Abstract
We recently hypothesized that T helper 17 (Th17) cells and their associated cytokines are involved in the development of arthritis following infection with Borrelia burgdorferi. Here, we show that interleukin-23 (IL-23), a survival factor for Th17 cells, is required for the induction of arthritis in mice vaccinated with B. burgdorferi strain 297 and challenged with “Borrelia bissettii.” When Borrelia-vaccinated and -challenged mice were given antibodies to the p19 subunit of IL-23, they failed to develop the histopathological changes observed in untreated vaccinated and challenged mice. In addition, viable B. bissettii organisms stimulated the secretion of IL-17 from Borrelia-immune lymph node cells during in vitro culture. When anti-IL-23 p19 antibody was included in cultures of B. bissettii organisms and Borrelia-immune lymph node cells, the production of IL-17 was reduced to levels observed in cultures containing immune cells alone. Taken together, these results support the hypothesis that Th17 cell-associated cytokines are involved in the development of Borrelia-mediated arthritis. These findings provide insight into previously overlooked immune mechanisms responsible for the development of Lyme arthritis.
The development of severe, destructive arthritis in animals vaccinated and challenged with Borrelia species (12, 29) is dependent upon the production of Borrelia-specific CD4+ T lymphocytes (28) and their cytokines. Recently, we showed that the proinflammatory cytokine interleukin-17 (IL-17) plays a major role in the induction of arthritis following Borrelia vaccination and challenge. Borrelia-vaccinated and -challenged mice given antibodies to IL-17 or to the IL-17 receptor failed to develop the histopathological changes observed in isotype control antibody-treated mice (10, 38). Taken together, these findings suggested that IL-17-producing CD4+ T cells augment the development of arthritis during the adaptive immune response to Borrelia infection.
Although naïve T cells respond to Borrelia infection, their differentiation into T helper 1 (Th1) and Th2 cells is promoted by IL-12 and IL-4, respectively. In the Borrelia vaccination and challenge model of arthritis, a different pathway of T-cell activation occurs because of the existence of memory T cells. It is known that a subset of activated, or memory, T cells distinct from Th1 and Th2 cells, Th17 cells, can produce IL-17 (42). Differentiation into Th17 cells relies on transforming growth factor β and inflammatory cytokines such as IL-6 (8, 31, 46), while the survival and propagation of Th17 cells are dependent on IL-23 (8). IL-23 is produced by activated dendritic cells and macrophages (7, 25) and is known to stimulate the release of IL-17 from activated CD4+ T cells (1). IL-23 is also required for the development of collagen-induced arthritis, and its absence correlates with a reduction in Th17 cells (14, 35) and mRNA encoding IL-17 (35). Therefore, we hypothesized that IL-23 plays a significant role in the IL-17-mediated development of arthritis in Borrelia-vaccinated and -challenged mice.
In this study, we showed that the treatment of Borrelia-vaccinated and -challenged mice with antibodies to the p19 subunit of IL-23 prevented the development of arthritis. In addition, we showed that the incubation of “Borrelia bissettii” organisms with lymph node cells obtained from Borrelia-vaccinated mice resulted in the release of IL-17. Moreover, we demonstrated that the release of IL-17 from these cells was inhibited in the presence of IL-23 neutralizing antibodies.
MATERIALS AND METHODS
Mice.
C57BL/6 inbred male mice weighing 20 to 30 g were obtained from Harlan (Indianapolis, IN). Six- to 12-week-old mice were housed at the animal facility located at the University of Wisconsin Medical School at an ambient temperature of 21°C. Food and acidified water were provided ad libitum during a light and dark cycle of 12 h. Experimental protocols were reviewed and approved by the Animal Care and Use Committee for the University of Wisconsin Medical School.
Organisms and preparation.
Low-passage-number (<10) virulent Borrelia burgdorferi 297 organisms (from human spinal fluid) and B. bissettii organisms (formerly referred to as B. burgdorferi strain C-1-11; from Microtus pennsylvanicus), representing two distinct seroprotective groups among isolates of B. burgdorferi sensu lato (30), were grown at 32°C in modified Barbour-Stoenner-Kelly (BSK) medium (provided by Gundersen Lutheran Medical Center, La Crosse, WI) until the cultures reached a concentration of approximately 107 spirochetes/ml. Samples of 500 μl were then dispensed into 1.5-ml screw-cap tubes (Sarstedt, Newton, NC) containing 500 μl of BSK medium supplemented with 10% glycerol (Sigma Chemical Co., St. Louis, MO). The tubes were sealed and stored at −70°C. Six days prior to the infection of mice, a frozen suspension of spirochetes was thawed, added to 4 ml of BSK medium, and incubated at 32°C. On the day of infection, the organisms were visualized by dark-field microscopy and enumerated using a Petroff-Hausser counting chamber.
Vaccine preparation.
Borrelia organisms were washed three times with phosphate-buffered saline (PBS; pH 7.4). The washed pellet was resuspended in 1% formalin (Sigma-Aldrich, St. Louis, MO), incubated at room temperature with periodic mixing for 30 min, washed three times by centrifugation with PBS (10,000 × g at 10°C for 15 min), and resuspended in PBS. Subsequently, the formalin-inactivated spirochetes were mixed with a sufficient volume of 1% aluminum hydroxide (Reheis, Berkeley Heights, NJ) to yield a concentration of 4 × 106 spirochetes/ml.
Vaccination of mice.
Mice were anesthetized with 15% isoflurane in mineral oil contained in a nose-and-mouth cup and were then injected subcutaneously in each inguinal region with 0.25 ml of the formalin-inactivated whole-cell vaccine preparation. Whole cells of Borrelia are not recommended for the development of a vaccine for humans, based on past concerns associated with other types of whole-cell vaccines (24). However, the ability of whole cells to consistently induce arthritis in mice allows the evaluation of the immunological mechanisms responsible for the arthritis (10, 12, 37, 38, 40).
Infection of vaccinated mice and justification.
