Abstract
An oral, microencapsulated anti-colonization factor 6 antigen (meCS6) vaccine, with or without heat-labile enterotoxin with mutation R192G (LTR192G) (mucosal adjuvant), against enterotoxigenic Escherichia coli (ETEC) was evaluated for regimen and adjuvant effects on safety and immunogenicity. Sixty subjects were enrolled into a three-dose, 2-week interval or four-dose, 2-day interval regimen. Each regimen was randomized into two equal groups of meCS6 alone (1 mg) or meCS6 with adjuvant (2 μg of LTR192G). The vaccine was well tolerated and no serious adverse events were reported. Serologic response to CS6 was low in all regimens (0 to 27%). CS6-immunogloublin A (IgA) antibody-secreting cell (ASC) responses ranged from 36 to 86%, with the highest level in the three-dose adjuvanted regimen; however, the magnitude was low. As expected, serologic and ASC LT responses were limited to adjuvanted regimens, with the exception of fecal IgA, which appeared to be nonspecific to LT administration. Further modifications to the delivery strategy and CS6 and adjuvant dose optimization will be needed before conducting further clinical trials with this epidemiologically important class of ETEC.
The significant worldwide burden of diarrheal disease due to enterotoxigenic Escherichia coli (ETEC) in children of developing countries has been well documented (5, 32, 39, 45). In addition, military and civilian travelers are at high risk of acquiring ETEC-associated diarrhea when visiting regions where it is endemic (1, 6, 15, 19, 21), which prompts ongoing vaccine development. Prevalence surveys have documented the significant contribution of CS6-ETEC (ETEC containing surface antigen 6; 10 to 37% of ETEC isolates) relative to the overall global ETEC burden (13, 29, 33, 36, 37, 42, 46, 47). Based on this information, this unique nonfimbrial colonization factor (CF) was selected as a target antigen for vaccine development (17, 18, 24, 25, 27, 32, 43, 48).
ETEC vaccine development has been based on two strategies: blocking adherence and/or toxin activity (32, 39). CFs are necessary for ETEC to adhere to the intestinal mucosal lining. After adherence, heat-labile toxin (LT), heat-stable toxin (ST), or both are expressed, resulting in watery diarrhea. The mucosal adjuvant LT is immunogenic but causes unacceptable gastrointestinal toxicity (3, 28). LTR192G is a mutant form of LT which retains immunogenicity and adjuvanticity with greatly reduced toxicity, as documented by in vitro assays, animal models, and clinical trials (3, 11, 14).
Incorporation of CFs into poly(dl-lactide-co-glycolide) (PLG) microspheres has been attempted in order to improve antigen delivery and uptake at mucosal inductive sites (30). In a previous study, microencapsulated CS6 (meCS6) was safe and well tolerated when delivered orally as either a 1- or 5-mg dose in a three-dose, 2-week interval regimen (23). The highest immune responses were observed in subjects (n = 5) receiving the 1-mg dose in a buffered solution, with 80% antibody-secreting cell (ASC) (median, 30 ASCs per 106 peripheral blood mononuclear cells [MNCs]), 80% serum immunoglobulin G (IgG), and 60% serum IgA responses. A dose effect on the immune response was not observed at the 5-mg dose, with only 40% of subjects having either ASC or serologic responses.
Our aim in this study was to evaluate the safety and immunogenicity of meCS6 (1 mg) with and without LTR192G (2 μg), comparing two different regimens. In addition, the LTR192G adjuvant effect on the CS6 immune response was investigated.
(This study was presented as a poster at the Ninth Annual Vaccine Conference, Baltimore, MD, 8 May 2006.)
MATERIALS AND METHODS
Volunteer eligibility and randomization.
Sixty healthy male and female subjects, 18 to 45 years of age, were recruited from the Washington, DC, metropolitan area. Exclusion criteria included residential, travel, or occupational factors in which a potential for exposure to ETEC, LT, or cholera toxin existed; abnormal bowel habits (defined as <3 stools per week or frequent liquid/loose stools); chronic gastrointestinal illness or major gastrointestinal surgery; more than weekly use of antidiarrheal, anticonstipation, or antacid therapy; positive serology results for human immunodeficiency virus 1, hepatitis B, or hepatitis C; immunosuppression or oral steroid medication; nursing or lactating women; and allergy to any vaccine. The subjects eligible for the study were randomized using block sizes of three into one of four treatment regimens as shown in Table 1.
TABLE 1.
Baseline characteristics of study participants
| Characteristic | Regimena
|
|||
|---|---|---|---|---|
| Day 0-14-28
|
Day 0-2-4-6
|
|||
| meCS6+ | meCS6− | meCS6+ | meCS6− | |
| No. of subjects | 15 | 15 | 15 | 15 |
| Ageb | 31 (18-45) | 36 (20-45) | 31 (20-44) | 30 (19-45) |
| Male genderc | 9 (60) | 10 (67) | 11 (73) | 6 (40) |
| Race/ethnicityc | ||||
| African-American | 8 (53) | 8 (53) | 4 (26) | 9 (60) |
| Asian-American | 1 (7) | 0 (0) | 0 (0) | 1 (7) |
| Caucasian | 5 (33) | 5 (33) | 7 (47) | 5 (33) |
| Hispanic, Caucasian | 0 (0) | 0 (0) | 1 (7) | 0 (0) |
| Hispanic, non-Caucasian | 1 (7) | 0 (0) | 2 (13) | 0 (0) |
| Pacific Islander | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
| Serologyd | ||||
| Anti-CS6 IgA | 5.6 | 5.2 | 6.6 | 7.1 |
| Anti-LT IgA | 25.7 | 25.7 | 16.2 | 13.8 |
| Anti-CS6 IgG | 7.2 | 7.2 | 28.8 | 38.9 |
| Anti-LT IgG | 69.2 | 104.7 | 49.0 | 63.1 |
| Fecal IgAe | ||||
| Anti-CS6 IgA | 2.6 | 3.3 | 3.4 | 4.6 |
| Anti-LT IgA | 3.2 | 4.5 | 6.7 | 5.9 |
| ASCsf | ||||
| Anti-CS6 IgA | 0.6 (5) | 2.2 (3) | 0.8 (2) | 1.0 (1) |
| Anti-LT IgA | 1.0 (4) | 18.8 (2) | 1.0 (3) | 1.5 (2) |
+, with adjuvant; −, without adjuvant.
