Abstract
Earlier studies have demonstrated in A/Sn mice highly susceptible to Chagas' disease protective immunity against lethal Trypanosoma cruzi infection elicited by vaccination with an open reading frame (ORF) expressed by amastigotes. In our experiments, we used this mouse model to search for other amastigote-expressed ORFs with a similar property. Fourteen ORFs previously determined to be expressed in this developmental stage were individually inserted into a eukaryotic expression vector containing a nucleotide sequence that encoded a mammalian secretory signal peptide. Immunization with 13 of the 14 ORFs induced specific antibodies which recognized the amastigotes. Three of those immune sera also reacted with trypomastigotes and epimastigotes. After a lethal challenge with Y strain trypomastigotes, the vast majority of plasmid-injected mice succumbed to infection. In some cases, a significant delay in mortality was observed. Only two of these ORFs provided protective immunity against the otherwise lethal infection caused by trypomastigotes of the Y or Colombia strain. These ORFs encode members of the trans-sialidase family of surface antigens related to the previously described protective antigen amastigote surface protein 2 (ASP-2). Nevertheless, at the level of antibody recognition, no cross-reactivity was observed between the ORFs and the previously described ASP-2 from the Y strain. In immunofluorescence analyses, we observed the presence of epitopes related to both proteins expressed by amastigotes of seven different strains. In conclusion, our approach allowed us to successfully identify two novel protective ORFs which we consider interesting for future studies on the immune response to Chagas' disease.
Trypanosoma cruzi is an obligate intracellular protozoan parasite and the etiologic agent of Chagas' disease. In spite of the significant reduction in transmission observed in several countries in the last 20 years, Chagas' disease is still a major health problem for many Latin American countries, afflicting millions of individuals and causing thousands of deaths every year (34). The poor prospect of treatment raises the possibility that immune interventions, such as immunization, could be used as an additional approach to improve disease prevention and treatment efficacy.
Based on the concept of immune interventions, independent researchers found that the immunization of mice with plasmids containing T. cruzi open reading frames (ORFs) generated not only immune responses mediated by antibodies, CD4+ and CD8+ type 1 T cells, but also remarkable protective immunity against otherwise lethal infection with T. cruzi (reviewed in references 15 and 32). Although prophylactic vaccination was performed in most studies, immunotherapy also proved feasible in certain experimental models (16).
Among the ORFs described as capable of eliciting protective immunity, there are members of the trans-sialidase (TS) family of antigens expressed on the surface of infective (trypomastigote) or intracellular (amastigote) stages of T. cruzi. Distinct protective ORFs expressed in trypomastigotes were described as encoding, for example, the enzymatic domain of T. cruzi TS (11, 21, 22, 27, 37), the trypomastigote surface antigens (16, 33, 45), or the complement regulatory protein (40). Other protective ORFs encoded amastigote surface protein 1 (ASP-1) or ASP-2 expressed in the intracellular stages of the parasite (2, 6, 10, 24, 43).
In addition to the members of the TS family of surface proteins, ORFs encoding other classes of antigens have also been reported for their ability to elicit protective immune responses against experimental mouse infection. Among those are, for example, the ORFs encoding cruzipain (9, 38), the LYT-1 antigen (21), the flagellar calcium-binding protein (Tc24) (16), and a fusion protein containing heat shock protein 70 (HSP70) and the paraflagellar rod protein 2 (PAR-2) (35) or HSP70 and KMP11 (36).
The examples shown above provided strong support to the fact that plasmid DNA immunization against T. cruzi infection can be a useful and relatively simple approach to identify protective target antigens in the mouse model. However, it is important to notice that most studies used C57BL/6 or BALB/c mice for the purpose of vaccination. Although these mice die when challenged with the infective trypomastigotes of certain parasite strains, they are not as susceptible to T. cruzi infection as other mouse strains, such as, for example, A/Sn mice. In order to study the antigens which provide the protective immunity required for vaccination, we have been using this mouse strain highly susceptible to Chagas' disease. Infection with relatively small doses of the parasites of the Y strain of T. cruzi leads to 100% death in a period of 30 days or less. Due to its high susceptibility, we believe that this experimental model is an interesting one to study antigens capable of generating a high degree of protective immunity against infection. In this mouse model, we have recently described how vaccination with a plasmid containing the ORF encoding an amastigote-specific antigen (ASP-2) generated specific CD4+ Th1 and CD8+ Tc1 immune responses. Most importantly, immunization with this plasmid promoted the survival of approximately 65% of the mice against a lethal T. cruzi infection (43). Protective immunity of this magnitude could not be duplicated by immunization with a plasmid encoding a trypomastigote-specific antigen (T. cruzi TS) (43, 44). Based on the data obtained following infection in this mouse model, we considered that perhaps antigens expressed by the intracellular amastigote forms of T. cruzi would be better targets for protective immune responses. Also, host cells containing amastigote nests are critically involved in chronic-phase Chagas' disease pathology. These infected cells stimulate inflammatory responses considered the main cause of chronic chagasic pathology and targets for host protective or, eventually, pathological immune responses (7, 41).
Based on our interest in the amastigote antigens, the present study had a dual purpose. First, we screened 14 ORFs/antigens putatively expressed by amastigotes of T. cruzi for their vaccination potential. Second, using a single experimental model of infection, we could compare the protective potentials of these different ORFs. The model selected for testing the protective activity of these ORFs was plasmid DNA immunization of A/Sn mice highly susceptible to Chagas' disease followed by infection with Y strain trypomastigotes.
Because T. cruzi proteome was not available when we initiated this project, we selected the ORFs to be used from three main source studies. The first study was based on the screening of a library obtained from amastigote cDNA which identified a number of T. cruzi ribosomal proteins, flagellar proteins, and HSPs specifically recognized by immunoglobulins (Igs) from Chagasic individuals (12). They were (i) ribosomal protein L7a-like protein, (ii) ribosomal protein S4 homolog, (iii) histone H2b, (iv) HSP, (v) mitochondrial HSP, (vi) elongation factor 2, (vii) flagellar calcium binding protein 3, and (viii) PAR-2.
At the time we selected those ORFs, only the flagellar calcium binding protein 3 (Tc24) ORF/antigen had been tested for its protective efficacy with extremely positive results (16). During the period we performed our study, Morell et al. (35) reported that PAR-2- and HSP (HSP70)-fused ORFs could also elicit a certain degree of immunity against late-stage infection in mice (35). The other ORFs have not been explored so far for the purpose of vaccination in experimental infection.
A second group of ORFs we selected was based on the study by Bhatia et al. (5). They described a number of T. cruzi sequences exhibiting characteristics of membrane-associated or -secreted proteins (5). Plasmid DNA immunization with some of these ORFs generated antibodies that reacted with the surface of amastigotes of T. cruzi (5). The ORFs we selected from this study were those coding for (i) protein G2, (ii) protein G4, (iii) elongation factor alpha G5, and (iv) AAA ATPase-like protein G8. Although the original publication indeed described plasmid DNA immunization and the induction of specific mouse antibody response by these ORFs/antigens, no data related to immune protection were published there or elsewhere.
Finally we searched in the recently published T. cruzi proteome/genome for proteins expressed in amastigote forms with possible identities to the protective antigen ASP-2 (3, 17, 18). Two ORFs were selected with the characteristics required for members of the TS family of surface antigens. The primary structure of each protein based on the predicted amino acid sequence and computational analysis indicated that both of them have the predicted signal peptide (SP) and a sialidase-like domain. One of them has an ASP box motif (SxDxGxTW), and the other has a putative glycosylphosphatidylinositol (GPI) addition anchor motif. Although we and others have previously shown that ASP-2-related ORFs can provide protective immunity following plasmid DNA immunization (2, 6, 10, 13, 24, 43), we considered that these genes are not identical; therefore, they constitute new ORFs that had never been tested in experimental vaccination studies.
