Abstract
Enterohemorrhagic Escherichia coli (EHEC) is the main cause of hemolytic-uremic syndrome, an endemic disease in Argentina which had an incidence in 2005 of 13.9 cases per 100,000 children younger than 5 years old. Cattle appear to be a major reservoir of EHEC, and a serological response to EHEC antigens has been demonstrated in natural and experimental infections. In the current study, antibodies against proteins implicated in EHEC's ability to form attaching and effacing lesions, some of which are exported to the host cell via a type three secretion system (TTSS), were identified in bovine colostrum by Western blot analysis. Twenty-seven (77.0%) of the 35 samples examined contained immunoglobulin G (IgG) antibodies against the three proteins assayed in this study: EspA, EspB, and the carboxy-terminal 280 amino acids of γ-intimin, an intimin subtype associated mainly with O157:H7 and O145:H- serotypes. Every colostrum sample was able to inhibit, in a range between 45.9 and 96.7%, the TTSS-mediated hemolytic activity of attaching and effacing E. coli. The inhibitory effect was partially mediated by IgG and lactoferrin. In conclusion, we found that early colostrum from cows contains antibodies, lactoferrin, and other unidentified substances that impair TTSS function in attaching and effacing E. coli strains. Bovine colostrum might act by reducing EHEC colonization in newborn calves and could be used as a prophylactic measure to protect non-breast-fed children against EHEC infection in an area of endemicity.
Enterohemorrhagic Escherichia coli (EHEC) is responsible for diseases in humans and animals whose clinical spectrum includes diarrhea, hemorrhagic colitis, and hemolytic-uremic syndrome (HUS), an endemic disease in Argentina, with an incidence in 2005 of 13.9 cases per 100,000 children younger than 5 years old. EHEC serotypes O157:H7 and O145:H- are associated worldwide with severe disease and are the most frequently isolated EHEC serotypes from HUS patients in Argentina (36, 37).
EHEC is characterized by Shiga toxin expression from integrated bacteriophages and other virulence-associated traits (11, 12). Many of these traits are encoded by the chromosomal pathogenicity island called the locus of enterocyte effacement (LEE) (5, 24, 43), which is implicated in EHEC's ability to colonize the intestinal mucosa of humans and animals with a histopathological lesion known as the attaching and effacing (A/E) lesion (28). This lesion is characterized by the destruction of intestinal microvilli and by the intimate adhesion of the bacterium to the enterocyte, with the formation of a pedestallike structure and the polymerization of cytoplasmic actin filaments beneath the attached bacteria. Most of the proteins responsible for the A/E lesion are delivered in the host cell via a type three secretion system (TTSS). The A/E lesion is also characteristic of enteropathogenic E. coli (EPEC) strains, another category of E. coli strains associated with diarrhea in children (28). The TTSS forms a needle made of multimers of E. coli secreted protein A (EspA), through which effector proteins are translocated into the host cell (14). Intimin, a bacterial outer membrane protein, binds to Tir, the translocated intimin receptor in the host cell membrane, leading to the formation of the A/E lesion. EspB contributes to the creation of a pore in the eukaryotic cell membrane and is, in turn, translocated for signal transduction into the cytoplasm.
Intimin, EspA, and EspB elicit an antibody response in serum during both human EHEC (18) and EPEC (23) infections, as well as in a murine model of infection with Citrobacter rodentium, a bacterium that harbors the LEE pathogenicity island (7). Antibodies against these proteins have also been detected in colostrum and milk from healthy women (20, 22, 30, 33).
Healthy cattle are recognized as the main source of human EHEC infections, although a limited number of serogroups have been associated with diarrhea in young calves (3, 10). On the other hand, several studies have shown that cattle are able to elicit an immunological response to some EHEC virulence factors. Both antibody reactivity and neutralizing activity against Shiga toxins have been detected in cattle sera and in immunoglobulin preparations derived from bovine colostrum (19, 34). Furthermore, Widiasih et al. (42) have shown that antibodies against lipopolysaccharides of serotypes O26, O111, and O157 are present in bovine colostrum and are passively transferred to newborn calves. It has also been demonstrated that colostrum from EPEC-vaccinated or nonvaccinated animals recognizes proteins with molecular weights that are consistent with that of intimin (32).
The aim of this work was to analyze the presence of antibodies against EHEC virulence factors in bovine colostrum. This knowledge may contribute to the ability to obtain stronger responses through vaccination with the purpose of producing nutraceutic colostrum or milk. The present study reports that immunoglobulin G (IgG) antibodies against EspA, EspB, and the highly specific C-terminal portion of the O157-associated γ-intimin, as well as other substances inhibiting the TTSS of attaching and effacing E. coli strains, are present in the colostrum of cows in Argentina.