Twenty-one days after vaccination with B. burgdorferi isolate 297 in alum, mice were anesthetized with isoflurane contained in a nose-and-mouth cup and injected subcutaneously using a 1-ml tuberculin syringe with a 27-gauge needle in both hind footpads with 50 μl of BSK medium containing 106 viable B. bissettii organisms. Some vaccinated mice were also challenged the following day. It was necessary to infect mice with B. bissettii because vaccination with B. burgdorferi isolate 297 induces protective antibodies that prevent the homologous infection from eliciting arthritis (13, 29). Other infectious Borrelia isolates, besides B. bissettii isolates, are also effective in eliciting the arthritis (13, 44). Controls included vaccinated mice injected with BSK medium alone. No swelling of the hind paws or histopathological changes in these mice have been observed.
The infection of young, genetically susceptible C3H mice with B. burgdorferi sensu stricto is accepted by many investigators as the model of choice for elucidating the mechanisms of Lyme arthritis in humans. Within days of infection with B. burgdorferi, mice exhibit an influx of neutrophils and other leukocytes into the joint capsule, resulting in the inflammation of the synovial lining, tendons, and ligaments (6). Two weeks following infection, these mice display a significant increase in neutrophilic infiltration, fibrin deposition, and synovial hyperplasia (6). Few lymphocytes are found in the tissue. The severity of arthritis peaks approximately 3 weeks after infection and rapidly decreases (6). However, humans do not develop arthritis immediately following infection, as occurs in the mouse model of infection.
Rather, humans develop arthritis several months after infection with B. burgdorferi. This period of time is required for the priming of T cells for the recognition of arthrogenic antigen(s) (e.g., outer surface protein A [OspA]) that induces the arthritis. Essentially, infection with B. burgdorferi vaccinates humans during this time. Although innate immune responses in the joints are involved at this period, the adaptive immune response, especially that of T cells, controls the induction and resolution of arthritis. In our model, we vaccinate mice with B. burgdorferi. Vaccination with Borrelia primes mouse T cells, and then we infect the mice with a heterologous strain of Borrelia that avoids the protective antibody response and yet is capable of activating T cells to elicit the arthritis. It is generally agreed that the arthritis in humans is T cell dependent (34, 49). However, the pathology observed in the murine Lyme arthritis model is mediated by innate immune cells. Indeed, the events of the innate immune response also play a vital role in the activation and expansion of the adaptive immune response. Our model allows for the investigation of both innate (3) and adaptive (38) immune responses. In addition, our vaccination-challenge model fulfills a key requirement (T cells) for representing the immunological events observed in human Lyme arthritis. However, it is important to realize that no mouse model of Lyme arthritis fully mimics the inflammatory events observed in humans infected with B. burgdorferi (39).
Administration of antibodies to the p19 subunit of IL-23.
Purified polyclonal goat antibody with specificity against the p19 subunit of mouse IL-23 was purchased from R & D Systems (Minneapolis, MN). The antibodies were resuspended in PBS (pH 7.4) that had been sterilized by passage through a 0.2-μm-pore-size filter (Acrodisk; Gelman Sciences, Ann Arbor, MI) to yield a concentration of 100 μg/ml. A dose of 0.05 ml of anti-IL-23 p19 antibody or a nonspecific immunoglobulin G1 (IgG1) isotype antibody was administered to mice subcutaneously in the hind paws.
Twenty-one days after vaccination, 16 mice each were infected in the hind paws with 106 viable B. bissettii organisms. One group of eight vaccinated and infected mice was injected in the hind paws with anti-IL-23 p19 antibody 1 h after challenge and daily thereafter for 5 days. In addition, a group of eight vaccinated and infected mice was injected with an IgG1 isotype antibody 1 h after challenge and daily thereafter for 5 days. Control groups included vaccinated, but unchallenged, mice given anti-IL-23 p19 antibody or IgG1 isotype antibody 1 h after their counterparts were challenged and daily thereafter for 5 days. These studies were repeated twice with different technical personnel.
Assessment of swelling.
Swelling was used to evaluate the edematous changes in the hind paws of mice. Hind paws were measured immediately prior to injection with Borrelia organisms, anti-IL-23 p19 antibodies, or IgG1 isotype control antibodies and every other day for 20 days with a digital caliper (Mitutoyo America Corporation, Aurora, IL) with a sensitivity of 0.01 mm. Measurements were obtained by anesthetizing each mouse with isoflurane and measuring the width and thickness of each hind paw joint. The caliper values were averaged to determine the mean caliper value for the severity of swelling.
Preparation of tissues for histological examination.
At 8 and 20 days after infection, mice were euthanized with isoflurane and their hind paws were amputated at midfemur. The paws were then fixed in 10% neutral buffered zinc formalin for 24 h. Subsequently, the paws were placed in decalcifying solution (Lerner Laboratories, Pittsburgh, PA) for 24 h, after which fresh decalcifying solution was added and the paws remained in the solution for another 48 h. Following decalcification, the legs were placed in tissue-embedding cassettes (Fisher Scientific), embedded in paraffin, and cut into 6-μm-thick sections. The sections were placed onto glass slides and stained with hematoxylin and eosin. Sections were cryptically coded, and an unbiased histopathological examination was performed by a board-certified pathologist (T.F.W.). The following terms were used to define the degree of neutrophilic infiltration into the tibiotarsal joint: mild (neutrophilic infiltration of the subsynovial tissues), moderate (neutrophilic infiltration of the subsynovial tissues, synovium, and synovial space), severe (profuse neutrophilic infiltration of the subsynovial tissues, synovium, and synovial space), and severe destruction (profuse neutrophilic infiltration of all tibiotarsal tissues, along with bone and/or cartilage destruction).
In vitro culture and ELISA.
Inguinal lymph node cells were harvested from mice 3 weeks after vaccination with B. burgdorferi. Single-cell suspensions of the lymph node cells were prepared by teasing apart the nodes with forceps and passing the nodes through a sterile nylon mesh screen (Fisher, Hanover Park, IL) into Dulbecco's modified Eagle's medium. Samples consisting of 105 immune lymph node cells were dispensed into each well of 24-well culture plates, and the plates were incubated at 37°C with or without 106 viable B. bissettii organisms per well and with or without 10 μg of anti-IL-23 p19 antibody per well. Eight and 24 h after incubation, cell cultures were centrifuged at 10,000 × g for 10 min. Supernatants were collected, and IL-17 levels were measured using a mouse IL-17A enzyme-linked immunosorbent assay (ELISA) Ready-SET-Go! kit (eBioscience, San Diego, CA). Values were expressed as optical densities at 450 nm after being adjusted to account for the background optical density at 570 nm. A standard curve was generated, and data were expressed as picograms of IL-17.
