Abstract
The present study aimed to evaluate the performance of three monoclonal antibodies (MAbs) in reverse enzyme-linked immunosorbent assays (ELISAs) for detecting immunoglobulin G (IgG), IgM, and IgA antibodies against Toxoplasma gondii in 175 serum samples from patients at different stages of T. gondii infection, as defined by both serological and clinical criteria, as follows: recent (n = 45), transient (n = 40), and chronic (n = 55) infection as well as seronegative subjects (n = 35). The results were compared with those obtained by indirect ELISA using soluble Toxoplasma total antigen (STAg). Our data demonstrated that MAb A3A4 recognizes a conformational epitope in SAG1-related-sequence (SRS) antigens, while A4D12 and 1B8 recognize linear epitopes defined as SAG2A surface antigen and p97 cytoplasmatic antigen, respectively. Reverse ELISA for IgG with A3A4 or A4D12 MAbs was highly correlated with indirect ELISA for anti-STAg IgG, whereas only A4D12 reverse ELISA showed high correlation with indirect ELISA for IgM and IgA isotypes. To our knowledge, this is the first report analyzing the performance of a reverse ELISA for simultaneous detection of IgG, IgM, and IgA isotypes active toward native SAG2A, SRS, and p97 molecules from STAg, using a panel of human sera from patients with recent and chronic toxoplasmosis. Thus, reverse ELISA based on the capture of native SAG2A and SRS antigens of STAg by MAbs could be an additional approach for strengthening the helpfulness of serological tests assessing the stage of infection, particularly in combination with highly sensitive and specific assays that are frequently used nowadays for diagnosis of toxoplasmosis during pregnancy or congenital infection in newborns.
Toxoplasma gondii is an obligate intracellular protozoan parasite from the phylum Apicomplexa and is able to infect humans and warm-blooded domestic and wild animals (6). Although the infection is asymptomatic in immunocompetent hosts, it can cause severe disease in immunocompromised subjects, like human immunodeficiency virus/AIDS patients, who usually suffer from toxoplasmic encephalitis, and fetuses who cannot develop an effective immune response against the parasite (23) when the parasite crosses the placenta during primary maternal infection, which can lead to spontaneous abortion, death of the fetus in utero, or severe congenital defects, such as hydrocephaly, mental retardation, or chorioretinitis (30, 31, 34).
The diagnosis of toxoplasmosis can be achieved by detecting specific antibodies in serum samples by using serological methods or by isolating the parasite DNA in biological samples, such as those from amniotic fluid, fetal tissue, blood, cerebrospinal fluid, and other clinical specimens, using the PCR method (8, 21). Several classical serological methods can detect immunoglobulin G (IgG) and IgM antibodies that are specific for T. gondii, such as enzyme-linked immunosorbent assays (ELISAs), indirect fluorescent antibody tests (IFATs), and Western blotting. When the confirmation of initial serology is required, a number of complementary tests are available, including the Sabin-Feldman dye test and tests for specific IgM or IgA, such as the capture ELISA, or other immunoglobulins, such as IgE and IgG avidity tests (1, 13, 14, 18, 22, 24).
The most commonly used serological method for detection of specific antibodies in serum samples is the indirect ELISA using soluble Toxoplasma antigen (STAg), excreted/secreted antigens, recombinant antigens, or purified antigens of T. gondii (10). These tests generate several false-positive and false-negative results, mostly for IgM and IgA antibodies, making the diagnosis of primary and congenital infections a challenging situation (26). In order to improve the diagnosis of toxoplasmosis, the development of highly sensitive and reproducible methods by use of monoclonal antibodies (MAbs) has been extensively researched. Among them, purified MAbs that recognize parasite epitopes can be used as the first antibody fixed to the polystyrene plates for the capture of parasite-specific molecules in soluble T. gondii extracts as a variation of the conventional ELISA. This approach was previously described to detect IgG antibodies to SAG1 antigen and other antigens from T. gondii in serum samples from pregnant women (19) and from cats (29), showing sensitivities equivalent to those of the conventional ELISA.
The purpose of the present study was to evaluate the diagnostic performance of three MAbs selected from a phage display library in reverse ELISAs for detecting IgG, IgM, and IgA antibodies specific for T. gondii in serum samples from patients at different stages of infection and compare the achieved results with those obtained by the conventional ELISA using total soluble antigen. The MAbs used in this study were A3A4, which recognizes the 30- and 60-kDa components from T. gondii SAG1-related sequences (SRSs) (p30-p60); A4D12, which recognizes another surface antigen of 22 kDa (SAG2A/p22); and 1B8, which recognizes an intracellular antigen of 97 kDa (p97).
MATERIALS AND METHODS
Patients and serum samples.
All serum samples used in this study came from immunocompetent individuals and were obtained from a well-characterized collection of sera with serological profiles previously determined by conventional assays as well as clinical information on the presence or absence of Toxoplasma infection. A total of 175 human serum samples was divided into four groups according to the following criteria: group I (recent infection) consisted of 45 serum samples from patients with clinical symptoms of infectious mononucleosis-like syndromes, such as fever, fatigue, and enlargement of the cervical lymph nodes, exhibiting IgM and IgA antibodies (titer ≥ 64) to T. gondii by capture ELISA and IgG antibodies (titer ≥ 256) by IFAT and ELISA; group II (transient infection) consisted of 40 serum samples from individuals who were asymptomatic but presented unclear results for laboratory assays, with IgM antibodies (titer ≥ 64) to T. gondii persistently positive for a long period of time but negative specific IgA antibodies, along with positive results for IgG (titer ≥ 64) antibodies in IFAT and ELISA; group III (chronic infection) consisted of 55 serum samples from individuals with asymptomatic toxoplasmosis, exhibiting T. gondii-specific IgG antibodies (titer ≥ 64) by IFAT and ELISA but negative results for specific IgM and IgA (titer < 16) antibodies by capture ELISA; and group IV (negative control) consisted of 35 serum samples collected from healthy individuals without clinical symptoms and with negative results for T. gondii-specific IgM, IgA, and IgG antibodies in the above-mentioned serological assays. This study was approved by the Ethical Committee from this institution.