MATERIALS AND METHODS
Colostrum samples.
Thirty-five colostrum samples were obtained from healthy dairy (n = 8) or beef (n = 27) cows within the first 24 to 72 h postpartum from four farms in Buenos Aires province, Argentina. All the farms were located in one of the most important dairy regions in the Central Pampas, an area endemic for HUS in children. Samples were obtained by random selection from cows with more than two labors. Colostrum samples were kept at −20°C until use. Before the assays, the samples were thawed and centrifuged at 13,000 × g to remove lipids. A pool of 15 randomly chosen colostrum samples was IgG depleted by passage through a protein G-Sepharose column (Amersham, NJ). The eluate was restored to the initial volume of the sample to maintain the concentration of the components not retained by the affinity column. An aliquot of the IgG-depleted pool was then adsorbed by affinity membrane chromatography to remove lactoferrin according to Wolman et al. (44). Briefly, 1 ml of colostrum was incubated overnight with polysulfone hollow-fiber microfiltration membranes modified by grafting a glycidyl methacrylate-dimethyl acrylamide copolymer and attaching the red HE-3B dye to them. Ninety-three percent of the lactoferrin from the pooled samples was adsorbed, as determined by an enzyme-linked immunosorbent assay (data not shown).
Escherichia coli strains and culture media.
Wild-type EHEC strains 146N4 (O157:H7) and 97/23A (O26:H11) were isolated from bovine feces at slaughter (8) and from a calf with bloody diarrhea (26), respectively. EPEC E2348/69 (17) was kindly provided by Marta Rivas, ANLIS-Instituto Nacional de Microbiología Carlos G. Malbrán, Buenos Aires. The E. coli EPEC E2348/69 ΔescN strain, a mutant that does not synthesize the TTSS, was kindly provided by Fernando Navarro García, CINVESTAV, Mexico.
Cloning procedures.
The EspA (579-bp) and EspB (945-bp) genes and the gene fragment (843 bp) encoding the carboxy-terminal 280 amino acids of γ-intimin (γ-intimin1-280) were obtained by PCR amplification from E. coli strains 97/23A (O26:H11) (EspA and EspB) and 146N4 (O157:H7) (γ-intimin) and cloned into pRSET-A (Invitrogen, Carlsbad, CA) in E. coli BL21(D3)/pLysS. The primers used for amplification contained 5′ extensions with appropriate restriction sites. The primer sequences and conditions for amplification are shown in Table 1. Template DNA was prepared by boiling one colony in 100 μl of distilled water for 5 min. The PCR was conducted in a 50-μl reaction mixture containing 10 μl of bacterial lysate, 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 1.5 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate (Promega, Madison, WI), 1 μM of each primer (Invitrogen, Carlsbad, CA), and 1.5 U Taq polymerase (Invitrogen, Brazil). The amplified DNA fragments were cleaved with restriction endonucleases BamHI and HindIII and cloned into a similarly cleaved His tag expression vector pRSET-A. The resulting constructs were transformed into chemically competent E. coli BL21(D3)/pLysS as described by the manufacturers. The constructs were sequenced at both ends to verify that the insertions resulted as expected.
TABLE 1.
Oligonucleotide sequences and PCR amplification conditions used to amplify EspA and EspB genes and the γ-intimin1-280 gene fragment from EHEC strains
| Gene or gene fragment | GenBank accession no. | Primer | Oligonucleotide sequence (5′-3′) | Positions | Amplification conditions |
|---|---|---|---|---|---|
| EspB | U65681 | espBup2 | GGATCCATGAATACTATTGATTATAC | 1-945 | 94°C, 2 min (1 cycle); 94°C, 1 min; 50°C, 1 min; 72°C, 1 min (30 cycles) |
| espBrev | AAGCTTTTAACCAGCTAAGCGAACCG | ||||
| EspA | AY223511 | espAfor | GGATCCATGGATACATCAACTGCAACATC | 1-579 | 94°C, 2 min (1 cycle); 94°C, 1 min; 55°C, 1 min; 72°C, 1 min (30 cycles) |
| espArev | AAGCTTTTATTTACCAAGGGATATTGCTG | ||||
| γ-Intimin1-280 | Z11541 | ctereaeup | GGATCCCAAACCAAGGCCAGCATTAC | 1962-2804 | 94°C, 1 min; 53°C, 1 min; 72°C, 1 min (30 cycles) |
| Grev | AAGCTTTTATTCTACACAAACCGCATAGA |
Preparation of His-tagged γ-intimin1-280, EspA, and EspB.
