Abstract
We found that the human intestinal isolate Bifidobacterium bifidum MIMBb75 strongly adhered to Caco-2 cells. Proteinase K and lithium chloride treatments showed that proteins play a key role in MIMBb75 adhesion to Caco-2 cells. By studying the cell wall-associated proteins, we identified a surface protein, which we labeled BopA. We purified the protein chromatographically and found that it functioned as an adhesion promoter on Caco-2 cells. In silico analysis of the gene coding for this protein and globomycin experiments showed that BopA is a cysteine-anchored lipoprotein expressed as a precursor polypeptide. A database search indicated that BopA appears to function biologically as an oligopeptide/tripeptide-solute-binding protein in the ABC transport system. We discovered a protein corresponding to BopA and its gene in eight other highly adherent B. bifidum strains. Finally, we found that B. bifidum MIMBb75 and BopA affected the production of interleukin-8 in Caco-2 epithelial cells. BopA is the first protein described to date to be directly involved in the adhesion of bifidobacteria to Caco-2 cells and to show immunomodulatory activity.
Bifidobacteria are autochthonous inhabitants of the human colon (6) and together with lactobacilli comprise most of the probiotics commercially available today (45).
Bifidobacterium bifidum is a species that is commonly detected in the feces of healthy adults and infants (25, 35). However, other species of the genus Bifidobacterium have been studied more, such as B. longum, B. adolescentis, and B. animalis subsp. lactis (1, 11, 12, 37, 43, 54).
Bifidobacterium bifidum strains have been reported as having several beneficial health effects: immunomodulation (29, 44), antibacterial activity (3, 52), bacteriocin production (60), improvement of the intestinal microbial balance in mice (10), and reduction of inflammation in chickens (14).
The ability to adhere to the intestinal epithelium may play an important role in gut colonization, as it prevents the peristaltic elimination of bacteria. Moreover, it promotes the modulation of the immune system (55) and prevents pathogens from attaching to the gut mucosa (48). On the other hand, adherence to the intestinal mucosa may increase the unlikely but potential risk of bacterial translocation and virulence (34). Several strains of Bifidobacterium bifidum have been observed to adhere particularly well to human intestinal cell lines (17, 39, 46), yet no studies have so far unequivocally demonstrated the bacterial molecular determinants involved in the adhesion mechanism.
Several in vitro methods have been developed to study the propensity of bacteria to adhere to the human intestinal epithelium. The use of the Caco-2 human intestinal epithelial cell line seems to be one of the most successful approaches (5, 7, 33, 39, 46). Caco-2 cells are human colonic adenocarcinoma cells that are able to express differentiation features characteristic of mature intestinal cells and, therefore, are valuable in vitro tools for studies related to intestinal cell function and differentiation (13) and for investigating the mechanisms underlying the interaction between bacterial cells and the human gut in vitro (10, 30, 47, 59).
In this study, we show how we identified a cell surface lipoprotein that we call BopA and how it helps the bacterial strain to adhere to a Caco-2 cell layer. Moreover, we demonstrated how B. bifidum MIMBb75 and BopA affect the production of interleukin-8 (IL-8) in Caco-2 epithelial cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Table 1 lists the bacterial strains used in this study. Bifidobacterium strains were grown overnight anaerobically at 37°C in MRS broth (Difco, Detroit, MI) supplemented with 0.05% l-cysteine hydrochloride (cMRS). Lactobacillus rhamnosus and Lactobacillus helveticus were cultivated overnight in MRS broth at 37°C and 42°C, respectively. Streptococcus thermophilus was cultivated overnight in M17 medium (Difco) at 37°C. Listeria monocytogenes ATCC 15313 was grown in brain hearth infusion broth (Difco) at 37°C under agitation.
TABLE 1.
