Abstract
In this study, we describe the isolation and characterization of a new exopolymer that exhibits high emulsifying activities against a range of oil substrates and demonstrates a differential capacity to desorb various mono-, di-, and trivalent metal species from marine sediment under nonionic and seawater ionic-strength conditions. This polymer, PE12, was produced by a new isolate, Pseudoalteromonas sp. strain TG12 (accession number EF685033), during growth in a modified Zobell's 2216 medium amended with 1% glucose. Chemical and chromatographic analysis showed it to be a high-molecular-mass (>2,000 kDa) glycoprotein composed of carbohydrate (32.3%) and protein (8.2%). PE12 was notable in that it contained xylose as the major sugar component at unusually high levels (27.7%) not previously reported for a Pseudoalteromonas exopolymer. The polymer was shown to desorb various metal species from marine sediment—a function putatively conferred by its high content of uronic acids (28.7%). Seawater ionic strength (simulated using 0.6 M NaCl), however, caused a significant reduction in PE12's ability to desorb the sediment-adsorbed metals. These results demonstrate the importance of electrolytes, a physical parameter intrinsic of seawater, in influencing the interaction of microbial exopolymers with metal ions. In summary, PE12 may represent a new class of Pseudoalteromonas exopolymer with a potential for use in biotechnological applications as an emulsifying or metal-chelating agent. In addition to the biotechnological potential of these findings, the ecological aspects of this and related bacterial exopolymers in marine environments are also discussed.
Surfactants and emulsifiers comprise a unique class of molecules that are distinguished for their capacity to interface between water-soluble and oil phases (4, 20, 66). This amphipathic quality is conferred by the presence of both polar and nonpolar moieties, endowing these molecules with a hydrophilic-hydrophobic nature. In this respect, surfactants and emulsifiers have been used extensively in almost every sector of modern industry today. As a large fraction is produced by organo-chemical synthesis, this raises concern over their potential toxicological effects to humans and the environment. Naturally produced amphipathic molecules, however, have gained increasing interest in recent years based on their associated lower levels of toxicity, higher degradability, and increased consumer demand for natural alternatives (4).
High-molecular-weight surface-active compounds (i.e., amphipathic biopolymers) are of particular interest for biotechnological and industrial applications (57, 65). Compared to their lower-molecular-weight counterparts, these biopolymers exhibit a number of advantages, such as possessing a large surface area on which multiple reactive groups may be expressed (58), the ability to confer texturizing and stabilizing properties to process and product formulations (37, 65), and the capacity to exhibit tensile strength and resistance to shear (23, 31). Some, particularly those that are anionic (e.g., the emulsans), have been shown to chelate cations (75), thus demonstrating their potential for use in the remediation of environmental sites contaminated with toxic metals (67). For commercial exploitation, microorganisms are recognized as a more reliable and sustainable source for producing these types of biopolymers than methods of organo-chemical synthesis. In addition, microbially derived polymers can exhibit enhanced performance and greater functional diversity than synthetic polymers. One typical example is xanthan gum, a commercial hydrocolloid produced by the plant-pathogenic bacterium Xanthomonas campestris, which, in addition to possessing interfacial properties (33), is also a viscosity builder, thus making it an important component of many healthcare products and food processing formulations (24).
In recent years, the marine environment has been recognized as a rich and relatively untapped source of microbial polymers (57, 72). Ecologically, these polymers serve important functions in marine environments, where they may be involved in microbial adhesion to solid surfaces and biofilm formation (21, 32, 47, 50, 53), the emulsification of hydrocarbon oils to enhance biodegradation (5, 60), or mediating the fate and mobility of heavy metals and trace metal nutrients (8, 13, 28). This wide spectrum of functional activity is reflected not merely in the complex chemistry of these biopolymers but also in the diversity of bacterial genera found producing them (59). In recent years, members within the genus Pseudoalteromonas have been recognized for producing exopolymers with properties of biotechnological interest. Although the chemical and physical properties of various Pseudoalteromonas exopolymers have been described (11, 44, 46, 49), to our knowledge no reports exist that describe their emulsifying activities or emulsion-stabilizing properties. There is limited information on the metal-binding properties of exopolymers produced by pseudoalteromonad species (39).
The objective of this study was to characterize a new exopolymer, PE12, produced by a new Pseudoalteromonas isolate, Pseudoalteromonas sp. strain TG12. This study examined the growth conditions for producing this exopolymer, its partial purification, chemical characterization, and functional properties that include emulsifying activities, emulsion-stabilizing properties, and the ability to desorb metal ions from marine sediment under nonionic and seawater ionic-strength conditions. As will be discussed, PE12 represents a new type of high-molecular-weight glycoprotein with an unusually high content of xylose. To our knowledge, only one other pseudoalteromonad, Pseudoalteromonas antarctica NF3 (originally identified as an Alteromonas), has previously been reported to produce an exopolymer characterized as a high-molecular-weight glycoprotein with specifically surfactant activity (11). The amphipathic nature of PE12, however, is characterized to be of primarily emulsifying quality. The results presented are discussed with respect to the physicochemical properties of this new polymer in relation to its emulsifying activity and capacity to facilitate the desorption of metals bound to marine sediment.
MATERIALS AND METHODS
Isolation and screening of emulsifying bacteria.
A seawater sample containing decaying seaweed was collected from the shores of Oban Bay (Scotland) and used to isolate bacteria based on their growth on a solid synthetic seawater medium (54) supplemented with NH4NO3 and n-hexadecane that had been supplied via the vapor phase as the sole carbon source. Colonies were grown in the dark at 28°C for 2 to 3 weeks. Isolates displaying distinct colony morphologies were streaked onto modified Zobell's 2216 marine (ZM/10) agar and stored at −80°C in ZM/1 broth supplemented with 20% glycerol. Both ZM/10 and ZM/1 media were prepared as previously described by Green et al. (27).
One isolate, strain TG12, was selected for its production of high emulsifying activity during growth in ZM/10 broth amended with glucose at 1% (wt/vol) concentration. During growth, samples were taken periodically for emulsification and tensiometric assays (see below). Washed cell pellets and cell-free supernatants (centrifuged at 13,000 × g for 10 min) were assayed. Viable cell counts were measured by plating out appropriate dilutions of culture broth onto ZM/10 agar plates. Growth experiments were repeated at least three times, and all analyses were performed in triplicate. Strain TG12 was identified by DNA sequencing of its 16S rRNA gene following amplification with a PCR using oligonucleotide primers 27f and 1492r (73), as described by Green et al. (27).
Production and extraction of exopolymer.
Strain TG12 was grown in 2-liter Erlenmeyer flasks containing 770 ml of ZM/10 medium amended with glucose (1%, wt/vol) and incubated (at 28°C and 150 rpm) for 45 to 50 h, at which point the cultures were then pooled together. The emulsifying fraction was then separated from the biomass by cross-flow filtration (0.2-μm-pore-size filter; Schleicher & Schuell). The cell-free permeate was then passed through a 100-kDa ultrafiltration membrane cassette (Schleicher & Schuell), and the retentate was dialysed with ∼5 liters of distilled water. KCl (7.5%, wt/vol) and 3 volumes of cold 99% ethanol were then added to precipitate the emulsifying fraction. After the precipitate was allowed to settle overnight at 10°C, it was recovered by centrifugation (4,500 × g for 10 min) and subsequently dialyzed extensively against distilled water and lyophilized. The resultant dried material, labeled PE12, was used in all subsequent chemical and physical characterization experiments.