Expression of the His-tagged proteins was performed as recommended by the manufacturers. Briefly, transformed E. coli BL21(D3)/pLysS cells carrying the plasmid pRSET-A containing the EspA, EspB, or γ-intimin1-280 fragment genes were grown overnight at 37°C with shaking in LB broth supplemented with ampicillin (100 μg/ml) and chloramphenicol (34 μg/ml). The overnight culture was diluted and grown under the same conditions in LB broth until an optical density at 600 nm (OD600) of 0.6 was reached. Recombinant gene expression was then induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) at a final concentration of 0.5 mM. After 4 h of incubation at 37°C, the cells were washed, lysed with 6 M guanidine HCl, and sonicated. The His-tagged proteins were purified from the clarified lysates by affinity chromatography in a column of Probond nickel-chelating resin (Invitrogen, Carlsbad, CA). The proteins were eluted by washing with buffers of decreasing pH (8 M urea, 20 mM sodium phosphate, 500 mM NaCl), including binding buffer (pH 7.8), wash buffer (pH 6.3), and elution buffer (pH 4.5), under denaturing conditions.
SDS-PAGE.
One-dimension sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out in a 12.0% polyacrylamide gel as described by Laemmli (16), with 1.5 μg protein loaded per lane. Protein samples were diluted in an equal volume of 2× sample buffer (2% SDS, 2% 2-mercaptoethanol, 20% glycerol, and 0.01% bromophenol blue in 0.0065 M Tris [pH 6.8]) and boiled for 5 min before being loaded onto gels. Molecular weights were estimated, and molecular markers (Bio-Rad, Hercules, CA) were included in each gel. Protein bands were revealed by Coomassie blue staining. The observed molecular masses in the SDS-PAGE gel were 35 kDa, 25 kDa, and 38 kDa for γ-intimin1-280, EspA, and EspB, respectively, which were in good agreement with the theoretical masses for γ-intimin1-280 (30 kDa), EspA (20.5 kDa), and EspB (33 kDa), if the addition of the polyhistidine trait was considered.
Immunoblotting.
Proteins were electrophoretically transferred from the gel onto nitrocellulose sheets (0.45 μm; Amersham-Pharmacia) for immunoblotting as described by Towbin et al. (40). Nitrocellulose strips were blocked with 5% nonfat dry milk in phosphate-buffered saline (PBS), pH 7.2, for 2 h under agitation, washed three times with PBS containing 0.2% Tween 20, and incubated for 2 h with either pure or 1:10, 1:50, or 1:100 diluted colostrum samples or with specific rabbit polyclonal antisera raised against EspA, EspB, and γ-intimin1-280. Following the three washes with PBS containing 0.2% Tween 20, the membranes were incubated for 2 h with horseradish peroxidase-conjugated rabbit anti-bovine IgG (Bioyeda, Rehovot, Israel) diluted 1:8,000 in PBS or with horseradish peroxidase-conjugated goat anti-rabbit IgG (Sigma Chemical Co., St. Louis, MO) diluted 1:10,000. The blots were revealed with 4-Cl-1-naphthol (Pierce, Rockford, IL). A pool of colostrum samples did not react when it was assayed against a nonrelated His-tagged protein, MPB64 from Mycobacterium bovis (data not shown).
Generation of specific rabbit antisera.
Female pathogen-free rabbits were immunized by subcutaneous and intramuscular routes on days 1, 14, and 28 with recombinant proteins (100 μg/dose) emulsified in Freund's incomplete adjuvant (Sigma Chemical Co., St. Louis, MO). Sera were collected on days 0, 13, 27, and 48. Sera were evaluated by Western blotting and pooled.
RBC lysis assay.
The possible inhibitory effect of colostrum on the hemolytic activity exhibited by TTSS-encoding E. coli strains (41) was evaluated. The EPEC E2348/69 strain was grown overnight in LB broth at 37°C without shaking and then diluted 1:100 in Dulbecco's modified Eagle medium (lacking phenol red; Gibco-BRL). A mixture of different quantities of colostrum and 2 ml of the diluted bacterial suspension was incubated for 1 h at 37°C. In turn, red blood cells (RBC) were separated by centrifugation from fresh, defibrinated sheep blood, washed three times with 10 mM PBS (pH 7.4), and resuspended at 5% in PBS. Then, 2 ml of the EPEC E2348/69 suspension preincubated with colostrum was mixed with 2 ml of the 5% suspension of RBC in PBS and incubated for 3 h at 37°C under a 5% CO2 atmosphere in 12-well plates. The suspension was removed from the plates and centrifuged at 12,000 × g for 1 min. Supernatants were monitored for the presence of released hemoglobin by measuring the OD543. The E. coli EPEC E2348/69 ΔescN strain, a mutant that does not synthesize the TTSS and does not produce lysis, was used as a negative control. The positive control consisted of the same incubation process but without the addition of colostrum. The percentage of inhibition was calculated as 100 − [(OD543 with colostrum/OD543 without colostrum) × 100].