Detection of anti-OspA borreliacidal antibodies.
Anti-OspA borreliacidal antibodies were detected by a flow cytometric procedure (11, 20). We showed previously that sera from Borrelia-vaccinated and -challenged animals (collected 0 to 3 weeks after infection) have no borreliacidal activity after absorption with recombinant OspA (20), demonstrating that the killing antibodies detected by borreliacidal antibody assays have specificity for OspA. Viable B. bissettii organisms in logarithmic growth phase were enumerated with a Petroff-Hausser counting chamber and diluted with fresh BSK medium to a concentration of approximately 105 organisms/ml. Concomitantly, serum samples were diluted 1:20 with BSK medium and sterilized by passage through a 0.2-μm-pore-size microcentrifuge filter (Costar, Cambridge, MA). The filtered serum samples were then transferred into sterile 1.5-ml screw-cap microcentrifuge tubes (Sarstedt, Newton, NC) and diluted serially (1:40 to 1:40,960) with BSK medium. Serum samples were heat inactivated at 56°C for 10 min, and a 100-μl aliquot of the spirochetes and 10 μl of sterile guinea pig serum (Sigma; 50% hemolytic component of ≥200 U/ml) were added. The assay mixtures were mixed thoroughly and incubated for 16 to 24 h at 35°C.
Following incubation, 100 μl of each assay suspension was transferred into a 12- by 75-mm polystyrene tube (Becton Dickinson, Franklin Lakes, NJ) containing 400 μl of PBS and 1 μg of acridine orange (Sigma) per ml. A FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA) was then used to detect anti-OspA borreliacidal activity. Spirochetes were isolated by gating using CellQuest software (Becton Dickinson) and analyzed for 1 to 2 min with the flow rate set at low. Anti-OspA borreliacidal antibodies kill the spirochete by inducing a complement cascade that disrupts the outer membrane and causes blebbing of the membrane. Anti-OspA borreliacidal antibodies were detected indirectly by monitoring the increased fluorescence intensity that occurs when the acridine orange intercalates into blebbed, nonviable spirochetes. A ≥13% shift in the mean fluorescence intensity compared to that of a normal serum control was considered to indicate positivity (11). The presence of blebbed, nonmotile B. burgdorferi organisms was then confirmed by dark-field microscopy.
Statistical analysis.
The degrees of swelling of the hind paws and anti-OspA borreliacidal antibody data among groups were tested by analysis of variance (45). The Fisher least-significant-difference test was used to examine pairs of means when a significant F ratio indicated reliable mean differences between the control and various test groups. The alpha level was set at 0.05 before the experiments were started. The standard error for the experiment was then determined.
RESULTS
Effects of anti-IL-23 p19 antibody on development and progression of hind-paw swelling.
Thirty-two mice were vaccinated subcutaneously in the inguinal region with B. burgdorferi isolate 297 contained in alum. Three weeks after vaccination, the mice were randomly divided into four groups. One group was given anti-IL-23 p19 antibody 3 weeks after vaccination and daily thereafter for 5 days, while a second group received no treatment. The remaining two groups of vaccinated mice were infected with 106 B. bissettii organisms. The mice in one of these vaccinated and challenged groups were injected subcutaneously in the hind paws with anti-IL-23 p19 antibody following infection and daily thereafter for 5 days, while the vaccinated and challenged mice in the remaining group were injected subcutaneously with an IgG1 isotype at the time of infection and daily thereafter for 5 days.
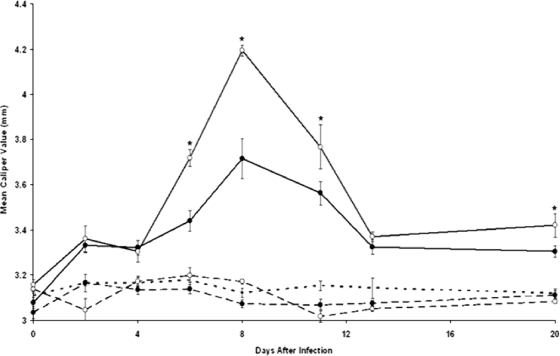
Significant (P ≤ 0.05) swelling of the hind paws of Borrelia-vaccinated and -infected mice was detected 6 days after infection. The swelling peaked on day 8 and rapidly decreased (Fig. 1). Although vaccinated and infected mice treated with anti-IL-23 p19 antibody also developed a similar trend in swelling of the hind paws, the severity of swelling was less (Fig. 1). No swelling of the hind paws of vaccinated mice with or without treatment with anti-IL-23 p19 antibody occurred. Finally, unvaccinated mice infected with B. bissettii also failed to develop swelling of the hind paws. Similar results were obtained when this experiment was repeated by different personnel.
FIG. 1.
Development of swelling of the hind paws of Borrelia-vaccinated (dashed lines) and Borrelia-vaccinated and -infected (solid lines) mice with (•) or without (○) treatment with anti-IL-23 p19 antibody. Three weeks after 32 mice were vaccinated with B. burgdorferi in alum, the mice were divided into four groups. One group was injected with anti-IL-23 p19 antibody 3 weeks after vaccination and daily thereafter for 5 days, while a second vaccinated group received no further treatment. The remaining two groups of vaccinated mice were infected with 106 B. bissettii organisms per mouse. One group of Borrelia-vaccinated and -infected mice received anti-IL-23 p19 antibody following infection and daily thereafter for 5 days, while the remaining group of vaccinated and infected mice received an IgG1 isotype antibody. Control groups included unvaccinated, unchallenged mice (dotted line). The study was terminated on day 20 after the infection of vaccinated mice with B. bissettii. Data are the means ± standard errors (error bars) for each treatment group. An asterisk denotes a significant (P ≤ 0.05) increase in swelling compared to that in Borrelia-vaccinated and -challenged control mice treated with anti-IL-23 p19 antibody.
Histopathological confirmation that anti-IL-23 p19 antibody treatment inhibited development of arthritis.