Chemical analysis.
For determination of the monosaccharide composition in the emulsifying extract PE12, triplicate samples (10 μl at 1% [wt/vol]) were dissolved in 500 μl of 2 M trifluoroacetic acid and hydrolyzed (at 100°C for 4 h). The samples were then prepared for analysis by high-performance anion exchange chromatography using a Dionex Carbopac PA-20 column and quantified using external standards. The total carbohydrate content was calculated from the individual amounts of monosaccharides.
For determination of amino acid composition, acid hydrolysis and derivatization were performed on triplicate samples (100 μl at 1% [wt/vol]) of PE12. Samples were hydrolyzed in 1 ml of acid (6 N HCl; 112°C for 22 h) and then analyzed using a Waters Alliance high-performance liquid chromatography (HPLC) system equipped with a Zorbax XDB C18 reverse-phase column. Quantification was performed using external standardization with amino acid standard mixtures. The total protein content was calculated from the individual amounts of amino acids.
Lipid analysis was performed on triplicate samples of PE12 and analyzed by gas chromatography, as described previously (16).
HPLC.
To evaluate the purity of the PE12 extract and to obtain an estimate of the molecular weight (Mr) of its constituent components, high-performance size-exclusion chromatography was performed with an Agilent 1100 chromatograph equipped with a refractometer and diode array UV detector. A BioSep-SEC-S 4000 column (300 by 7.8 mm) was used at 30°C. The eluent was 0.1 M NaNO3 (pH 8), and the flow rate was 0.6 ml/min. Dextran standards of Mrs ranging from 12,000 to 2,000,000 were used to calibrate the column for Mr estimation.
Emulsification assays.
Production of the emulsifier during growth was determined using a modified version of the method described by Cooper and Goldenberg (17). Samples of culture broth with cells removed (by centrifugation at 13,000 × g for 5 min) were mixed with an equal volume of n-hexadecane in acid-washed (0.1N HCl) screw-cap glass tubes (100 by 13 mm), manually shaken (15 s), vortexed at 2,200 rpm (15 s) to homogeneity, left to stand for 10 min, and shaken as before; the height of the emulsion layer (emulsification index at 24 h [EI24]) was measured after allowing the mixture to stand for 24 h at 21°C. This same assay was also used to measure the EI24 produced by solutions of the extracted emulsifier (PE12) against n-hexadecane.
The ability of the extracted emulsifier (PE12) to form oil-in-water emulsions against different food oils was based on a modified version of the method described by Cirigliano and Carman (15). For this, 2.5-ml aqueous volumes of the test emulsifier (0.02%, wt/vol) were mixed with 0.4 ml of the test oil in the same way as described above. The solutions were allowed to stand for 10 min before the turbidity of the lower aqueous layer was measured using a spectrophotometer at 540 nm. The emulsifying activity (A540) was measured after the emulsions were allowed to stand undisturbed at room temperature for 24 h. All A540 values were expressed as the average from triplicate experiments. The emulsifiers xanthan gum and gum arabic (Sigma) were used as commercial controls. The food oils tested were olive (Filippo Berio, Italy), sunflower (Tesco, United Kingdom), rapeseed (Tesco, United Kingdom), walnut (Tesco, United Kingdom), and vegetable (Tesco, United Kingdom). Activities were compared under neutral (0.1 M phosphate-buffered saline, pH 7.5) and acidic (0.1 M sodium acetate buffer, pH 3.5) conditions.
Desorption of metals from marine sediment.
The ability of the PE12 polymer to desorb various metal ions (Al3+, Fe2+/3+, K+, Mg2+, Mn2+, Na+, Si4+, Ca2+, Ba2+, Cu2+, Li+, Sr2+, and Zn2+) from a marine sediment sample was evaluated in deionized water and in 0.6 M NaCl to simulate seawater ionic-strength conditions. The water used in both treatments was of 18 MΩ/cm quality. The sediment sample used in these experiments was obtained from Garroch Head, a site located in the river Clyde near Glasgow (Scotland), which for many years had been a dumping ground for sewage. The sediment was air dried and ground to a fine powder. Selected characteristics of the sediment were as follows: dry bulk density, 0.435 g/cm3; porosity, 0.829; salt content, 6.67%; organic carbon, 3.85% ± 0.14%; organic nitrogen, 0.39% ± 0.01%; elemental C/N ratio, 11.46.
For the experiment, a series of 15-ml polypropylene vials was prepared containing 0.5 g of sediment. Five-milliliter volumes of the PE12 polymer at a concentration of 1 mg/ml dissolved in either deionized water or 0.6 M NaCl were added to the sediment samples, and the mixtures were incubated in the dark overnight with shaking (150 rpm at 21°C). The vials were then centrifuged (at 10,000 × g for 10 min), and 3 ml of supernatant was removed for metal analysis, which was performed by inductively coupled plasma-atomic emission spectrometry, using a Perkin Elmer 4300DV spectrometer equipped with an AS93 Autosampler (Perkin Elmer Life and Analytical Sciences, Inc., MA). Multielement calibration solutions were prepared in-house from 10,000 μg/ml single-element solutions (CPI International, Amsterdam, The Netherlands). The samples were diluted in 5% nitric acid solution and determined axially (Al, Fe, Mn, Si, Ca, Ba, Cu, Li, and Zn) or radially (K, Mg, Na, and Sr). The total metal concentrations in the native polymer and untreated sediment were also analyzed. All experiments were conducted in triplicate for each treatment (i.e., in deionized water or 0.6 M NaCl).
Nonspectral interference from high sodium concentrations has been widely reported in the literature on inductively coupled plasma-atomic emission spectrometry (12, 43, 69). This was overcome by “matrix matching” the calibration solutions to the samples (51) with respect to the sodium chloride concentration. Thus, two independent calibration sequences were prepared, one in an appropriate concentration of sodium chloride solution and the other in water, which were then applied to the respective samples.
Tensiometry.
A Nima DST-9005 tensiometer was used with the du Noüy ring method to measure surfactant production by strain TG12 during growth in liquid medium and to determine if the PE12 exopolymer, at different concentrations, could reduce the surface tension of water.
Statistical analysis.
For the emulsification assays, a three-way analysis of variance (ANOVA) was used to assess the significance of the emulsifying activities compared to the control when averaged over all oil types and both pHs (Dunnett test). One-way ANOVA was used for each pH and oil type combination (Tukey's test) to determine if any significant difference (P < 0.05) in the emulsifying activities was produced by the three polymers.
Nucleotide sequence accession number.
The sequence of strain TG12 has been deposited in GenBank under accession number EF685033.
RESULTS
Characteristics of strain TG12.