Mean (range).
Frequency (percent).
Geometric mean titer.
Vaccine-specific IgA per total IgA (ELISA units/μg).
Median number of ASCs per million MNCs among volunteers with baseline values of >0 (number of volunteers with baseline values of >0).
Vaccines. (i) Vaccine composition.
The test articles were meCS6, containing the putative colonization factor CS6, and LTR192G, a mucosal adjuvant and modified ETEC enterotoxin. meCS6 consists of purified CS6 antigen encapsulated in PLG microspheres, produced using current good manufacturing practices (cGMP) at the Walter Reed Army Institute of Research (WRAIR), Silver Spring, MD. The four genes required for CS6 expression were cloned into an ETEC strain E8775 plasmid vector pUC19 with ampicillin resistance replaced by kanamycin resistance (48). The plasmid was transformed into E. coli HB101, with the resulting strain, M346, used to prepare the vaccine master cell bank. Bulk purified CS6 was loaded into PLG microspheres by the solvent evaporation technique (7). The final product was lyophilized in single-dose vials containing 81.3 mg total dry weight of microspheres with 0.951 mg CS6 protein. The recombinant E. coli strain JM83(pLC326), used as a source of LTR192G, was constructed at Tulane University in the laboratory of John Clements (11). LTR192G was manufactured under cGMP at the WRAIR. The final product was lyophilized in vials containing 1,000 μg of protein per vial. The test articles were delivered in CeraVacxII (Cera Products, LLC, Jessup, MD), an oral electrolyte solution (osmolality, 270 mmol/liter), containing 1.5 g of sodium bicarbonate, 0.375 g of trisodium citrate, and 5.25 g of rice syrup solids in the administered volume. This product was manufactured under cGMP and USDA/FDA guidelines. CeraVacxII (derivative of CeraVacx) was designed and formulated as a buffer to optimize the maintenance of a favorable pH throughout the gastric digestion process, increase gastric emptying, and increase absorption of buffer after completing its course through the stomach (35).
(ii) Vaccine administration.
Single-dose vials of meCS6 were reconstituted with 10 ml of CeraVacxII and then mixed with 140 ml of CeraVacxII to a final volume of 150 ml per dose. On each vaccination day, LTR192G was reconstituted with sterile water and diluted in phosphate-buffered saline (PBS) to achieve a dose of 2 μg per 0.4 ml solution and added just prior to ingestion (within 30 min). Subjects fasted for 90 min pre- and postvaccination.
(iii) Postvaccination follow-up.
Safety monitoring included the following: 30-minute postvaccination observation, 24-hour telephone clinical checks, and diaries (capturing the number and grade of bowel movements). Additional clinical visits occurred through postvaccination day 28. For the three-dose, 2-week dosing interval (Day 0-14-28 regimen), diaries were provided for a 7-day period after each dose. For the four-dose, 2-day dosing interval regimen (Day 0-2-4-6 regimen), diaries were provided during the 6-day vaccination period and for 7 days after the final dose. Safety laboratory assessments (complete blood count and analysis of serum aspartate aminotransferase, alanine aminotransferase, and creatinine) were checked on days 7 and 35 for the Day 0-14-28 regimen and days 9 and 21 for the Day 0-2-4-6 regimen. Adverse events (AEs) were graded as mild (transient discomfort that did not interfere in a significant manner with the subject's normal function), moderate (produced limited impairment of function and could require therapeutic intervention but produced no sequelae), or severe (resulted in a marked impairment of function and could lead to temporary inability to resume usual life pattern; might also produce sequelae that require prolonged therapeutic intervention).
Laboratory methods. (i) Serology assays.
The Day 0-14-28 group had their serum assayed for CS6- and LT-specific IgA and IgG antibodies at baseline, 2 weeks postdose for the first two doses, and 4 weeks postdose for the third dose. The Day 0-2-4-6 group serology assays were done at baseline and 3, 7, 14, and 28 days after the dose series. Anti-CS6 antibodies were measured by enzyme-linked immunosorbent assay (ELISA) (2, 16, 20). Individual microtiter wells (Nunc immunoplates) were coated with 100 μl of a 1.0-μg/ml PBS solution of CS6 antigen, incubated overnight at 37°C, washed with PBS, and blocked with 0.1% bovine serum albumin (BSA) (Sigma). The CS6 antigen was generated at bench scale using the identical fermentation and purification process as for the cGMP (vaccine) lot, with a purity of >95% as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and densitometric scanning (10). Threefold serial dilutions of sera were made (starting dilution of 1:5), and plates were incubated at room temperature (RT) for 90 minutes. Rabbit anti-human IgA horseradish peroxidase (HRP) conjugate (Jackson) or rabbit anti-human IgG HRP conjugate (Jackson) were added for 90 minutes of incubation at RT. The wells were developed for 20 minutes with orthophenylene diamine (Sigma), followed by optical density (OD) determination at 450 nm in a Titertek spectrophotometer. The end-point titer was determined as the reciprocal of the interpolated dilution giving an A450 of 0.4 OD units above background. Anti-LT antibodies were assayed by GM1-ELISA (38, 40). Microtiter wells were coated with 100 μl of a 0.5-μg/ml PBS solution of GM1 ganglioside (Sigma), incubated at RT overnight, washed with PBS, and blocked with BSA, followed by coating with 100 μl of a 0.5-μg/ml solution of LTR192G (cGMP vaccine lot) in PBS. The procedure continued in the same manner as for anti-CS6 antibodies. Serum samples that demonstrated a ≥2-fold rise in titer above the baseline were considered to indicate seroconversion, provided that the postimmunization titer was ≥10.