Statistical analysis.
Data are expressed as means ± standard deviations. Statistical analysis of paired data was carried out using Student's t test, with a 95% confidence limit; a probability value (P) of <0.05 was considered significant.
RESULTS
Reactivity of colostrum with γ-intimin1-280, EspA, and EspB.
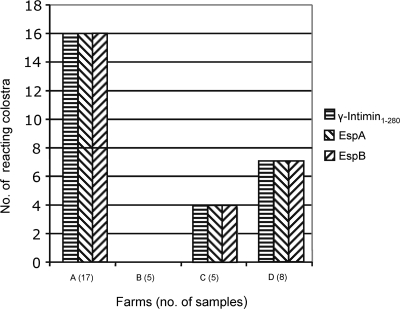
Twenty-seven (77.0%) of 35 individually examined colostrum samples contained IgG antibodies against the three proteins assayed, γ-intimin1-280, EspA, and EspB, as determined by immunoblotting with the respective His-tagged antigens. The reacting colostrum samples belonged to three of the four farms sampled. Five of eight negative samples were taken 24 h after birth, when the antibody titer begins to fall drastically (Fig. 1) (15, 27).
FIG. 1.
Farm distribution of colostrum samples with positive reactions in the immunoblotting assay for recombinant EspA, EspB, and γ-intimin1-280 proteins.
Most of the samples showed a strong reactivity with the EspB band in the 1:10 dilution. Antibodies against EspA and γ-intimin1-280 were present at a lower level than those against EspB and were detected only when the samples were undiluted. No reactivity against the three proteins was detected with dilutions of 1:50 and 1:100. Figure 2 shows an example of the recognition of EspA, EspB, and γ-intimin1-280 by a representative colostrum sample (sample 283). Western blotting of bovine samples developed with anti-bovine IgA conjugates did not show visible bands (data not shown).
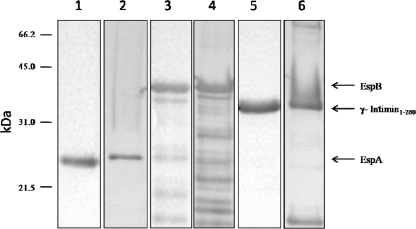
FIG. 2.
Immunoblotting recognition of EspA, EspB, and γ-intimin1-280 by colostrum sample 283. The binding of colostrum to EspA, EspB, and γ-intimin1-280 (lanes 1, 3, and 5, respectively) and the binding of rabbit polyclonal anti-EspA, anti-EspB, and anti-γ-intimin1-280 antisera to the corresponding proteins (lanes 2, 4, and 6, respectively) are shown.
Colostrum inhibition of TTSS-induced hemolysis.
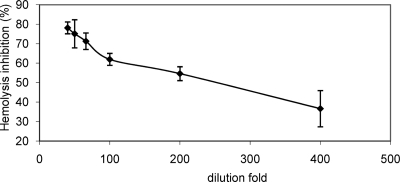
A colostrum sample positive for antibodies against γ-intimin1-280, EspA, and EspB was selected to analyze the effect of colostrum on the TTSS-mediated RBC lysis. Colostrum sample 283 inhibited the RBC hemolysis in a dose-dependent manner, with a maximum of 77.3% inhibition when the EPEC strain was preincubated with a 1:40 colostrum dilution (Fig. 3). This dilution was chosen for further inhibition assays with individual or pooled samples. All the individually assayed colostrum samples reduced hemolysis by 45.9% to 96.7% (data not shown). No significant differences were observed between samples positive for the presence of EspA-, EspB-, and γ-intimin1-280-specific antibodies (77.1% ± 13.7% inhibition; n = 28) and negative samples (66.3% ± 12.0% inhibition; n = 7) (P < 0.065), thus suggesting the presence of TTSS-inhibitory substances other than anti-EspA, -EspB, and -intimin IgG antibodies in bovine colostrum.
FIG. 3.
Dose-response curve of the inhibitory activity of colostrum sample 283 on RBC lysis by EPEC E2348/69.
Contribution of IgG and bovine lactoferrin to the inhibition of TTSS-induced hemolysis.
