previously described panel of bovine sera (M. T. Collins et al., Clin. Diagn. Lab. Immunol. 12:685-692, 2005) that included 444 paratuberculosis cases and 412 controls. The new assay, called JTC-ELISA, had a significantly higher diagnostic sensitivity and an equivalent specificity compared to those of five commercial paratuberculosis ELISA kits. By receiver-operating characteristic analysis, the JTC-ELISA had the highest area under the curve of the six assays evaluated. The JTC-ELISA was particularly sensitive at detecting low-level fecal shedders of Mavium subsp. paratuberculosis (40%; the sensitivity of the commercial kits was 20%). The JTC-ELISA works effectively on both serum and milk samples for the detection of cattle with subclinical M. avium subsp. paratuberculosis infections, providing a cost-effective diagnostic tool to support paratuberculosis control programs in cattle herds.
Paratuberculosis (Johne's disease) is a prevalent and economically important chronic inflammatory bowel disease of ruminants caused by Mycobacterium avium subsp. paratuberculosis (3, 12). The control of this infection within cattle herds requires both herd management changes to limit fecal-oral infection spread and diagnostic testing to identify infectious adult cattle for segregation or removal (10). Although the detection of M. avium subsp. paratuberculosis organisms in clinical samples by culture or PCR affords a definitive diagnosis, these diagnostic methods are slow, laborious, and/or expensive. Serum antibody diagnostic tests avoid these problems but suffer from low diagnostic sensitivity (5). Nonetheless, economic decision analysis modeling indicates that low-cost tests are the most cost-effective for commercial dairy herds despite their low sensitivity, provided appropriate actions are taken in a timely fashion based on enzyme-linked immunosorbent assay (ELISA) results (4, 7, 17).
ELISA platforms provide inexpensive and readily automated techniques for high sample throughput, an important consideration for diagnostic laboratories. Improvements in ELISAs for bovine paratuberculosis require design changes that increase assay sensitivity while retaining high (≥99%) specificity. Among four commercial bovine paratuberculosis ELISA kits with high specificity, diagnostic sensitivity for the detection of fecal culture-positive cattle was <30% for the detection of clinically normal M. avium subsp. paratuberculosis fecal culture-positive cattle (5).
Early secreted proteins of M. avium subsp. paratuberculosis are recognized as important antigens for the diagnosis of bovine paratuberculosis (1, 19). Cho et al. demonstrated that serum antibodies from naturally M. avium subsp. paratuberculosis-infected cows react more strongly and to more secreted antigens than cellular extract (CE) antigens, but antibody binding patterns for individual cattle were highly variable (1). It is not uncommon for cattle to test strongly positive in one commercial assay but negative in another, providing additional evidence of the individual variation in antibody responses to M. avium subsp. paratuberculosis antigens (5). ELISAs based on the expression and purification of selected secreted antigens failed to produce ELISAs of greater accuracy than that of crude culture filtrates (CF) (2). This potentially is due both to changes in proteins during cloning and to the high variability in antibody response to M. avium subsp. paratuberculosis proteins among cattle (5). Therefore, our studies to develop and improve a bovine paratuberculosis ELISA focused on the use of a composite of secreted antigens from M. avium subsp. paratuberculosis.
MATERIALS AND METHODS
Antigen production.
CF M. avium subsp. paratuberculosis antigens were produced from strain JTC303 by the inoculation of 100 μl of a seedlot culture containing 109 CFU/ml into 35 ml of Watson-Reid broth medium, which was modified by supplementation with 2 μg/ml mycobactin (mWR) (15). Strain JTC303 originates from the ileum tissue of a Holstein bull with paratuberculosis that was submitted to the Johne's Testing Center in December 1999. The identity of JTC303 was verified by IS900 PCR and passaged in vitro a few times, and stock cultures were maintained at −80°C.
CF antigens were derived from early- to mid-log-phase cultures with 8 to 10 weeks of incubation at 37°C. CF antigens were harvested and concentrated as previously described (1). Briefly, M. avium subsp. paratuberculosis cells grown in mWR were removed by centrifugation at 10,000 × g for 30 min. After filtration through a 0.2-μm-pore-size filter (Nalge Nunc International, Rochester, NY), the filtrate was concentrated 40- to 50-fold using a Centricon Plus-80 (molecular weight cutoff, 5,000; Amicon, Bevery, MA) and dialyzed five times in 10 mM phosphate-buffered saline (PBS), pH 7.2, using a Slide-A-Lyzer dialysis cassette (Pierce, Rockford, IL). The concentration of soluble protein was determined using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL).