Bacterial strains included in the study
| Species | Strain | Source | Identification methodc | Reference |
|---|---|---|---|---|
| B. bifidum | MIMBb75a | Adult feces | DNA homology | |
| DSM20456T | Infant feces | |||
| IAB26b | Elderly feces | ARDRA | ||
| MPB41b | Elderly feces | ARDRA | ||
| MPB34b | Elderly feces | ARDRA | ||
| NAB1b | Elderly feces | DNA homology | 8 | |
| NCC390 | Adult feces | 57 | ||
| SAB20b | Elderly feces | ARDRA | ||
| SAB23b | Elderly feces | ARDRA | ||
| B. adolescentis | NAA38b | Elderly feces | DNA homology | 8 |
| B. catenulatum | ATCC 27539T | Adult feces | ||
| B. longum | NAL8b | Elderly feces | DNA homology | 21 |
| NCC2705 | Adult feces | 54 | ||
| B. pseudocaenulatum | NAP32b | Elderly feces | DNA homology | 8 |
| B. animalis subsp. lactis. | Bb12 | Commercial fermented milk | 43 | |
| L. helveticus | MIMLh22a | Dairy natural starter | DNA homology | |
| L. rhamnosus | GG | Adult feces | 43 | |
| S. thermophilus | DSM20617T | Yogurt | ||
| L. monocytogenes | ATCC 15313T |
From the Industrial Microbiology Culture Collection, DiSTAM, University of Milan, Milan, Italy.
From the MAAE culture collection, DiSTAM, University of Milan, Milan, Italy.
ARDRA, amplified rRNA gene restriction analysis.
Bacterial adhesion to Caco-2 cells.
Caco-2 cells were routinely grown in 3-cm petri plates on microscopy cover glasses in Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) heat-inactivated (30 min at 56°C) fetal calf serum, 100 U ml−1 penicillin, 100 mg ml−1 streptomycin, 0.1 mM nonessential amino acids, and 2 mM l-glutamine and incubated at 37°C in a water-jacketed incubator in an atmosphere of 95% air and 5% carbon dioxide. The culture medium was changed twice weekly. For adhesion assays, cells were used 15 days after confluence (fully differentiated cells). Cell monolayers were carefully washed twice with phosphate-buffered saline (PBS) (pH 7.3) before bacterial cells were added. The bacterial cell concentration of a culture grown overnight was determined microscopically after DAPI (4′,6′-diamidino-2-phenylindole) staining. Approximately 2 × 108 cells of each strain resuspended in PBS (pH 7.3) were incubated with a monolayer of fully differentiated Caco-2 cells. After 1 h at 37°C in anaerobic conditions, all monolayers were washed three times with PBS to release unbound bacteria. Cells were then fixed with 3 ml of methanol and incubated for 8 min at room temperature. After methanol was removed, cells were stained with 3 ml of Giemsa stain solution (1:20) (Carlo Erba, Milan, Italy) and left for 30 min at room temperature. Wells were then washed until no color was observed in the washing solution and dried in an incubator for 1 h. Microscopy cover glasses were then removed from the petri plate and examined microscopically (magnification, ×100), immersed in oil. Adherent bacteria in 20 randomly selected microscopic fields were counted and averaged. An unpaired Student t test was run for statistically significant differences.
Preparation of bacterial cell wall extract.
Bacterial cells from 0.2 liters of liquid culture were harvested by centrifugation and processed according to methods described previously by Mattarelli et al. (36), with a modified use of the French press (12,000 lb/in2) for breaking cells.
SDS-PAGE and N-terminal sequence analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described previously by Laemmli (32), using a 4% stacking gel and a 10% resolving gel. For BopA sequence analysis, proteins were electrotransferred onto a polyvinylidene difluoride membrane (ProBlott; Applied Biosystems, Foster City, CA). The membrane was first stained with Coomassie blue, and the band corresponding to BopA was then excised. The sample was desalted using a ProSpin cartridge (Applied Biosystems) and placed onto a Polybrene-coated and precycled glass fiber filter, followed by sequencing with an Applied Biosystems model 477A protein sequencer equipped with an online model 120A PTH amino acid analyzer. For internal sequencing, the sample was subjected to digestion with trypsin (sequencing grade; Promega, Milan, Italy) as described previously by Fernandez et al. (16). The peptides were separated with a high-performance liquid chromatograph equipped with a Vydac C18 column (2.1 mm by 150 mm). One of the tryptic peptides was applied onto glass fiber filters and sequenced as described above.
