Abstract
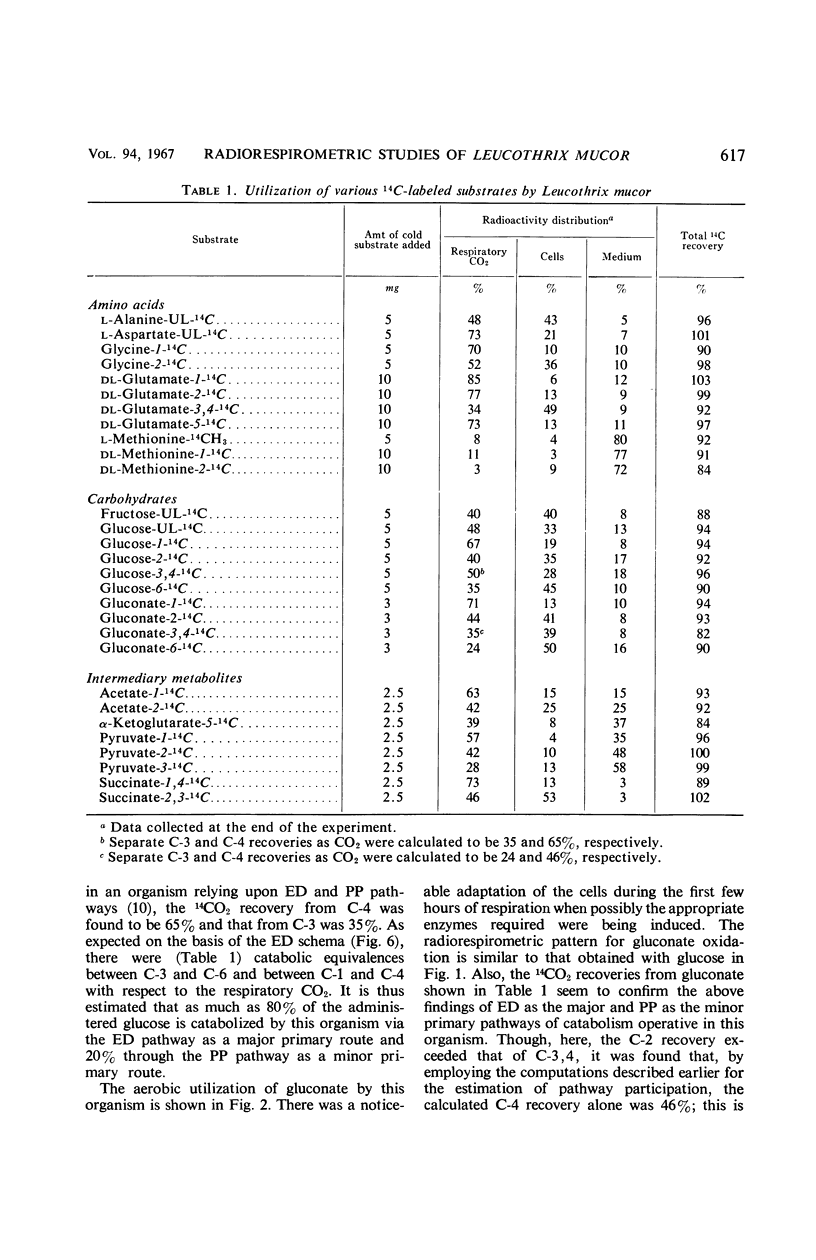
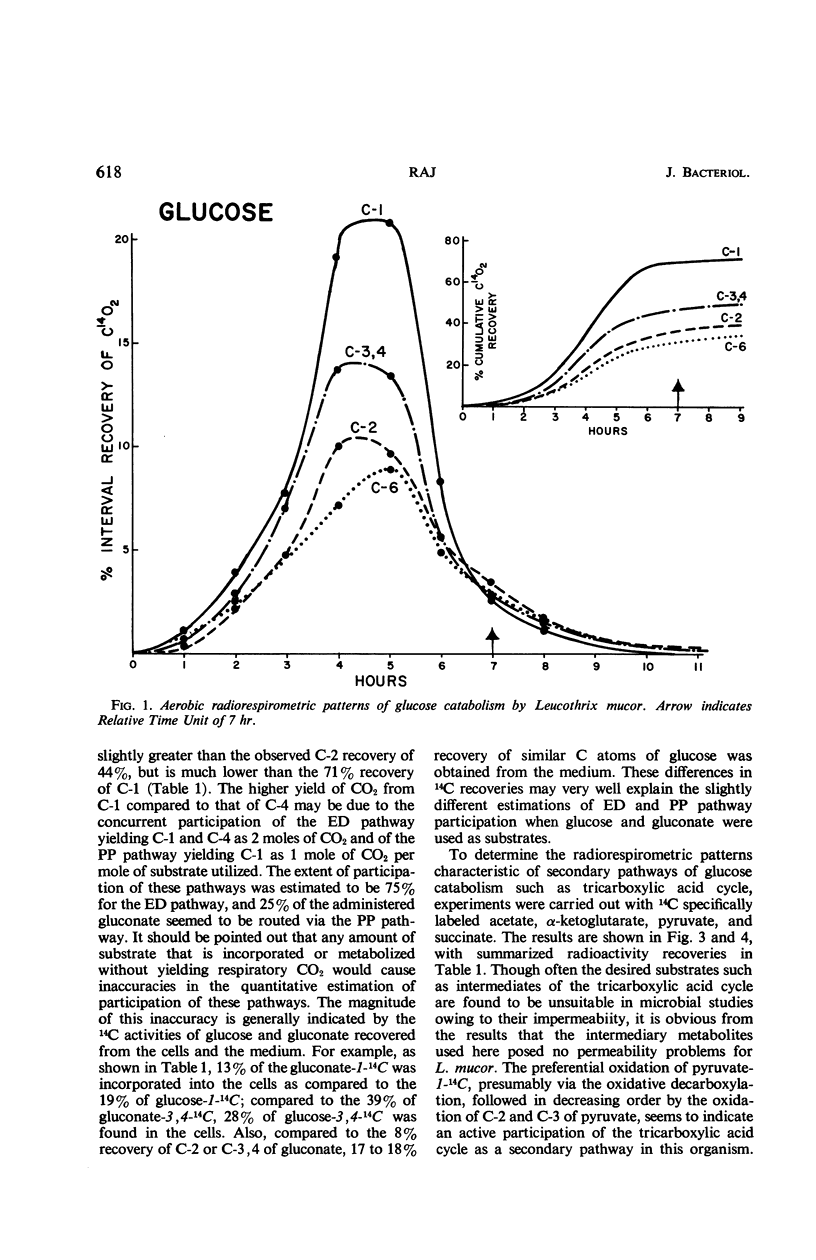
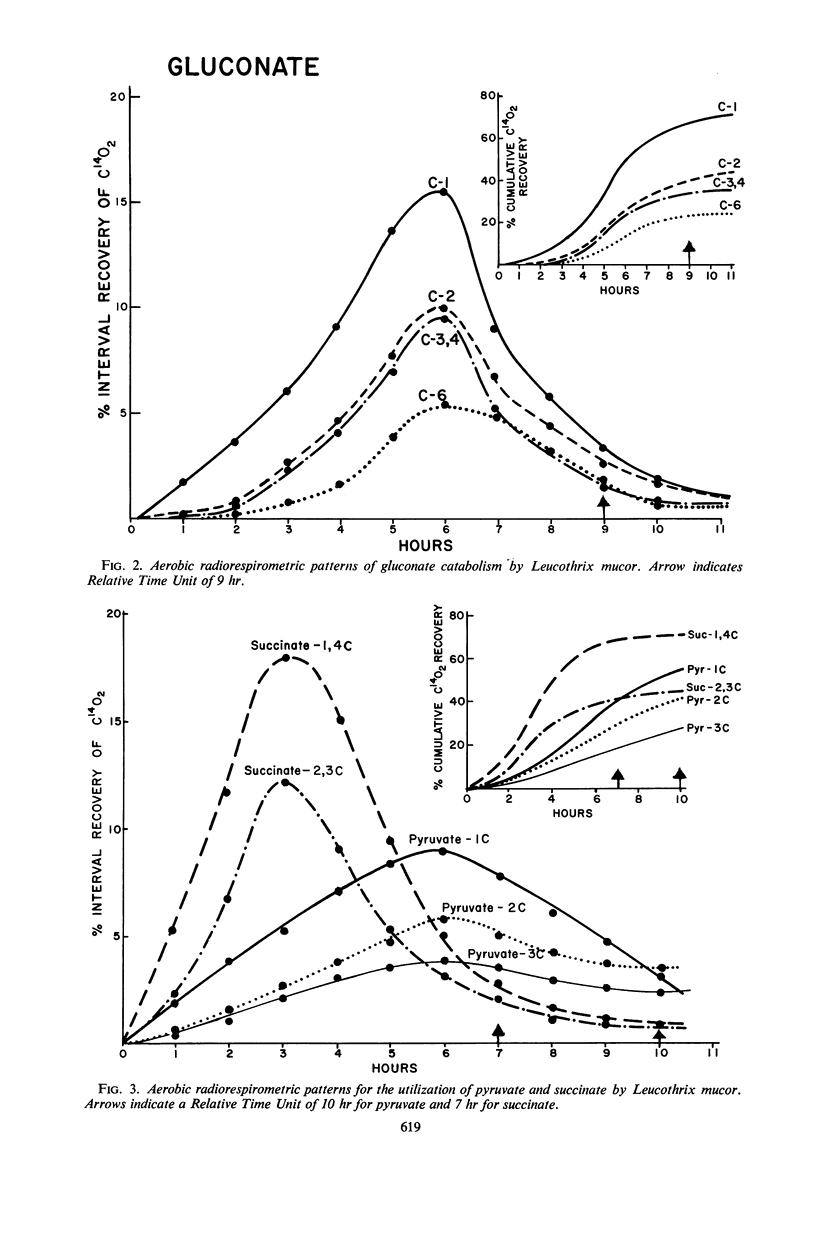
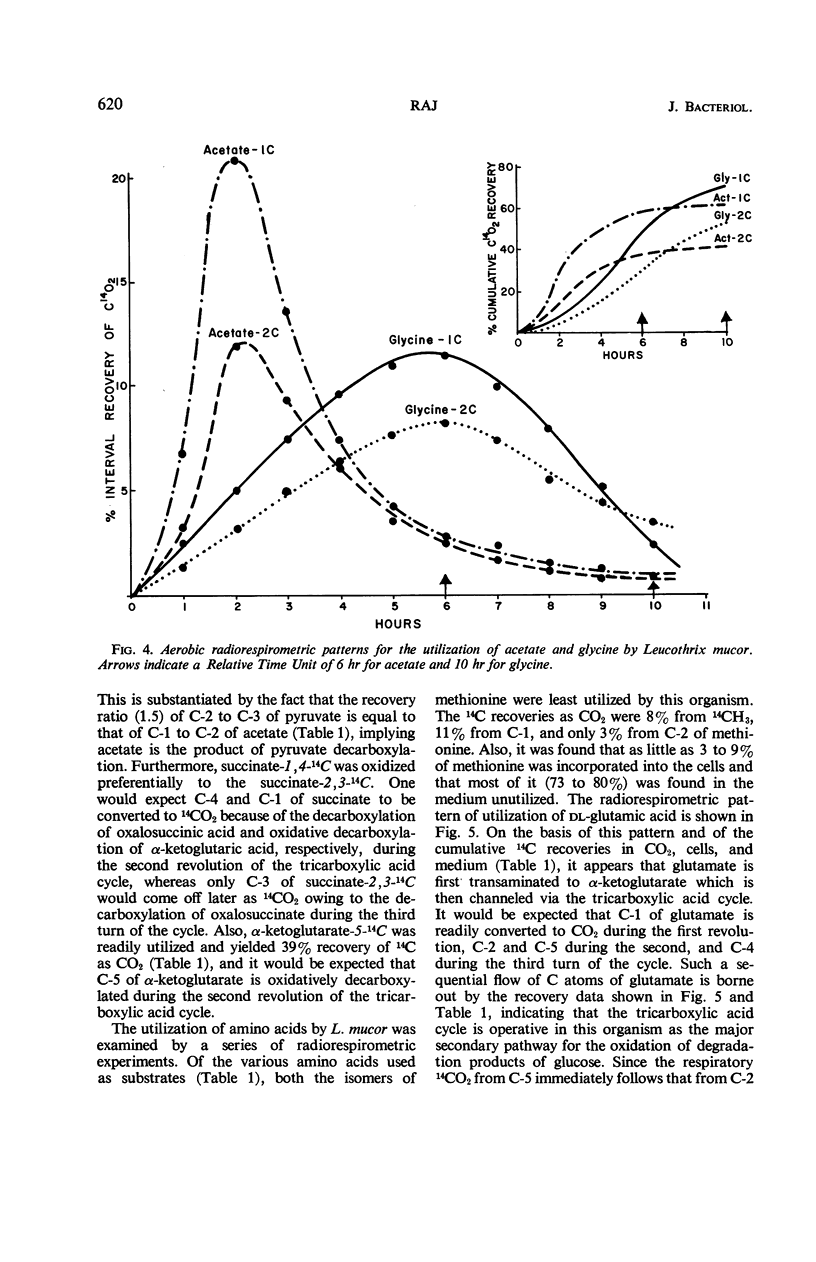
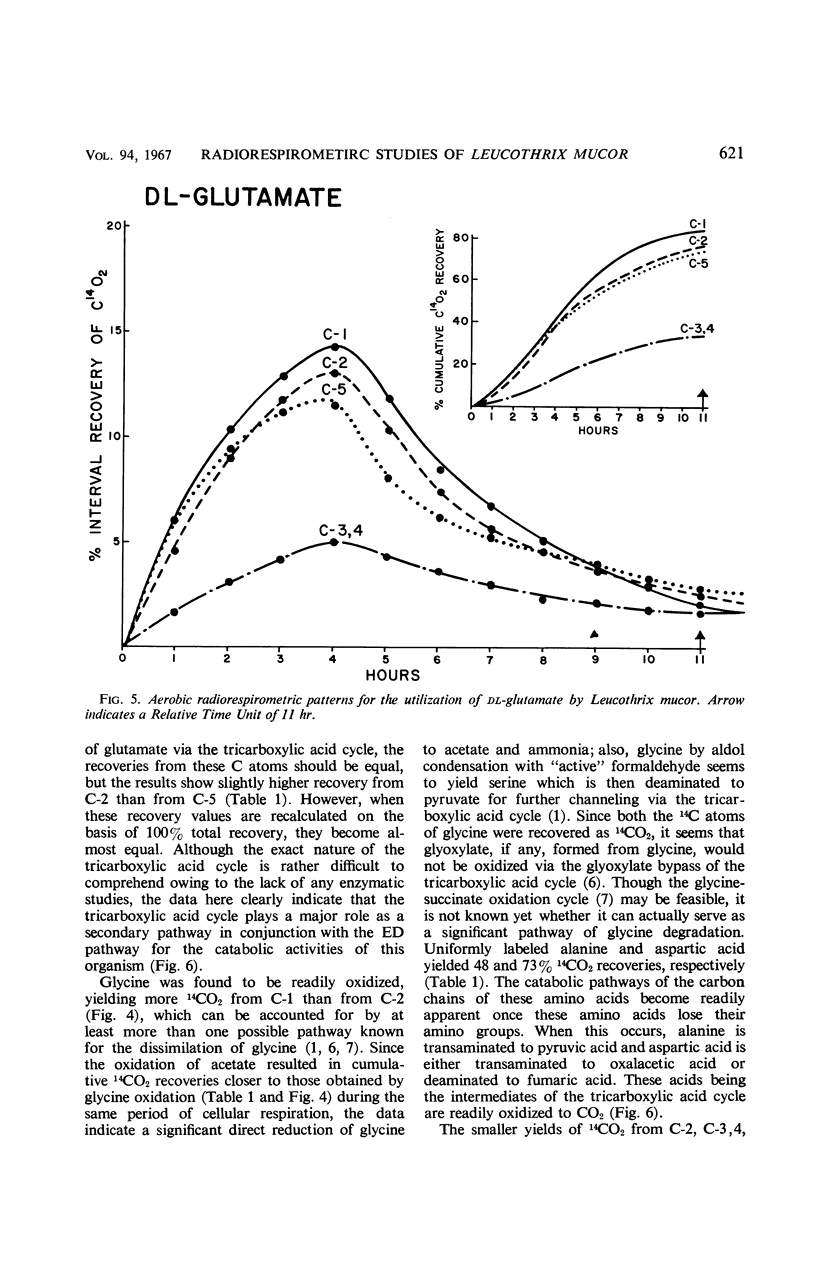
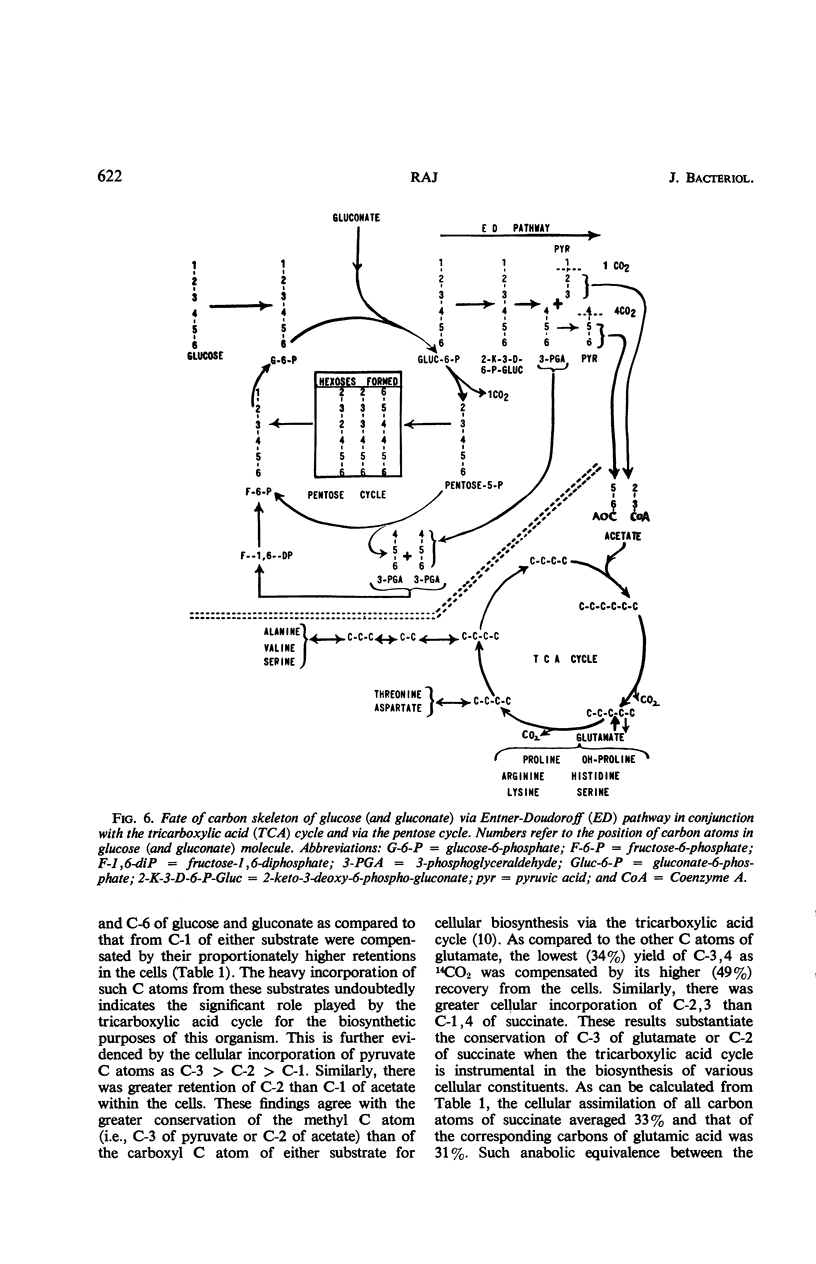
Catabolic capabilities of Leucothrix mucor were studied by radiorespirometric technique with a liquid scintillation spectrometer. Through measurement of relative rates and total percentages of 14CO2 produced, together with the determination of the