Screening of a number of isolates for production of surface-active agents identified one isolate, strain TG12, which was selected for further study based on its ability to produce stable water-in-oil emulsions with n-hexadecane. Strain TG12 grew well in both ZM/10 and ZM/1 media, although maximum emulsification activities (EI24 of 60%) were obtained in the low-nutrient medium (ZM/10) when supplemented with 1% (wt/vol) glucose. Emulsification assays performed on retentate and filtrate fractions derived after passage of the cell-free spent medium through Amicon YM filters (30 to 100 kDa) revealed the emulsifying agent to have a molecular mass of >100 kDa (data not shown). 16S rRNA gene sequencing identified strain TG12 to be a member of the genus Pseudoalteromonas.
Growth and emulsifier production.
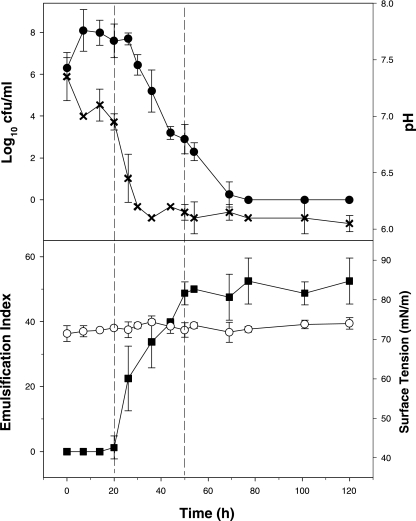
Fig. 1 shows the growth of strain TG12 in ZM/10 broth amended with glucose and production of its extracellular emulsifying agent. Exponential growth commenced immediately after inoculation, reaching a maximum viable cell count (1.26 × 108 CFU/ml) after only 7 h. The cells remained in the stationary phase for the next 19 h, after which they entered a death phase which was marked by a rapid decrease in the viable cell count. Viability was zero after 74 h. This decrease in cell viability was coupled to an almost proportional decrease in the measured pH of the medium, from an initial value of 7.4 to 6.1 after 36 h. Emulsifier production, as detected in the cell-free culture broth, commenced after 20 h, coinciding with the end of the stationary phase of growth, and reached highest levels (EI24 of 50 to 60%) well into the death phase of growth at 50 h (Fig. 1). These emulsions remained stable for months without displaying any signs of phase separation or droplet coalescence (results not shown). Washed cell suspensions were also observed to exhibit good emulsifying activity (data not shown), indicating that cells of strain TG12 also expressed a cell-bound emulsifying agent. No reduction to the surface tension of the culture medium (72.1 ± 0.5 mN/m) was detected at any time during the growth experiments. From an initial volume of 10 liters of ZM/10 broth amended with 1% glucose, the average dry-weight yield of the emulsifying extract PE12 recovered during the phase of maximal emulsifying activity (50 to 60 h) was 8.08 ± 2.08 mg/liter.
FIG. 1.
Growth and emulsifier production by Pseudoalteromonas sp. strain TG12 in ZM/10 broth amended with 1% (wt/vol) glucose. EI and surface tension values were derived from cell-free culture broth after removal of the cells by centrifugation. •, log10 CFU/ml; X, pH; ○, surface tension; ▪, EI.
Chemical composition and molecular mass.
The carbohydrate content of PE12 was 32.3% ± 2.3% (Table 1) of the total weight of dried polymer recovered. Monosaccharide analysis showed that hexoses (rhamnose, fucose, galactose, glucose, and mannose), amino sugars (galactosamine, glucosamine, and muramic acid), uronic acids (galacturonic and glucuronic acid), and the pentose xylose were present. Glucose (10.4% ± 0.7%), glucosamine (24.8% ± 0.4%), xylose (27.7% ± 0.2%), and galacturonic acid (23.1% ± 1.8%) were the most abundant, while all other monosaccharides were each present at less than 10% and together contributed about 14.0% ± 0.7% to the total carbohydrate content. The total uronic acid content of PE12 was 28.7%, as contributed by galacturonic and glucuronic acids.
TABLE 1.
Monosaccharide profile of the extracellular emulsifier PE12
| Component | Mean mol% compositionb |
|---|---|
| Rhamnose | 1.2 ± 0.1 |
| Fucose | 0.5 ± 0.0 |
| Galactose | 0.9 ± 0.0 |
| Galactosaminea | 0.7 ± 0.1 |
| Glucose | 10.4 ± 0.7 |
| Glucosaminea | 24.8 ± 0.4 |
| Mannose | 4.8 ± 0.4 |
| Xylose | 27.7 ± 0.2 |
| Muramic acid | 0.3 ± 0.0 |
| Galacturonic acid | 23.1 ± 1.8 |
| Glucuronic acid | 5.6 ± 0.1 |
| Totalc | 32.3 ± 2.3 |
N-Acetylgalactosamine and N-acetylglucosamine are de-N-acetylated during the acid hydrolysis and are detected as galactosamine and glucosamine.
Values are the means of triplicate samples ± standard deviations.
The total is expressed as the mean percentage of total dry weight of the polymer from triplicate determinations.
The total amino acid content of PE12 was 8.2% ± 0.3% (Table 2) of the total weight of dried polymer. Amino acid analysis of hydrolyzed samples identified the presence of four major amino acids—aspartic acid, glutamic acid, glycine, and alanine—that in total contributed 46.4% ± 1.4% to the total amino acid content. The percent contribution of hydrophobic nonpolar amino acids to the total amino acid content was 38.8%, whereas that of polar amino acids was 61.2%. Lipid analysis did not reveal any fatty acids.
TABLE 2.
Amino acid composition of the extracellular emulsifier PE12
| Component | Mean mol% compositiona |
|---|---|
| Asp | 13.4 ± 0.6 |
| Glu | 10.9 ± 0.1 |
| Ser | 7.1 ± 0.2 |
| Gly | 11.8 ± 0.1 |
| His | 1.2 ± 0.0 |
| Thr | 5.3 ± 0.1 |
| Arg | 4.2 ± 0.1 |
| Ala | 10.3 ± 0.6 |
| Pro | 3.8 ± 0.1 |
| Tyr | 2.5 ± 0.3 |
| Cys | 0.4 ± 0.6 |
| Val | 6.3 ± 0.2 |
| Met | 2.5 ± 0.0 |
| Ile | 5.0 ± 0.1 |
| Leu | 7.2 ± 0.2 |
| Lys | 4.4 ± 0.1 |
| Phe | 3.7 ± 0.1 |
| Totalb | 8.2 ± 0.3 |
Values are the means of triplicate samples ± standard deviations.
The total is expressed as the mean percentage of total dry weight of the polymer from triplicate determinations.
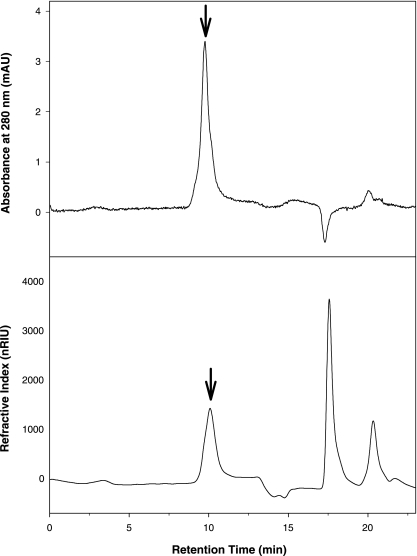
Chromatography of the PE12 extract on a BioSep-SEC-S4000 column with 0.1 M NaNO3 (pH 8) as the mobile phase further confirmed the glycoprotein composition of the PE12 polymer (Fig. 2). Three distinct refractive index peaks were eluted at retention times 10.0 min, 17.6 min, and 20.3 min. Only the peak at 10.0 min (Fig. 2) displayed a distinct corresponding UV peak with an absorption maximum at 280 nm. Analysis of fractions collected for each of these three peaks proved this peak to be the major component in the PE12 extract, and all of the emulsifying activity was associated with this peak. The molecular mass of the active fraction (i.e., the peak at 10.0 min) was determined to be a molecule of >2,000 kDa. The other two peaks that eluted later (at 17.6 and 20.3 min) were outside of the lower exclusion limit of the column and could be oligomeric degradation products. Chromatography of PE12 on a YMC-Pack Polyamine II column did not reveal the presence of any free monosaccharides (result not shown).