Partial purification of BopA.
An equal volume of 5 M LiCl was added to the bacterial cell wall preparations, and after extensive vortexing, the suspensions were incubated for 2 h at 37°C. After centrifugation, the supernatant was recovered, and the buffer was exchanged to 50 mM Tris-HCl buffer (pH 8.0) using an Amicon Ultra (Millipore, Billerica, MA) centrifugal filter device (5,000-molecular-weight membrane cutoff). Aliquots of 200 μl of the resulting solution were applied to a MonoQ 5/50 GL column (0.46 cm by 25 cm) (GE Healthcare, Milan, Italy) equilibrated in 50 mM Tris-HCl buffer (pH 8.0) using an high-performance liquid chromatography apparatus (Water) at a flow rate of 1 ml min−1. The unbound fraction containing the BopA protein was collected and concentrated 15 times by ultrafiltration using the same device and conditions described above. The protein fraction retained by the chromatographic column was eluted with the equilibration buffer containing 1 M NaCl and then discarded. Protein purity was determined by SDS-PAGE analysis.
Competitive adhesion assay.
The effect of BopA on bacterial adhesion was examined by incubating a Caco-2 cell monolayer with purified BopA (37.5 and 375 mg ml−1 in PBS as final concentrations) at 37°C for 1 h; 1 mg ml−1 bovine serum albumin (BSA) was used as a control. Subsequently, a bacterial adhesion assay was run as described above. A Student t test was run for statistically significant differences.
Stimulation of Caco-2 monolayers and enzyme-linked immunosorbent assay measurement of cytokine production.
For all assays, a known number of Caco-2 cells were seeded into 24-well plates and grown as described above. Probiotic bacteria (final concentration of 108 bacterial cells ml−1) and purified BopA protein (final concentrations of 0.1, 10, and 20 μg ml−1) were added to monolayers of Caco-2 cells in 0.5 ml of fresh antibiotic-free Dulbecco's modified Eagle's medium and incubated overnight at 37°C in 5% CO2. Cytokine IL-1β (1 ng ml−1), casein (10 μg ml−1), and BSA (10 μg ml−1) were used as controls. To test the effect of Listeria monocytogenes, Caco-2 cells were incubated with 108 bacterial cells ml−1 at 37°C for 1 h. After that, microtiter plates were kept at −70°C for 4 h to completely disrupt the Caco-2 cells. The supernatant and disrupted Caco-2 cells were then collected, and phenylmethylsulfonyl fluoride was added (20 mM final concentration). After centrifugation at a relative centrifugal force of 14,000 for 1 min, levels of IL-6 and IL-8 in the supernatants were determined by using RayBio Human IL enzyme-linked immunosorbent assay kits (RayBiotech, Inc., Norcross, GA) as instructed by the manufacturer. Each sample was processed in duplicate in two independent experiments.
PCR amplification of the DNA region coding for BopA.
Each 25-μl reaction mixture contained 200 μM of deoxynucleoside triphosphate, 2.5 μl of 10× reaction buffer (Fermentas, Vilnius, Lithuania), 2.5 mM of MgCl2, each primer at a concentration of 0.5 μM, 0.5 U of Taq polymerase (Fermentas), and 1 μl of bacterial DNA solution (containing about 100 ng of DNA). The initial denaturation step at 95°C for 2 min was followed by 35 cycles of denaturation at 94°C for 45 s and annealing at 54°C to 62°C for 45 s, depending on the primer, with an extension step at 72°C for 1 min. The final cycle was followed by an additional 7-min elongation period at 72°C. The primers employed were as follows: InvXf1 (5′-GTGTGTACACGCTGACCA-3′), InvXrS (5′-TCTTCTCGGTCCATTCGAT-3′), InvXr2 (5′-GGAAGGTGGAGAACAGCT-3′), InvXfD (5′-CCTCGACGTGTCGATTCA-3′), and InvXr1 (5′-GCCACCCTGCTTCAGCT-3′).