MATERIALS AND METHODS
Mice and parasites.
Female 5- to 8-week-old A/Sn mice used in this study were purchased from Centro de Desenvolvimento de Modelos Experimentais (Universidade Federal de São Paulo). Mouse experiments were performed in accordance with the guidelines approved by the ethics committee of Universidade Federal de São Paulo. Parasites of the Y, Sylvio X10/4, Dm28c, CL-Brener, Tulahuen, G, and Colombian strains of T. cruzi were used (10, 14). All strains were molecularly typed based on small subunit D7-24Sα ribosomal DNA sequences to confirm their origin (8). Epimastigotes and trypomastigotes were obtained as described previously (10). Extracellular amastigotes were generated by incubation of trypomastigotes derived from infected LLC-MK2 cells in LIT medium for 24 to 48 h at 37°C (1).
Infection with bloodstream trypomastigotes of the Y strain or Colombia strain and parasitemia were performed as described previously (43). Each mouse was inoculated intraperitoneally (i.p.) with 150 (Y strain) or 250 (Colombia strain) trypomastigotes. The values of peak parasitemia of each individual mouse were log transformed before being compared by one-way analysis of variance followed by Tukey's honestly significant difference tests. The log rank test was used to compare mouse survival rates after challenge with T. cruzi. The differences were considered significant when the P value was <0.05.
ORF cloning and sequencing.
DNA was prepared from T. cruzi epimastigotes of the Y or CL-Brener strains as described previously (31). PCRs were performed using the enzyme Platinum Pfx DNA polymerase, and the annealing temperature varied from 52°C to 58°C.
The oligonucleotides used for each ORF are described in Table 1. DNA fragments were excised from agarose gels, in the DNA products were cloned into pMOSBlue vector (General Electrics). Automatic sequencing of double-stranded DNA was carried out using the BigDye terminator cycle sequencing kit (Perkin-Elmer) on an ABI Prism 377 sequencer (Perkin-Elmer) using initially the U19 and T7 oligonucleotides and appropriate oligonucleotides designed to cover the entire sequence.
TABLE 1.
Oligonucleotides used in the present study
| ORF | Oligonucleotide sequence
|
|
|---|---|---|
| Forward | Reverse | |
| ribpS4 | GGAGGTACCATGACCAAG AAGCACCTG | CAGAATTCCCTATTTTCGTGCCTTGCG |
| rpl7a | GGAGGTACCATGCCCGGCAAGGAAGTG | CAGAATTCCTCACATTACGGCGGCAGC |
| h2b | GGAGGTACCATGGCCACCCCCAAGAGC | CAGAATTCCCTAACTAGAGGCGTGCGA |
| hsp70 | GGAGGTACCATGACGTACGAGGGAGCC | CAGAATTCCTCAGTCCACTTCCTCCAC |
| mtp70 | GGAGGTACCATGTTCGCTCGTCGTTTG | CAGAATTCCTTACTGCTTCTGCTGATC |
| ef2 | GGAGGTACCCATGGCAAG TCGACTTG | CAGAATTCCCCAGTGGTCAAAGACACA |
| tc24 | GGAGGTACCATGGGTGCTTGTGGGTCG | CAGAATTCCTCACGCGCTCTCCGGCAC |
| par2 | GGAGGTACCATGAGCTACAAGGAGGCA | CAGAATTCC TTACTGTGTGATCTGCTG |
| tcg2 | GGAGGTACCATGTCGCTTTCATTTATC | CAGAATTCCTTTGTACTCCTTTTGCCAAC |
| tcg4 | GGAGGTACCATGTCAGCCAAGGCTCCC | CAGAATTCCCTACTTTCCAAGCGCCTT |
| tcg5 | GGAGGTACCATGGGGAAGGAAAAGGTG | CAGAATTCCTCACTTCTTAGCGGCCTT |
| tcg8 | GGAGGTACCATGTCCGATAACCATCAA | AGCGGCCGCTCACTGTGGTACAACGCT |
| asp-3(5340) | GGAGGTACCGATGGAAGTAGTGGGGCA | CAGAATTCCCCCCAGAAGCTCTGCGTC |
| asp-4(7015) | GAATTCGAGAACAGTGTTTCAGGG | GCCGCTCGAACCGACACCCGTTGCTCC |
| asp-3(5340) (recombinant protein) | GGGGGATCCGATGGAAGTAGTGGGGCA | GGGGAATTCCCCCAGAAGCTCTGCGTC |
DNA and predicted amino acid sequences were analyzed using the DNAStar package, version 5.00 (DNAStar, Inc.). Alignments were produced through the ClustalW program. Analysis for potential secretory SP and a putative GPI addition anchor motif were performed at the available websites (http://www.cbs.dtu.dk/services/SignalP or http://mendel.imp.ac.at/sat/gpi/gpi_server.html).
Eukaryotic expression vector: plasmid generation, purification, and immunization.
Commercially available plasmid pcDNA3 (Invitrogen, San Diego, CA) was used as the mammalian expression vector. To this plasmid, we added the nucleotide sequence encoding the mouse Ig κ chain SP (IgSP) (6). Isolated T. cruzi ORFs were subcloned in frame with the IgSP. ORFs ribpS4, rpL7a, h2b, tc24, par2, and tcg4 and asp-3(5340) were cloned after digestion with KpnI and EcoRI. ORFs hsp70 and ef2 were cloned after digestion with KpnI and XbaI. ORFs mtp70, tcg2, tcg5, and tcg8 were cloned after digestion with KpnI and BamHI. ORF asp-4(7015) was cloned after digestion with BamHI and XbaI. Other plasmids of T. cruzi ASP-2 (pIgSP-clone9) of the Y strain or Colombian strain (pIgSP-clone22) were generated as described earlier (6, 10).
Plasmids were produced in E. coli DH5α and purified on cesium chloride density gradients according to standard protocols. Each plasmid DNA was diluted in sterile phosphate-buffered saline to a concentration of 1.5 mg/ml, and A/Sn mice were immunized according to a protocol described earlier (11). Seventy-five micrograms of plasmid DNA was injected intramuscularly at the same sites used for a cardiotoxin injection given 5 days earlier. Two boosting immunizations consisted of the same amount of plasmid DNA injected intramuscularly 3 and 5 weeks after the first dose. One to 2 weeks after the last dose, mice were bled or challenged with bloodstream trypomastigotes.
IFA.
HeLa cells (1.5 × 104) were plated in RPMI medium containing 10% fetal bovine serum (Invitrogen) in 24-well plates (Costar) containing 13-mm round coverslips at 37°C in a humidified atmosphere containing 5% CO2. Ten parasites per target cell was the dose used for infection with trypomastigotes of the Y strain. After 48 h, HeLa cells fixed with a paraformaldehyde solution (2% [vol/vol]) for 20 min at room temperature. Parasite fixation and indirect immunofluorescence assay (IFA) staining were performed exactly as described earlier (6, 10). IFA images were acquired with a Zeiss Axiovert microscope attached to a Bio-Rad 1024UV confocal microscope using a 100× 1.4 differential interference contrast PlanApo objective.
Bacterial recombinant proteins: plasmid generation, E. coli expression, and purification.
As a bacterial expression vector, we used the commercial vector pET28A (Novagen, Madison, WI). A plasmid was generated based on the sequence of ASP-3(5340). The asp-3(5340) gene was amplified by PCR using the forward and reverse oligonucleotides indicated in Table 1. The amplified PCR products were cloned into the pMOSBlue vector, removed by treatment with restriction enzymes BamHI and EcoRI, and ligated into the pET28A vector treated with the same enzymes.