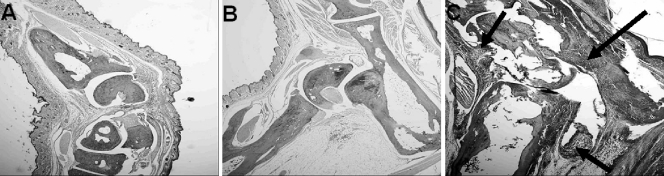
Although Borrelia-vaccinated and -infected mice with or without treatment with anti-IL-23 p19 antibody showed edematous changes in the hind paws, major histopathological differences between these two groups of mice were detected. Vaccinated and infected mice given an IgG1 isotype antibody showed severe inflammation of the tibiotarsal joints, including the synovial space, synovial lining, and perisynovium, 8 days after infection (Fig. 2C). Many neutrophils were present, especially in the perisynovial tissues. In contrast, vaccinated and infected mice treated with anti-IL-23 p19 antibody showed little evidence of histopathological changes, except edema and minor infiltration of neutrophils into the synovial lining and perisynovial tissues (Fig. 3). The controls, including vaccinated mice with (Fig. 2A) or without (Fig. 2B) treatment with anti-IL-23 p19 antibody, were also free of significant adverse histopathological responses.
FIG. 2.
Histopathology of the tibiotarsal joints of groups of control mice. All mice were euthanized on the same day (day 8 after the infection of vaccinated mice with B. bissettii). No histopathological changes were detected in the joints of Borrelia-vaccinated mice treated with (A) or without (B) anti-IL-23 p19 antibody. By contrast, severe inflammation of the perisynovial and synovial tissues, along with the infiltration of neutrophils into the joint space, was detected in Borrelia-vaccinated and -infected mice given an IgG1 isotype antibody (C). Arrows in panel C mark areas of inflammation in the tibiotarsal joints. Each mouse in this group (a total of eight mice) developed arthritis. Original magnification, ×40.
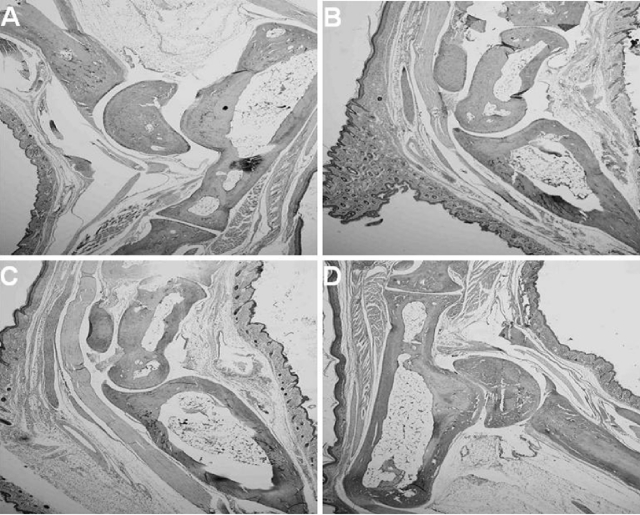
FIG. 3.
Histopathology of the tibiotarsal joints of four individual Borrelia-vaccinated and -infected mice (A to D) treated with anti-IL-23 p19 antibody immediately after infection and daily thereafter for 5 days. Mice were euthanized on the same day (day 8 after the infection of vaccinated mice with B. bissettii). No histopathological changes in the joints were detected. Anti-IL-23 p19 antibody treatment of Borrelia-vaccinated and -infected mice prevented the development of arthritis. Original magnification, ×40.
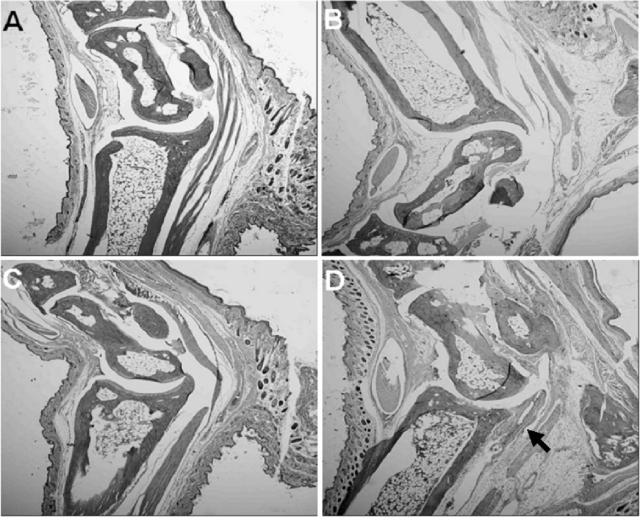
Twenty days after the infection of vaccinated mice, considerable infiltration of neutrophils into the synovial space, synovial lining, and perisynovial tissues was detected (Fig. 4D). In fact, the histopathological responses were more severe than those observed in Borrelia-vaccinated and -challenged mice on day 8 after infection (Fig. 2C). In contrast, no histopathological changes in Borrelia-vaccinated and -infected mice treated with anti-IL-23 p19 antibody were detected, despite the termination of antibody treatment approximately 2 weeks before the mice were euthanized (Fig. 4C). Moreover, no histopathological changes in Borrelia-vaccinated mice with (Fig. 4A) or without (Fig. 4B) treatment with anti-IL-23 p19 antibody were detected.
FIG. 4.
Histopathology of the tibiotarsal joints of Borrelia-vaccinated mice with (A) or without (B) treatment with anti-IL-23 p19 antibody and Borrelia-vaccinated and -infected mice with (C) or without (D) treatment with anti-IL-23 p19 antibody. All groups of mice were euthanized on the same day (day 20 after vaccinated mice were infected with B. bissettii). No histopathological changes were detected in Borrelia-vaccinated mice with (A) or without (B) treatment with anti-IL-23 p19 antibody. Likewise, no histopathological changes were detected in Borrelia-vaccinated and -infected mice treated with anti-IL-23 p19 antibody, even after the termination of anti-IL-23 p19 antibody treatment approximately 2 weeks before the mice were euthanized (C). The development of arthritis was prevented. However, Borrelia-vaccinated and -infected mice given an IgG1 isotype antibody had severe inflammation of the perisynovial and synovial tissues, with the infiltration of neutrophils into the joint space (D). The arrow in panel D marks an area of inflammation in the tibiotarsal joint. Each mouse in this group (a total of eight mice) developed arthritis. Original magnification, ×40.
Anti-IL-23 p19 antibody inhibits the production of IL-17 by lymph node cells obtained from Borrelia-vaccinated mice.