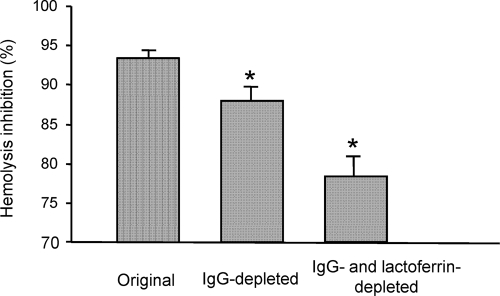
When a pool of 15 randomly chosen colostrum samples was depleted of IgG through passage of the samples across a protein G-Sepharose column, a reduction from 93.4% ± 0.9% to 87.9% ± 1.7% (P < 0.01) in the TTSS-mediated hemolysis was observed (Fig. 4). In some individual samples, the contribution of IgG to the inhibitory activity observed reached 40% (data not shown). The subsequent absorption of lactoferrin from IgG-depleted colostrum by affinity membrane chromatography produced an additional level of reduction in hemolysis, with an inhibitory effect of 78.3% ± 2.6% (P < 0.01). In accordance with these results, purified bovine lactoferrin (44) produced a reduction of 76.9% ± 1.6% in RBC lysis at a concentration of 1 mg/ml.
FIG. 4.
Inhibitory activity on RBC lysis by EPEC E2348/69 observed with the original, IgG-depleted, and IgG- and lactoferrin-depleted colostrum pools. *, P value of <0.01.
DISCUSSION
Our research indicates that bovine colostrum contains antibodies and other substances that recognize proteins involved in the intestinal adherence and damage produced by EHEC, in accordance with previous studies which have found antilipopolysaccharide or anti-Stx antibodies in bovine colostrum (19, 34, 42). We observed a high frequency of colostrum antibodies against γ-intimin1-280, EspA, and EspB proteins among dairy cows from Argentinean herds. Recently, Bretschneider et al. (1) have demonstrated that cattle respond serologically to E. coli O157:H7 intimin and EspB during the course of an experimental infection. The immunogenicity of the LEE-encoded proteins studied here was also observed in patients infected with E. coli O157:H7 or other EHEC serotypes, as well as in their household contacts (13, 18) or children with EPEC-associated diarrhea (23). Furthermore, several studies have shown that colostrum and milk from healthy women from both industrialized and developing countries contain IgA antibodies reactive to the LEE-encoded proteins (20, 22, 30, 33).
The immunoblotting assays showed a higher reactivity of bovine colostrum for EspB than for γ-intimin1-280 and EspA. This difference could be attributed to the cross-reactivity of the alpha, beta, and gamma EspB subtypes presently identified in LEE-bearing strains (21). On the other hand, antibodies against EspA and the nonconserved carboxy-terminal region of γ-intimin are serotype specific, with little or no cross-reactivity (4, 29, 38). The strong reactivity of colostrum samples with EspB can also be attributed to a higher persistence of EspB-specific antibodies in bovine sera, as demonstrated in an E. coli O157:H7 experimental infection (1).
In an attempt to demonstrate whether colostrum can inhibit the functionality of the TTSS, we used an LEE-dependent RBC lysis assay. Warawa et al. (41) have shown that EPEC strains exhibit a TTSS secretion-dependent hemolytic activity requiring the EspA, EspB, and EspD proteins. In this work, bovine colostrum inhibited the TTSS-mediated action of EPEC 2348/69 on RBC in a dose-dependent manner. This effect was partially related to anti-EHEC IgG antibodies, since a significant reduction in the inhibitory activity was obtained with IgG-depleted colostrum. However, colostrum retained a high level of hemolysis inhibition, thus suggesting that it contains other active substances.
Lactoferrin, an iron-binding glycoprotein present in colostrum, milk, and other body fluids, has antimicrobial, anti-inflammatory, and immunomodulatory functions (2). Recently, Ochoa et al. (31) have demonstrated that the lactoferrin present in human milk inhibits TTSS-mediated EPEC adherence to mammalian cells. Based on this report, we looked at the possibility that bovine lactoferrin might also impair TTSS function. The significant reduction in the TTSS-inhibitory activity observed when colostrum was depleted of lactoferrin by affinity membrane chromatography confirmed this hypothesis. This is in agreement with the observation that inhibition of EPEC adherence by bovine colostrum is mediated by a high-molecular-weight fraction (32). On the other hand, low concentrations of other specific immunoglobulin isotypes or unknown substances could be responsible for the remaining inhibitory activity observed with IgG- and lactoferrin-depleted colostrum.
Earlier studies have clearly shown that different Shiga toxin-producing E. coli serotypes colonize healthy cattle and that only a small percentage of these isolates possess intimin (8, 25). The presence of antibodies against γ-intimin1-280 seems to indicate previous or current exposure to and the colonization of cattle with either EHEC O157:H7 or O145:H-, two of the most virulent EHEC strains for humans and the serotypes prevalent in human cases of HUS in Argentina (35).