Mycobacterium phlei ATCC 11758 was cultivated in mWR broth for 4 weeks at 37°C to prepare CE antigens for absorption and the removal of serum antibodies that cross-react with other Mycobacterium spp. as previously described (6, 20).
Bovine serum and milk samples.
Paratuberculosis cases were defined as fecal culture-positive cows from known infected herds. The sera were from a previously described bovine serum sample collection (5) and included 444 fecal culture-positive cows from seven different herds and 412 control cows resident in seven Midwest dairy herds that were free of paratuberculosis (5). The accuracy of the novel ELISA based on the JTC303 strain, termed JTC-ELISA, was compared to previously published results from five commercial paratuberculosis ELISA kits (HerdCheck, ParaCheck, AntelBio, SERELISA, and Pourquier) on the same sera (5). Negative control sera for the assay were collected from healthy dairy cows in a herd free of M. avium subsp. paratuberculosis infection (Dairy Teaching Herd, School of Veterinary Medicine, University of Wisconsin—Madison).
An additional panel of paired serum and milk samples (n = 296) was used to compare the results of testing serum and milk on the same individuals. It consisted of 78 (for JTC-ELISA) or 84 (for the other tested kits) bovine paratuberculosis cases and 196 (for ID Screen and JTC-ELISA) or 214 (for the other kits) controls (based on the culture of feces for M. avium subsp. paratuberculosis) from multiple dairy cattle herds. For milk samples, a 1:20 sample dilution was used instead of the 1:50 dilution used for the testing of sera.
JTC-ELISA procedure.
M. avium subsp. paratuberculosis CF antigens (2 μg/ml) were diluted in coating buffer (KPL, Gaithersburg, MD), and 100 μl was added to each well in 96-well microtiter plates (Maxisorp, Nalge Nunc International, Rochester, NY) and incubated at 4°C overnight. After being washed, the wells were blocked with 10% normal goat serum (Sigma, St. Louis, MO) at room temperature for 2 h. Cross-reactive antibodies were absorbed from each serum sample by mixing 100 μl of 1:25 serum diluted in 10 mM PBS (pH 7.2) with 100 μl of 500 μg/ml M. phlei CE antigens (final serum dilution, 1:50; final M. phlei CE antigen concentration, 250 μg/ml). For milk antibody detection, the absorbed milk samples were prepared by mixing 100 μl of 1:10 milk diluted in 10 mM PBS (pH 7.2) with 100 μl of 500 μg/ml M. phlei CE antigens (final milk dilution, 1:20; final M. phlei CE antigen concentration, 250 μg/ml). The absorbed serum or milk (100 μl) was added to each microtiter plate well and incubated for 30 min at room temperature with shaking at 600 rpm. Wells then were washed three times with washing buffer (KPL, Gaithersburg, MD). Mouse monoclonal anti-bovine immunoglobulin G (IgG) antibody conjugated with biotin (Sigma, St. Louis, MO) and sheep polyclonal anti-bovine IgG conjugated with biotin (KPL, Gaithersburg, MD) at dilutions of 1:10,000 and 1:20,000, respectively, were added to all wells and incubated for 30 min at room temperature. Plates were washed five times with washing buffer (KPL, Gaithersburg, MD), and 100 μl of streptavidin (1:10,000 dilution; SeroTec Inc., Raleigh, NC) was added to all wells. Plates then were incubated for 30 min at room temperature. After being washed five times with wash buffer (KPL, Gaithersburg, MD), 100 μl of tetramethylbenzidine substrate (TMBE-500; Moss Inc., Pasadena, MD) was added to each well, followed by plate incubation for 1 min at room temperature. The reaction was stopped by adding 100 μl of stop solution (KPL, Gaithersburg, MD). The optical density (OD) of the final reaction in each well was measured at 450 nm by an ELISA plate reader (μQuant; Bio-Tek Instruments Inc., Winooski, VT). On each ELISA plate, sera from two M. avium subsp. paratuberculosis-infected cows, called Eyvette and Palmer B (the infections were verified by histopathologic and microbiologic examination of tissues), were used as positive controls, and sera from two noninfected cows, Olive and Charmany, were used as negative controls. All sera were tested in duplicate. ELISA OD values were transformed to sample/positive (S/P) ratios by the following equation:
 |
Mean S/P values for each serum were used for data analysis. Assays were considered valid only when the mean OD of positive control sera was >0.90 and the mean OD of negative control sera was <0.15. Invalid assays were repeated.