Plasmids were transformed into E. coli BL21 expression host cells (Novagen). Protein expression of His-tagged 65-kDa protein (His-65kDa; ASP-2, Y strain) or His-63kDa [ASP-asp-3(5340)] was obtained as described previously (2, 6, 10, 13). The purity of recombinant protein was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). An SDS molecular weight marker kit (Sigma) and a Benchmarker prestained protein ladder (Invitrogen) were used as protein standards.
ELISA and immunoblotting.
Antibodies were detected by enzyme-linked immunosorbent assay (ELISA) using polystyrene microtiter plates (high binding; Costar) coated with the recombinant protein His-65kDa for ASP-2 (6) or His-63kDa for ASP-3(5340). The experimental procedures have been previously described in detail in references 6 and 10.
For immunoblotting, extracellular amastigotes of T. cruzi were obtained as described above. The parasite concentration was adjusted to 109 parasites per ml. Parasite extracts were obtained by treating the parasites with a solution of PBS containing a final concentration of 1% (vol/vol) NP-40 and proteolytic enzyme inhibitors (1 mM phenylmethylsulfonyl fluoride and 1 mM iodoacetamide Σ). After centrifugation, the supernatants were collected, mixed with SDS-PAGE sample buffer containing 2-mercaptoethanol, and boiled. The amount of liquid corresponding to 107 parasites was loaded for SDS-PAGE and transferred to nitrocellulose membranes (Millipore). Antibody staining was performed with a pool of sera from mice immunized with plasmid DNA containing ORF asp-3(5340) diluted 1:500 or asp-4(7015) diluted 1:800. Bound antibodies were detected by incubating the immunoblot membranes with goat anti-mouse IgG coupled to peroxidase diluted 1:1,200 [asp-3(5340)] or 1:1,600 [asp-4(7015)] (Invitrogen) followed by development of the chemoluminescent reaction with Super Signal West Pico chemiluminescent substrate (Pierce), as described by the manufacturer, and exposure to Hyperfilm.
Nucleotide sequence accession numbers.
Nucleotide sequence data reported are available in the Third Party Annotation section of the DDBJ/EMBL/GenBank databases under the accession numbers BK006544 to BK006557 (Table 2).
TABLE 2.
Summary of the ORFs used in the present study
| ORF | Product description | GenBank accession no.
|
Amino acid identity (%) | References | |
|---|---|---|---|---|---|
| Previous | This study | ||||
| ribpS4 | Ribosomal protein S4 homolog | AF005904 | BK006552 | 100 | 25 |
| rpl7a | Ribosomal protein L7a-like protein | AF316150 | BK006549 | 98 | 12 |
| h2b | Histone H2b | X60982 | BK006545 | 100 | 23 |
| hsp70 | HSP | M26595 | BK006546 | 98 | 19 |
| mtp70 | Mitochondrial HSP | M73627 | BK006547 | 99 | 20 |
| ef2 | Elongation factor 2 | D50806 | BK006544 | 99 | |
| tc24 | Flagellar calcium binding protein 3 | AF192980 | BK006553 | 99 | |
| par2 | Paraflagellar rod protein 2 | M97548 | BK006548 | 99 | 4 |
| tcg2 | Protein G2 | AY727915 | BK006557 | 96 | 5 |
| tcg4 | Protein G4 | AY727917 | BK006554 | 98 | 5 |
| tcg5 | Elongation factor alpha G5 | AY727921 | BK006555 | 99 | 5 |
| tcg8 | AAA ATPase-like protein G8 | AY727920 | BK006556 | 99 | 5 |
| asp-3(5340) | Strain CL-Brener trans-sialidase | XM_804480 | BK006550 | 96 | 3, 17 |
| asp-4(7015) | Strain CL-Brener trans-sialidase | XM_803731 | BK006551 | 97 | 3, 17 |
| asp-2(Y strain) | Amastigote surface protein 2 | AY186572 | 6 | ||
RESULTS
As an attempt to characterize new antigens expressed by Y strain amastigotes of T. cruzi, we selected several ORFs based on previous studies of immunogenicity performed either with mice or humans with T. cruzi immunity (5, 12). A detailed rationale for the selected ORFs is provided in the introduction.
Thirteen ORFs were PCR amplified from genomic DNA isolated from the Y strain. One ORF (gene identification no. 5340.t00006 and GenBank accession no. XM-804480) could not be isolated from the genomic DNA of the Y strain. In this case, we used genomic DNA from the CL-Brener strain. In many cases, computational analyses were used to determine whether the predicted amino acid sequences of these ORFs had a highly probable SP. For these ORFs, we prepared 5′ oligonucleotides which excluded the sequence coding for the putative SP. As depicted in Table 2, each predicted amino acid sequence showed a high degree of identity to the previously described ORF, varying from 96 to 100%.
These 14 ORFs were subcloned into the eukaryotic expression vector pcDNA3 containing the nucleotide sequence encoding the mouse IgSP (6). We used this vector based on experiments in which we found that the presence of a eukaryotic signal peptide improved the immunogenicity of several ORFs when delivered by genetic immunization with plasmids or viruses (M. M. Rodrigues, unpublished observations). Groups of A/Sn mice were immunized with each of these plasmids. We decided to immunize each mouse group with a single plasmid, not a pool of plasmids, due to a previous observation that DNA vaccination with a mixture of plasmids may interfere with the immune response of the mice (39). Although this approach was more laborious, the results were more decisive.
After the third immunizing dose, the animals were bled and their sera were used to react by IFA with cells of the different stages of T. cruzi. As shown in Table 3, except for the mice immunized with the control empty vector or the par-2 ORF, all other immune sera recognized amastigotes. The lack of immune reactivity of the sera from par-2-immunized mice cannot be attributed to the absence of specific antibodies because they clearly recognized the other two forms of the parasite (trypomastigotes and epimastigotes).
TABLE 3.
Reactivity by IFA of sera from plasmid DNA-immunized A/Sn mice to different developmental stages of T. cruzi
| T. cruzi ORF with plasmid pIgSPa | Reactivity to developmental stage:
|
||
|---|---|---|---|
| Epimastigote | Amastigote | Trypomastigote | |
| Empty | Negative | Negative | Negative |
| ribpS4 | Negative | Positive | Negative |
| rpl7a | Negative | Positive | Negative |
| h2b | Negative | Positive | Negative |
| hsp70 | Positive | Positive | Positive |
| mtp70 | Positive | Positive | Positive |
| ef2 | Negative | Positive | Negative |
| tc24 | Positive | Positive | Positive |
| par2 | Positive | Negative | Positive |
| tcg2 | Negative | Positive | Negative |
| tcg4 | Negative | Positive | Negative |
| tcg5 | Negative | Positive | Negative |
| tcg8 | Negative | Positive | Negative |
| asp-3(5340) | Negative | Positive | Negative |
| asp-4(7015) | Negative | Positive | Negative |
| asp-2 (Y strain) | Negative | Positive | Negative |
Sera from mice immunized three times with the indicated ORFs were used for the IFA reaction.
Immune sera from mice immunized with three other ORFs (tc24, hsp70, and mtp70) recognized all three developmental forms of T. cruzi. Immunization with the other 11 ORFs elicited stage-specific antibodies to amastigotes. We concluded that the ORFs we selected were in most cases amastigote stage specific or contain predominantly amastigote stage-specific epitopes.
The presence of T. cruzi-specific antibodies was taken as a strong indication that the antigens had been produced and were available to the immune system. DNA-immunized mice were then challenged i.p. with a lethal dose of Y strain trypomastigotes. A group of mice injected with empty pcDNA3 was always used as a control, and their parasitemia and mortality were considered for statistical comparison. As shown in Table 4, all control mice succumbed to infection. Their deaths occurred less than 30 days after challenge in 98.39% of the cases. pcDNA3-injected mice challenged in parallel with each plasmid were used to establish the statistical significance of the other mouse groups based on the log rank test for survival rates after T. cruzi infection. Complete protection was only considered for animals that survived more than 60 days after challenge. The mice which did not die after this period were sacrificed.