Bovine colostrum may play a role in preventing EHEC colonization in humans. Clinical studies have shown that immunoglobulin preparations from healthy cows ameliorate diarrhea in children infected with diarrheagenic Escherichia coli (9, 39). It has also been demonstrated that bovine colostrum contains immunoglobulins that neutralize Shiga toxins and EHEC hemolysin of E. coli O157:H7 in vitro (19) and protects mice against an oral challenge with EHEC O157:H7 (6). Moreover, Zhao et al. (45) have reported that EHEC fecal shedding in calves increases after weaning; this observation strongly suggests that colostrum protects calves from early EHEC infection. In the current work, we showed that bovine colostrum contains IgG antibodies against proteins involved in the colonization of attaching and effacing E. coli and blocks the TTSS function of these strains by means of IgG antibodies, lactoferrin, and other unidentified substances.
In conclusion, we found that early bovine colostrum impairs TTSS function in attaching and effacing E. coli strains by different mechanisms. Thus, bovine colostrum could naturally reduce EHEC colonization in newborn calves. These results encourage us to think that bovine colostrum could be used as a prophylactic measure to protect non-breast-fed children against EHEC infection in an area of endemicity.
Acknowledgments
We are grateful to Osvaldo Cascone and Federico J. Wolman (Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires) for the lactoferrin depletion assay and Viviana Parreño, Gisella Marcoppido, and Ana María Elizondo for providing colostrum samples. We are indebted to Ana María Elizondo and Laura González for their invaluable technical assistance.
This work was supported in part by a grant from the Agencia Nacional de Promoción Científica y Tecnológica, PICTO INTA 2002 12923. D. A. Vilte is a Ph.D. student supported by the Agencia Nacional de Promoción Científica y Tecnológica. M. Larzábal is a Ph.D. student and A. Cataldi is a Ph.D. fellow of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Footnotes
Published ahead of print on 18 June 2008.
REFERENCES
- 1.Bretschneider, G., E. M. Berberov, and R. A. Moxley. 2007. Isotype-specific antibody responses against Escherichia coli O157:H7 locus of enterocyte effacement proteins in adult beef cattle following experimental infection. Vet. Immunol. Immunopathol. 118:229-238. [DOI] [PubMed] [Google Scholar]
- 2.Brock, J. H. 2002. The physiology of lactoferrin. Biochem. Cell Biol. 80:1-6. [DOI] [PubMed] [Google Scholar]
- 3.Caprioli, A., S. Morabito, H. Brugere, and E. Oswald. 2005. Enterohaemorrhagic Escherichia coli: emerging issues on virulence and modes of transmission. Vet. Res. 36:289-311. [DOI] [PubMed] [Google Scholar]
- 4.Crepin, V. F., R. Shaw, S. Knutton, and G. Frankel. 2005. Molecular basis of antigenic polymorphism of EspA filaments: development of a peptide display technology. J. Mol. Biol. 350:42-52. [DOI] [PubMed] [Google Scholar]
- 5.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 6.Funatogawa, K., T. Ide, F. Kirikae, K. Saruta, M. Nakano, and T. Kirikae. 2002. Use of immunoglobulin enriched bovine colostrum against challenge with enterohaemorrhagic Escherichia coli O157:H7 in mice. Microbiol. Immunol. 46:761-766. [DOI] [PubMed] [Google Scholar]
- 7.Ghaem-Maghami, M., C. P. Simmons, S. Daniell, M. Pizza, D. Lewis, G. Frankel, and G. Dougan. 2001. Intimin-specific immune responses prevent bacterial colonization by the attaching-effacing pathogen Citrobacter rodentium. Infect. Immun. 69:5597-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gioffré, A., L. Meichtri, E. Miliwebsky, A. Baschkier, G. Chillemi, M. I. Romano, S. Sosa Estani, A. Cataldi, R. Rodríguez, and M. Rivas. 2002. Detection of Shiga toxin-producing Escherichia coli by PCR in cattle in Argentina. Evaluation of two procedures. Vet. Microbiol. 87:301-313. [DOI] [PubMed] [Google Scholar]
- 9.Huppertz, H. I., S. Rutkowski, D. H. Busch, R. Eisebit, R. Lissner, and H. Karch. 1999. Bovine colostrum ameliorates diarrhea in infection with diarrheagenic Escherichia coli, Shiga toxin-producing E. coli, and E. coli expressing intimin and hemolysin. J. Pediatr. Gastroenterol. Nutr. 29:452-456. [DOI] [PubMed] [Google Scholar]