Milk antibody detection.
The JTC-ELISA was tested on pairs of milk and serum samples. All results were compared to those of three commercial ELISA kits, HerdCheck (IDEXX Laboratories, Inc. Westbrook, ME), ParaCheck (Prionics AG, Zurich, Switzerland), and ID Screen (ID-Vet, Montpellier, France). The ParaCheck and ID Screen kits have specific instructions for testing milk. The HerdCheck kit does not have specific instructions for testing bovine milk, so procedures similar to those for the other two kits were followed. Specifically, milk was diluted 1:20 in the kit's dilution buffer, and results were interpreted as for assays on sera following the manufacturer's instructions.
Data analysis.
The ELISA results were subjected to receiver-operating characteristic (ROC) curve analysis (8, 9). This method estimates the sensitivity and specificity of the ELISA at every possible S/P interpretation cutoff and provides an overall measure of test accuracy as the area under the ROC curve (AUC). Differences in mean ODs for bovine sera from infected and noninfected cattle also were evaluated by the Mann-Whitney test. Differences were considered significant at P < 0.05, and lower P values were considered indicative of more valuable ELISA antigens. ROC curve analysis was used for the comparison between JTC-ELISA and other commercial ELISA kits, and significant differences were reported when P < 0.05. The JTC-ELISA sensitivity by the level of fecal shedding of M. avium subsp. paratuberculosis was evaluated for four shedding levels, designated slight (n = 229), light (n = 68), moderate (n = 36), and heavy (n = 82). These designations corresponded to the mean fecal shedding levels of ≤1.0, 1.1 to 2.0, 2.1 to 3.0, and >3.0, respectively, on a 0 to 4 scoring system, as determined by fecal culture results from three independent laboratories on aliquots of the same unfrozen fecal sample, as previously described (5). Overall agreement between serum and milk samples for each ELISA was evaluated by the kappa statistic. In a similar pair-wise fashion, the correlation (r2) between serum and milk sample results was reported using linear regression. Most statistical analyses were performed using statistical software (GraphPad Prism version 4.03 for Windows; GraphPad Software, San Diego, CA). ELISA AUCs were compared by a manual calculation using established methods, and those with significantly higher AUCs were considered more accurate (8, 9). Likelihood ratios with 95% confidence intervals (CI) were computed using MedCalc (http://www.medcalc.be/).
RESULTS
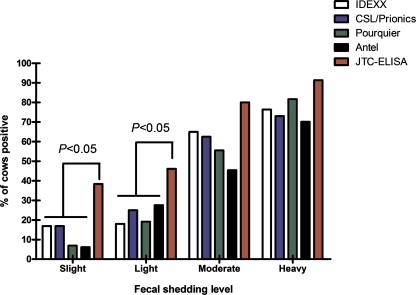
The optimal cutoff for the JTC-ELISA was S/P ≥ 0.15. At this cutoff, using 856 bovine sera previously described from well-defined paratuberculosis cases and controls (5), the JTC-ELISA had the highest overall accuracy (sensitivity plus specificity) and the highest AUC compared to those of five commercially available bovine paratuberculosis ELISAs (Table 1). The JTC-ELISA was particularly more sensitive than commercial ELISAs at detecting cows that were slight or light fecal shedders of M. avium subsp. paratuberculosis: 40 and 20%, respectively (Fig. 1). If the JTC-ELISA was evaluated against a single laboratory fecal culture case definition (i.e., paratuberculosis cases are defined as fecal culture-positive cows, which was determined by using a conventional culture on Herrold's egg yolk agar slants and the sedimentation fecal processing technique as performed at the Minnesota Veterinary Diagnostic Laboratory), the JTC-ELISA had a 74% (95% CI, 65.5 to 83.4) sensitivity and 99% (95% CI, 96.4 to 99.9) specificity, while all of the commercial ELISA kits had roughly a 50% sensitivity on the same sera using the same case definition (5). Likelihood ratios for the three levels of JTC-ELISA above the assay optimal S/P cutoff were >9, while those in the S/P range just below the cutoff (0.11 to 0.15) were 4.52 (Table 2).
TABLE 1.