Parasites and antigens.
T. gondii (RH strain) tachyzoites were maintained in Swiss mice by serial passage for 48 to 72 h (18). A parasite suspension was treated with protease inhibitors (10 μg/ml aprotinin, 50 μg/ml leupeptin, and 1.6 mM phenylmethylsulfonyl fluoride) and then submitted to freeze-thaw and sonication cycles (25). After centrifugation (10,000 × g, 15 min, 4°C), the supernatant was collected, the protein content was determined (15), and aliquots were stored at −20°C until they were used as STAg in ELISAs. Alternatively, the parasite suspension was adjusted to a final concentration of 1 × 108 tachyzoites/ml and lysed in sample buffer (0.1 M Tris-HCl, pH 6.8, 4% sodium dodecyl sulfate [SDS], 20% glycerol, 0.2% bromophenol blue). Solubilized parasites were spun (10,000 × g, 10 min, 4°C), and the supernatant was recovered and used as a T. gondii lysate antigen in the immunoblotting assays. Whole T. gondii antigens were also prepared for use in immunolocalization assays by IFAT, as described elsewhere (2). Parasites were washed in phosphate-buffered saline (PBS), treated with 1% formaldehyde for 30 min at room temperature, fixed in microscopic slides, and stored at −20°C.
MAb production, purification, and epitope mapping based on phage display.
A3A4 and A4D12 MAbs were generated by intraperitoneal immunization of BALB/c mice with T. gondii (RH strain) tachyzoites treated with 30% acetone in PBS at 4°C for 72 h, followed by a booster 1 month later by intravenous injection. Hybridoma was produced by mixing (1:1) splenocytes with SP2O/Ag14 murine myeloma cells in the presence of polyethylene glycol 1500 (11). The cloned hybridomas were amplified, isotyped (IgG1 κ chain), and stored in liquid nitrogen. Supernatants of MAb 1B8 (IgG2b κ chain) were kindly provided by Lloyd H. Kasper (Dartmouth Medical School, Lebanon, NH). The MAbs were purified in a protein G-Sepharose affinity column (Sigma Chemical Co.) according to the manufacturer instructions, and purified IgG fractions were concentrated and dialyzed in Amicon (Millipore, Billeria, MA), using membranes with 30-kDa cutoff. The protein content of each purified MAb was determined by the Lowry method (15). MAbs A3A4 and A4D12 were further characterized by epitope mapping based on phage display (Ph.D.-12 peptide library kit; New England BioLabs Inc., Beverly, MA) as previously described (5). The amino acid deduction from phage DNA sequences revealed peptide core consensus around PSWW-VI (MAb A3A4) and DGSSA (MAb A4D12) sequences. BLASTp analysis of a database with peptide sequences resulted in considerable alignments with p30-p60 (SRS) and p22 (SAG2A) surface antigens from T. gondii.
Immunoblotting and immunolocalization.
The T. gondii lysate antigen was boiled for 3 min in the presence (reducing conditions) or absence (nonreducing conditions) of 10% 2-mercaptoethanol (2ME) and resolved in an SDS-polyacrylamide gel electrophoresis gel at 10% as previously described (12). Proteins were electrotransferred to nitrocellulose membranes (32), and strips were blocked with 0.01 M PBS (pH 7.2) containing 0.05% Tween 20 (PBS-T) plus 5% nonfat skim milk (PBS-TM) for 1 h at 37°C. After being washed with PBS-T, the membranes were incubated with A3A4, A4D12, or 1B8 MAbs for 2 h at 37°C. After the wash, peroxidase-labeled goat anti-mouse IgG (1:1,000; Sigma Chemical Co.) was added and incubated for 1 h at 37°C. After the final wash, the reaction was developed with 3,3′diaminobenzidine tablet sets (Sigma Fast; Sigma Chemical Co.). For immunolocalization by IFAT, antigen slides of formolized T. gondii tachyzoites were incubated with MAbs A3A4, A4D12, and 1B8 for 45 min at 37°C. As a negative control, an irrelevant mouse IgG isotype was used. After the wash, fluorescein isothiocyanate-labeled goat anti-mouse IgG (Sigma Chemical Co.) diluted 1:50 in PBS plus 0.01% Evans blue was added and incubated for 30 min at 37°C. The slides were overlaid with carbonate-buffered glycerol (pH 8.5) and a coverslip and examined under fluorescence microscopy.
Indirect ELISA.