(ii) Fecal IgA assay.
The Day 0-14-28 groups had assays done for CS6- and LT-specific fecal IgA at baseline and weekly thereafter (except for day 49) until day 56. The Day 0-2-4-6 groups had assays at baseline and at days 4, 6, 9, 14, 21, and 34. Stool samples were accepted for up to 8 h after defecation, if properly stored and transported to the study site by the subjects (in a transport container with an ice pack). The samples were frozen at −80°C until the time of assay. Fecal extracts were assayed based on methods previously described (2). For total IgA, Nunc immunoplate wells were coated with 100 μl goat anti-human F(ab′)2 IgG (Jackson) in a 1.0-μg/ml PBS solution. The procedure was continued in the same manner as the serum antibody measurements, using threefold dilutions of fecal extracts and 100 μl/well of goat anti-human IgA HRP (Jackson; 1:3,000 dilution). Anti-CS6 and -LT IgA determinations were done in the same fashion, using CS6 (1.0 μg/ml in PBS) and GM1 (0.5 μg/ml in PBS) (Sigma) followed by LTR192G as a coating antigen and 100 μl/well of rabbit antihuman IgA HRP diluted 1:2,500 in 0.1% BSA-PBS-0.05% Tween as the secondary antibody. For CS6 and LT, BSA well OD values were subtracted from the test antigen well OD values to adjust for background. A positive response was defined as a ≥2-fold increase in vaccine-specific-IgA antibody titer per total IgA between baseline and postvaccine samples. Samples were excluded if the total IgA content was <10 μg/ml, if the difference in total IgA concentration between baseline and postvaccine specimens was >10-fold, or if specific baseline titers were ±2 standard deviations above the group mean (unless there was a significant difference between baseline and postvaccine titers).
(iii) IgA-ASC assay.
Whole blood was collected in EDTA-containing tubes at baseline and 1 week after each dose of vaccine for the Day 0-14-28 groups. For the Day 0-2-4-6 groups, specimens were obtained at baseline and on days 6, 9, 14, 21, and 34. An enzyme-linked immunospot assay was initially conducted in real time using fresh EDTA-blood specimens according to previously described methods (23, 44). Due to technical difficulties in interpreting ASC well counts, ASCs were assayed with cryopreserved cells from the same volunteer specimens. MNCs were isolated by using a Ficoll-Hypaque density gradient and cryopreserved in a commercially available freezing medium (C-6164; Sigma Chemicals) at a controlled rate and stored in liquid nitrogen until assayed (4). Antigen-specific IgA-ASCs were determined using an enzyme-linked immunospot assay. Briefly, after thawing and washing, viability was determined using Guava ViaCount reagent (Guava Technologies; 4000-0041). Viable cells (average viability, 91.1% ± 3.2%; n = 277) were adjusted to 3.3 × 106/ml in medium containing 10% fetal calf serum, 2 mM l-glutamine, and 50 μg/ml gentamicin (complete medium). Multiscreen immunoplates (Millipore S2EM004M99) were coated with antigens. After blocking with 5% fetal calf serum in RPMI, 100 μl of complete medium containing 3.3 × 105 MNCs was added in triplicate. Plates were incubated for 4 to 5 hours at 37°C in a 5% CO2 environment, followed by an additional 2-hour incubation at 37°C after addition of 0.025 μg of alkaline phosphate-conjugated goat anti-human IgA (KPL). Spots were developed using Nitro Blue Tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate substrate (SigmaFast; Sigma Chemicals). After an additional 15 minutes of incubation, the plates were extensively rinsed with water and air dried. Spots were counted using a CTL spot analyzer (Cellular Technology Ltd.). For each antigen, data were expressed as the number of ASCs per 106 MNCs. A positive response was defined as a ≥2-fold increase over the baseline value of antigen-specific ASCs per 106 MNCs, when the number of ASCs was ≥0.5 per 106 MNCs in the baseline sample and ≥1.0 ASCs per 106 MNCs if preimmune ASCs were <0.5 per 106 MNCs.
(iv) Statistical analysis.
The primary safety outcome was enterotoxicity that was possibly or probably related to the test articles. Enterotoxicity was indicated by postimmunization gastrointestinal AEs, including loose stools, diarrhea (defined as ≥3 loose/liquid stools in a 24-hour period), abdominal cramps, nausea, and vomiting. Gastrointestinal AEs with onset within 3 days postvaccination were considered probably related to the test articles, and those with onset within 4 to 6 days were considered possibly related. Rates of all AEs were analyzed by Pearson's chi-square or Fisher's exact test, as appropriate, to compare regimens. Safety data were analyzed for all subjects who received at least one dose of a test article. The primary immunologic outcomes were direct and indirect mucosal immune responses (fecal IgA and ASCs) and systemic (serologic) immune responses (IgG and IgA) directed against both vaccine antigens. Immunologic outcomes were compared between groups receiving the two dosing regimens and between vaccine subsets (2 μg of LTR192G or no LTR192G). The outcomes analyzed were responder rates, geometric mean antibody titers, median peak fold rises (PFRs) in antibodies, and median maximum (or peak) ASC counts. Between-group comparisons were made with nonparametric tests (Kruskal-Wallis test for continuous data and Fisher's exact test for categorical data) unless assumptions were fulfilled for the Student's t test or chi-square test. Paired t tests were used to compare individual postvaccination responses to baseline responses within each treatment group. All statistical tests were performed using standard statistical software programs and were interpreted in a two-tailed fashion using a P of <0.05 to indicate a significant difference.
RESULTS
Demographics and study conduct.