Overall comparison of JTC-ELISA to five widely used commercial kitsa
| Kit | % Sensitivity | % Specificity | Accuracyb | AUC |
|---|---|---|---|---|
| HerdCheck | 28.9 | 95.30 | 124.2 | 0.619 |
| ParaCheck | 28.4 | 99.70 | 128.1 | 0.795 |
| Pourquier | 28.0 | 100.00 | 128.0 | 0.709 |
| SERELISA | 44.5 | 84.90 | 129.4 | 0.706 |
| AntelBio (milk) | 28.9 | 99.70 | 128.6 | 0.632 |
| JTC-ELISA | 56.3* | 99.00 | 155.3 | 0.894* |
*, P < 0.05.
Sensitivity plus specificity.
FIG. 1.
Percentage of cows that tested positive by JTC-ELISA and by commercial bovine paratuberculosis ELISA kits on sera from 444 M. avium subsp. paratuberculosis-infected cows according to the level of fecal shedding.
TABLE 2.
Likelihood ratios for five S/P levels of the JTC-ELISA result
| S/P range | No. (%) of infected cows | No. (%) of noninfected cows | Likelihood ratio (95% CI) |
|---|---|---|---|
| <0.10 | 158 (35.6) | 400 (97.1) | 0.37 (0.32-0.42) |
| 0.11-0.15 | 39 (8.8) | 8 (1.9) | 4.52 (2.14-9.56) |
| 0.16-0.20 | 39 (8.8) | 4 (0.9) | 9.05 (3.26-25.10) |
| 0.21-0.30 | 39 (8.8) | 0 (0) | ∞ (4.46-∞) |
| >0.30 | 169 (38.1) | 0 (0) | ∞ (19.60-∞) |
| Total | 444 | 412 |
On paired serum and milk samples, the JTC-ELISA showed the highest overall accuracy, highest sensitivity, and highest correlation of results between sample pairs compared to results for three commercial ELISA kits for bovine paratuberculosis (Table 3).
TABLE 3.
Evaluation of JTC-ELISA for antibody detection in milk samples
| Kit | No. of sample pairs | No. of cases + no. of controls | % Sensitivitya | % Overall serum/milk agreement | Kappa statistic | r2 |
|---|---|---|---|---|---|---|
| HerdCheck | 296 | 82 + 214 | 14.10 | 32.90 | 0.07 | 0.281 |
| ParaCheck | 296 | 82 + 214 | 88.00 | 92.70 | 0.85 | 0.704 |
| ID Screen | 278 | 82 + 196 | 72.50 | 92.70 | 0.85 | 0.671 |
| JTC-ELISA | 274 | 78 + 196 | 91.10* | 93.60 | 0.87 | 0.779 |
Sensitivity indicates the percentage of serum ELISA-positive cows detected by milk ELISA by using the same ELISA kit. *, P < 0.05.
DISCUSSION
M. avium subsp. paratuberculosis antigens found in CF were superior solid-phase ELISA antigens to those used in commercial ELISA kits for bovine paratuberculosis. The diagnostically best antigens were produced by a specific strain of M. avium subsp. paratuberculosis, JTC303, grown at pH 6.0 in mWR medium (data on strain and medium comparisons not shown). The optimal time for CF antigen harvest was when cultures were in the early to mid-log growth phase, i.e., 8 to 10 weeks of incubation, using a total inoculum of 108 frozen M. avium subsp. paratuberculosis cells for 35 ml of pH 6.0 mWR medium. A combined monoclonal and polyclonal anti-bovine IgG conjugate and the avidin-biotin signal amplification system enhanced the JTC-ELISA's sensitivity. As with other paratuberculosis ELISAs, the absorption of bovine sera using a cellular extract of M. phlei prior to testing was important for high JTC-ELISA specificity.
This is the first report on the use of secreted M. avium subsp. paratuberculosis antigens in a serodiagnostic test. Other ELISAs have focused on cellular antigens from stationary-phase cultures of M. avium subsp. paratuberculosis, in part because of the much higher protein yield from cells of this very slow-growing fastidious pathogen. In the present study, CE antigens from none of the study strains were superior to their respective CF antigens regarding ELISA sensitivity and specificity (data not shown), confirming our previous report (1).
The reactivity of antibodies in sera from M. avium subsp. paratuberculosis-infected cattle to CF antigens from early-log-phase versus stationary-phase cultures was striking (data not shown). Specific immunodominant M. avium subsp. paratuberculosis antigens apparently were expressed to a greater degree in early- to mid-log-phase cultures, and/or these antigens were degraded as the cultures aged. Alternatively, this observation was due simply to the proportion of relevant antigens in young versus older cultures, i.e., the absolute concentration of critical antigens may be the same, but they are only a small proportion of the total protein found in CF antigens from stationary-phase cultures. Similar observations have been reported for M. tuberculosis. The expression of M. tuberculosis proteins such as alpha-crystallin, antigen 85 complex, PstS-1, l-alanine dehydrogenase, and the 65-kDa antigen qualitatively, but not quantitatively, varied according to the culture growth phase (14, 16, 18).