TABLE 4.
Results of A/Sn mice vaccination with the different ORFs and challenge with blood trypomastigotes from the Y strain of T. cruzia
| T. cruzi ORF with plasmid pIgSP | No. of immunized animals | % Survival at:
|
P value (log rank test)b | |
|---|---|---|---|---|
| 30 days | 60 days | |||
| Empty | 62 | 1.61 | 0 | |
| ribpS4 | 6 | 0 | 0 | 0.0055 |
| rpl7a | 6 | 0 | 0 | 0.0024 |
| h2b | 6 | 0 | 0 | 0.2759 |
| hsp70 | 6 | 0 | 0 | 0.1787 |
| mtp70 | 6 | 0 | 0 | 0.0619 |
| ef2 | 6 | 0 | 0 | 0.4579 |
| tc24 | 6 | 33.33 | 16.66 | 0.0265 |
| par2 | 6 | 16.66 | 0 | 0.0519 |
| tcg2 | 6 | 0 | 0 | 0.3450 |
| tcg 4 | 4 | 0 | 0 | 0.5520 |
| tcg 5 | 5 | 0 | 0 | 0.0014 |
| tcg 8 | 6 | 16.66 | 0 | 0.0271 |
| asp-3(5340) | 6 | 83.33 | 50 | 0.0005 |
| asp-4(7015) | 6 | 83.33 | 66.66 | 0.0061 |
| asp-2 (Y strain) | 7 | 71.43 | 57.14 | 0.0007 |
A/Sn mice were immunized as described in Materials and Methods with pIgSP plasmids containing the indicated T. cruzi ORFs. Results are representative of two or more experiments performed with each plasmid.
Kaplan-Meier curves for survival of mice immunized with the different plasmids were compared to those for pcDNA3-injected animals using the log rank test. Data are representative of two or more experiments performed with each plasmid. Boldface numbers represent statistically significant differences (P < 0.05).
Seven of the 15 ORFs tested failed to induce any degree of immunity as determined by the survival rates after infection (P > 0.05, log rank test [Table 4]). Five other ORFs provided a certain degree of protective immunity, as indicated by a significant delay in mortality compared to control pcDNA3-immunized animals challenged in parallel. These ORFs were (i) rpl7a (P < 0.0024), (ii) ribpS4 (P < 0.0055), (iii) tcg5 (P < 0.0014), (iv) tcg8 (P < 0.0271), and (v) tc24 (P < 0.0265). However, only a few mice survived more than 60 days after challenge.
Immunization with two ORFs generated higher immunity, not only by delaying cumulative mortality significantly but also by allowing 50% or more of the challenged animals to survive. These proteins were previously denominated ASP-related proteins (gene identification no. 5340.t00006 or 7015.t00004 [2] and GenBank accession no. XM_804480 or XM_803731, respectively). As mentioned above, based on the proteome analysis, these two proteins were expressed by amastigotes of T. cruzi. Through their predicted amino acid sequences, both genes encode members of the TS family containing 674 or 777 amino acids (aa), respectively (3). We denominated these antigens ASP-3(5340) and ASP-4(7015) due to their presence on amastigotes and their previous gene identification. In Fig. 1, we show a schematic representation of the primary structure of each protein based on the predicted amino acid sequence and computational analysis. Both of them have predicted SP and a sialidase-like domain. ASP-3(5340) has an ASP box motif (SxDxGxTW) between aa 344 and 351, and asp-4(7015) has a putative GPI addition anchor motif (Fig. 1A and C, respectively). On the same figure, we also show the regions of each ORF that were subcloned into our eukaryotic expression vector. We removed the nucleotide sequences encoding the predicted T. cruzi SP or GPI addition anchor motifs (Fig. 1B and D). These new ORFs were then subcloned in frame with the sequence encoding mouse IgSP.
FIG. 1.
Schematic representation of the predicted amino acid sequences encoded by ORFs asp-3(5340) and asp-4(7015). Panels A and C show the schematic representation based on the predicted amino acid sequences of ORFs initially described (GenBank accession no. XM_804480.1 and XM_803731). Panels B and D show the predicted amino acid sequences of ORFs that we subcloned into the pcDNA3 eukaryotic expression vector.
Table 5 shows the identities found when the ORFs' nucleotide and predicted amino acid sequences were compared with selected sequences of previously described ORFs of members of the TS family expressed by amastigotes of T. cruzi. At the nucleotide or amino acid level, asp-3(5340) displayed a high degree of identity to isoforms of asp-2 recently cloned from the cDNA of amastigotes of the Sylvio X10/4 or G strain, respectively (10). The nucleotide identities between asp-3(5340) and the asp-2 from the Y strain (clone 9 or clone 13) were 58.2 and 59.8%, respectively (6). At the level of predicted amino acid sequence, the identities between asp-3(5340) and the asp-2 from the Y strain (clone 9 or clone 13) were 43.8 and 45.8%, respectively. Finally, the identities at both nucleotide and predicted amino acid levels to asp-4(7015) were very limited.
TABLE 5.
Estimation of nucleotide and predicted amino acid identities among products of selected ORFs expressed by T. cruzi amastigotes
| Strain and protein | % Nucleotide or predicted amino acid sequence identitya
|
|||||
|---|---|---|---|---|---|---|
| CL-Brener ASP-3(5340)b | Sylvio X10/4 ASP-2c | G ASP-2d | Y ASP-2 (clone 9)e | Y ASP-2 (clone 13)f | ASP-4(7015)g | |
| CL-Brener ASP-3(5340) | 93.8 | 91.8 | 58.2 | 59.8 | 28.1 | |
| Sylvio X10/4 ASP-2 | 87.7 | 94.7 | 58.9 | 61.5 | 25.5 | |
| G ASP-2 | 72.8 | 77.3 | 56.8 | 59.2 | 24.3 | |
| Y ASP-2 (clone 9) | 43.8 | 44.7 | 40.1 | 79.4 | 28.9 | |
| Y ASP-2 (clone 13) | 45.4 | 48.1 | 43.2 | 69.9 | 27.5 | |
| ASP-4(7015) | 21.7 | 18.4 | 15.2 | 22.3 | 23.2 | |
Identities were calculated by the ClustalW alignment. Boldface values represent nucleotide sequence identity; lightface values represent predicted amino acid identity.
GenBank accession no. BK006550.
GenBank accession no. EF579923.1 (10).
GenBank accession no. EF579923.1 (10).
GenBank accession no. AY186572.1 (6).
GenBank accession no. BK006551.
asp-4(7015) displays a large number of copies of similar ORFs in the parasite genome (17). Twenty of the putative ORFs have an identity at the level of amino acid predicted sequences of ≥80%. The amino acid identity to the other asp was limited, varying from 15.2% [asp-2, G strain] to 23.2% (asp-2, clone 13, Y strain [Table 5]). It is important to note that asp-4(7015) contains the epitope TsKb-21 (ANYNFTLV), which has been shown to be highly recognized during experimental infection of C57BL/6 mice with the Brazil strain of T. cruzi (Fig. 1C and D and reference 30).