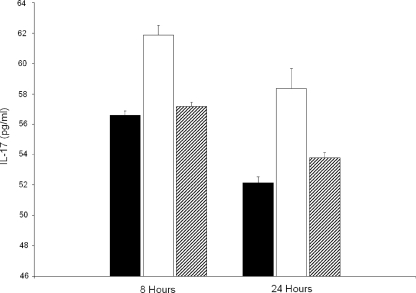
Inguinal lymph node cells were obtained from Borrelia-vaccinated mice on day 21 after vaccination. The lymph node cells were incubated with or without 106 viable B. bissettii organisms and with or without anti-IL-23 p19 antibody for 8 and 24 h. Controls included medium alone, medium with lymph node cells, medium with B. bissettii, and medium with anti-IL-23 p19 antibody alone. Figure 5 shows that lymph node cells obtained from Borrelia-vaccinated mice and incubated in the absence of viable B. bissettii organisms produced approximately 57 pg of IL-17/ml after incubation for 8 h. The coculture of immune lymph node cells and viable B. bissettii organisms increased the production of IL-17 by nearly 10%. By contrast, the addition of anti-IL-23 p19 antibody to cultures of immune lymph node cells and viable B. bissettii spirochetes reduced the production of IL-17 to levels nearly equal to those by immune lymph node cells alone. Similar results were obtained when IL-17 production was measured 24 h after incubation. All the controls produced between 9 and 57 pg of IL-17/ml.
FIG. 5.
The concentrations (in picograms per milliliter) of IL-17 in the supernatants of cultures of lymph node cells obtained from Borrelia-vaccinated mice and incubated with (open bars) or without (closed bars) 106 viable B. bissettii organisms were determined. In addition, the IL-17 concentrations in the supernatants of lymph node cells obtained from Borrelia-vaccinated mice and cultured with 106 viable B. bissettii organisms and anti-IL-23 p19 antibody were determined (hatched bars). The concentrations of IL-17 were determined 8 and 24 h after the initiation of the cultures with an IL-17 detection ELISA. Additional controls included supernatants obtained from medium with lymph node cells in the presence of anti-IL-23 p19 antibody and medium without lymph node cells in the presence or absence of anti-IL-23 p19 antibody. No IL-17 or less than 57 pg of IL-17/ml was detected in these cultures.
Treatment with anti-IL-23 p19 antibody failed to alter the anti-OspA borreliacidal antibody response.
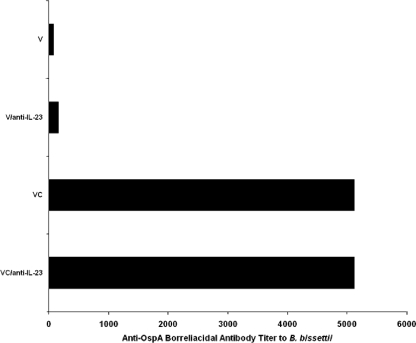
Sera from Borrelia-vaccinated mice with or without infection with B. bissettii and with or without treatment with anti-IL-23 p19 antibody were obtained on day 8 after infection or day 28 after vaccination alone. Figure 6 shows that no significant differences in titers of anti-OspA borreliacidal antibody against B. bissettii in Borrelia-vaccinated and -infected mice with or without treatment with anti-IL-23 p19 antibody were detected. Likewise, anti-IL-23 p19 antibody treatment had no effect on the anti-OspA borreliacidal antibody in sera of vaccinated mice.
FIG. 6.
Titers of anti-OspA borreliacidal antibodies directed against B. bissettii obtained for sera from vaccinated mice (V), vaccinated mice treated with anti-IL-23 p19 antibody (V/anti-IL-23), vaccinated and challenged mice given an IgG1 isotype antibody (VC), and vaccinated and challenged mice treated with anti-IL-23 p19 (VC/anti-IL-23). Sera were collected on day 8 after infection. The titers are the reciprocals of the last dilutions with anti-OspA borreliacidal activity. A shift of ≥13% in the mean antibody fluorescence compared to that of the control serum was considered to indicate a positive result by the flow cytometric method. The same titers were obtained when the flow cytometric borreliacidal assays were repeated.
DISCUSSION
Recently, we proposed that the paradigm of B. burgdorferi-induced arthritis as solely a Th1 cytokine-mediated response may require modification to include Th17 cells and their associated cytokines (39). In support of this proposal, arthritis following Borrelia infection of naïve (9) or Borrelia-vaccinated (10, 12, 38) mice progresses despite a genetic deficiency in gamma interferon (IFN-γ) and its receptor. In addition, the treatment of Borrelia-vaccinated and -challenged mice with antibodies to IL-17 or to the IL-17 receptor prevents the induction of arthritis observed in untreated Borrelia-vaccinated and -challenged mice (10, 38). The establishment of a role for IL-23, a survival factor for Th17 cells (8), in the development of Borrelia-induced arthritis would provide further support that the events of the adaptive immune response leading to the development of arthritis following Borrelia infection are influenced by Th17 cells and their associated cytokines.
In this study, we showed that IL-23 plays an important role in the development of arthritis in mice following infection with B. burgdorferi. When Borrelia-vaccinated and -challenged mice were given antibodies to the p19 subunit of IL-23, they failed to develop the histopathological changes observed in untreated Borrelia-vaccinated and -challenged mice. In addition, we showed that the blockage of IL-23 in cultures of B. bissettii and lymph node cells isolated from Borrelia-vaccinated mice inhibited the production of IL-17. Taken together, these findings suggest that IL-23 is a key mediator in the development of Borrelia-associated arthritis and affects the production of IL-17.
These findings are important because they reveal inflammatory pathways previously unconsidered in the investigation of Borrelia-mediated arthritis. Traditionally, the development of arthritis following Borrelia infection has been attributed to the actions of Th1 cells producing IFN-γ after the stimulation of cytokines like IL-12. For example, Anguita et al. (4) demonstrated that Borrelia-infected mice given antibodies to IL-12 develop less severe histopathological changes in the knees and tibiotarsal joints than infected control mice do. They attributed these findings in anti-IL-12 antibody-treated mice in part to a decrease in the production of IFN-γ (4). However, recent reports have shown that IFN-γ is not required for the development of Borrelia-mediated arthritis (9, 12). This apparent contradiction suggests that the IL-12-IFN-γ axis is not totally responsible for the induction of arthritis. The investigation of IL-23, a cytokine with structural similarity to IL-12, may address this paradox.