Sixty subjects were enrolled and received at least one dose of vaccine. Volunteer demographics overall were an age range of 18 to 45 years (mean 32), 60% male, and ethnicity as follows: African-American, 48%; Caucasian, 37%; Hispanic, 7%; Asian, 5%; and other, 3%. Table 1 characterizes volunteer demographics by study group (regimen). The four groups were similar in demographic makeup. Fifty-six subjects (93%) completed the entire vaccination series, and 58 (97%) complied with safety monitoring at least 28 days after receipt of their last vaccine dose. Three of the subjects were unable to comply with the study schedule, and one volunteer did not receive vaccine after the first dose due to an AE not related to the test articles.
Safety profile.
Overall, the vaccine was well tolerated regardless of dosing regimen or receipt of LTR192G, and no serious AEs occurred. Table 2 shows the frequency of surveyed symptoms possibly or probably related to the test articles. Intermittent loose stool was the most-common surveyed symptom (37% of subjects), although only two subjects met the definition for diarrhea. Abdominal cramping (13% of subjects) and nausea (12% of subjects) were the next most commonly reported symptoms; a headache and malaise were each reported by one volunteer. There was no significant difference in the frequency (or number, in the case of number of loose stools) of these events across vaccine groups.
TABLE 2.
Frequency of volunteers experiencing AEsa
| AE | No. (%) for regimenb:
|
|||
|---|---|---|---|---|
| Day 0-14-28
|
Day 0-2-4-6
|
|||
| meCS6+ | meCS6− | meCS6+ | meCS6− | |
| Diarrheac | 0 (0) | 1 (7) | 1 (7) | 0 (0) |
| Loose stoolsd | 5 (33) | 5 (33) | 5 (33) | 5 (33) |
| Abdominal cramps | 1 (7) | 2 (13) | 4 (27) | 1 (7) |
| Nausea | 0 (0) | 4 (27) | 1 (7) | 2 (13) |
Limited to actively monitored events determined to be possibly, probably, or definitely related to the vaccine (determined by temporal relationship and no other clearly evident cause). No vaccine-related vomiting or fever was noted. One person in the Day 0-2-4-6 meCS6− group had a headache and malaise that were possibly vaccine related.
+, with adjuvant; −, without adjuvant.
Three or more loose liquid stools per 24 h.
Two or less loose or liquid stools per 24 h. Does not include subjects with diarrhea.
One subject meeting the diarrhea definition reported four grade 4 stools (without associated symptoms) within 24 hours after receiving the fourth dose of meCS6 plus adjuvant in the Day 0-2-4-6 regimen. The diarrhea did not interfere with daily activities and resolved spontaneously. The other subject meeting the diarrhea definition reported four grade 3 stools over a 4-hour period (with associated moderate upper abdominal pain and nausea) 6 days after the first dose of meCS6 without adjuvant (Day 0-14-28 regimen). Resolution of all symptoms occurred within 24 hours, with no symptoms with subsequent doses.
Another subject reported abdominal cramps precluding daily activities in the evening following the first dose of meCS6 with adjuvant (Day 0-2-4-6 regimen). The cramps lasted 90 min, during which time two grade 3 stools were passed, with spontaneous resolution. Reports of moderate grade AEs included two subjects with short-lived abdominal cramps. All other symptoms possibly or probably related to the test articles were graded mild. No clinically significant changes from baseline levels in hematology or chemistry safety laboratory values occurred in any group. Fifty-one (85%) subjects were available for phone follow-ups 6 months after the last vaccination. None reported any serious health problems or hospitalizations since study completion.
Immune responses.
A total of 57 subjects (95%) received at least two (Day 0-14-28) or three (Day 0-2-4-6) vaccine doses required for inclusion of data in the primary immunologic analyses. Table 3 presents summary response rates and PFRs or maximum numbers of cells (ASCs) for all groups.
TABLE 3.
Frequency of responses to vaccine antigens by group
| Antigen | Response type | Result for regimena:
|
|||
|---|---|---|---|---|---|
| Day 0-14-28
|
Day 0-2-4-6
|
||||
| meCS6+ | meCS6− | meCS6+ | meCS6− | ||
| CS6 | IgA (feces) | 14 (50.4) | 21 (5.1) | 43 (8.2) | 40 (7.2) |
| IgA (serum) | 7 (4.4) | 0 (n/a)b | 14 (11.0) | 20 (15.0) | |
| IgG (serum) | 21 (3.9) | 0 (n/a) | 21 (4.6) | 27 (3.1) | |
| IgA-ASC | 86 (3.0) | 36 (1.7) | 57 (2.7) | 47 (2.5) | |
| LT | IgA (feces) | 50 (10.0) | 57 (8.3) | 71 (7.7) | 53 (6.0) |
| IgA (serum) | 57 (4.1) | 0 (n/a) | 36 (7.4) | 0 (n/a) | |
| IgG (serum) | 43 (9.7) | 7 (2.3) | 36 (5.4) | 0 (n/a) | |
| IgA-ASC | 71 (5.5) | 0 (n/a) | 57 (3.3) | 7 (2.3) | |
Serology responses are summarized as the percentages of volunteers meeting the responder definition and the median PFRs from the baseline titer among responders (in parentheses). ASC responses are summarized as the percentages of volunteers meeting the responder definition and the median maximum numbers of ASCs among responders (in parentheses). +, with adjuvant; −, without adjuvant.
n/a, not applicable.
Serology.