Indirect ELISA was carried out to detect IgG, IgM, and IgA antibodies to T. gondii as described elsewhere (18). High-binding microtiter plates (Corning Laboratories Inc., New York, NY) were coated with STAg (10 μg/ml) in 0.06 M carbonate buffer (pH 9.6) overnight at 4°C. The plates were washed three times with PBS-T and blocked with PBS-TM for 1 h at room temperature. Subsequently, the wells were incubated with serum samples diluted at 1:64 (for detecting IgG and IgM) or 1:16 (for detecting IgA) for 1 h at 37°C. After the wash, peroxidase-labeled goat anti-human IgG (1:2,000), anti-human IgM (1:1,000) or anti-human IgA (1:500) (Sigma Chemical Co.) was added and incubated for 1 h at 37°C. The assay was developed by adding the enzyme substrate (0.03% H2O2) and chromogen (0.01 M 2,2′-azino-bis-3-ethyl-benzothiazolinesulfonic acid [ABTS; Sigma Chemical Co.]) in 0.07 M citrate-phosphate buffer (pH 4.2). The optical density (OD) was read at 405 nm with a plate reader (Titertek Multiskan Plus spectrophotometer; Flow Laboratories). The cutoff of the reaction was determined as the mean OD of negative control sera plus 3 standard deviations. Antibody titers were arbitrarily expressed as ELISA index (EI) values, according to the formula ODsample/ODcutoff, as described previously (28). Samples with EI values of >1.2 were considered positive, and borderline EI reactivity values close to 1.0 were excluded.
Capture ELISA for IgM and IgA.
Microtiter plates (Immulon 2; Dynex Technologies, Chantilly, VA) were coated overnight at 4°C with goat anti-human IgM or anti-human IgA antibodies (10 μg/ml) (Sigma Chemical Co.) diluted in 0.06 M carbonate buffer (pH 9.6). The plates were washed with PBS-T and blocked with PBS-TM for 1 h at room temperature. Subsequently, the wells were incubated with serum samples diluted at 1:16 for 2 h at 37°C and then with STAg (100 μg/ml) for 2 h at 37°C. The bound antigen was detected with a peroxidase-labeled rabbit F(ab′)2 anti-T. gondii conjugate (33) diluted 1:50 and incubated for 1 h at 37°C. The subsequent steps of the reaction were developed as described for indirect ELISA.
Inhibition enzyme immunoassay.
An inhibition ELISA, as described elsewhere (7, 9), with some modifications was carried out in order to evaluate the immunodominant properties of the epitopes recognized by A3A4, A4D12, and 1B8 MAbs. A panel of 20 human sera (10 seropositive and 10 seronegative to T. gondii) was used, and the serological statuses of the samples were determined by indirect ELISA for IgG to T. gondii, using STAg. Microtiter plates (NUNC maxisorpRT; Nalge Nunc International Co., Rochester, NY) were coated with STAg (10 μg/ml) in 0.06 M carbonate buffer (pH 9.6) overnight at 4°C. The plates were washed four times with PBS-T and incubated with the human serum samples (in quadruplicate) diluted 1:64 in 0.02 M Tris-buffered saline containing 0.05% Tween 20 (TBS-T) or a control (TBS-T only) for 30 min at room temperature. After being washed, the plates were incubated with purified MAbs (10 μg/ml in TBS-T) in duplicate wells for 30 min at room temperature. After four additional washes, peroxidase-labeled goat anti-mouse IgG (1:1,000; Sigma Chemical Co.) was added and incubated for 30 min at room temperature. The assay was developed by adding the enzyme substrate (0.03% H2O2) and chromogen (0.01 M ABTS). ODs were determined at 405 nm and the results expressed as percentages of inhibition of binding of MAbs to the STAg relative to the level for the positive control (uninhibited MAb by serum samples, which were replaced by TBS-T only).
Reverse enzyme immunoassay.
A reverse ELISA was developed by using MAbs A3A4, A4D12, and 1B8 as capture antibodies to bind STAg, as previously described (27). Optimization of the reaction was established in preliminary experiments through block titration of the reagents. High-binding microtiter plates (Corning Laboratories Inc.) were separately coated with purified A3A4, A4D12, or 1B8 MAbs at 10 μg/ml in 0.06 M carbonate buffer (pH 9.6) overnight at 4°C. As a control for the MAbs, plates were coated in parallel with an irrelevant mouse IgG1 isotype (eBioscience, San Diego, CA) at the same concentration. The plates were washed three times with PBS-T and blocked with PBS-TM for 1 h at room temperature. After the plates were blocked, the wells were washed and subsequently incubated with STAg (20 μg/ml in PBS-TM) for 1 h at 37°C. The plates were washed six times and then incubated with serum samples diluted 1:64 (for detecting IgG and IgM) or 1:16 (for detecting IgA) for 1 h at 37°C. The subsequent steps of the reaction were developed as described for indirect ELISA.
Statistical analysis.
The seropositivity percentages found in the different assays were compared by the χ2 test. Inhibition percentages were expressed in 95% confidence intervals (CI). Correlations between levels of IgG, IgM, and IgA antibodies to T. gondii as determined by indirect ELISA using STAg and reverse ELISA using MAbs were analyzed by the Spearman correlation test. P values of <0.05 were considered statistically significant.
RESULTS
Immunolocalization and immunoblotting.