Sequence determination of the DNA region coding for BopA.
Starting from the N-terminal amino acid sequence of the whole BopA and its trypsin fragment, we designed a pair of degenerated primers according the codon use of Bifidobacterium longum NCC2705. The primers used were BIFOPf (5′-GGCAACTCCAACATIGGCWSSGCSGGCAA-3′) and BIFOPr (5′-CTTYTCGTAGTAGCCGTCSSWGTT-3′). PCR experiments run with BIFOP primers yielded a 1,191-bp fragment that was cloned into the pGEM-T vector (Promega) and sequenced. Subsequent self-ligation and inverse PCR experiments were run as follows. For self-ligation experiments, 5 μg of chromosomal DNA was digested overnight with 30 U of BamHI or SalI restriction enzyme (Fermentas). Digested DNA was purified by phenol-chloroform extraction and used in an overnight ligation reaction at 15°C in a volume of 0.5 ml with 5 U of T4 DNA ligase (Fermentas). Different amounts of self-ligated DNA were used as a template in inverse PCRs with DyNAzyme Ext DNA polymerase (Finnzymes, Helsinki, Finland).
Sequence analysis.
We used the Basic Local Alignment Search Tool (BLAST) for searching for similarities against the GenBank and EMBL sequence databases and predicted the promoter by using Neural Network Promoter Prediction (http://www.fruitfly.org/seq_tools/promoter.html). A putative signal peptide sequence was found with SignalP software (http://www.cbs.dtu.dk/services/SignalP-2.0/). We also used DOLOP database software (http://www.mrc-lmb.cam.ac.uk/genomes/dolop/analysis.shtml) to predict bacterial lipoproteins.
Nucleotide sequence accession number.
The sequence data for the 3,419-bp DNA fragment from Bifidobacterium bifidum MIMBb75 have been deposited in the EMBL database under accession number AM710395.
RESULTS
Bifidobacterium bifidum MIMBb75 adheres to the Caco-2 human colonic cell line.
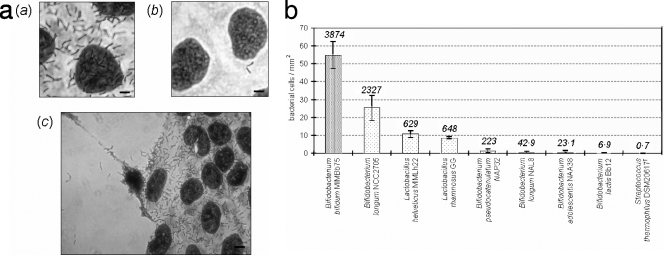
The human fecal isolate Bifidobacterium bifidum MIMBb75 was tested and found to be well adherent to Caco-2 human colonic cells (Fig. 1a). After extensive washing with PBS, a significant proportion of cells of this bacterial strain remained attached to the Caco-2 monolayer, providing evidence that the adhesion was not only nonspecific physical entrapment. In particular, about 55 bacterial cells adhered to a 1-mm2 epithelial cell monolayer, resulting in an adhesion index (bacterial cells/100 Caco-2 cells) of 3,874 (Fig. 1b), which is statistically the highest among the strains tested (P < 0.026). Also, Bifidobacterium longum NCC2705, Lactobacillus helveticus MIMLh22, and Lactobacillus rhamnosus GG adhered well to Caco-2 cells, resulting in adhesions of about 25, 11, and 9 bacterial cells per mm2, respectively (adhesion indexes of 2,327, 629, and 648, respectively). The remaining strains, Bifidobacterium adolescentis NAA38, Bifidobacterium longum NAL8, Bifidobacterium pseudocatenulatum NAP32, and Streptococcus thermophilus DSM20617T, adhered to Caco-2 cell surfaces with less than 2 cells per mm2 (Fig. 1b).
FIG. 1.