- 10.Hussein, H. S., and L. M. Bollinger. 2005. Prevalence of Shiga toxin-producing Escherichia coli in beef cattle. J. Food Prot. 68:2224-2241. [DOI] [PubMed] [Google Scholar]
- 11.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 12.Karch, H., P. I. Tarr, and M. Bielaszewska. 2005. Enterohemorrhagic Escherichia coli in human medicine. Int. J. Med. Microbiol. 295:405-418. [DOI] [PubMed] [Google Scholar]
- 13.Karpman, D., Z. D. Békássy, A. C. Sjögren, M. S. Dubois, M. A. Karmali, M. Mascarenhas, K. G. Jarvis, L. J. Gansheroff, A. D. O'Brien, G. S. Arbus, and J. B. Kaper. 2002. Antibodies to intimin and Escherichia coli secreted proteins A and B in patients with enterohemorrhagic Escherichia coli infections. Pediatr. Nephrol. 17:201-211. [DOI] [PubMed] [Google Scholar]
- 14.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic Escherichia coli (EPEC) transfer its receptor for intimate adherence into mammalian cells. Cell 91:511-520. [DOI] [PubMed] [Google Scholar]
- 15.Korhonen, H., P. Marnila, and H. S. Gill. 2000. Milk immunoglobulins and complement factors. Br. J. Nutr. 84(Suppl. 1):S75-S80. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119-1122. [DOI] [PubMed] [Google Scholar]
- 18.Li, Y., E. Frey, A. M. R. Mackenzie, and B. B. Finlay. 2000. Human response to Escherichia coli O157:H7 infection: antibodies to secreted virulence factors. Infect. Immun. 68:5090-5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lissner, R., H. Schmidt, and H. Karch. 1996. A standard immunoglobulin preparation produced from bovine colostra shows antibody reactivity and neutralization activity against Shiga-like toxins and EHEC-hemolysin of Escherichia coli O157:H7. Infection 24:378-383. [DOI] [PubMed] [Google Scholar]
- 20.Loureiro, I., G. Frankel, J. Adu-Bobie, G. Dougan, L. R. Trabulsi, and M. M. Carneiro-Sampaio. 1998. Human colostrum contains IgA antibodies reactive to enteropathogenic Escherichia coli virulence-associated proteins: intimin, BfpA, EspA, and EspB. J. Pediatr. Gastroenterol. Nutr. 27:166-171. [DOI] [PubMed] [Google Scholar]
- 21.Lu, Y., C. Toma, Y. Honma, and M. Iwanaga. 2002. Detection of EspB using reversed passive latex agglutination: application to determination of enteropathogenic Escherichia coli. Diagn. Microbiol. Infect. Dis. 43:7-12. [DOI] [PubMed] [Google Scholar]
- 22.Manjarrez-Hernandez, H. A., S. Gavilanes-Parra, E. Chavez-Berrocal, A. Navarro-Ocaña, and A. Cravioto. 2000. Antigen detection in enteropathogenic Escherichia coli using secretory immunoglobulin A antibodies isolated from human breast milk. Infect. Immun. 68:5030-5036. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez, M. B., C. R. Taddei, A. Ruiz-Tagle, L. R. Trabulsi, and J. A. Girón. 1999. Antibody response of children with enteropathogenic Escherichia coli infection to the bundle-forming pilus and locus of enterocyte effacement-encoded virulence determinants. J. Infect. Dis. 179:269-274. [DOI] [PubMed] [Google Scholar]
- 24.McDaniel, T. K., K. G. Jarvis, M. S. Donnenberg, and J. B. Kaper. 1995. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl. Acad. Sci. USA 92:1664-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meichtri, L., A. Gioffré, E. Miliwebsky, I. Chinen, A. Baschkier, G. Chillemi, B. E. Guth, M. O. Masana, A. Cataldi, H. R. Rodríguez, and M. Rivas. 2004. Shiga toxin-producing Escherichia coli in healthy young beef steers from Argentina: prevalence and virulence properties. Int. J. Food Microbiol. 96:189-198. [DOI] [PubMed] [Google Scholar]
- 26.Mercado, E. C., A. Gioffré, S. M. Rodríguez, A. Cataldi, K. Irino, A. M. Elizondo, A. L. Cipolla, M. I. Romano, R. Malena, and M. A. Mendez. 2004. Non-O157 Shiga toxin-producing Escherichia coli isolated from diarrhoeic calves in Argentina. J. Vet. Med. B 51:82-88. [DOI] [PubMed] [Google Scholar]
- 27.Moore, M., J. W. Tyler, M. Chigerwe, M. E. Dawes, and J. R. Middleton. 2005. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 226:1375-1377. [DOI] [PubMed] [Google Scholar]