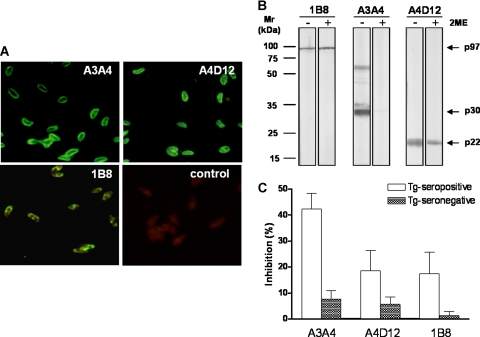
The cellular localizations of the antigens recognized by A3A4, A4D12, and 1B8 MAbs in T. gondii tachyzoites were examined in IFAT (Fig. 1A). A bright and linear peripheral fluorescence of the parasite surfaces was observed when MAbs A3A4 and A4D12 were used. In contrast, the reaction developed with MAb 1B8 showed a heterogeneous granular intracellular staining, as expected. These results confirm that MAbs A3A4 and A4D12 recognize T. gondii surface proteins while MAb 1B8 (anti-p97) recognizes an intracellular antigen.
FIG. 1.
(A) Immunolocalization of p30-p60, p22, and p97 antigens in T. gondii tachyzoites by A3A4, A4D12, and 1B8 MAbs, respectively, in IFAT. As negative control, an irrelevant mouse IgG isotype was used instead of MAbs, followed by addition of fluorescein isothiocyanate-labeled goat anti-mouse IgG. (B) Immunoblotting of T. gondii total lysate separated in 10% SDS-polyacrylamide gel electrophoresis gel in the absence (−) or presence (+) of 10% 2ME and probed with 1B8, A3A4, and A4D12 MAbs. Standards of molecular mass (Mr) expressed in kilodaltons (kDa) are indicated on the left. Antigens (p97, p30-p60, and p22) recognized by respective MAbs are shown on the right. (C) Inhibition ELISA performed with A3A4, A4D12, or 1B8 MAbs in STAg-coated microplates incubated with a panel of seropositive or seronegative human sera previously analyzed by indirect ELISA for IgG to T. gondii, evaluating the capacities of these samples to compete with MAbs.
The apparent molecular masses of the antigens and the type of epitope recognized by the A3A4, A4D12, and 1B8 MAbs were determined by immunoblotting with T. gondii lysate antigen in the presence or absence of 2ME (Fig. 1B). MAb A4D12 recognized a component of 22 kDa under both reducing and nonreducing conditions. A similar profile of reactivity was observed under both conditions with MAb 1B8, which recognizes a 97-kDa T. gondii antigen, since it has been previously characterized as a non-conformation-sensitive antibody. On the other hand, MAb A3A4 showed reactivity to 30/60-kDa (monomer/dimer) polypeptides under nonreducing conditions while no reactivity was detected when the T. gondii lysate antigen was run under reducing conditions. These data indicate that MAb A3A4 is a conformation-sensitive antibody and recognizes a conformational epitope in the p30-p60 antigen and/or the SRS family, while MAbs A4D12 and 1B8 are non-conformation-sensitive antibodies that recognize linear epitopes in the p22 and p97 antigens, respectively.
Inhibition ELISA.
The abilities of A3A4, A4D12, and 1B8 MAbs to recognize p30-p60, p22, and p97 antigens, respectively, in STAg were analyzed by an inhibition ELISA, evaluating the capacities of these MAbs to compete with a panel of human sera with serological statuses previously established by indirect ELISA for IgG to T. gondii. As shown in Fig. 1C, positive sera showed higher percentages of inhibition by A3A4 MAb (95% CI = 36% to 48%), while negative sera showed lower inhibition rates (95% CI = 4% to 11%). Regarding A4D12 MAb, a wide-ranging inhibition was found for positive sera (95% CI = 11% to 26%), but negative sera displayed a more restricted inhibition range (95% CI = 3% to 8%). In a similar manner, 1B8 MAb showed varied inhibition percentages for positive sera (95% CI = 9% to 26%), while negative sera exhibited the lowest inhibition rates (95% CI = 0% to 3%). These results demonstrate that, even though A3A4 MAb showed the highest competition capability in comparison with A4D12 and 1B8 MAbs, the epitopes recognized by all of them belong to antigenic regions that may be characterized as immunodominant.
Reverse ELISA versus indirect ELISA.
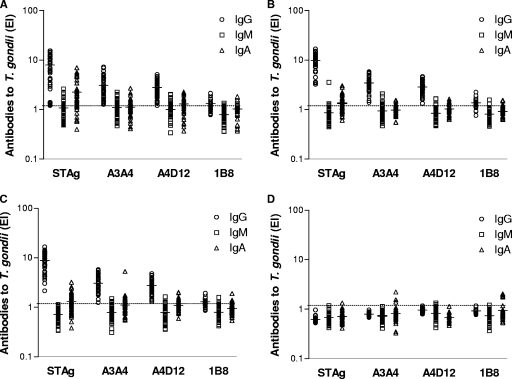
Levels of IgG, IgM, and IgA antibodies to T. gondii as determined by indirect ELISA using STAg were compared with those obtained from reverse ELISA using MAbs (Fig. 2). All groups with IgG-positive samples showed a total concordance in IgG positivity rates (100%) between indirect ELISA and reverse ELISA using MAbs A3A4 and A4D12 (Fig. 2A, B, and C). However, in reverse ELISA for IgG with MAb 1B8, positivity percentages were significantly lower, ranging from 60% to 70% for all three groups (P < 0.0001). All assays showed a total concordance of negative results for IgG antibodies in the group of seronegative samples (Fig. 2D).
FIG. 2.