Adherence of bacterial strains to a Caco-2 cell monolayer. (a) Adhesion of Bifidobacterium bifidum MIMBb75 (a and c) and Bifidobacterium pseudocatenulatum NAP32 (b) to a Caco-2 cell monolayer as observed with Giemsa staining under a light microscope. MIMBb75 adhesion was specific to Caco-2 cells: no adhesion was detected on the cover glass underlying Caco-2 cells (c, left). Bars, 5 μm in a and b and 10 μm in c. (b) Quantification of adhesion ability. The numbers above each column refer to the adhesion index (bacterial cells adhered to 100 Caco-2 cells). The data represent the means of at least two independent experiments conducted in duplicate. The vertical bars indicate standard deviations. Bifidobacterium lactis stands for Bifidobacterium animalis subsp. lactis.
Proteins are involved in the adhesion of B. bifidum MIMBb75 to Caco-2 cells.
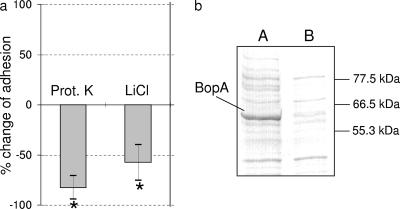
We tested adhesion after incubating B. bifidum MIMBb75 with proteinase K (0.5 mg ml−1) for 30 min at 37°C. The bacterial strain was now dramatically less adherent to Caco-2 cells (83% less adherent), suggesting that surface proteinaceous compounds may promote the strain's binding to epithelial cells (Fig. 2a).
FIG. 2.
Role of proteins in B. bifidum MIMBb75 adhesion to Caco-2 cells. (a) Percent changes in adhesion after incubation of MIMBb75 cells in a proteinase K (Prot. K) solution and after extraction of surface proteins with 5 M LiCl. *, statistically significant difference compared to untreated cells (P < 0.05). (b) SDS-PAGE analysis of MIMBb75 cell wall-associated proteins grown under standard conditions (lane A) and after treatment with 5 M LiCl (lane B). The molecular masses (in kDa) of the standard proteins are indicated at right.
The cell wall-associated proteins of B. bifidum MIMBb75 were extracted and separated through SDS-PAGE. Their electrophoretic profiles revealed the presence of a predominant protein with an apparent molecular mass of about 60 kDa (Fig. 2b, lane A). When the cell surface proteins were removed by extraction with 5 M LiCl, the band corresponding to the dominant protein appeared drastically reduced by SDS-PAGE (Fig. 2b, lane B), confirming the protein's outside location. At the same time, treatment with LiCl significantly reduced the adhesion of B. bifidum MIMBb75 (58% reduced adhesion) (Fig. 2a). According to data reported previously by Mattarelli et al. (36), we named the protein BopA (bifidobacterial outer protein).
BopA competes with MIMBb75 adhesion.
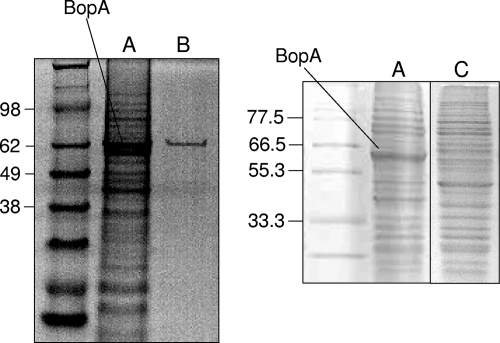
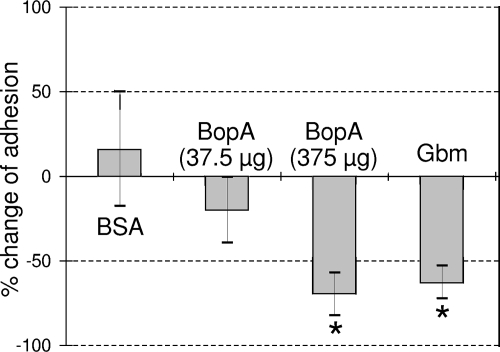
BopA was chromatographically purified from bacterial cell wall extracts to apparent homogeneity as observed by SDS-PAGE (Fig. 3). Purified BopA was then used in competition experiments through a Caco-2 adhesion assay. Caco-2 cells were incubated with 375 μg of purified BopA for 1 h at 37°C in anaerobic condition. After this treatment, B. bifidum MIMBb75 was markedly less adherent (70% less adherent) (Fig. 4), suggesting that BopA may have interfered with the strain's adhesion mechanism with regard to Caco-2 cells. On the other hands, its adhesion was not significantly affected by BSA or 37.5 μg of BopA (Fig. 4).