IL-23 is a heterodimeric protein composed of a p19 subunit in addition to a p40 subunit shared with IL-12 (41). With the development of anti-IL-23 p19 antibody and IL-23 p19− p40+ knockout mice, the divergent roles of these two cytokines have become clearer. As a result, models of some inflammatory diseases, such as collagen-induced arthritis and experimental autoimmune encephalomyelitis, which had been attributed previously to the actions of IL-12, have been updated to implicate IL-23 instead (14, 35). Curiously, collagen-induced arthritis and experimental autoimmune encephalomyelitis, like Lyme arthritis, were purported to require IL-12 (4, 27, 33) but not the cytokine it induces, IFN-γ (9, 15, 47). These observations can be attributed to IL-23. IL-23 and IL-12 show a degree of structural similarity such that investigators intending to neutralize IL-12 with an anti-p40 antibody likely neutralized IL-23 as well, possibly confounding the interpretation of the role of IL-12 in inflammation. Anguita et al. (4) reported a reduction in the severity of murine Lyme arthritis after treatment with anti-IL-12 antibodies. The anti-IL-12 antibodies used in this study may not have distinguished between IL-12 and IL-23. Moreover, IL-23 does not induce the production of IFN-γ from activated T cells (26). This fact would explain why various arthritides (18, 47), including Borrelia-induced arthritis (9, 10), can develop in the absence of IFN-γ. Taken together, these data indicate that IL-23 is an excellent candidate for the investigation of inflammatory pathways previously attributed to Th1-associated cytokines, including Borrelia-induced arthritis. In support of this statement, we showed that Borrelia-vaccinated and -challenged mice treated with anti-IL-23 p19 antibody failed to develop the histopathological changes observed in untreated vaccinated and challenged control mice.
The establishment of a role for IL-23 in the development of Borrelia-mediated arthritis provides more insight into the events that contribute to the development of pathology following Borrelia infection. Initially, the infection of vaccinated mice elicits IL-23 from innate immune cells, such as dendritic cells and macrophages (7, 25). IL-23 then acts as a survival factor for IL-17-producing memory CD4+ T cells (8) and drives their replication for the production of IL-17. In addition, the innate immune cells release another cytokine, IL-15 (36, 43). We showed previously that the treatment of Borrelia-vaccinated and -challenged mice with anti-IL-15 antibody also prevents arthritis (3). IL-15 may assist in IL-23-mediated IL-17 production by upregulating the expression of IL-23 receptors on memory cells (48) and enabling these cells to produce IL-17. IL-17, in turn, induces the production of various inflammatory mediators, including IL-1β (21), tumor necrosis factor alpha (21), IL-6 (23), nitric oxide (5), and prostaglandin E2 (16). These inflammatory agents contribute to the development of arthritis, likely until the spirochete burden is reduced to levels insufficient for the further stimulation of Th17 cells. However, we showed that the production of anti-outer surface protein A (OspA) borreliacidal antibody was not affected by the administration of anti-IL-23 antibodies. This finding suggests that the development of arthritis continues, even after the development of anti-OspA antibody that can eliminate B. bissettii.
In support of this mechanism, borrelial lipoproteins have been shown previously to stimulate the production of IL-17 from helper T cells in vitro (17). Borrelia organisms also induce IL-23, which is required for the production of IL-17 (1, 8), from bone marrow-derived dendritic cells (25). In addition, IL-15, which is present in the sera of patients with Lyme borreliosis (19), synergizes with IL-23 to augment IL-17 production (48). Finally, we showed that the blockage of each of these cytokines (3, 10; present study) in Borrelia-vaccinated and -challenged mice prevents the development of arthritis observed in untreated control mice. Collectively, these findings provide strong support for the hypothesis that IL-23 and related cytokines play an important role in the IL-17-dependent development of arthritis following infection with Borrelia organisms.
We have established a role for IL-23 in the development of arthritis following Borrelia infection by using the Borrelia vaccination and challenge model of arthritis. However, the infection of unvaccinated C57BL/6 mice with Borrelia organisms results in only minimal inflammatory changes in the hind paws (10), despite the likely production of IL-23 by innate immune cells such as dendritic cells and macrophages (7, 25). It is known that the expression of the IL-23 receptor on naïve T cells is low to absent (32), suggesting that IL-23 (and presumably IL-17) exerts minimal pathological effects following the infection of naïve mice with B. burgdorferi. Indeed, the histopathology of the tibiotarsal joints of Borrelia-infected mice treated with anti-IL-17 antibody is indistinguishable from that of infected mice given an isotype control antibody (unpublished data). By contrast, the infection of animals following vaccination induces arthritis (12, 29) which is mediated by activated CD4+ T cells (28) and ameliorated by the administration of anti-IL-17 (10, 38), anti-IL-15 (3), or anti-IL-23 antibodies. These results suggest that different mechanisms are involved after the infection of unvaccinated mice with B. burgdorferi and the infection of Borrelia-vaccinated mice with Borrelia organisms. These findings may apply to human Lyme arthritis. Human Lyme arthritis develops months after infection and depends on the presence of primed or immune cells specific to arthrogenic proteins, such as OspA (2, 22). Therefore, the Borrelia vaccination and challenge model of Lyme arthritis is appropriate for studying the development of arthritis.
In summary, we have shown that IL-23 plays a significant role in the development of arthritis following Borrelia infection. The blockage of IL-23 in Borrelia-vaccinated and -infected mice prevents the development of arthritis observed in untreated vaccinated and challenged mice. In addition, the neutralization of IL-23 prevents the in vitro release of IL-17 from Borrelia-stimulated immune lymph node cells. These findings provide support for our hypothesis that the induction of Borrelia-mediated arthritis is influenced by Th17 cells and their associated cytokines (39). In support of this statement, Knauer et al. (25) also showed that the IL-17-dependent events resulting in Lyme arthritis are influenced primarily by IL-23. Further investigation of this previously overlooked inflammatory pathway will provide new insights into the immune mechanisms responsible for the development of Lyme arthritis.
Acknowledgments
This study was supported by the Wisconsin State Laboratory of Hygiene, the public health laboratory for the state of Wisconsin, Madison, and the Gundersen Medical Foundation, La Crosse, WI.