The analytical sensitivity of the JTC-ELISA was enhanced by using a commercial avidin-biotin conjugate system. Pooling two conjugates also enhanced the assay sensitivity. Eight sera from M. avium subsp. paratuberculosis-infected cattle were ELISA negative using only mouse monoclonal anti-bovine IgG but were strongly positive using polyclonal sheep anti-bovine IgG. Conversely, nine sera from infected cows were reactive, albeit at low ELISA ODs, using mouse monoclonal anti-bovine IgG, but were ELISA negative using polyclonal sheep anti-bovine IgG. Thus, a 10.4% gain in ELISA sensitivity was achieved by using a pool of these two conjugates (data not shown).
Milk samples are collected from all lactating dairy cattle in most herds on a monthly or bimonthly basis for the assessment of milk quality. Utilizing the same milk sample for the diagnosis of paratuberculosis by ELISA is less costly (with a net cost to producers of ∼$5.00/cow) than doing ELISAs on sera by the avoidance of veterinary fees for sample collection and shipment to the laboratory (with a net cost to producers of ∼$9.00/cow). These so-called milk ELISAs for paratuberculosis have been shown to correlate with the M. avium subsp. paratuberculosis fecal shedding status of cattle and have an overall diagnostic sensitivity that is equivalent to that of ELISAs done on serum samples (5, 13). Lombard et al. also showed a significant positive correlation between the percentages of five environmental fecal samples collected on dairy farms that were culture positive for M. avium subsp. paratuberculosis and the proportion of the herd that was milk ELISA positive for paratuberculosis (11). A cost-benefit analysis of paratuberculosis control programs in commercial dairy herds indicates that low-cost tests such as the milk ELISA are more economically advantageous than tests of higher accuracy and higher cost, assuming appropriate actions are taken on test results in a timely fashion (7). The JTC-ELISA detected a higher percentage of 78 serum ELISA-positive cows, 91.1%, than did three other paratuberculosis ELISA kits, and it also had the highest agreement, kappa statistic, and linear regression values compared to the ELISA results of other kits on simultaneously collected serum samples (Table 3).
The JTC-ELISA is more sensitive for the detection of fecal culture-positive cattle than commercially available ELISA kits for bovine paratuberculosis and has a diagnostic specificity equivalent to that of the highest-specificity kits available. The assay can be used on either bovine serum or milk and, by changing conjugates, can be used on other ruminant species (data not shown). This, coupled with the low cost and high-throughput capability inherent in ELISA technology, offers the best testing option for the support of paratuberculosis control programs in commercial cattle herds (4).
Acknowledgments
This work was funded by the Johne's Testing Center, School of Veterinary Medicine, University of Wisconsin—Madison, by a grant from Korea Science and Engineering Foundation through the Infection Signaling Network Research Center (R13-2007-020-01000-0) at Chungnam National University, and by the Korea Science and Engineering Foundation through MOST grant R01-2007-000-10702-0.
We are grateful for the donation of strains of M. avium subsp. paratuberculosis by S. Naser, University of Central Florida, the statistical analysis advice of Ian Gardner, and the manuscript editorial assistance of E. J. B. Manning.
Footnotes
Published ahead of print on 11 June 2008.