- 28.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neves, B. C., R. K. Shaw, G. Frankel, and S. Knutton. 2003. Polymorphisms within EspA filaments of enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 71:2262-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noguera-Obenza, M., T. J. Ochoa, H. F. Gomez, M. L. Guerrero, I. Herrera-Insua, A. L. Morrow, G. Ruiz-Palacios, L. K. Pickering, C. A. Guzman, and T. G. Cleary. 2003. Human milk secretory antibodies against attaching and effacing Escherichia coli antigens. Emerg. Infect. Dis. 9:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ochoa, T. J., M. Noguera-Obenza, F. Ebel, C. A. Guzman, H. F. Gomez, and T. G. Cleary. 2003. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 71:5149-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmeira, P., S. B. Carbonare, M. L. Silva, L. R. Trabulsi, and M. M. Carneiro-Sampaio. 2001. Inhibition of enteropathogenic Escherichia coli (EPEC) adherence to Hep-2 cells by bovine colostrum and milk. Allergol. Immunopathol. (Madrid) 29:229-237. [DOI] [PubMed] [Google Scholar]
- 33.Parissi-Crivelli, A., J. M. Parissi-Crivelli, and J. A. Girón. 2000. Recognition of enteropathogenic Escherichia coli virulence determinants by human colostrum and serum antibodies. J. Clin. Microbiol. 38:2696-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pirro, F., L. H. Wieler, K. Failing, R. Bauerfeind, and G. Baljer. 1995. Neutralizing antibodies against Shiga-like toxins from Escherichia coli in colostra and sera of cattle. Vet. Microbiol. 43:131-141. [DOI] [PubMed] [Google Scholar]
- 35.Repetto, H. A. 2005. Long-term course and mechanisms of progression of renal disease in hemolytic uremic syndrome. Kidney Int. Suppl. 97:S102-S106. [DOI] [PubMed] [Google Scholar]
- 36.Rivas, M., E. Miliwebsky, I. Chinen, C. D. Roldan, L. Balbi, B. Garcia, G. Fiorilli, S. Sosa-Estani, J. Kincaid, J. Rangel, and P. M. Griffin. 2006. Characterization and epidemiologic subtyping of Shiga toxin-producing Escherichia coli strains isolated from hemolytic uremic syndrome and diarrhea cases in Argentina. Foodborne Pathog. Dis. 3:88-96. [DOI] [PubMed] [Google Scholar]
- 37.Rivas, M., E. Miliwebsky, I. Chinen, N. Deza, and G. A. Leotta. 2006. The epidemiology of hemolytic uremic syndrome in Argentina. Diagnosis of the etiologic agent, reservoirs and routes of transmission. Medicina (Buenos Aires) 66(Suppl. 3):27-32. (In Spanish.) [PubMed] [Google Scholar]
- 38.Son, W.-G., T. A. Graham, and V. P. J. Gannon. 2002. Immunological characterization of Escherichia coli O157:H7 intimin γ1. Clin. Diagn. Lab. Immunol. 9:46-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tawfeek, H. I., N. H. Najim, and S. Al-Mashikhi. 2003. Efficacy of an infant formula containing anti-Escherichia coli colostral antibodies from hyperimmunized cows in preventing diarrhea in infants and children: a field trial. Int. J. Infect. Dis. 7:120-128. [DOI] [PubMed] [Google Scholar]
- 40.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warawa, J., B. B. Finlay, and B. Kenny. 1999. Type III secretion-dependent hemolytic activity of enteropathogenic Escherichia coli. Infect. Immun. 67:5538-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Widiasih, D. A., I. Matsuda, K. Omoe, D. L. Hu, S. Sugii, and K. Shinagawa. 2004. Passive transfer of antibodies to Shiga toxin-producing Escherichia coli O26, O111 and O157 antigens in neonatal calves by feeding colostrum. J. Vet. Med. Sci. 66:213-215. [DOI] [PubMed] [Google Scholar]
- 43.Wieler, L. H., T. K. McDaniel, T. S. Whittam, and J. B. Kaper. 1997. Insertion site of the locus of enterocyte effacement in enteropathogenic and enterohemorrhagic Escherichia coli differs in relation to the clonal phylogeny of the strains. FEMS Microbiol. Lett. 156:49-53. [DOI] [PubMed] [Google Scholar]
- 44.Wolman, F. J., D. González Maglio, M. Graselli, and O. Cascone. 2007. One-step lactoferrin purification from bovine whey and colostrum by affinity membrane chromatography. J. Membr. Sci. 288:132-138. [Google Scholar]
- 45.Zhao, T., M. P. Doyle, J. Shere, and L. Garber. 1995. Prevalence of enterohemorrhagic Escherichia coli O157:H7 in a survey of dairy herds. Appl. Environ. Microbiol. 61:1290-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]