We also thank Dean Jobe for performing borreliacidal antibody assays and Heather Cushing for assistance with ELISA.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Aggarwal, S., N. Ghilardi, M. H. Xie, F. J. de Sauvage, and A. L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910-1914. [DOI] [PubMed] [Google Scholar]
- 2.Akin, E., G. L. McHugh, R. A. Flavell, E. Fikrig, and A. C. Steere. 1999. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect. Immun. 67:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amlong, C. A., D. T. Nardelli, S. Heil Peterson, T. F. Warner, S. M. Callister, and R. F. Schell. 2006. Anti-interleukin-15 prevents arthritis in Borrelia-vaccinated and -infected mice. Clin. Vaccine Immunol. 13:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anguita, J., D. H. Persing, M. Rincón, S. W. Barthold, and E. Fikrig. 1996. Effect of anti-interleukin 12 treatment of murine Lyme borreliosis. J. Clin. Investig. 97:1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attur, M. G., R. N. Patel, S. B. Abramson, and A. R. Amin. 1997. Interleukin-17 up-regulation of nitric oxide production in human osteoarthritis cartilage. Arthritis Rheum. 40:1050-1053. [DOI] [PubMed] [Google Scholar]
- 6.Barthold, S. W. 1996. Lyme borreliosis in the laboratory mouse. J. Spirochetal Tick-Borne Dis. 3:22-44. [Google Scholar]
- 7.Becker, C., S. Wirtz, M. Blessing, J. Pirhonen, D. Strand, O. Bechthold, J. Frick, P. R. Galle, I. Autenrieth, and M. F. Neurath. 2003. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J. Clin. Investig. 112:693-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal development pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235-238. [DOI] [PubMed] [Google Scholar]
- 9.Brown, C. R., and S. L. Reiner. 1999. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect. Immun. 67:3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burchill, M. A., D. T. Nardelli, D. M. England, D. J. DeCoster, J. A. Christopherson, S. M. Callister, and R. F. Schell. 2003. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect. Immun. 71:3437-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callister, S. M., D. A. Jobe, R. F. Schell, C. S. Pavia, and S. D. Lovrich. 1996. Sensitivity and specificity of the borreliacidal-antibody test during early Lyme disease: a “gold standard”? Clin. Diagn. Lab. Immunol. 3:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christopherson, J. A., E. L. Munson, D. M. England, C. L. Croke, M. C. Remington, M. L. Molitor, D. J. DeCoster, S. M. Callister, and R. F. Schell. 2003. Destructive arthritis in vaccinated interferon gamma-deficient mice challenged with Borrelia burgdorferi: modulation by tumor necrosis factor alpha. Clin. Diagn. Lab. Immunol. 10:44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croke, C. L., E. L. Munson, S. D. Lovrich, J. A. Christopherson, M. C. Remington, D. M. England, S. M. Callister, and R. F. Schell. 2000. Occurrence of severe destructive Lyme arthritis in hamsters vaccinated with outer surface protein A and challenged with Borrelia burgdorferi. Infect. Immun. 68:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cua, D. J., J. Sherlock, Y. Chen, C. A. Murphy, B. Joyce, B. Seymour, L. Lucian, W. To, S. Kwan, T. Churakova, S. Zurawski, M. Wiekowski, S. A. Lira, D. Gorman, R. A. Kastelein, and J. D. Sedgwick. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421:744-748. [DOI] [PubMed] [Google Scholar]
- 15.Ferber, I. A., S. Brocke, C. Taylor-Edwards, W. Ridgway, C. Dinisco, L. Steinman, D. Dalton, and C. G. Fathman. 1996. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol. 156:5-7. [PubMed] [Google Scholar]
- 16.Fossiez, F., O. Djossou, P. Chomarat, L. Flores-Romo, S. Ait-Yahia, C. Maat, J.-J. Pin, P. Garrone, E. Garcia, S. Saeland, D. Blanchard, C. Gaillard, B. D. Mahapatra, E. Rouvier, P. Golstein, J. Bachereau, and S. Lebecque. 1996. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Infante-Duarte, C., H. F. Horton, M. C. Byrne, and T. Kamradt. 2000. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 165:6107-6115. [DOI] [PubMed] [Google Scholar]
- 18.Irmler, I., M. Gajda, and R. Bräuer. 2007. Exacerbation of antigen-induced arthritis in IFN-γ-deficient mice as a result of unrestricted IL-17 response. J. Immunol. 179:6228-6236. [DOI] [PubMed] [Google Scholar]
- 19.Jablonska, E., M. Marcinczyk, L. Tabarek, S. Pancewicz, T. Hermanowska-Szpakowicz, and J. Jablonski. 2003. IL-15 in the culture supernatants of PMN and PBMC and the serum of patients with Lyme disease. Rokz. Akad. Med. Bialymst. 48:78-81. [PubMed] [Google Scholar]
- 20.Jensen, J. R., B. K. DuChateau, E. L. Munson, S. M. Callister, and R. F. Schell. 1998. Inhibition of the production of anti-OspA borreliacidal antibody with T cells from hamsters vaccinated against Borrelia burgdorferi. Infect. Immun. 66:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jovanovic, D. V., J. A. Di Battista, J. Martel-Pelletier, F. C. Jolicoeur, Y. He, M. Zhang, F. Mineau, and J. P. Pelletier. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1β and TNF-α, by human macrophages. J. Immunol. 160:3513-3521. [PubMed] [Google Scholar]
- 22.Kalish, R. A., J. M. Leong, and A. C. Steere. 1993. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect. Immun. 61:2774-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz, Y., O. Nadiv, and Y. Beer. 2001. Interleukin-17 enhances tumor necrosis factor α-induced synthesis of interleukin 1, 6 and 8 in skin and synovial fibroblasts. Arthritis Rheum. 44:2176-2184. [DOI] [PubMed] [Google Scholar]
- 24.Keitel, W. A. 1999. Cellular and acellular pertussis vaccines in adults. Clin. Infect. Dis. 28(Suppl. 2):S118-S123. [DOI] [PubMed] [Google Scholar]