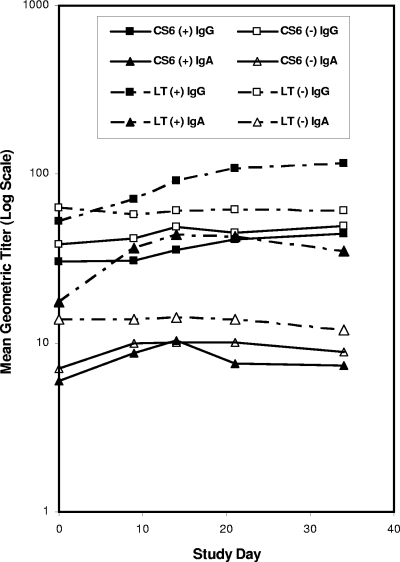
Response rates and serology kinetics for anti-CS6 and -LTR192G antigens are presented in Table 3 and Fig. 1 and 2. Baseline titers (Table 1) of anti-CS6 IgG in the Day 0-2-4-6 group were comparable to those in the D. E. Katz study (unpublished data), while those in the Day 0-14-28 group were significantly lower (P < 0.0001). Response rates to CS6 were similar (21 to 27%) in three groups, with no responders in the fourth group (unadjuvanted Day 0-14-28). The IgG response rate to CS6 in the Day 0-2-4-6 group without adjuvant trended toward being significant compared with the Day 0-14-28 group without adjuvant (Fisher's exact test; P = 0.10), with titer PFRs significantly higher (Wilcoxon rank sum test; P = 0.04). Subjects in the Day 0-2-4-6 regimen had the highest anti-CS6 IgG titers, with levels three to four times those in the Day 0-14-28 regimen, regardless of responder status, and almost 10 times higher when restricted to responders alone. Baseline anti-CS6 IgA titers, response rates, and PFRs were similar across all groups. No significant differences in IgG or IgA response rates to LTR192G occurred between the two groups that received this test article. One volunteer who did not receive LTR192G was classified as a responder.
FIG. 1.
Serologic responses for Day 0-14-28 regimen. +, with adjuvant; −, without adjuvant.
FIG. 2.
Serologic responses for Day 0-2-4-6 regimen. +, with adjuvant; −, without adjuvant.
Fecal IgA.
The response rate to CS6 was higher in the Day 0-2-4-6 groups (12 of 29; 41%) than in the Day 0-14-28 groups (5 of 28; 18%), regardless of the receipt of LTR192G (Table 3) (Fisher's exact test; P = 0.08). The highest overall titers and PFRs occurred in the Day 0-14-28 group receiving LTR192G. No significant difference in response rates or PFRs occurred for anti-LT fecal IgA, regardless of receipt of LTR192G.
IgA-ASCs.
LTR192G adjuvanted the CS6-specific response rate in the Day 0-14-28 regimen (86% in the adjuvanted group versus 36% in the unadjuvanted group [Fisher's exact test; P = 0.02]), with significantly higher median peak ASCs per 106 MNCs among responders (Table 3) (Wilcoxon rank-sum test; P = 0.04). The ASC response rate was also higher (not significant) in the Day 0-2-4-6 adjuvanted group versus the unadjuvanted group. The median peak number of anti-CS6 ASCs per 106 MNCs was ≤3 across all groups. Forty-two percent of anti-CS6 and 60% of anti-LT peak responses were seen after the third vaccine dose in the Day 0-14-28 adjuvanted regimen. In all other groups, maximum ASC counts were evenly distributed across all vaccine doses.
DISCUSSION
Our study demonstrated that meCS6, with or without LTR192G, was safe when administered in two different regimens (four-dose, 2-day interval and three-dose, 2-week interval), with an anti-CS6 ASC response rate of 86% when adjuvanted in the three-dose regimen. One episode of diarrhea occurred in 1 of 30 subjects, which was consistent with LTR192G-related enterotoxicity (four-dose adjuvanted group). In contrast, in a Helicobacter pylori killed whole-cell vaccine study (placebo controlled), with and without LTR192G (25 μg), diarrhea occurred in 6 of 31 (19%) subjects, all from adjuvanted vaccine groups (26). Loose stools not meeting the definition of a diarrhea episode, a more-sensitive indicator of enterotoxicity, were evenly distributed across the vaccine groups in the current study.
PLG-encapsulated and nonencapsulated CS6 antigens have been evaluated for safety and immunogenicity in recent studies of purified CFs and enterotoxoids. In the study by Güereña-Burgueño et al., nonencapsulated CS6 was administered transcutaneously with and without LT (500 μg) at 0, 1, and 3 months with good anti-CS6 serologic and ASC responses in the adjuvanted groups (14).
The rationale for incorporating CS6, an antigen intended to target the mucosal immune system, into a microparticle system was informed by encouraging preclinical studies testing oral immunization using PLG vaccines (7, 31). In humans, there has been limited experience with enteric vaccines incorporated into PLG microspheres (41). PLG-encapsulated and nonencapsulated CS6 antigens were administered orally in the study by Katz et al. (23). In five subjects who received 1 mg of unadjuvanted meCS6 with buffer, three were anti-CS6 IgA responders (PFRs of 17 to 77), four were anti-CS6 IgG responders (PFRs of 4 to 153), and four were IgA-ASC responders (peak of 6 to 41 spots per 106 MNCs).
In comparing results in the unadjuvanted Day 0-14-28 group (n = 14) in this study with those for the smaller group (n = 5) in the Katz et al. study (no adjuvant used), which received the same regimen, we note considerable differences in qualitative and quantitative responses to CS6. A factor that may have accounted for the difference in immunogenicity in our study is a new lot of meCS6 with some differences in various parameters compared to the lot used by Katz et al. Two characteristics of the two lots for consideration are the proportion of meCS6 with the desired particle size of 4 to 11 μg (94%) in this lot versus the proportion (52%) in the previous lot and the in vitro release of CS6 at 24 h (39%) in this lot versus the release (53%) in the previous lot. The core antigen load of meCS6 was similar in both lots (1.17% in this lot versus 1.01% in the previous lot). Whether any of these differences in vaccine characteristics affected the immunogenicity of the vaccine in humans is not known. The effects of the microencapsulation process on specific epitopes of the CS6 bulk-purified protein in relation to the specific uptake in the human small intestine are unknown. Preclinical immunogenicity measures done on both meCS6 lots demonstrated good immune responses when the vaccine was given subcutaneously to rabbits and intranasally to mice. Mice did not respond to vaccine delivered orally (8). The same lots and clinical formulations used in this study were also tested for safety and immunogenicity in Aotus nancymae monkeys using the same doses and schedules as in the clinical trial. The Day 0-14-28 regimen was more immunogenic (only serologic responses were measured). Six of seven (86%) monkeys given 1 mg of meCS6 in buffer orally without adjuvant had serum IgG responses with mean PFRs of 78.7 ± 7.8 (22). Currently, the sensitivity of in vitro and animal immunogenicity tests for predicting CS6 immune responses in humans is unknown. The vaccination procedures had what are seemingly minor differences. In the Katz et al. study, the vaccine, reconstituted in 100 ml of CeraVacx, was ingested, followed by 50 ml of CeraVacx without vaccine within 5 min. In our study, the vaccine was reconstituted in 150 ml of CeraVacxII (same buffering formula as CeraVacx, with a small amount of flavoring added), with no postdose buffer.