FIG. 2.
HPLC of the PE12 extract on a BioSep-SEC-S4000 size-exclusion column. Only the refractive index peak at 10 min (arrow) displayed a distinct corresponding UV peak with an absorption maximum at 280 nm. nRIU, refractive index units.
Emulsification of food oils.
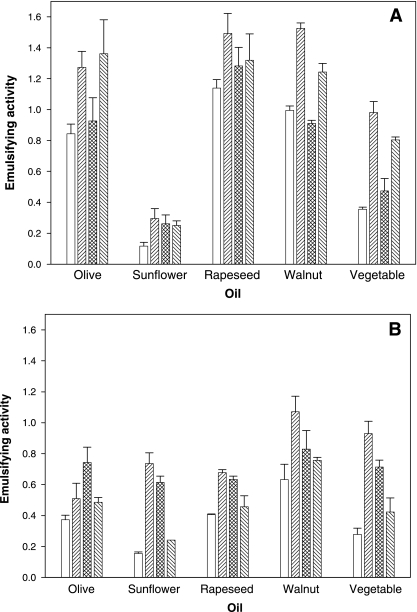
Concentrations of the isolated polymer, PE12, at 0.01 to 0.2% (wt/vol) did not have any effect on the surface tension of water (72.1 mN/m at 21°C). However, the polymer was found to effectively emulsify a range of food oils. Figure 3A shows the emulsifying activities of the different emulsifiers under neutral pH conditions when tested against five different food oils. A three-way factorial ANOVA showed that the main effect of polymer type was highly significant (P < 0.001) (data not shown). Based on average results for all oil types and both pH values, all three polymers (PE12, xanthan gum, and gum arabic) produced emulsifying activities that were significantly different from that of the control.
FIG. 3.
Emulsifying activity at pH 7.5 (A) and pH 3.5 (B) for oil-in-water emulsions prepared using PE12 compared to commercial emulsifiers. □, untreated control; ▒, PE12; ▩, xanthan gum; ▧, gum arabic.
Multiple one-way ANOVA was used to identify significant differences (P < 0.05) in emulsification activities as contributed by the type of oil used and pH treatment. Under neutral pH conditions (Fig. 3A), PE12 and gum arabic produced similar activities against all the oils, except walnut oil, where the activity of PE12 was significantly higher than activities of both gum arabic and xanthan gum. Compared to the control, only PE12 and gum arabic produced significantly higher activities against olive, walnut, and vegetable oils. Against rapeseed oil, only PE12 produced a significantly higher activity compared to the control. None of the polymers showed any significant emulsifying activity toward sunflower oil. Under acidic conditions (Fig. 3B), the emulsifying activities of PE12 and xanthan gum were similar when tested against both sunflower and vegetable oil but significantly higher than the control. Against walnut oil, PE12 exhibited the highest emulsifying effect, whereas xanthan gum was the better emulsifier against olive oil. None of the polymers showed any significant emulsification of rapeseed oil.
Desorption of sediment-adsorbed metals.
The observation that PE12 had a high uronic acid content (ca. 28%) suggested that it may be able to bind metals. The data in Table 3 show the amount of each metal ion that was desorbed from the sediment under nonionic (i.e., deionized water) and ionic (i.e., 0.6 M NaCl) conditions. Initial analysis of the polymer showed that it had several metal species intrinsically bound to it; i.e., these metal concentrations were not removed by dialysis during polymer purification. Ca2+ was present at the highest concentration (141.5 mg/g of polymer), and of the other metals analyzed, Sr2+ and Fe2+/3+ were present at 2.7 mg and 0.14 mg per gram of polymer, respectively.
TABLE 3.
Profile of metals bound to PE12 and ability of the polymer to desorb sediment-adsorbed metals under nonionic (deionized water) and ionic (0.6 M NaCl) strength conditions
| Metal | Polymer-associated metal (mg/g of polymer)a | Desorbed metal (mg/g of polymer) inb:
|
|
|---|---|---|---|
| Deionized water | 0.6 M NaCl | ||
| Al3+ | BDL | 1.0 ± 0.1 | <0.26 |
| Fe2+/3+ | 0.14 ± 0.03 | 0.9 ± 0.1 | <0.08 |
| K+ | BDL | 10.6 ± 2.0 | 1.2 ± 1.7 |
| Mg2+ | BDL | 31.0 ± 6.0 | 5.6 ± 3.5 |
| Mn2+ | <0.02 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Na+ | BDL | 154.5 ± 52.4 | |
| Si4+ | BDL | 3.4 ± 0.4 | 0.6 ± 0.1 |
| Ca2+ | 141.5 ± 26.2 | <0.06 | <0.23 |
| Ba2+ | BDL | <0.003 | <0.004 |
| Cu2+ | BDL | <0.13 | <0.11 |
| Li+ | <0.005 | <0.001 | <0.001 |
| Sr2+ | 2.7 ± 0.5 | <0.01 | <0.01 |
| Zn2+ | BDL | <0.05 | <0.04 |
Nondialyzable metal concentration associated with the polymer.
Values represent the amount of metal desorbed by the polymer after subtracting any background metal concentrations contributed by the polymer or sediment to the aqueous phase. BDL, below detection limit.
In deionized water, PE12 showed the ability to desorb Al3+, Fe2+/3+, K+, Mg2+, Na+, and Si4+ from the sediment. This was particularly evident with the group I alkali (K+ and Na+) and group II (Mg2+) alkaline earth metals, with 10.6 mg, 154.5 mg, and 31.0 mg desorbed per gram of polymer, respectively. However, ionic conditions significantly decreased PE12's ability to desorb these metals from the sediment. Due to the presence of Na+ at high concentrations in the 0.6 M NaCl experiments, concentrations of this metal ion could not be accurately quantified in these treatments and are thus not shown. Interestingly, PE12 desorbed the same quantity of Mn2+ (0.1 mg/g polymer) from the sediment under both treatment conditions. The polymer, however, did not exhibit any effect on the desorption of Ca2+, Ba2+, Cu2+, Li+, Sr2+, and Zn2+ from the sediment under either ionic or nonionic conditions.