- 25.Knauer, J., S. Siegemund, U. Müller, S. Al-Robaiy, R. A. Kastelein, G. Alber, and R. K. Straubinger. 2007. Borrelia burgdorferi potently activates bone marrow-derived conventional dendritic cells for production of IL-23 required for IL-17 release by T cells. FEMS Immunol. Med. Microbiol. 49:353-363. [DOI] [PubMed] [Google Scholar]
- 26.Langrish, C. L., Y. Chen, W. M. Blumenschein, J. Mattson, B. Basham, J. D. Sedgwick, T. McClanahan, R. A. Kastelein, and D. J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard, J. P., K. E. Waldburger, and S. J. Goldman. 1995. Prevention of experimental autoimmune encephalomyelitis by antibodies against interleukin-12. J. Exp. Med. 181:381-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, and R. F. Schell. 1995. Borrelia burgdorferi-specific T lymphocytes induce severe destructive Lyme arthritis. Infect. Immun. 63:1400-1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, J. R. Creson, S. D. Lovrich, S. M. Callister, D. A. Jobe, and R. F. Schell. 1994. Development of destructive arthritis in vaccinated hamsters challenged with Borrelia burgdorferi. Infect. Immun. 62:2825-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lovrich, S. D., S. M. Callister, L. C. L. Lim, and R. F. Schell. 1993. Seroprotective groups among isolates of Borrelia burgdorferi. Infect. Immun. 61:4367-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangan, P. R., L. E. Harrington, D. B. O'Quinn, W. S. Helms, D. C. Bullard, C. O. Elson, R. D. Hatton, S. M. Wahl, T. R. Schoeb, and C. T. Weaver. 2006. Transforming growth factor-β induces development of the TH17 lineage. Nature 441:231-234. [DOI] [PubMed] [Google Scholar]
- 32.McGeachy, M. J., and D. J. Cua. 2007. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin. Immunol. 19:372-376. [DOI] [PubMed] [Google Scholar]
- 33.McIntyre, K. W., D. J. Shuster, K. M. Gillooly, R. R. Warrier, S. E. Connaughton, L. B. Hall, L. H. Arp, M. K. Gately, and J. Magram. 1996. Reduced incidence and severity of collagen-induced arthritis in interleukin-12-deficient mice. Eur. J. Immunol. 26:2933-2938. [DOI] [PubMed] [Google Scholar]
- 34.McKisic, M. D., W. L. Redmond, and S. W. Barthold. 2000. Cutting edge: T cell-mediated pathology in murine Lyme borreliosis. J. Immunol. 164:6096-6099. [DOI] [PubMed] [Google Scholar]
- 35.Murphy, C. A., C. L. Langrish, Y. Chen, W. Blumenschein, T. McClanahan, R. A. Kastelein, J. D. Sedgwick, and D. J. Cua. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musso, T., L. Calosso, M. Zucca, M. Millesimo, D. Ravarino, M. Giovarelli, F. Malavasi, A. N. Ponzi, R. Paus, and S. Bulfone-Paus. 1999. Human monocytes constitutively express membrane-bound, biologically active, and interferon-γ-up-regulated interleukin-15. Blood 93:3531-3539. [PubMed] [Google Scholar]
- 37.Nardelli, D. T., J. P. Cloute, K. H. K. Luk, J. Torrealba, T. F. Warner, S. M. Callister, and R. F. Schell. 2005. CD4+ CD25+ T cells prevent arthritis associated with Borrelia vaccination and infection. Clin. Diagn. Lab. Immunol. 12:786-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nardelli, D. T., M. A. Burchill, D. M. England, J. Torrealba, S. M. Callister, and R. F. Schell. 2004. Association of CD4+ CD25+ T cells with prevention of severe destructive arthritis in Borrelia burgdorferi-vaccinated and challenged interferon gamma-deficient mice treated with anti-interleukin-17 antibody. Clin. Diagn. Lab. Immunol. 11:1075-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nardelli, D. T., S. M. Callister, and R. F. Schell. 2008. Lyme arthritis: current concepts and a change in paradigm. Clin. Vaccine Immunol. 15:21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nardelli, D. T., T. F. Warner, S. M. Callister, and R. F. Schell. 2006. Anti-CD25 antibody treatment of mice vaccinated and challenged with Borrelia spp. does not exacerbate arthritis but inhibits borreliacidal antibody production. Clin. Vaccine Immunol. 13:884-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oppmann, B., R. Lesley, B. Blom, J. C. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. S. Abrams, K. W. Moore, D. Rennick, R. de Waal-Malefyt, C. Hannum, J. F. Bazan, and R. A. Kastelein. 2000. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13:715-725. [DOI] [PubMed] [Google Scholar]
- 42.Park, H., Z. Li, X. O. Yang, S. H. Chang, R. Nurieva, Y. H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruckert, R., K. Brandt, E. Bulanova, F. Mirghomizadeh, R. Paus, and S. Bulfone-Paus. 2003. Dendritic cell-derived IL-15 controls the induction of CD8 T cell immune responses. Eur. J. Immunol. 33:3493-3503. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz, J. L., R. F. Schell, A. Hejka, D. M. England, and L. Konick. 1998. Induction of Lyme arthritis in LSH hamsters. Infect. Immun. 56:2336-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steel, R. G. D., and J. H. Torrie. 1960. Principles and procedures of statistics with special reference to the biological sciences, p. 99-276. McGraw-Hill Book Co., New York, NY.
- 46.Veldhoen, M., R. J. Hocking, C. J. Atkins, R. M. Locksley, and B. Stockinger. 2006. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179-189. [DOI] [PubMed] [Google Scholar]
- 47.Vermeire, K., H. Heremans, M. Vandeputte, S. Huang, A. Billiau, and P. Matthys. 1997. Accelerated collagen-induced arthritis in IFN-γ receptor-deficient mice. J. Immunol. 158:5507-5513. [PubMed] [Google Scholar]
- 48.Yoshihara, K., H. Yamada, A. Hori, T. Yajima, C. Kubo, and Y. Yoshikai. 2007. IL-15 exacerbates collagen-induced arthritis with an enhanced CD4+ T cell response to produce IL-17. Eur. J. Immunol. 37:2744-2752. [DOI] [PubMed] [Google Scholar]
- 49.Yssel, H., M.-C. Shanafelt, C. Sodoerberg, P. V. Schneider, J. Anzola, and G. Peltz. 1991. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J. Exp. Med. 174:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]