Sample size differences between the two studies (statistical effects) may explain some of the difference in the results in the two studies. The impressive serologic and IgA-ASC response rates and the magnitude of responses seen in the small (n = 5) group in the Katz et al. study were unique to that group compared to results for the other groups of three to five subjects, even those who received 5 mg of meCS6 with buffer. Potential differences in the study populations' prior immunity may explain part of the variability in results. Demographic factors in our study populations were comparable to those of the first meCS6 study by Katz et al. (23): residence in the Washington, DC, metropolitan region; age range of 21 to 44 years (mean, 40); 66% male; ethnicity (African-American, 60%; Caucasian, 21%; Hispanic, 3%; other, 8%); and 80% of subjects completing the entire vaccination series.
There is limited information about the immune response to CS6-ETEC in natural disease. Immune responses to CS6-ETEC infections have been measured in Department of Defense personnel with diarrhea in Incirlik, Turkey. In 28 cases of ETEC diarrheal illness with CS6 as the only CF and with no other enteric pathogens isolated, mean peak anti-CS6 IgA titers were 20, mean peak anti-CS6 IgG titers were 240, and median peak IgA-ASC counts were 5 spots per 106 MNCs (C. Porter, unpublished data). These immune responses may have been blunted by antibiotic treatment given upon presentation to medical facilities. While there are no well-established immune protective correlates against ETEC infections, naturally acquired IgG anti-CFA/I antibody levels were protective against subsequent infections with CFA/I-ETEC in young children (34). In an experimental infection model, CS6-ETEC strain B7A (serotype 0148H:28/LT+ST+) immune responses were similar to those seen in our study (9).
When our Day 0-14-28 adjuvanted group immune responses are compared to all CS6 subunit vaccine responses, the response rate is at the high end of the spectrum for ASC response (but with a lower magnitude, in general) and the lower end for serologic rates and magnitudes of response (14, 23). This group had higher ASC responses than subjects challenged with ETEC strain B7A, with similar magnitudes in responders and similar serologic responses. The magnitude of response in our study group is similar to that seen in natural CS6-ETEC infections. The ASC response rate and magnitudes in this group are also comparable to those reported against other CFs (CFA/I, CS1/3, CS2, and CS4) present in an inactivated ETEC whole-cell vaccine. Except for anti-CFA/I, serum IgA responses were also similar compared to these other CFs (2).
The immune responses observed in our study were lower in all groups than prospectively determined thresholds we considered necessary for further testing of the current formulation of meCS6. While an 86% anti-CS6 ASC response was observed in the Day 0-14-28 adjuvanted regimen, low peak ASC counts and poor serologic responses accompanied this result. Our study may have been limited by a suboptimal dose of meCS6 or LTR192G, a suboptimal ratio of meCS6 to LTR192G, or by not having both test articles coformulated in the microspheres. The microencapsulation technology itself may need further refinements before this delivery system can be taken forward into advanced development, such as targeting ligands or surface exposing the antigen (12, 30). Further concerns are the lack of information known about CS6 compared to better-characterized CFs such as CFA/I.
In conclusion, although CS6 is a logical vaccine candidate, the product was not as adequately immunogenic in the current formulation as a microsphere vaccine. However, CS6-ETEC strains are prevalent in many areas where ETEC is endemic, and they are often the only pathogen isolated from diarrheal cases. More research is needed to characterize the role of CS6 in the pathogenicity of ETEC infections in order to develop an effective broad-spectrum ETEC vaccine.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy of the Department of the Navy, Department of Defense, or the U.S. Government.
This work was supported and funded by the Military Infectious Disease Research Program, NMRC Work Unit Number A0055. The study protocol was approved by the Naval Medical Research Center Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects.