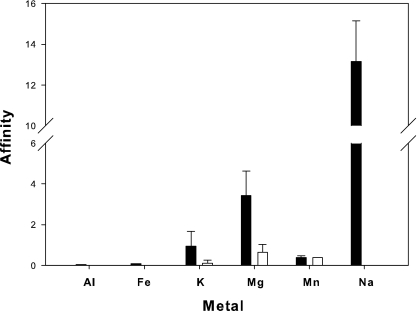
The affinity of the polymer for desorption of Al3+, Fe2+/3+, K+, Mg2+, Mn2+, and Na+ from the sediment is shown in Fig. 4, where metal affinity is expressed as the ratio of metal ion desorbed (per gram of PE12) to the amount of metal ion associated initially with the sediment. Overall, PE12 exhibited a higher affinity for all the metals when dissolved in deionized water. The preferred metal ion was Na+, followed by Mg2+, K+, Mn2+, Fe2+/3+, and then Al3+. In 0.6 M NaCl, the polymer exhibited a high affinity for Mg2+, followed by Mn2+ and then K+. As noted above, values for Na+ in the 0.6 M NaCl treatment were not calculated because this metal ion was present at concentrations that exceeded accurate quantification. Affinity values for Si4+ could not be calculated because original concentrations of this metal in the sediment were not available.
FIG. 4.
Affinity of the PE12 polymer for sediment-adsorbed metals under nonionic (▪, deionized water) and ionic (□, 0.6 M NaCl)-strength conditions.
DISCUSSION
To our knowledge this study represents the first description of a polymeric emulsifying agent produced by a pseudoalteromonad, including an evaluation of its capacity to absorb metal ions. This exopolymer, PE12, was found to be a high-molecular-weight glycoprotein with a monosaccharide profile that is typical of bacterial exopolymers (35). Its high uronic acid (28.7%) and low protein (8.2%) contents are chemical features that have been reported in exopolymers derived from other Pseudoalteromonas/Alteromonas species (14, 44, 55, 62). However, although a number of polysaccharides and oligosaccharides from various species of Pseudoalteromonas have been chemically and structurally characterized to reveal the presence of unusual sugars, including those with uncommon nonsugar substituents (49), to our knowledge, this is the first report describing an exopolysaccharide (EPS) from a Pseudoalteromonas species containing xylose at significantly high levels. Other studies describing EPS from Pseudoalteromonas species have reported xylose as a minor component (<1% of total carbohydrate) or as absent altogether (46, 49, 63). As xylose is rarely found in bacterial EPS (35), its high abundance in PE12 is an interesting finding whose significance remains unclear to us at present.
The glycoprotein composition of PE12 was confirmed by size-exclusion chromatography, as shown by a close coupling between the refractive index and UV absorbance at 280 nm of the high-molecular-weight component (Fig. 2). The two lower-molecular-mass components (estimated at <2.5 kDa) identified in the HPLC chromatogram were not expected since during their extraction the cell-free spent medium had been subjected to ultrafiltration through a 100-kDa molecular mass cutoff membrane, followed by immediate treatment with ethanol to isolate and precipitate only high-molecular-weight species. Based on this extraction method, it is unlikely that these low-molecular-weight contaminants are degradation products from enzyme activity but are, instead, rather loosely associated oligomeric components that dissociated as a result of the change in ionic conditions during downstream processing or chromatographic analysis. Interestingly, almost 60% of the polymer was not accounted for by our chemical analysis. This refractory nature is not uncommon and has been described for other bacterial exopolysaccharides (52) and may possibly be due to the presence of uronic acids (1, 6) or glycosidic linkages of hexosamines (10). PE12's high levels of galacturonic acid (23.1% ± 1.8%) and glucosamine (24.8% ± 0.4%) may also explain its high resistance to acid hydrolysis conditions used in its chemical analysis. Interestingly, a large amount of Ca2+ (141.5 mg/g polymer) was found intrinsically associated with the polymer compared to that bound by other bacterial exopolymers (25, 48). Whether this high Ca2+ loading contributes in some way to PE12's refractory nature is also as yet unknown.
It has been reported that uronic acids (pKa of ∼3) can confer a net negative charge to EPS polymers above pH 3 (18). If we assume that the uronic acid component of PE12 is responsible for the inherent surface activity of this polymer, we would expect acidic conditions (i.e., pH 3.5) to have no inhibitory effect on its emulsifying activity. However, this was not the case since, overall, acidic conditions appeared to decrease the emulsifying potential of PE12 as well as the commercial emulsifiers tested (Fig. 3B). Although further work will be needed to better understand these effects, the protonation of carboxyl groups may provide a mechanism to explain the decreased emulsifying activities under low pH conditions (61). Since the untreated controls (i.e., no emulsifier added) also exhibited emulsifying activities, likely due to the intrinsic presence of free fatty acids in the oils (42), protonation of these fatty acids could also have contributed to the reduced activities observed under acidic pH.
Overall, the emulsifying ability of PE12 compared well to the commercial emulsifiers against all the oils and both pH conditions. Interestingly, even very low concentrations (0.02%) of PE12 produced stable emulsions with the various food oils. This apparently “high-yield” value is an advantageous property both in terms of process economics and potential biotechnological applications (64). This may be attributed to certain functional groups on the PE12 polymer, such as 6-deoxyhexoses, or increased substitution by acetyl moieties, either of which can render polymeric compounds amphipathic in nature (19, 26). As no lipids were detected, the proteinaceous component of PE12 may have played a role in its interaction with the oils, possibly by mediating the steric adsorption of the polymer to oil droplets, as has been reported with gum arabic (74), emulsan (34), and other microbially derived polysaccharides (24). Alanine was identified as the major nonpolar amino acid (10.3%) in PE12. As a hydrophobic amino acid, it may contribute to the polymer's ability to interact with oil droplets. Carboxylate and methoxycarbonyl groups of polyuronates have also been reported to contribute emulsifying qualities to polymers (34, 70, 71), such as polysaccharide fractions of pectins, which can contain up to 44 to 60% uronic acids (19).
It has been suggested that uronic acids provide exopolymers with an ability to complex with metal species. The observation that the PE12 glycoprotein contains a high uronic acid content (ca. 28%) prompted us to investigate its capacity to absorb metal species, which is of biotechnological interest and, more broadly, has ecological implications for the role of these polymers in situ. Metal-binding experiments performed in deionized water showed that PE12 could effectively mediate the desorption of various sediment-adsorbed metals, particularly Na+ (154.5 mg/g polymer), Mg2+ (31.0 mg/g polymer), and K+ (10.6 mg/g polymer). Compared to the binding of Mg2+ by other bacterial polymers reported in the literature (7, 25, 48, 56), PE12 quantitatively removed at least 75% more of this metal per gram of polymer. Notably, PE12 exhibited an apparently high specificity and affinity for Na+, which may be an intrinsic property of marine polymers. As Na+ and Mg2+ are the most abundant metal ions in the ocean and, unlike Fe2+, are by no means limiting, their binding to charged marine bacterial exopolymers like PE12 may be a common feature that warrants further investigation to define its significance. Interestingly, the presence of 0.6 M NaCl drastically reduced the polymer's ability to desorb these metals from sediment. In a study by Bhaskar and Bhosle (9), concentrations of NaCl (0.17 to 0.6 M) were observed to reduce the ability of a Marinobacter exopolymer to bind Cu2+ and Pb2+. Similarly, increasing salinity has been shown to decrease the capacity of dissolved organic matter for binding metal ions (41). To better understand the metal desorption capacity of PE12, its metal affinity for different metal species was calculated as a ratio of the amount of metal desorbed (per gram of polymer) to the initial amount of metal associated with the sediment (Fig. 4). Under nonionic conditions, Na+ was desorbed with the highest affinity, followed by Mg2+, K+, Mn2+, Fe2+/3+, and Al3+. The polymer's affinity for all the other metals, except Mn2+, was severely affected under ionic-strength conditions (i.e., 0.6 M NaCl) and may be explained by competition from Na+ for binding sites on the polymer. This is supported by the polymer's high affinity for these ions, as measured in the deionized water treatment. Thus, our data indicate that marine bacterial exopolymers, such as PE12, have the capacity to sequester metals, suggesting their potential for use in biotechnological applications such as the remediation of contaminated effluent, soil, or sediment.