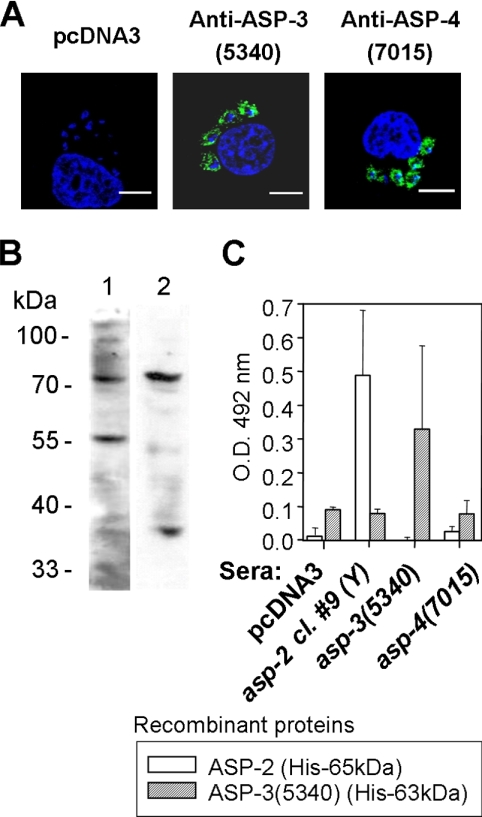
With IFA, we observed that sera from asp-3(5340) or asp-4(7015) DNA-immunized mice reacted with Y strain amastigotes grown in cultured HeLa cells (Fig. 2A). An immunoblot showed that sera from asp-3(5340) DNA-immunized mice recognized bands of approximately 72 kDa and 57 kDa when reacted with extracts of extracellular amastigotes of the Y strain of T. cruzi. The immune sera from mice immunized with asp-4(7015) probed with the same parasite extract recognized polypeptides of approximately 74 kDa and 37 kDa (Fig. 2B). Control sera from immunized pcDNA3 failed to recognize any amastigote protein by immunoblotting (data not shown).
FIG. 2.
IFA, immunoblotting, and ELISA using antibodies specific for ASP-3(5340) and ASP-4(7015). (A) Intracellular amastigotes were analyzed by IFA using antibodies specific for ASP-3(5340) and ASP-4(7015). HeLa cells infected for 48 h with parasites of the Y strain of T. cruzi were incubated with a pool of sera derived from mice immunized with plasmids containing ORF asp-3(5340) or asp-4(7015) and imaged under fluorescence. Blue, DAPI staining; green, fluorescein isothiocyanate-labeled goat anti-mouse Ig. Magnification bars, 10 μm. (B) Extracellular amastigote extracts (equivalent to 107 parasites) of the Y strain were loaded into each lane for an SDS-PAGE performed under reducing conditions. Immunoblot strips were incubated with a pool of sera derived from mice immunized with plasmids containing ORF asp-3(5340) or asp-4(7015) diluted 1:500 or 1:800, respectively. (C) Pools of sera derived from mice immunized with plasmids containing ORF asp-2, asp-3(5340), or asp-4(7015) were diluted 1:400. Results are expressed as the average ± standard deviation of three independent experiments.
Due to the limited identity in the predicted primary amino acid sequence between asp-3(5340), asp-4(7015), and asp-2 (Y strain), we predicted that cross-reactivity at the immunological level would be restricted. To test this hypothesis, we used pooled sera from mice immunized with each ORF to react with two recombinant proteins in ELISA. As shown in Fig. 2C, sera from A/Sn mice immunized with asp-2 reacted with recombinant ASP-2 (His-65kDa) but not with recombinant ASP-3(5340) (His-63kDa). Inversely, sera from mice immunized with asp-3(5340) reacted with recombinant ASP-3(5340) (His-63kDa) but not with recombinant ASP-2 (His-65kDa). Sera from mice immunized with asp-4(7015) failed to react with either recombinant protein. The lack of reactivity cannot be attributed to low antibody concentrations because these sera recognized amastigotes by IFA (Table 3). We concluded that although all three proteins are members of the TS family, containing a sialidase-like domain, and are expressed as amastigote-specific antigens, they share limited antigenic cross-reactivity, at least at the level of antibody recognition.
We also sought to determine whether sera from mice immunized with ORF asp-3(5340) or asp-4(7015) could recognize amastigotes of different T. cruzi strains. For that purpose, IFA was carried out using amastigotes of seven distinct strains as targets. The results, summarized in Table 6, show that immune sera from asp-3(5340), asp-4(7015), or asp-2 DNA-immunized mice recognized amastigotes of all different strains, indicating the presence of the antigen or cross-reactive epitopes.
TABLE 6.
Results of the IFA using sera from plasmid DNA-vaccinated mice against amastigotes of different T. cruzi strains
| Amastigote strain | IFA result for T. cruzi ORF:
|
|||
|---|---|---|---|---|
| asp-3(5340) | asp-4(7015) | asp-2 | Empty | |
| Y | Positive | Positive | Positive | Negative |
| Colombian | Positive | Positive | Positive | Negative |
| Sylvio X10/4 | Positive | Positive | Positive | Negative |
| G | Positive | Positive | Positive | Negative |
| CL Brener | Positive | Positive | Positive | Negative |
| Tulahuén | Positive | Positive | Positive | Negative |
| Dm28c | Positive | Positive | Positive | Negative |
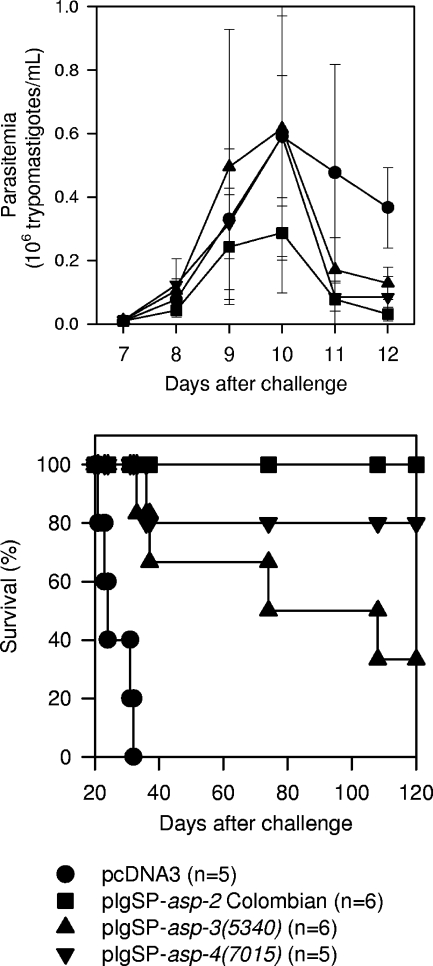
Finally, we determined whether protective immunity elicited by these two ORFs could also be obtained against a challenge with a second strain of T. cruzi. For that purpose, A/Sn mice were vaccinated with plasmids containing asp-3(5340), asp-4(7015), or asp-2 (clone 22, Colombian strain). Parasitemia and mortality were monitored after challenge with trypomastigotes of the Colombia strain (14). Only mice vaccinated with asp-2 displayed a significant reduction of the parasitemia compared to pcDNA3-injected animals (Fig. 3A; P < 0.01). However, the mortality rates of asp-3(5340), asp-4(7015), and asp-2 DNA-vaccinated mice were significantly delayed in comparison to those of animals injected with pcDNA3 (Fig. 3B; P = 0.0007, P = 0.0018, and P = 0.0007, respectively). No difference was observed among the groups that received each asp (P > 0.05, log rank test). We concluded from this experiment that vaccination with plasmids containing asp-3(5340) or asp-4(7015) indeed provided a certain degree of protective immunity to A/Sn mice highly susceptible to Chagas' disease following a lethal challenge with a second unrelated strain of T. cruzi.
FIG. 3.