Levels of IgG, IgM, and IgA antibodies to T. gondii as determined by indirect ELISA using STAg or reverse ELISA using MAbs (A3A4, A4D12, and 1B8) in four groups of human sera: (A) recent infection defined by positive IgG, IgM, and IgA to T. gondii (n = 45); (B) transient infection defined by positive IgG and IgM but negative IgA to T. gondii (n = 40); (C) chronic infection defined by positive IgG but negative IgM and IgA to T. gondii (n = 55); and (D) negative IgG, IgM, and IgA to T. gondii (n = 35). Antibody levels are expressed as EI values, the dashed lines indicate the cutoff value (EI > 1.2), and the horizontal bars represent the mean of EI values.
Upon analysis of IgM antibodies to T. gondii as determined by indirect or reverse ELISA for IgM, no significant difference in the seropositivity levels of the groups with IgM-positive samples (Fig. 2A and B) was found between the ELISAs when A3A4 or A4D12 was used. Again, reverse ELISA for IgM with MAb 1B8 displayed significantly lower positivity rates (4.4%; P = 0.0042) in the group of patients with recent infection (Fig. 2A). In the groups with IgG-positive samples only (Fig. 2C) and seronegative samples (Fig. 2D), IgM seropositivity was below 11% in all assays.
Regarding the T. gondii-specific IgA antibodies, indirect ELISA for IgA with STAg showed significantly higher positivity rates (78%) than reverse ELISA for IgA with A3A4 (38%; P = 0.0006) or 1B8 (29%; P = 0.0001) but not that for IgA with A4D12 (60%) in the group of patients with recent infection (Fig. 2A). However, in the group of patients with transient (Fig. 2B) or chronic (Fig. 2C) infections, reverse ELISA for IgA with MAbs, particularly A3A4 and 1B8, displayed significantly lower positivity rates (12% to 25%) than indirect ELISA for IgA (50% to 55%) (P < 0.05). In the seronegative group (Fig. 2D), no significant difference was found for IgA seropositivity (<15%) in any assay.
Correlation between reverse and indirect ELISAs.
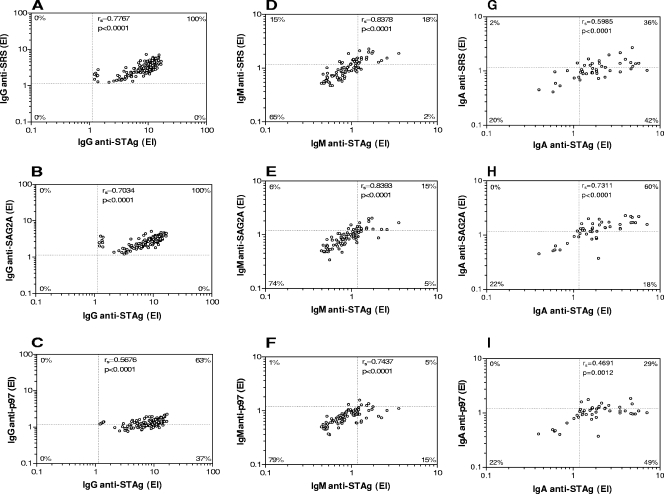
Correlation indices between indirect and reverse ELISAs were performed, taking into account all IgG-positive samples (groups I, II, and III), all IgM-positive samples (groups I and II), and all IgA-positive samples (group I) for each antibody isotype. As shown in Fig. 3, a strong and significant positive correlation was observed between levels of anti-STAg IgG and anti-SRS (Fig. 3A) or anti-SAG2A (Fig. 3B) IgG, with 100% of double-positive samples. However, a moderate positive correlation was found for anti-p97 IgG (Fig. 3C), with 37% of serum samples being positive for anti-STAg IgG only (P < 0.0001). For the IgM isotype, high positive correlations were observed in all assays, particularly for anti-SAG2A IgM, which showed similar percentages of single-positive samples (Fig. 3E). However, a significantly higher percentage of single-positive samples was found for anti-SRS IgM (15%; P = 0.0120) (Fig. 3D), with a significantly lower one for anti-p97 IgM only (1%; P = 0.0037) (Fig. 3F). For the IgA isotype, the highest positive correlation was seen for anti-SAG2A IgA (Fig. 3H), with 60% of double-positive samples, although 18% were positive for anti-STAg IgA only (P = 0.0124). However, anti-SRS IgA (Fig. 3G) and anti-p97 IgA (Fig. 3I) showed significantly lower percentages of double-positive samples (36% and 29%, respectively), with considerable numbers of single-positive samples for anti-STAg IgA only (42% and 49%, respectively) (P < 0.0001). Overall, these observations suggest that reverse ELISA for IgG with A3A4 (anti-SRS) or A4D12 (anti-SAG2A) MAbs is highly correlated with indirect ELISA for anti-STAg IgG, and for IgM and IgA isotypes in particular, the reverse ELISA using A4D12 showed the highest correlation.
FIG. 3.
Correlation between the levels of IgG, IgM, and IgA antibodies to T. gondii as determined by indirect ELISA using STAg and reverse ELISA using anti-SRS (A, D, G), anti-SAG2A (B, E, H), and anti-p97 (C, F, I) MAbs in human sera. A total of 140 serum samples (groups I, II, and III) were analyzed for IgG, 85 serum samples (groups I and II) for IgM, and 35 serum samples (group I) for IgA. The correlation coefficients (rs) were calculated by the Spearman correlation test. The dashed lines indicate the cutoff values (EI > 1.2) for each assay. The percentages of double-positive, double-negative, and single-positive samples for each antibody isotype are indicated on the corresponding corners.