While our original rationale in this study and others (29, 30) was to search for biotechnologically interesting natural polymers, our finding of a number of marine bacteria producing glycoprotein exopolymers with significant emulsifying activity and metal-binding properties raises fundamental questions of why these organisms produce such polymers and what their role might be in relation to the producer strain and, more widely, to ecosystem functioning. It is recognized that marine bacterial exopolymers contribute to the organic matter continuum in seawater and act as “hot spots” of microbial diversity and activity (3). An exopolymer endowed with amphipathic qualities, such as PE12, may be expected to interact with suspended hydrophobic material in seawater, thereby potentially increasing its solubility and bioavailability to flux into the “microbial loop.” Notably, marine bacterial exopolymers are recognized for their high uronic acid content (36), which is comparably higher than that found in exopolymers derived from marine planktonic algae (8) and nonmarine bacteria (22). This endows these macromolecules with a polyanionic nature—a property that has been proposed to enable these polymers to complex cationic species (38, 39, 68). Based on evidence from field studies in polar marine environments showing a correlation between enhanced phytoplankton activity and high levels of bacterial exopolymeric substances (2), it has been suggested that these polymers may play a role in the binding of trace metals, like iron, possibly leading to an increase in their availability to phytoplankton (3, 8, 45). To our knowledge, however, the binding of iron by marine bacterial exopolymers has not previously been investigated. In the present study, we found PE12 to contain an intrinsic amount of iron (0.14 mg/g polymer) already complexed to the polymer prior to exposure to sediment. Although seawater ionic-strength conditions inhibited PE12's ability to desorb iron from the sediment, this concentration remained an intrinsic component of the polymer. This indicates that moieties on the polymer have specific and comparatively high affinity for iron because the complexation of this metal species involves specific structural coordination. Whether the affinity for iron is conferred by the uronic acid or protein moieties or by a combination of both is unknown at present. Additional work in our laboratory has demonstrated the ability of other types of marine bacterial exopolymers to effectively mediate the desorption of high concentrations of iron from marine sediment (T. Gutierrez, T. Shimmield, and D. H. Green, unpublished data). Thus, our data support the hypothesis that marine bacterial exopolymers can contribute to maintaining a pool of iron in the water column for potential uptake by phytoplankton (40).
In the present study, we have described a new type of Pseudoalteromonas exopolymer, PE12, characterized as a high-molecular-weight glycoprotein with an unusually high content of xylose. Compared to commercial emulsifiers, PE12 displayed excellent emulsifying activities against a range of food oils, indicating its potential as an emulsifying agent in biotechnological and industrial applications. PE12 also showed an ability to mediate the desorption of various metal ions from marine sediment, a property attributed to its polyanionic nature due to the presence of multivalent sites (i.e., carboxyl groups of uronic acids) that are likely to be localized on exposed areas of the polymer's macromolecular structure. However, the significant effect of 0.6 M NaCl on this process raises the importance of taking into account ionic-strength conditions in experiments designed to shed light on the interactions of organic macromolecules with metals in the field. Further work to investigate the metal-binding properties of other marine bacterial exopolymers is currently in progress in our laboratory with the expectation that it will reveal greater understanding of key organisms playing a role in the fate and mobility of metals in the marine water column.
Acknowledgments
This work was supported by grants from the Argyll and the Islands Enterprise (CID 15905) and Natural Environment Research Council (NE/E523272/1).
We appreciate and thank Heather Orr for conducting the fatty acid analysis.
Footnotes
Published ahead of print on 13 June 2008.
REFERENCES
- 1.Anton, J., I. Meseguer, and F. Rodriguez-Valera. 1988. Production of an extracellular polysaccharide by Haloferax mediterranei. Appl. Environ. Microbiol. 54:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apollonio, S., M. Pennington, and G. F. Cota. 2002. Stimulation of phytoplankton photosynthesis by bottom-ice extracts in the Arctic. Polar Biol. 25:350-354. [Google Scholar]
- 3.Azam, F. 1998. Microbial control of oceanic carbon flux: the plot thickens. Science 280:694-696. [Google Scholar]
- 4.Banat, I. M., R. S. Makkar, and S. S. Cameotra. 2000. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 53:495-508. [DOI] [PubMed] [Google Scholar]
- 5.Barkay, T., S. Navon-Venezia, E. Z. Ron, and E. Rosenberg. 1999. Enhancement of solubilization and biodegradation of polyaromatic hydrocarbons by the bioemulsifier alasan. Appl. Environ. Microbiol. 65:2697-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bejar, V., C. Calvo, J. Moliz, F. Diaz-Martinez, F. Quesada, and E. Quesada. 1996. Effect of growth conditions on the rheological properties and chemical composition of Volcaniella eurihalina exopolysaccharide. Appl. Biochem. Biotechnol. 59:77-86. [Google Scholar]
- 7.Beveridge, T. J., and S. F. Koval. 1981. Binding of metals to cell envelopes of Escherichia coli K-12. Appl. Environ. Microbiol. 42:325-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhaskar, P. V., and N. B. Bhosle. 2005. Microbial extracellular polymeric substances in marine biogeochemical processes. Curr. Sci. 88:45-53. [Google Scholar]
- 9.Bhaskar, P. V., and N. B. Bhosle. 2006. Bacterial extracellular polymeric substances (EPS): a carrier of heavy metals in the marine food-chain. Environ. Int. 32:191-198. [DOI] [PubMed] [Google Scholar]
- 10.Biermann, C. J. 1988. Hydrolysis and other cleavages of glycosidic linkages in polysaccharides. Adv. Carbohydr. Chem. Biochem. 46:251-271. [Google Scholar]
- 11.Bozal, N., A. Manresa, J. Castellvi, and J. Guinea. 1994. A new bacterial strain of Antarctica, Alteromonas sp., that produces a heteropolymer slime. Polar Biol. 14:561-567. [Google Scholar]
- 12.Brenner, I. B., A. Zander, M. Cole, and A. Wiseman. 1997. Comparison of axially and radially viewed inductively coupled plasmas for multi-element analysis: effect of sodium and calcium. J. Anal. Atomic Spectrom. 12:897-906. [Google Scholar]
- 13.Buffle, J., K. J. Wilkinson, S. Stoll, M. Fillela, and J. Zhang. 1998. A generalized description of aquatic colloidal interactions: the three-colloidal component approach. Environ. Sci. Technol. 32:2887-2899. [Google Scholar]
- 14.Cambon-Bonavita, M. A., G. Raguenes, J. Jean, P. Vincent, and J. Guezennec. 2002. A novel polymer produced by a bacterium isolated from a deep-sea hydrothermal vent polychaete annelid. J. Appl. Microbiol. 93:310-315. [DOI] [PubMed] [Google Scholar]