J.A.L. is a military service member. This work was prepared as part of her official duties.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Adkins, H., B. Merrell, T. O'Rourke, and P. Echeverria. 1990. Travelers' diarrhea among U.S. Navy and Marine Corps personnel during a Western Pacific deployment. Mil. Med. 155:111-116. [PubMed] [Google Scholar]
- 2.Åhrèn, C., M. Jertborn, and A. M. Svennerholm. 1998. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect. Immun. 66:3311-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee, S., A. Medina-Fatimi, R. Nichols, D. Tendler, M. Michetti, J. Simon, C. P. Kelly, T. P. Monath, and P. Michetti. 2002. Safety and efficacy of low dose Escherichia coli enterotoxin adjuvant for urease based oral immunisation against Helicobacter pylori in healthy volunteers. Gut 51:634-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baqar, S., A. A. Nour El Din, D. A. Scott, A. L. Bourgeois, A. S. Mourad, M. T. Kleinosky, M. J. Oplinger, and J. R. Murphy. 1997. Standardization of measurement of immunoglobulin-secreting cells in human peripheral circulation. Clin. Diagn. Lab. Immunol. 4:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black, R. E. 1993. Epidemiology of diarrhoeal disease: implications for control by vaccines. Vaccine 11:100-106. [DOI] [PubMed] [Google Scholar]
- 6.Black, R. E. 1990. Epidemiology of travelers' diarrhea and relative importance of various pathogens. Rev. Infect. Dis. 12(Suppl. 1):S73-S79. [DOI] [PubMed] [Google Scholar]
- 7.Byrd, W., A. DeLorimier, Z. R. Zheng, and F. J. Cassels. 2005. Microencapsulated subunit vaccine approach to enterotoxigenic Escherichia coli and other mucosal pathogens. Adv. Drug Deliv. Rev. 57:1362-1380. [DOI] [PubMed] [Google Scholar]
- 8.Byrd, W., and F. J. Cassels. 2006. Intranasal immunization of BALB/c mice with enterotoxigenic Escherichia coli colonization factor CS6 encapsulated in biodegradable poly(DL-lactide-co-glycolide) microspheres. Vaccine 24:1359-1366. [DOI] [PubMed] [Google Scholar]
- 9.Coster, T. S., M. K. Wolf, E. R. Hall, F. J. Cassels, D. N. Taylor, C. T Liu, F. C. Trespalacios, A. DeLorimier, D. R. Angleberger, and C. E. McQueen. 2007. Immune response, ciprofloxacin activity, and gender differences after human experimental challenge by two strains of enterotoxigenic Escherichia coli. Infect. Immun. 75:252-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLorimier, A. J., W. Byrd, E. R. Hall, W. M. Vaughn, D. Tang, Z. J. Roberts, C. E. McQueen, and F. J. Cassels. 2003. Murine antibody response to intranasally administered enterotoxigenic Escherichia coli colonization factor CS6. Vaccine 21:2548-2555. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson, B. L., and J. D. Clements. 1995. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect. Immun. 63:1617-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Florence, A. T. 1997. The oral absorption of micro- and nanoparticulates: neither exceptional nor unusual. Pharm. Res. 14:259-266. [DOI] [PubMed] [Google Scholar]
- 13.Gaastra, W., and A. M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 14.Güereña-Burgueño, F., E. R. Hall, D. N. Taylor, F. J. Cassels, D. A. Scott, M. K. Wolf, Z. J. Roberts, G. V. Nesterova, C. R. Alving, and G. M. Glenn. 2002. Safety and immunogenicity of a prototype enterotoxigenic Escherichia coli vaccine administered transcutaneously. Infect. Immun. 70:1874-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haberberger, R. L., and D. A. Scott. 1994. Diarrheal disease aboard a U.S. Navy ship after a brief port visit to a high risk area. Mil. Med. 159:445-448. [PubMed] [Google Scholar]
- 16.Hall, E. R., T. F. Wierzba, C. Åhrèn, M. R. Rao, S. Bassily, W. Francis, F. Y. Girgis, M. Safwat, Y. J. Lee, A. M. Svennerholm, J. D. Clements, and S. J. Savarino. 2001. Induction of systemic anti-fimbrial and anti-toxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenic Escherichia coli plus cholera toxin B-subunit vaccine. Infect. Immun. 69:2853-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Helander, A., G. C. Hansson, and A. M. Svennerholm. 1997. Binding of enterotoxigenic Escherichia coli to isolated enterocytes and intestinal mucus. Microb. Pathog. 23:335-346. [DOI] [PubMed] [Google Scholar]
- 18.Helander, A., H. M. Grewal, W. Gaastra, and A. M. Svennerholm. 1997. Detection and characterization of the coli surface antigen 6 of enterotoxigenic Escherichia coli strains by using monoclonal antibodies. J. Clin. Microbiol. 35:867-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyams, K., and A. Bourgeois. 1991. Diarrheal disease during Operation Desert Shield. N. Engl. J. Med. 325:1423-1428. [DOI] [PubMed] [Google Scholar]
- 20.Jertborn, M., C. Åhrèn, J. Holmgren, and A. M. Svennerholm. 1998. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine 16:255-260. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, Z. D., B. Lowe, M. P. Verenkar, D. Ashley, R. Steffin, N. Tornieporth, F. von Sonnenburg, P. Waiyaki, and H. L. Dupont. 2002. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay). J. Infect. Dis. 185:497-502. [DOI] [PubMed] [Google Scholar]
- 22.Jones, F. R., E. R. Hall, D. Tribble, S. J. Savarino, F. J. Cassels, C. Porter, G. Nunez, N. Espinoza, M. Salazar, R. Luckett, and D. Scott. 2005. The New World primate, Aotus nancymae, as a model for examining the immunogenicity of a prototype enterotoxigenic Escherichia coli subunit vaccine. Vaccine 24:3786-3792. [DOI] [PubMed] [Google Scholar]
- 23.Katz, D. E., A. J. DeLorimier, M. K. Wolf, E. R. Hall, F. J. Cassels, J. E. van Hamont, R. Newcomer, M. A. Davachi, D. M. Taylor, and C. E. McQueen. 2003. Oral immunization of adult volunteers with microencapsulated enterotoxigenic Escherichia coli (ETEC) CS6 antigen. Vaccine 21:341-346. [DOI] [PubMed] [Google Scholar]