cellular incorporation of 14C from different carbon atoms of various labeled substrates, such as simple carbohydrates, intermediary metabolites, and amino acids, the essential characteristics of the primary and secondary pathways operative in this organism were examined. These substrates appeared to be degraded mainly via enzymes of the Entner-Doudoroff pathway in conjunction with the tricarboxylic acid cycle. Estimation of concurrent participation of pathways indicated that 20 to 25% of the administered carbohydrate was catabolized via the pentose phosphate pathway. There was no evidence of the Embden-Meyerhof-Parnas pathway operating in this organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENZIMAN M., SAGERS R. D., GUNSALUS I. C. L-serine specific dehydrase from Clostridium acidi-urici. J Bacteriol. 1960 Apr;79:474–479. doi: 10.1128/jb.79.4.474-479.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK T. D. KNOTS IN LEUCOTHRIX MUCOR. Science. 1964 May 15;144(3620):870–872. doi: 10.1126/science.144.3620.870. [DOI] [PubMed] [Google Scholar]
- HAROLD R., STANIER R. Y. The genera Leucothrix and Thiothrix. Bacteriol Rev. 1955 Jun;19(2):49–64. doi: 10.1128/br.19.2.49-64.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITOS P. A., WANG C. H., MOHLER B. A., KING T. E., CHELDELIN V. H. Glucose and gluconate dissimilation in Acetobacter suboxydans. J Biol Chem. 1958 Dec;233(6):1295–1298. [PubMed] [Google Scholar]