- 15.Cirigliano, M. C., and G. M. Carman. 1984. Isolation of a bioemulsifier from Candida lipolytica. Appl. Environ. Microbiol. 48:747-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook, E. J., M. V. Bell, K. D. Black, and M. S. Kelly. 2000. Fatty acid compositions of gonodal material and diets of the sea urchin, Psammechinus miliaris: trophic and nutritional implications. J. Exp. Mar. Biol. Ecol. 255:261-274. [DOI] [PubMed] [Google Scholar]
- 17.Cooper, D. G., and B. G. Goldenberg. 1987. Surface-active agents from two Bacillus species. Appl. Environ. Microbiol. 53:224-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corpe, W. A. 1970. An acid polysaccharide produced by primary film forming bacteria. Dev. Ind. Microbiol. 16:249-255. [Google Scholar]
- 19.Dea, I. C. M., and J. K. Madden. 1986. Acetylated pectic polysaccharides of sugar beet. Food Hydrocolloids 1:71-88. [Google Scholar]
- 20.Desai, J. D., and I. M. Banat. 1997. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 61:47-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fletcher, M., and G. D. Floodgate. 1973. An electron microscopic demonstration of an acidic polysaccharide involved in adhesion of a marine bacterium to solid surfaces. J. Gen. Microbiol. 74:325-334. [Google Scholar]
- 22.Ford, T., E. Sacco, J. Black, T. Kelley, R. C. Goodacre, R. C. W. Berkeley, and R. Mitchell. 1991. Characterization of exopolymers of aquatic bacteria by pyrolysis-mass spectrometry. Appl. Environ. Microbiol. 57:1595-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garti, N. 1999. What can nature offer from an emulsifier point of view: trends and progress? Colloids Surf. 152:125-146. [Google Scholar]
- 24.Garti, N., and M. E. Leser. 1999. Natural hydrocolloids as food emulsifiers, p. 104-145. In D. R. Karsa (ed.), Design and selection of performance surfactants, vol. 2. Wiley-Blackwell, Oxford, United Kingdom. [Google Scholar]
- 25.Geddie, J. L., and I. W. Sutherland. 1993. Uptake of metals by bacterial polysaccharides. J. Appl. Bacteriol. 74:467-472. [Google Scholar]
- 26.Graber, M., A. Morin, F. Duchiron, and P. F. Monsan. 1988. Microbial polysaccharides containing 6-deoxysugars. Enzyme Microbiol. Technol. 10:198-206. [Google Scholar]
- 27.Green, D. H., L. E. Llewellyn, A. P. Negri, S. I. Blackburn, and C. J. S. Bolch. 2004. Phylogenetic and functional diversity of the cultivable bacterial community associated with the paralytic shellfish poisoning dinoflagellate Gymnodinium catenatum. FEMS Microbiol. Ecol. 47:345-357. [DOI] [PubMed] [Google Scholar]
- 28.Guo, L., C. C. Hung, P. H. Santschi, and I. D. Walsh. 2002. 234Th scavenging and its relationship to acid polysaccharide abundance in the Gulf of Mexico. Mar. Chem. 78:103-119. [Google Scholar]
- 29.Gutierrez, T., B. Mulloy, C. Bavington, K. Black, and D. H. Green. 2007. Partial purification and chemical characterization of a glycoprotein (putative hydrocolloid) emulsifier produced by a marine Antarctobacter species. Appl. Microbiol. Biotechnol. 76:1017-1026. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez, T., B. Mulloy, K. Black, and D. H. Green. 2007. Glycoprotein emulsifiers from two marine Halomonas species: chemical and physical characterization. J. Appl. Microbiol. 103:1716-1727. [DOI] [PubMed] [Google Scholar]
- 31.Hasenhuettle, G. L., and R. W. Hartel. 1997. Food emulsifiers and their applications. Chapman and Hall, New York, NY.
- 32.Holmstrom, C., and S. Kjelleberg. 1999. Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol. Ecol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 33.Ikegami, S., P. A. Williams, and G. O. Phillips. 1992. Interfacial properties of xanthan gum, p. 371-377. In G. O. Phillips, D. J. Wedlock, and P. A. Williams (ed.), Gums and stabilizers for the food industry, vol. 6. IRL, Oxford, United Kingdom. [Google Scholar]
- 34.Kaplan, N., Z. Zosim, and E. Rosenberg. 1987. Reconstitution of emulsifying activity of Acinetobacter calcoaceticus BD4 emulsan by using pure polysaccharide and protein. Appl. Environ. Microbiol. 53:440-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenne, L., and B. Lindberg. 1983. Bacterial polysaccharides, p. 287-363. In G. O. Aspinall (ed.), The polysaccharides. Academic Press, New York, NY.
- 36.Kennedy, A. F. D., and I. W. Sutherland. 1987. Analysis of bacterial exopolysaccharides. Biotechnol. Appl. Biochem. 9:12-19. [PubMed] [Google Scholar]
- 37.Klekner, V., and N. Kosaric. 1993. Biosurfactants for cosmetics. Surfactant Sci. Ser. 48:373-389. [Google Scholar]
- 38.Loaec, M., R. Olier, and J. Guezennec. 1997. Uptake of lead, cadmium and zinc by a novel bacterial exopolysaccharide. Water Res. 31:1171-1179. [Google Scholar]
- 39.Loaec, M., R. Olier, and J. Guezennec. 1998. Chelating properties of bacterial exopolysaccharides from deep-sea hydrothermal vents. Carbohydr. Polymers 35:65-70. [Google Scholar]
- 40.Longhurst, A., S. Sathyendranath, T. Platt, and C. Caverhill. 1995. An estimate of global primary production in the ocean from satellite radiometer data. J. Plankton Res. 17:1245-1271. [Google Scholar]
- 41.Lores, E. M., and J. R. Pennock. 1998. The effect of salinity on binding of Cd, Cr, Cu and Zn to dissolved organic matter. Chemosphere 37:861-874. [Google Scholar]
- 42.Ma, F., and M. A. Hanna. 1999. Biodiesel production: a review. Bioresour. Technol. 70:1-15. [Google Scholar]
- 43.Maestre, S., J. Mora, and J.-L. Todolí. 2002. Studies about the origin of the non-spectroscopic interferences caused by sodium and calcium in inductively coupled plasma atomic emission spectrometry. Influence of spray chamber design. Spectrochim. Acta B 57:1753-1770. [Google Scholar]
- 44.Mancuso Nichols, C. A., S. Garon, J. P. Bowman, G. Raguenes, and J. Guezennec. 2004. Production of exopolysaccharides by Antarctic marine bacterial isolates. J. Appl. Microbiol. 96:1057-1066. [DOI] [PubMed] [Google Scholar]
- 45.Mancuso Nichols, C. A., J., Guezennec, and J. P. Bowman. 2005. Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: a review. Mar. Biotechnol. 7:253-271. [DOI] [PubMed] [Google Scholar]
- 46.Mancuso Nichols, C., S. G. Lardiere, J. P. Bowman, P. D. Nichols, J. A. E. Gibson, and J. Guezennec. 2005. Chemical characterization of exopolysaccharides from Antarctic marine bacteria. Microb. Ecol. 49:578-589. [DOI] [PubMed] [Google Scholar]
- 47.Marshall, K. C., R. Stout, and R. Mitchell. 1971. Mechanism of the initial events in the sorption of marine bacteria to surfaces. J. Gen. Microbiol. 68:337-348. [Google Scholar]
- 48.McLean, R. J., D. Beauchemin, L. Clapham, and T. J. Beveridge. 1990. Metal-binding characteristics of the gamma-glutamyl capsular polymer of Bacillus licheniformis ATCC 9945. Appl. Environ. Microbiol. 56:3671-3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nazarenko, E. L., N. A. Komandrova, R. P. Gorshkova, S. V. Tomshich, V. A. Zubkov, M. Kilcoyne, and A. V. Savage. 2003. Structures of polysaccharides and oligosaccharides of some gram-negative marine Proteobacteria. Carbohydr. Res. 338:2449-2457. [DOI] [PubMed] [Google Scholar]
- 50.Neu, T. R. 1996. Significance of bacterial surface-active compounds in interaction of bacteria with interfaces. Microbiol. Rev. 60:151-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nölte, J. 2003. ICP emission spectrometry. A practical guide. Wiley-VCH, Weinheim, Germany.