- 24.Knutton, S., M. M. McConnell, B. Rowe, and A. S. McNeish. 1989. Adhesion and ultrastructural properties of human enterotoxigenic Escherichia coli producing colonization factor antigens III and IV. Infect. Immun. 57:3364-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knutton, S., D. R. Lloyd, and A. S. McNeish. 1987. Identification of a new fimbrial structure in enterotoxigenic Escherichia coli (ETEC) serotype O148:H28 which adheres to human intestinal mucosa: a potentially new human ETEC colonization factor. Infect. Immun. 55:86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotloff, K. L., M. B. Sztein, S. S. Wasserman, G. A. Losonsky, S. C. DiLorenzo, and R. I. Walker. 2001. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect. Immun. 69:3581-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McConnell, M. M., L. V. Thomas, G. A. Willshaw, H. R. Smith, and B. Rowe. 1988. Genetic control and properties of coli surface antigens of colonization factor antigen IV (PCF8775) of enterotoxigenic Escherichia coli. Infect. Immun. 56:1974-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michetti, P., C. Kreiss, K. L. Kotloff, N. Porta, J. L. Blanco, D. Bachmann, M. Herranz, P. F. Saldinger, I. Corthésey-Theulaz, G. Lazonsky, R. Nichols, J. Simon, M. Stolte, S. Ackerman, T. P. Monath, and A. L. Blum. 1999. Oral immunization with urease and Escherichia coli heat-labile enterotoxin is safe and immunogenic in Helicobacter pylori-infected adults. Gastroenterology 116:804-812. [DOI] [PubMed] [Google Scholar]
- 29.Nirdnoy, W., O. Serichantalergs, A. Cravioto, C. LeBron, M. Wolf, C. W. Hoge, A. M. Svennerholm, D. N. Taylor, and P. Echeverria. 1997. Distribution of colonization factor antigens among enterotoxigenic Escherichia coli strains isolated from patients with diarrhea in Nepal, Indonesia, Peru, and Thailand. J. Clin. Microbiol. 35:527-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Hagan, D. T., and N. M. Valiante. 2003. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2:727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Hagan, D. T. 1998. Microparticles and polymers for the mucosal delivery of vaccines. Adv. Drug Deliv. Rev. 34:305-320. [DOI] [PubMed] [Google Scholar]
- 32.Qadri, F., A. M. Svennerholm, A. S. Faruque, and R. B. Sack. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qadri, F., S. K. Das, A. S. Faruque, G. J. Fuchs, M. J. Albert, R. B. Sack, and A. Svennerholm. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J. Clin. Microbiol. 38:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao, M. R., T. F. Wierzba, S. J. Savarino, R. Abu-Elyazeed, N. El-Ghoreb, E. R. Hall, A. Naficy, I. Abdel-Messih, R. W. Frenck, Jr., A. M. Svennerholm, and J. D. Clemens. 2005. Serologic correlates of protection against enterotoxigenic Escherichia coli diarrhea. J. Infect. Dis. 191:562-570. [DOI] [PubMed] [Google Scholar]
- 35.Sack, D. A., J. Shimko, R. B. Sack, J. G. Gomes, K. MacLeod, D. O'Sullivan, and D. Spriggs. 1997. Comparison of alternative buffers for use with a new live oral cholera vaccine, Peru-15, in outpatient volunteers. Infect. Immun. 65:2107-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaheen, H. I., S. B. Khalil, M. R. Rao, R. Abu-Elyazeed, T. F. Wierzba, L. F. Peruski, S. Putnam, J. Navarro, B. Z. Morzy, A. Cravioto, J. D. Clemens, A. M. Svennerholm, and S. J. Savarino. 2004. Phenotypic profiles of enterotoxigenic Escherichia coli associated with early childhood diarrhea in rural Egypt. J. Clin. Microbiol. 42:5588-5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinsland, H., P. Valentiner-Branth, M. Perch, F. Diaz, T. K. Fischer, P. Aaby, K. Mølbak, and H. Sommerfelt. 2002. Enterotoxigenic Escherichia coli infections and diarrhea in a cohort of young children in Guinea-Bissau. J. Infect. Dis. 186:1740-1747. [DOI] [PubMed] [Google Scholar]
- 38.Stoll, B. J., A. M. Svennerholm, L. Gothefors, D. Barua, S. Huda, and J. Holmgren. 1986. Local and systemic antibody responses to naturally acquired enterotoxigenic Escherichia coli diarrhea in an endemic area. J. Infect. Dis. 153:527-534. [DOI] [PubMed] [Google Scholar]
- 39.Svennerholm, A. M., and D. Steele. 2004. Microbial-gut interactions in health and disease. Progress in enteric vaccine development. Best Pract. Res. Clin. Gastroenterol. 18:421-445. [DOI] [PubMed] [Google Scholar]
- 40.Svennerholm, A. M., J. Holmgren, R. Black, M. Levine, and M. Merson. 1983. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J. Infect. Dis. 147:514-522. [DOI] [PubMed] [Google Scholar]
- 41.Tacket, C. O., R. H. Reid, E. C. Boedeker, G. Losonsky, J. P Nataro, H. Bhagat, and R. Edelman. 1994. Enteral immunization and challenge of volunteers given enterotoxigenic E. coli CFA/II encapsulated in biodegradable microspheres. Vaccine 12:1270-1274. [DOI] [PubMed] [Google Scholar]
- 42.Viboud, G. I., M. J. Jouve, N. Binsztein, M. Vergara, M. Rivas, M. Quiroga, and A. M. Svennerholm. 1999. Prospective cohort study of enterotoxigenic Escherichia coli infections in Argentinean children. J. Clin. Microbiol. 37:2829-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Viboud, G. I., M. M. McConnell, A. Helander, and A. M. Svennerholm. 1996. Binding of enterotoxigenic Escherichia coli expressing different colonization factors to tissue-cultured Caco-2 cells and to isolated human enterocytes. Microb. Pathog. 21:139-147. [DOI] [PubMed] [Google Scholar]
- 44.Wenneras, C., A. M. Svennerholm, C. Åhrẽn, and C. Czerkinsky. 1992. Antibody-secreting cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect. Immun. 60:2605-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO. 2006. State of the art of new vaccines, research and development. WHO, Geneva, Switzerland. http://www.who.int/vaccines-documents/DocsPDF06/814.pdf.
- 46.Wolf, M. K. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf, M. K., D. N. Taylor, E. C. Boedeker, K. C. Hyams, D. R. Maneval, M. M. Levine, K. Tamura, R. A. Wilson, and P. Echeverria. 1993. Characterization of enterotoxigenic Escherichia coli isolated from U.S. troops deployed to the Middle East. J. Clin. Microbiol. 31:851-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf, M. K., L. A. de Haan, F. J. Cassels, G. A. Wilshaw, R. Warren, E. C. Boedecker, and W. Gaastra. 1997. The CS6 colonization factor of human enterotoxigenic Escherichia coli contains two heterologous major subunits. FEMS Microbiol. Lett. 148:35-42. [DOI] [PubMed] [Google Scholar]