Trypomastigote-induced parasitemia and mortality in A/Sn mice immunized with plasmids containing asp-related ORFs expressed in amastigotes of T. cruzi. A/Sn mice were immunized with plasmid pIgSP-asp-3(5340), pIgSp-asp-4(7015), pIgSP-clone22 (Colombian strain), or pcDNA3. Fourteen days after the last immunization, mice were challenged i.p. with 250 bloodstream trypomastigotes of the Colombia strain. In the top panel, the course of infection was estimated by the number of trypomastigotes per ml of blood. Results represent the mean ± standard deviation obtained from five or six mice immunized with each plasmid. At the peak of infection, the parasitemias of mice immunized with each plasmid were compared by one-way analysis of variance and Tukey's honestly significant difference tests. The peak parasitemias of mice immunized with pIgSP-asp-3(5340) or pIgSp-asp-4(7015) were not different from those of animals injected with pcDNA3 (P > 0.05 in both cases). However, a significant difference was detected when we compared the peak parasitemias of mice immunized with pcDNA3 or pIgSP-clone22 (Colombian strain) (P < 0.01). In the bottom panel, Kaplan-Meier curves for the survival of mice immunized with pIgSP-asp-3(5340), pIgSp-asp-4(7015), pIgSP-clone22 (Colombian strain), or pcDNA3. The results of the comparison were as follows: (i) pcDNA3 × pIgSP-asp-3(5340), P = 0.0007; (ii) pcDNA3 × pIgSP-asp-4(7015), P = 0.0018; (iii) pcDNA3 × pIgSP-clone22 (Colombian strain), P = 0.0007; (iv) pIgSP-asp-4(7015) × pIgSP-asp-3(5340), P > 0.05; (v) pIgSP-asp-3(5340) × pIgSP-clone22, P > 0.05; and (vi) pIgSP-asp-4(7015) × pIgSP-clone22, P > 0.05. Data are representative of two experiments performed with similar results.
DISCUSSION
The aims of the present study were first to screen 14 ORFs/antigens putatively expressed by amastigotes of T. cruzi for their vaccination potential and second to compare the protective potential of these different ORFs following plasmid DNA immunization of A/Sn mice highly susceptible to Chagas' disease challenged with Y strain trypomastigotes.
Using the immune sera of plasmid DNA-vaccinated mice, we confirmed that in fact most of the antigens selected were stage-specific amastigote antigens. Only one ORF/antigen was not expressed by amastigotes (par-2). Three other antigens were expressed in the three developmental stages tested. The presence of specific antibodies served as a guide to confirm successful protein expression and immunogenicity. Although protein expression and antigenic priming were observed, many of these ORFs failed to provide a detectable degree of protective immunity. These ORFs were h2b, hsp70, mtp70, ef2, par2, tcg2, and tcg4. Our observations confirm and extend a recent publication from Morell et al. (35), who reported that par2 or hsp70 ORFs in mice failed to promote a detectable degree of protective immunity when injected individually. However, when these authors used a fused gene containing both ORFs for vaccination, immunity to late-stage infection was observed (35). Together with our results, they indicated that perhaps plasmid DNA immunization with par-2 ORF individually is not as effective as a previously described immunization method using recombinant protein PAR-2 (28, 46).
The most relevant part of our study was the fact that after a lethal challenge with Y strain trypomastigotes of T. cruzi, we observed that several antigens induced a certain degree of immunity, as measured by a delay in mouse mortality. Immunization with plasmids containing ORFs RpL7a, RibpS4, TcG5, Tc24, and TcG8 caused a significant delay in mouse mortality (Table 4). Among those, only ORF Tc24 had been previously described for its ability to elicit protective immunity against a lethal T. cruzi infection (16). Therefore, our results are the first to suggest that these other ORFs/antigens can generate a certain degree of immunity against experimental T. cruzi infection. Nevertheless, it is important to note that in rare cases, the mice survived more than 30 days after challenge. The fact that the protective potential of these five ORFs is limited in this mouse model of infection should not be seen as a completely negative result when evaluating the importance of ORFs as a target of protective immunity. Perhaps they can be further explored using other genetically distinct mouse or parasite strains. Also, the use of stronger genetic vectors, such as adenovirus, or the use of recombinant proteins and Toll-like receptor agonist adjuvants, such as CpG oligodeoxynucleotides, might help to increase their immune-protective potential (2, 13, 27, 29).
Two ORFs/antigens elicited protective immunity capable of delaying mouse mortality and providing complete protection to a fraction of mice infected with two distinct strains of T. cruzi. These antigens are members of the TS family of surface proteins, and their immunological potential has been explored here for the first time. Although one of them, ASP-3(5340), shares a certain degree (43.8 to 45.4%) of identity at the level of primary structure with a previously described stage-specific antigen (ASP-2 of the Y strain), we did not observe cross-reactivity between both as recognized by specific antibodies (Fig. 2C). Also, there was no recognition of the 83-kDa antigen by immunoblot analysis (Fig. 2B). The primary structure of the second antigen, ASP-4(7015), shares very limited identity at the level of primary structure with the other ASP, and sera from immune mice failed to recognize either of the recombinant proteins representing them (Fig. 2C).
Analyses with IFA showed that amastigotes from seven parasite strains express epitopes related to each of these new antigens as recognized by immune sera. Although these results are suggestive of their expression in other parasite strains, until these ORFs are cloned and sequenced, it will not be possible to establish their structural relationship with certainty.
The results of protective immunity obtained following vaccination with these two ORFs are important because in this mouse model only vaccination with the asp-2 ORF had been successful so far (2, 13, 43). In contrast, vaccination with a plasmid containing the ORF encoding the catalytic domain of T. cruzi TS failed to promote a similar degree of protective immunity (43, 44). The same plasmid has been shown in many instances to be highly protective in BALB/c mice challenged with parasites of the Y or Tulahuen strain of T. cruzi (11, 22, 27).
In the present study, we did not delve more deeply into the nature of the immunological mechanisms operating in DNA-vaccinated mice. Nevertheless, it is plausible to propose that they are most likely related to T-cell-mediated immune responses, as antibodies have never been described as being efficient against the intracellular form of T. cruzi. In contrast, CD4 Th1 and CD8 Tc1 are often seen as important mechanisms of immunity following infection with or vaccination against T. cruzi (26, 27, 29, 30, 37, 42, 43). Nevertheless, it is very important to notice that in this mouse model even a strong CD4+ Th1 response against an important antigen such as the catalytic domain of TS did not confer a significant degree of protective immunity (43, 44). Antigen-specific CD4+ T cells capable of producing considerable amounts of gamma interferon and proliferating in vitro failed to protect these animals (44). Therefore, other mechanisms such as CD8+ T cells might be critical for the protection of these highly susceptible A/Sn mice. Indeed protected A/Sn mice vaccinated with either plasmid DNA or a recombinant protein expressing the ASP-2 antigen became completely susceptible to infection when depleted of CD8+ T cells (2, 43). Our data clearly point to an essential role for CD8+ T cells for protective immunity. We believe that the appropriate experimental approach to initiate studies on the mechanisms of immunity generated by these new ORFs/antigens would be in vivo depletion of CD4+ or CD8+ T cells before challenge. Immunological analyses based on antibody titers and CD4+ T-cell activation (proliferative responses and gamma interferon secretion in vitro) have not added much to the understanding of this experimental model so far (44).
In summary, our study shows that the availability of information on the amastigote-expressed ORFs of T. cruzi coupled with plasmid DNA immunization can be an alternative method to screen and compare for new antigen targets of protective immune responses against experimental infection. These antigens can then be used in different immunological studies to aid in understanding the complex immunopathology observed during Chagas' disease.
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo, The Millennium Institute for Vaccine Development and Technology (CNPq-420067/2005-1), and The Millennium Institute for Gene Therapy. F.A.B.H., L.G.Z., and C.C. are recipients of fellowships from FAPESP. E.L.V.S. and M.M.R. are recipients of fellowships from CNPq.
Footnotes
Published ahead of print on 25 June 2008.