- 52.Ogawa, H., Y. Amagai, I. Koike, K. Kaiser, and R. Benner. 2001. Production of refractory dissolved organic matter by bacteria. Science 292:917-920. [DOI] [PubMed] [Google Scholar]
- 53.Paerl, H. W. 1975. Microbial attachment to particles in marine and freshwater ecosystems. Microb. Ecol. 2:73-83. [DOI] [PubMed] [Google Scholar]
- 54.Passeri, A., M. Schmidt, T. Haffner, V. Wray, S. Lang, and F. Wagner. 1992. Marine biosurfactants. IV. Production, characterization and biosynthesis of an anionic glucose lipid from the marine bacterial strain MM1. Appl. Microbiol. Biotechnol. 37:281-286. [Google Scholar]
- 55.Raguenes, G., M. A. Cambon-Bonavita, J. F. Lohier, C. Boisset, and J. Guezennec. 2003. A novel, highly viscous polysaccharide excreted by an Alteromonas isolated from a deep-sea hydrothermal vent shrimp. Curr. Microbiol. 46:448-452. [DOI] [PubMed] [Google Scholar]
- 56.Rayman, M. K., and R. A. MacLeod. 1975. Interaction of Mg2+ with peptidoglycan and its relation to the prevention of lysis of a marine pseudomonad. J. Bacteriol. 122:650-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosenberg, E. 1993. Microbial diversity as a source of useful biopolymers. J. Ind. Microbiol. 11:131-137. [DOI] [PubMed] [Google Scholar]
- 58.Rosenberg, E., and E. Z. Ron. 1997. Bioemulsans: microbial polymeric emulsifiers. Curr. Opin. Biotechnol. 8:313-316. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberg, E., and E. Z. Ron. 1999. High- and low-molecular-mass microbial surfactants. Appl. Microbiol. Biotechnol. 52:154-162. [DOI] [PubMed] [Google Scholar]
- 60.Rosenberg, E., T. Barkay, S. Navon-Venezia, and E. Z. Ron. 1999. Role of Acinetobacter bioemulsans in petroleum degradation, p. 171-180. In R. Fass, Y. Flashner, and S. Reuevny (ed.), Novel approaches for bioremediation of organic pollution. Kluwer/Plenum Publishers, New York, NY.
- 61.Rosenberg, E., A. Zuckerberg, C. Rubinovitz, and D. Gutnick. 1979. Emulsifier of Arthrobacter RAG-1: isolation and emulsifying properties. Appl. Environ. Microbiol. 37:402-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rougeaux, H., R. Pichon, N. Kervarec, G. H. C. Raguenes, and J. G. Guezennec. 1996. Novel bacterial exopolysaccharides from deep-sea hydrothermal vents. Carbohydr. Polymers 31:237-242. [Google Scholar]
- 63.Rougeaux, H., J. Guezennec, R. W. Carlson, N. Kervarec, R. Pichon, and P. Talaga. 1999. Structural determination of the exopolysaccharide of Pseudoalteromonas strain HYD 721 isolated from a deep-sea hydrothermal vent. Carbohydr. Res. 315:273-285. [DOI] [PubMed] [Google Scholar]
- 64.Sanderson, G. R. 1990. The functional properties and application of microbial polysaccharides—a supplier's view, p. 333-339. In G. O. Phillips, D. J. Wedlock, and P. A. Williams (ed.), Gums and stabilizers for food industry, vol. 5. IRL Press, Oxford, United Kingdom. [Google Scholar]
- 65.Shepherd, R., J. Rockey, I. W. Sutherland, and S. Roller. 1995. Novel bioemulsifiers from microorganisms for use in foods. J. Biotechnol. 40:207-217. [DOI] [PubMed] [Google Scholar]
- 66.Singh, P., and S. S. Cameotra. 2004. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 22:142-146. [DOI] [PubMed] [Google Scholar]
- 67.Singh, P., and S. S. Cameotra. 2004. Enhancement of metal bioremediation by use of microbial surfactants. Biochem. Biophys. Res. Commun. 319:291-297. [DOI] [PubMed] [Google Scholar]
- 68.Steiner, A. E., D. A. McLaren, and C. F. Forster. 1976. The nature of activated sludge flocs. Water Res. 10:25-30. [Google Scholar]
- 69.Todolí, J.-L., L. Gras, V. Hernandis, and J. Mora. 2002. Elemental matrix effects in ICP-AES. J. Anal. Atomic Spectrom. 17:142-169. [Google Scholar]
- 70.Tolstogusov, V. B. 1991. Functional properties of food proteins and the role of protein-polysaccharide interaction. Food Hydrocolloids 4:429-468. [Google Scholar]
- 71.Tolstogusov, V. B. 1994. Some physico-chemical aspects of protein processing in foods, p. 115-124. In G. O. Phillips, P. A. Williams, and D. J. Wedlock (ed.), Gums and stabilizers for the food industry, vol. 7. IRL Press, Oxford, United Kingdom. [Google Scholar]
- 72.Weiner, R. M. 1997. Biopolymers from marine prokaryotes. Mar. Biotechnol. 15:390-394. [DOI] [PubMed] [Google Scholar]
- 73.Weisburg, W. G., S. M. Barns, D. A. Pelletier, and D. J. Lane. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams, P. A., G. O. Phillips, and R. C. Randall. 1990. Structure-function relationships of gum arabic, p. 25-36. In G. O. Phillips, D. J. Wedlock, and P. A. Williams (ed.), Gums and stabilizers for the food industry, vol. 5. IRL Press, Oxford, United Kingdom. [Google Scholar]
- 75.Zosim, Z., D. Gutnick, and E. Rosenberg. 1983. Uranium binding by emulsan and emulsanosols. Biotechnol. Bioeng. 25:1725-1735. [DOI] [PubMed] [Google Scholar]