DISCUSSION
The serological diagnosis of toxoplasmosis can be based on criteria that distinguish serologic profiles in T. gondii infection (3). For a long time, the main marker of recent infection was the presence of specific IgM antibodies in parallel with a rapid ascension of IgG (profile I). A serological phase of transition was determined when IgG antibodies were detected in high titers, together with an absence or low levels of IgM antibodies (profile II). Profile III was characterized by the presence of IgG antibodies in low titers and the complete absence of IgM as detected by classical serological assays, such as immunofluorescence and hemagglutination tests. However, in recent years important progress has been made in acquiring knowledge concerning the antigenic and genomic structure of T. gondii (4). For example, detection of other isotypes, as IgA and IgE, or functional affinity (avidity) of IgG is resulting in an availability of new immunoassays that may allow a better definition of the stage of infection (26). It has been shown that such definition is very easy in the presence of seroconversion or persistent negative serology, but some challenges still persist, such as how to interpret the detection of specific IgM in pregnant women. IgM antibodies arise within the first week of infection, rapidly increase, and thereafter decline and disappear at highly variable rates. False-positive results and persistence of positive titers even years after initial infection hamper correct interpretation of results obtained in IgM antibody tests. The greatest value of testing for IgM lies in the fact that a negative test essentially may rule out recently acquired infection (20). However, results obtained with some commercial kits used to detect IgM antibodies are sometimes unreliable, with false-positive rates as high as 60%. Therefore, at this time, important pieces of information concerning serodiagnosis of toxoplasmosis are still lacking, and the results of all-new serological tests should be added to the puzzle and sent to a reference laboratory (26).
As the purpose of the present study was to evaluate the diagnostic performance of three MAbs in reverse ELISAs for detecting IgG, IgM, and IgA antibodies specific for T. gondii in serum samples from patients at different stages of infection, the achieved results were compared with those obtained by conventional ELISA using total soluble antigen. Our data clearly demonstrated that the A3A4 MAb recognizes a conformational epitope in SRS family surface antigen, while the A4D12 and 1B8 MAbs recognize linear epitopes in the SAG2A surface and p97 cytoplasmatic antigens, respectively. Upon performance of inhibition assays, our results demonstrate that the A3A4 MAb, rather than the A4D12 and 1B8 MAbs, showed significant competition with positive human sera, confirming that the epitopes recognized by these MAbs belong to the antigen immunodominant regions.
The specificities of the A3A4 and A4D12 MAbs were also investigated for cross-reactivity against a closely related Apicomplexa parasite, Neospora caninum, by IFAT and immunoblotting. Both MAbs reacted with tachyzoites from both genotypes I and II of T. gondii (RH and Me49, respectively), but no reactivity was observed on N. caninum tachyzoites, indicating that these MAbs are species- but not Toxoplasma strain-specific antibodies (data not shown). Concerning the specificity of the 1B8 MAb, it was already described that this MAb recognizes a p97 cytosolic structure from genotypes I, II, and III of T. gondii as well as from closely related Apicomplexa parasites, such as Besnoitia jellisoni, Plasmodium falciparum (17), and Neospora caninum (16), but not from Leishmania donovani or Leishmania mexicana (17), even though 1B8 epitope localization in this molecule was not identified so far by phage display.
As the serum samples analyzed in the present study were selected based on the results obtained by conventional assays, i.e., indirect ELISA for IgG and capture ELISA for IgM and IgA, comparisons were carried out among these tests in order to observe the performance of the proposed reverse ELISAs. When MAbs A3A4 and A4D12 were used, all groups with IgG-positive samples showed total concordance in positivity rates between indirect ELISA for IgG and reverse ELISA for IgG. However, for reverse ELISA for IgG with MAb 1B8, positivity percentages were significantly lower, ranging from 60% to 70%, in the three groups of seropositive samples. In the group with IgG-seronegative samples, both indirect ELISA for IgG and reverse ELISA for IgG showed total concordance of negative results.
Upon analysis of IgM antibodies to T. gondii as determined by indirect or reverse ELISA for IgM, no significant difference in the seropositivity levels of the groups with IgM-positive samples was found between the ELISAs when A3A4 or A4D12 was used, although reverse ELISA for IgM with A3A4 showed a tendency to detect higher numbers of IgM-positive samples. Also, reverse ELISA for IgM with MAb 1B8 displayed significantly lower positivity rates. In the group with samples showing positivity for IgG only and in the seronegative group, IgM seropositivity was low in all assays. Indirect ELISA for IgM with STAg showed a low ability to detect IgM antibodies in the two groups with recent and transient infections, previously defined as IgM-positive samples by capture ELISA for IgM.
Concerning the detection of T. gondii-specific IgA antibodies, indirect ELISA for IgA with STAg showed significantly higher positivity rates than reverse ELISA for IgA with A3A4 or 1B8 but not that for IgA with A4D12 in the group of recent-infection-presenting, IgA-positive samples. Nevertheless, in the group of transient or chronic infections previously defined as presenting IgA-negative samples, reverse ELISA for IgA with MAbs, particularly A3A4 and 1B8, displayed significantly lower positivity rates than indirect ELISA for IgA. In the seronegative group, no significant difference was found for IgA seropositivity in any assay.