REFERENCES
- 1.Andrews, N. W., K. S. Hong, E. S. Robbins, and V. Nussenzweig. 1987. Stage-specific surface antigens expressed during the morphogenesis of vertebrate forms of Trypanosoma cruzi. Exp. Parasitol. 64:474-484. [DOI] [PubMed] [Google Scholar]
- 2.Araujo, A. F. S., B. C. G. de Alencar, J. R. C. Vasconcelos, M. I. Hiyane, C. R. F. Marinho, M. L. O. Penido, S. B. Boscardin, D. F. Hoft, R. T. Gazzinelli, and M. M. Rodrigues. 2005. CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2. Infect. Immun. 73:6017-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atwood, J. A., III, D. B. Weatherly, T. A. Minning, B. Bundy, C. Cavola, F. R. Opperdoes, R. Orlando, and R. L. Tarleton. 2005. The Trypanosoma cruzi proteome. Science 309:473-476. [DOI] [PubMed] [Google Scholar]
- 4.Beard, C. A., J. L. Saborio, D. Tewari, K. G. Krieglstein, A. H. Henschen, and J. E. Manning. 1992. Evidence for two distinct major protein components, PAR 1 and PAR 2, in the paraflagellar rod of Trypanosoma cruzi. Complete nucleotide sequence of PAR-2. J. Biol. Chem. 267:21656-21662. [PubMed] [Google Scholar]
- 5.Bhatia, V., M. Sinha, B. Luxon, and N. Garg. 2004. Utility of the Trypanosoma cruzi sequence database for identification of potential vaccine candidates by in silico and in vitro screening. Infect. Immun. 72:6245-6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boscardin, S. B., S. S. Kinoshita, A. E. Fujimura, and M. M. Rodrigues. 2003. Immunization with cDNA expressed by amastigotes of Trypanosoma cruzi elicits protective immune response against experimental infection. Infect. Immun. 71:2744-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandariz, S., A. Schijman, C. Vigliano, P. Arteman, R. Viotti, C. Beldjord, and M. J. Levin. 1995. Detection of parasite DNA in Chagas' heart disease. Lancet 346:1370-1371. [DOI] [PubMed] [Google Scholar]
- 8.Briones, M. R. S., R. P. Souto, B. Stolf, and B. Zingales. 1999. The evolution of two Trypanosoma cruzi subgroups inferred from rRNA genes can be correlated with the interchange of American mammalian faunas in the Cenozoic and has implications to pathogenicity and host specificity. Mol. Biochem. Parasitol. 104:219-232. [DOI] [PubMed] [Google Scholar]
- 9.Cazorla, S. I., P. D. Becker, F. M. Frank, T. Ebensen, M. J. Sartori, R. S. Corral, E. L. Malchiodi, and C. A. Guzmán. 2008. Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi. Infect. Immun. 76:324-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claser, C., N. M. Espindola, G. Sasso, A. J. Vaz, S. B. Boscardin, and M. M. Rodrigues. 2007. Immunologically relevant strain polymorphism in the amastigote surface protein 2 of Trypanosoma cruzi. Microbes Infect. 9:1011-1019. [DOI] [PubMed] [Google Scholar]
- 11.Costa, F., G. Franchin, V. L. Pereira-Chioccola, M. Ribeirão, S. Schenkman, and M. M. Rodrigues. 1998. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine 16:768-774. [DOI] [PubMed] [Google Scholar]
- 12.DaRocha, W. D., D. C. Bartholomeu, C. D. Macedo, M. F. Horta, E. Cunha-Neto, J. E. Donelson, and S. M. Teixeira. 2002. Characterization of cDNA clones encoding ribonucleoprotein antigens expressed in Trypanosoma cruzi amastigotes. Parasitol. Res. 88:292-300. [DOI] [PubMed] [Google Scholar]
- 13.de Alencar, B. C., A. F. Araújo, M. L. Penido, R. T. Gazzinelli, and M. M. Rodrigues. 2007. Cross-priming of long lived protective CD8+ T cells against Trypanosoma cruzi infection: importance of a TLR9 agonist and CD4+ T cells. Vaccine 25:6018-6027. [DOI] [PubMed] [Google Scholar]
- 14.Dost, C. K., S. Albuquerque, V. Hemleben, W. Engels, and J. C. Prado, Jr. 2002. Molecular genetic characterization of different Trypanosoma cruzi strains and comparison of their development in Mus musculus and Calomys callosus. Parasitol. Res. 88:609-616. [DOI] [PubMed] [Google Scholar]
- 15.Dumonteil, E. 2007. DNA vaccines against protozoan parasites: advances and challenges. J. Biomed. Biotechnol. 2007:90520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumonteil, E., J. Escobedo-Ortegon, N. Reyes-Rodriguez, A. Arjona-Torres, and M. J. Ramirez-Sierra. 2004. Immunotherapy of Trypanosoma cruzi infection with DNA vaccines in mice. Infect. Immun. 72:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Sayed, N. M., P. J. Myler, D. C. Bartholomeu, D. Nilsson, G. Aggarwal, A. N. Tran, E. Ghedin, E. A. Worthey, A. L. Delcher, G. Blandin, S. J. Westenberger, E. Caler, G. C. Cerqueira, C. Branche, B. Haas, A. Anupama, E. Arner, L. Aslund, P. Attipoe, E. Bontempi, F. Bringaud, P. Burton, E. Cadag, D. A. Campbell, M. Carrington, J. Crabtree, H. Darban, J. F. da Silveira, P. de Jong, K. Edwards, P. T. Englund, G. Fazelina, T. Feldblyum, M. Ferella, A. C. Frasch, K. Gull, D. Horn, L. Hou, Y. Huang, E. Kindlund, M. Klingbeil, S. Kluge, H. Koo, D. Lacerda, M. J. Levin, H. Lorenzi, T. Louie, C. R. Machado, R. McCulloch, A. McKenna, Y. Mizuno, J. C. Mottram, S. Nelson, S. Ochaya, K. Osoegawa, G. Pai, M. Parsons, M. Pentony, U. Pettersson, M. Pop, J. L. Ramirez, J. Rinta, L. Robertson, S. L. Salzberg, D. O. Sanchez, A. Seyler, R. Sharma, J. Shetty, A. J. Simpson, E. Sisk, M. T. Tammi, R. Tarleton, S. Teixeira, S. Van Aken, C. Vogt, P. N. Ward, B. Wickstead, J. Wortman, O. White, C. M. Fraser, K. D. Stuart, and B. Andersson. 2005. The genome sequence of Trypanosoma cruzi, etiologic agent of Chagas disease. Science 309:409-415. [DOI] [PubMed] [Google Scholar]
- 18.El-Sayed, N. M., P. J. Myler, G. Blandin, M. Berriman, J. Crabtree, G. Aggarwal, E. Caler, H. Renauld, E. A. Worthey, C. Hertz-Fowler, E. Ghedin, C. Peacock, D. C. Bartholomeu, B. J. Haas, A. N. Tran, J. R. Wortman, U. C. Alsmark, S. Angiuoli, A. Anupama, J. Badger, F. Bringaud, E. Cadag, J. M. Carlton, G. C. Cerqueira, T. Creasy, A. L. Delcher, A. Djikeng, T. M. Embley, C. Hauser, A. C. Ivens, S. K. Kummerfeld, J. B. Pereira-Leal, D. Nilsson, J. Peterson, S. L. Salzberg, J. Shallom, J. C. Silva, J. Sundaram, S. Westenberger, O. White, S. E. Melville, J. E. Donelson, B. Andersson, K. D. Stuart, and M. Hall. 2005. Comparative genomics of trypanosomatid parasitic protozoa. Science 309:404-409. [DOI] [PubMed] [Google Scholar]
- 19.Engman, D. M., S. C. Fehr, and J. E. Donelson. 1992. Specific functional domains of mitochondrial hsp70s suggested by sequence comparison of the trypanosome and yeast proteins. Mol. Biochem. Parasitol. 51:153-155. [DOI] [PubMed] [Google Scholar]
- 20.Engman, D. M., S. R. Sias, J. D. Gabe, J. E. Donelson, and E. A. Dragon. 1989. Comparison of HSP70 genes from two strains of Trypanosoma cruzi. Mol. Biochem. Parasitol. 37:285-287. [DOI] [PubMed] [Google Scholar]
- 21.Fralish, B. H., and R. L. Tarleton. 2003. Genetic immunization with LYT1 or a pool of trans-sialidase genes protects mice from lethal Trypanosoma cruzi infection. Vaccine 21:3070-3080. [DOI] [PubMed] [Google Scholar]