REFERENCES
- 1.Cho, D., and M. T. Collins. 2006. Comparison of the proteosomes and antigenicities of secreted and cellular proteins produced by Mycobacterium paratuberculosis. Clin. Vaccine Immunol. 13:1155-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho, D., S. J. Shin, A. M. Talaat, and M. T. Collins. 2007. Cloning, expression, purification and serodiagnostic evaluation of fourteen Mycobacterium paratuberculosis proteins. Protein Expr. Purif. 53:411-420. [DOI] [PubMed] [Google Scholar]
- 3.Cocito, C., P. Gilot, M. Coene, K. M. de, P. Poupart, and P. Vannuffel. 1994. Paratuberculosis. Clin. Microbiol. Rev. 7:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins, M. T., I. A. Gardner, F. B. Garry, A. J. Roussel, and S. J. Wells. 2006. Consensus recommendations on diagnostic testing for the detection of paratuberculosis in cattle in the United States. J. Am. Vet. Med. Assoc. 229:1912-1919. [DOI] [PubMed] [Google Scholar]
- 5.Collins, M. T., S. J. Wells, K. R. Petrini, J. E. Collins, R. D. Schultz, and R. H. Whitlock. 2005. Evaluation of five antibody detection tests for diagnosis of bovine paratuberculosis. Clin. Diagn. Lab. Immunol. 12:685-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox, J. C., D. P. Drane, S. L. Jones, S. Ridge, and A. R. Milner. 1991. Development and evaluation of a rapid absorbed enzyme immunoassay test for the diagnosis of Johne's disease in cattle. Aust. Vet. J. 68:157-160. [DOI] [PubMed] [Google Scholar]
- 7.Dorshorst, N. C., M. T. Collins, and J. E. Lombard. 2006. Decision analysis model for paratuberculosis control in commercial dairy herds. Prev. Vet. Med. 75:92-122. [DOI] [PubMed] [Google Scholar]
- 8.Gardner, I. A., and M. Greiner. 2006. Receiver-operating characteristic curves and likelihood ratios: improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet. Clin. Pathol. 35:8-17. [DOI] [PubMed] [Google Scholar]
- 9.Hanley, J. A., and B. J. McNeil. 1983. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839-843. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy, D. J., and G. Benedictus. 2001. Control of Mycobacterium avium subsp. paratuberculosis infection in agricultural species. Rev. Sci. Tech. 20:151-179. [DOI] [PubMed] [Google Scholar]
- 11.Lombard, J. E., B. A. Wagner, R. L. Smith, B. J. McCluskey, B. N. Harris, J. B. Payeur, F. B. Garry, and M. D. Salman. 2006. Evaluation of environmental sampling and culture to determine Mycobacterium avium subspecies paratuberculosis distribution and herd infection status on US dairy operations. J. Dairy Sci. 89:4163-4171. [DOI] [PubMed] [Google Scholar]
- 12.Manning, E. J., and M. T. Collins. 2001. Mycobacterium avium subsp. paratuberculosis: pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. 20:133-150. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen, S. S., Y. T. Grohn, and C. Enevoldsen. 2002. Variation of the milk antibody response to paratuberculosis in naturally infected dairy cows. J. Dairy Sci. 85:2795-2802. [DOI] [PubMed] [Google Scholar]
- 14.Pheiffer, C., J. Betts, P. Lukey, and P. van Helden. 2002. Protein expression in Mycobacterium tuberculosis differs with growth stage and strain type. Clin. Chem. Lab. Med. 40:869-875. [DOI] [PubMed] [Google Scholar]
- 15.Sung, N., and M. T. Collins. 2003. Variation in resistance of Mycobacterium paratuberculosis to acid environments as a function of culture medium. Appl. Environ. Microbiol. 69:6833-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tullius, M. V., G. Harth, and M. A. Horwitz. 2001. High extracellular levels of Mycobacterium tuberculosis glutamine synthetase and superoxide dismutase in actively growing cultures are due to high expression and extracellular stability rather than to a protein-specific export mechanism. Infect. Immun. 69:6348-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber, M. F., M. Nielen, A. G. Velthuis, and H. J. van Roermund. 2008. Milk quality assurance for paratuberculosis: simulation of within-herd infection dynamics and economics. Vet. Res. 39:12. [DOI] [PubMed] [Google Scholar]
- 18.Wiker, H. G., M. Harboe, S. Nagai, and J. Bennedsen. 1990. Quantitative and qualitative studies on the major extracellular antigen of Mycobacterium tuberculosis H37Rv and Mycobacterium bovis BCG. Am. Rev. Respir. Dis. 141:830-838. [DOI] [PubMed] [Google Scholar]
- 19.Willemsen, P. T., J. Westerveen, A. Dinkla, D. Bakker, F. G. van Zijderveld, and J. E. Thole. 2006. Secreted antigens of Mycobacterium avium subspecies paratuberculosis as prominent immune targets. Vet. Microbiol. 114:337-344. [DOI] [PubMed] [Google Scholar]
- 20.Yokomizo, Y., R. S. Merkal, and P. A. Lyle. 1983. Enzyme-linked immunosorbent assay for detection of bovine immunoglobulin G1 antibody to a protoplasmic antigen of Mycobacterium paratuberculosis. Am. J. Vet. Res. 44:2205-2207. [PubMed] [Google Scholar]