To our knowledge, this is the first report analyzing the performance of a reverse ELISA for the detection of IgG, IgM, and IgA isotypes active toward native SAG2A, SRS p30-p60, and p97 molecules from T. gondii tachyzoites, using a panel of human sera from patients with recent and chronic toxoplasmosis. There are in the literature only two studies describing a MAb capture-based assay for IgG alone in human or stray cat sera (19, 29). In the present study, it was demonstrated that reverse ELISA for IgG with A3A4 or A4D12 is highly correlated with indirect ELISA for anti-STAg IgG, whereas the reverse ELISA using A4D12 for IgM and IgA showed the highest correlation with indirect ELISAs for both isotypes. As the recommended strategy for toxoplasmosis involves the sequential or concomitant combination of more than one of the currently available methods and since no method on its own can guarantee an adequate level of accuracy, we believe that reverse ELISA could be used in combination with highly sensitive and specific assays. In this context, PCR constitutes the gold standard method for diagnosis of Toxoplasma infection, especially in cases of congenital toxoplasmosis or infection during pregnancy, even though it is not a routine procedure, because the recovery of the parasite from biological samples is often impracticable due to the parasite life cycle (27).
Overall, the results presented herein add a new piece of information among important issues related to the serological diagnosis of toxoplasmosis. For example, it may be possible to perform immunoenzymatic assays capturing immunodominant components instead of using crude extract, a strategy that obviates the antigenic purification processes that are time-consuming and have serious problems of yield and solubility due to the hydrophobic characteristics of these glycosylphosphatidylinositol-anchored components. Also, it may be possible to work with well-characterized, native, immunodominant components instead of recombinant components obtained mainly from prokaryotic systems that differ substantially from native molecules due to posttranslational events, such as glycosylation pathways. Finally, the important findings concerning IgG isotype, with 100% concordance with STAg, open new possibilities for investigating IgG subclasses, as well as IgG avidity, by using the same approach described in the present study. In conclusion, the present study demonstrated that reverse ELISA based on the capture of native SAG2A, SRS p30-p60, and p97 antigens from the soluble crude extract of T. gondii tachyzoites by MAbs constitutes an additional approach for strengthening the helpfulness of conventional serological tests, particularly those used to determine stage of infection.
Acknowledgments
We thank L. H. Kasper from Dartmouth Medical School, Lebanon, NH, for providing MAb 1B8.
This study was supported by Brazilian funding agencies (CNPq, FAPEMIG, and CAPES).
Footnotes
Published ahead of print on 18 June 2008.
REFERENCES
- 1.Ashburn, D., A. W. Joss, T. H. Pennington, and D. O. Ho-Yen. 1998. Do IgA, IgE, and IgG avidity tests have any value in the diagnosis of toxoplasma infection in pregnancy? J. Clin. Pathol. 51:312-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Camargo, M. E. 1964. Improved technique of indirect immunofluorescence for serological diagnosis of toxoplasmosis. Rev. Inst. Med. Trop. Sao Paulo 12:117-118. [PubMed] [Google Scholar]
- 3.Camargo, M. E., A. W. Ferreira, J. R. Mineo, C. K. Takiguti, and O. S. Nakahara. 1978. Immunoglobulin G and immunoglobulin M enzyme-linked immunosorbent assays and defined toxoplasmosis serological patterns. Infect. Immun. 21:55-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Contini, C., M. Giuliodori, R. Cultrera, and S. Seraceni. 2006. Detection of clinical-stage specific molecular Toxoplasma gondii gene patterns in patients with toxoplasmic lymphadenitis. J. Clin. Microbiol. 55:771-774. [DOI] [PubMed] [Google Scholar]
- 5.Cunha-Júnior, J. P. 2005. Caracterização molecular dos mimetopos H2 (SAG2A) e B12 (SRS6) de moléculas de superfície de Toxoplasma gondii. Ph.D. thesis. Federal University of Uberlandia, Uberlandia, Brazil.
- 6.Dubey, J. P., N. L. Miller, and J. K. Frenkel. 1970. The Toxoplasma gondii oocyst from cat feces. J. Exp. Med. 132:636-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortier, B., D. Rolland, F. Ajana, J. F. Dubremetz, and A. Vernes. 1991. Detection of specific antibodies to Toxoplasma gondii by a competitive enzyme immunoassay using a monoclonal antibody against the P30 antigen. Eur. J. Clin. Microbiol. Dis. 10:38-40. [DOI] [PubMed] [Google Scholar]
- 8.Foulon, W., J. M. Pinon, B. Stray-Pedersen, A. Pollak, M. Lappalainen, A. Decoster, I. Villena, P. A. Jenum, M. Hayde, and A. Naessens. 1999. Prenatal diagnosis of congenital toxoplasmosis: a multicenter evaluation of different diagnostic parameters. Am. J. Obstet. Gynecol. 181:843-847. [DOI] [PubMed] [Google Scholar]
- 9.Graille, M., E. A. Stura, M. Bossus, B. H. Muller, O. Letourneur, N. Battail-Poirot, G. Sibai, M. Gauthier, D. Rolland, M. H. Le Du, and F. Ducancel. 2005. Crystal structure of the complex between the monomeric form of Toxoplasma gondii surface antigen 1 (SAG1) and a monoclonal antibody that mimics the human immune response. J. Mol. Biol. 354:447-458. [DOI] [PubMed] [Google Scholar]
- 10.Joynson, D. H., and E. C. Guy. 2001. Laboratory diagnosis of Toxoplasma infection, p. 296-318. In D. H. Joynson and T. G. Wreghitt (ed.), Toxoplasmosis: a comprehensive clinical guide. Cambridge University Press, Cambridge, United Kingdom.