- 22.Fujimura, A. E., S. S. Kinoshita, V. L. Pereira-Chioccola, and M. M. Rodrigues. 2001. DNA sequences encoding CD4+ and CD8+ T-cell epitopes are important for efficient protective immunity induced by DNA vaccination with a Trypanosoma cruzi gene. Infect. Immun. 69:5477-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Salcedo, J. A., J. L. Oliver, R. P. Stock, and A. Gonzalez. 1994. Molecular characterization and transcription of the histone H2B gene from the protozoan parasite Trypanosoma cruzi. Mol. Microbiol. 13:1033-1043. [DOI] [PubMed] [Google Scholar]
- 24.Garg, N., and R. L. Tarleton. 2002. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 70:5547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez, R., S. Palacios, J. Herrera, S. Martinez-Calvillo, and I. Lopez. 1998. The deduced primary structure of a ribosomal protein S4 from Trypanosoma cruzi. Biochim. Biophys. Acta 1395:321-325. [DOI] [PubMed] [Google Scholar]
- 26.Hoft, D. F., and C. S. Eickhoff. 2005. Type 1 immunity provides both optimal mucosal and systemic protection against a mucosally invasive, intracellular pathogen. Infect. Immun. 73:4934-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoft, D. F., C. S. Eickhoff, O. K. Giddings, J. R. Vasconcelos, and M. M. Rodrigues. 2007. trans-Sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic Trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming. J. Immunol. 179:6889-6900. [DOI] [PubMed] [Google Scholar]
- 28.Luhrs, K. A., D. L. Fouts, and J. E. Manning. 2003. Immunization with recombinant paraflagellar rod protein induces protective immunity against Trypanosoma cruzi infection. Vaccine 21:3058-3069. [DOI] [PubMed] [Google Scholar]
- 29.Machado, A. V., J. E. Cardoso, C. Claser, M. M. Rodrigues, R. T. Gazzinelli, and O. Bruna-Romero. 2006. Long-term protective immunity induced against Trypanosoma cruzi infection after vaccination with recombinant adenoviruses encoding amastigote surface protein-2 and trans-sialidase. Hum. Gene Ther. 17:898-908. [DOI] [PubMed] [Google Scholar]
- 30.Martin, D. L., D. B. Weatherly, S. A. Laucella, M. A. Cabinian, M. T. Crim, S. Sullivan, M. Heiges, S. H. Craven, C. S. Rosenberg, M. H. Collins, A. Sette, M. Postan, and R. L. Tarleton. 2006. CD8+ T-cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina-Acosta, E., and G. A. M. Cross. 1993. Rapid isolation of DNA from trypanosomatid protozoa using a simple “mini-prep” procedure. Mol. Biochem. Parasitol. 59:327-330. [DOI] [PubMed] [Google Scholar]
- 32.Miyahira Y. 2008. Trypanosoma cruzi infection from the view of CD8+ T cell immunity—an infection model for developing T cell vaccine. Parasitol. Int. 57:38-48. [DOI] [PubMed] [Google Scholar]
- 33.Miyahira, Y., M. Katae, K. Takeda, H. Yagita, K. Okumura, S. Kobayashi, T. Takeuchi, T. Kamiyama, Y. Fukuchi, and T. Aoki. 2003. Activation of natural killer T cells by α-galactosylceramide impairs DNA vaccine-induced protective immunity against Trypanosoma cruzi. Infect. Immun. 71:1234-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morel, C. M., and J. Lazdins. 2003. Chagas disease. Nat. Rev. Microbiol. 1:14-15. [DOI] [PubMed] [Google Scholar]
- 35.Morell, M., M. C. Thomas, T. Caballero, C. Alonso, and M. C. López. 2006. The genetic immunization with paraflagellar rod protein-2 fused to the HSP70 confers protection against late Trypanosoma cruzi infection. Vaccine 24:7046-7055. [DOI] [PubMed] [Google Scholar]
- 36.Planelles, L., M. C. Thomas, C. Alonso, and M. C. López. 2001. DNA immunization with Trypanosoma cruzi HSP70 fused to the KMP11 protein elicits a cytotoxic and humoral immune response against the antigen and leads to protection. Infect. Immun. 69:6558-6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues, M. M., M. Ribeirão, V. Pereira-Chioccola, L. Renia, and F. Costa. 1999. Predominance of CD4 Th1 and CD8 Tc1 cells revealed by characterization of the cellular immune response generated by immunization with a DNA vaccine containing a Trypanosoma cruzi gene. Infect. Immun. 67:3855-3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnapp, A. R., C. S. Eickhoff, D. Sizemore, R. Curtiss III, and D. F. Hoft. 2002. Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice. Infect. Immun. 70:5065-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedegah, M., Y. Charoenvit, L. Minh, M. Belmonte, V. F. Majam, S. Abot, H. Ganeshan, S. Kumar, D. J. Bacon, A. Stowers, D. L. Narum, D. J. Carucci, and W. O. Rogers. 2004. Reduced immunogenicity of DNA vaccine plasmids in mixtures. Gene Ther. 11:448-456. [DOI] [PubMed] [Google Scholar]
- 40.Sepulveda, P., M. Hontebeyrie, P. Liegeard, A. Mascilli, and K. A. Norris. 2000. DNA-based immunization with Trypanosoma cruzi complement regulatory protein elicits complement lytic antibodies and confers protection against Trypanosoma cruzi infection. Infect. Immun. 68:4986-4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarleton, R. L. 2001. Parasite persistence in the aetiology of Chagas' disease. Int. J. Parasitol. 31:550-554. [DOI] [PubMed] [Google Scholar]
- 42.Tzelepis, F., B. C. G. de Alencar, M. L. O. Penido, R. T. Gazzinelli, P. M. Persechini, and M. M. Rodrigues. 2006. Distinct kinetics of effector CD8+ cytotoxic T cells after infection with Trypanosoma cruzi in naive or vaccinated mice. Infect. Immun. 74:2477-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasconcelos, J. R., M. I. Hiyane, C. R. F. Marinho, C. Claser, A. M. Vieira-Machado, R. T. Gazinelli, O. Bruña-Romero, J. M. Alvarez, S. B. Boscardin, and M. M. Rodrigues. 2004. Protective immunity against Trypanosoma cruzi infection in a highly susceptible mouse strain following vaccination with genes encoding the amastigote surface protein-2 and trans-sialidase. Hum. Gene Ther. 15:878-886. [DOI] [PubMed] [Google Scholar]
- 44.Vasconcelos, J. R., S. B. Boscardin, M. I. Hiyane, S. S. Kinoshita, A. E. Fujimura, and M. M. Rodrigues. 2003. A DNA-priming protein-boosting regimen significantly improves type 1 immune response but not protective immunity to Trypanosoma cruzi infection in a highly susceptible mouse strain. Immunol. Cell Biol. 81:121-129. [DOI] [PubMed] [Google Scholar]
- 45.Wizel, B., N. Garg, and R. L. Tarleton. 1998. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect. Immun. 66:5073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wrightsman, R. A., and J. E. Manning. 2000. Paraflagellar rod proteins administered with alum and IL-12 or recombinant adenovirus expressing IL-12 generates antigen-specific responses and protective immunity in mice against Trypanosoma cruzi. Vaccine 18:1419-1427. [DOI] [PubMed] [Google Scholar]