- NAKADA H. I., FRIEDMANN B., WEINHOUSE S. Pathways of glycine catabolism in rat liver. J Biol Chem. 1955 Oct;216(2):583–592. [PubMed] [Google Scholar]
- NEMETH A. M., RUSSELL C. S., SHEMIN D. The succinate-glycine cycle. II. Metabolism of delta-aminolevulinic acid. J Biol Chem. 1957 Nov;229(1):415–422. [PubMed] [Google Scholar]
- RAJ H. D., DURYEE F. L., DEENEY A. M., WANG C. H., ANDERSON A. W., ELLIKER P. R. Utilization of carbohydrates and amino acids by Micrococcus radiodurans. Can J Microbiol. 1960 Jun;6:289–298. doi: 10.1139/m60-033. [DOI] [PubMed] [Google Scholar]
- STERN I. J., WANG C. H., GILMOUR C. M. Comparative catabolism of carbohydrates in Pseudomonas species. J Bacteriol. 1960 Apr;79:601–611. doi: 10.1128/jb.79.4.601-611.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler W. J., Gilmour C. M. Biochemistry of nitrate respiration in Pseudomonas stutzeri. I. Aerobic and nitrate respiration routes of carbohydrate catabolism. J Bacteriol. 1966 Jan;91(1):245–250. doi: 10.1128/jb.91.1.245-250.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