- 11.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 12.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 13.Lappalainen, M., and K. Hedman. 2004. Serodiagnosis of toxoplasmosis. The impact of measurement of IgG avidity. Ann. Ist. Super. Sanita 40:81-83. [PubMed] [Google Scholar]
- 14.Liesenfeld, O., J. G. Montoya, S. Kinney, C. Press, and J. S. Remington. 2001. Effect of testing IgG avidity in the diagnosis of primary infection with Toxoplasma gondii infection in pregnant women: experience in a US reference laboratory. J. Infect. Dis. 183:1248-1253. [DOI] [PubMed] [Google Scholar]
- 15.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 16.Matsuura, T., and L. H. Kasper. 1997. Molecular analysis and characterization of a protein involved in the replication of intracellular Toxoplasma gondii. Mol. Biochem. Parasitol. 90:403-413. [DOI] [PubMed] [Google Scholar]
- 17.Mineo, J. R., I. A. Khan, and L. H. Kasper. 1994. Toxoplasma gondii: a monoclonal antibody that inhibits intracellular replication. Exp. Parasitol. 79:351-361. [DOI] [PubMed] [Google Scholar]
- 18.Mineo, J. R., M. E. Camargo, and A. W. Ferreira. 1980. Enzyme-linked immunosorbent assay for antibodies to Toxoplasma gondii polysaccharides in human toxoplasmosis. Infect. Immun. 27:283-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moleón, I., T. González, R. Machín, J. R. Molina, and C. A. García. 1993. Inmunoensayo de “captura” para la detección de IgG humana anti proteína P30 de Toxoplasma gondii. Rev. Lat. Am. Microbiol. 35:309-314. [PubMed] [Google Scholar]
- 20.Montoya, J. G., and O. Liesenfeld. 2004. Toxoplasmosis. Lancet 363:1965-1976. [DOI] [PubMed] [Google Scholar]
- 21.Pelloux, H., J. Weiss, J. Simon, F. Muet, H. Fricker-Hidalgo, A. Goullier-Fleuret, and P. Ambroise-Thomas. 1996. A new set of primers for the detection of Toxoplasma gondii in amniotic fluid using polymerase chain reaction. FEMS Microbiol. Lett. 138:11-15. [DOI] [PubMed] [Google Scholar]
- 22.Remington, J. S., P. Thulliez, and J. G. Montoya. 2004. Recent developments for diagnosis of toxoplasmosis. J. Clin. Microbiol. 42:941-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remington, J. S., R. McLeod, P. Thulliez, and G. Desmonts. 2001. Toxoplasmosis, p. 205-346. In J. S. Remington and J. Klein (ed.), Infectious diseases of the fetus and newborn infant, 5th ed. W. B. Saunders, Philadelphia, PA.
- 24.Roberts, A., K. Hedman, V. Luyasu, J. Zufferey, M. H. Bessieres, R. M. Blatz, E. Candolfi, A. Decoster, G. Enders, U. Gross, E. Guy, M. Hayde, D. Ho-Yen, J. Johnson, B. Lecolier, A. Naessens, H. Pelloux, P. Thulliez, and E. Petersen. 2001. Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur. J. Clin. Microbiol. Infect. Dis. 20:467-474. [DOI] [PubMed] [Google Scholar]
- 25.Scott, P., E. Pearce, P. Natovitz, and A. Sher. 1987. Vaccination against cutaneous leishmaniasis in murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J. Immunol. 139:221-227. [PubMed] [Google Scholar]
- 26.Sensini, A. 2006. Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin. Microbiol. Infect. 12:504-512. [DOI] [PubMed] [Google Scholar]
- 27.Silva, D. A. O., A. M. Gervásio, M. C. Sopelete, E. Arruda-Chaves, L. K. Arruda, M. D. Chapman, S. S. Sung, and E. A. Taketomi. 2001. A sensitive reverse ELISA for the measurement of specific IgE to Der p 2, a major Dermatophagoides pteronyssinus allergen. Ann. Allergy Asthma Immunol. 86:545-550. [DOI] [PubMed] [Google Scholar]
- 28.Silva, D. A. O., N. M. Silva, T. W. P. Mineo, A. A. Pajuaba Neto, E. A. Ferro, and J. R. Mineo. 2002. Heterologous antibodies to evaluate the kinetics of the humoral immune response in dogs experimentally infected with Toxoplasma gondii RH strain. Vet. Parasitol. 107:181-195. [DOI] [PubMed] [Google Scholar]
- 29.Sohn, W. M., and H. W. Nam. 1999. Western blot analysis of stray cat sera against Toxoplasma gondii and the diagnostic availability of monoclonal antibodies in sandwich-ELISA. Korean J. Parasitol. 37:249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sukthana, Y. 2006. Toxoplasmosis: beyond animals to humans. Trends Parasitol. 22:137-142. [DOI] [PubMed] [Google Scholar]
- 31.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson, M. B., and P. K. Nakane. 1978. Recent developments in the periodate method of conjugating horseradish peroxidase (HRPO) to antibodies, p. 215-224. In W. Knapp, K. Holubar, and G. Wick (ed.), Immunofluorescence and related staining techniques. Elsevier North Holland Biomedical Press, Amsterdam, The Netherlands.
- 34.Wong, S. Y., and J. S. Remington. 1994. Toxoplasmosis in pregnancy. Clin. Infect. Dis. 18:853-861. [DOI] [PubMed] [Google Scholar]