FIG. 3.
(A) SDS-PAGE analysis of B. bifidum MIMBb75 cell wall-associated proteins grown under standard conditions. (B and C) Purified BopA (B) and cell wall proteins after growth in 50 μg ml−1 globomycin (C) are also shown. The molecular masses (in kDa) of the standard proteins are indicated at left.
FIG. 4.
Percent changes in adhesion to Caco-2 cells of B. bifidum MIMBb75 cells in competition with BSA or BopA proteins and after growth of bacterial cells in 50 μg ml−1 globomycin (Gbm). The data represent the means of at least two independent experiments conducted in duplicate. The vertical bars indicate standard deviations. *, statistically significant difference compared to an adhesion assay carried out under standard conditions (P < 0.05).
Identification and characterization of the gene coding for BopA.
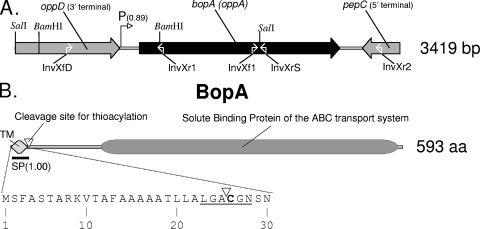
We determined the N-terminal amino acids of BopA (X-G-N-S-N-N-G-S-A-G-N [X refers to an undetermined amino acid]) and a trypsin-generated BopA peptide (N-S-D-G-Y-Y-E-K). Starting from these sequences, via PCR experiments, we determined a 3,419-bp genomic region (Fig. 5a). This nucleotidic sequence contains the gene of BopA and a putative ribosomal binding site in a purine-rich region (AGGAGAGGAA) upstream of the translational start codon ATG. The deduced polypeptide sequence of BopA starts with an N-terminal signal sequence of 25 amino acids, which meets all the requirements of a transmembrane helix, and contains the lipobox motif (L-X-Z-C-Y-Z). This motif functions as a recognition signal for lipid modification by thioacylation made on the conserved and essential cysteine residue. The lipid residue covalently linked to the cysteine moiety is thought to allow for the anchoring of proteins in a plasma membrane (49).
FIG. 5.
(A) Schematic representation of the genome region including bopA in Bifidobacterium bifidum MIMBb75. The number in parentheses after the putative promoter refers to the Neural Network Promoter Prediction probability. The white open arrows refer to the target of the primers indicated below. (B) Putative domain architecture of BopA from B. bifidum MIMBb75 shown with the first 30 amino acids of BopA and containing the signal peptide and the lipobox (underlined). The cleavage site for thioacylation, between Ala-25 and Cys-26, is indicated with a white triangle. The number in parentheses refers to the SignalP-HMM probability of the signal peptide. TM, transmembrane region. A BLAST search was used to assign putative biological roles to the genes or domains.
The mature BopA has a calculated molecular mass of 62 kDa and an isoelectric point of 5.03. The molecular mass correlates well with that estimated by SDS-PAGE.
A database search with the BopA protein sequence revealed significant homology with the solute-binding protein of the ABC transport system from high-GC gram-positive bacteria (Arthrobacter, Propionibacterium, Mycobacterium, Streptomyces, Nocardia, and Brevibacterium) (Fig. 5b). The closest similarity occurred with genes encoding the core domain of the ABC-type tripeptide/oligopeptide binding protein (CDD accession numbers COG0747 and COG4166) of Arthrobacter sp. strain FB24 (GenBank accession number ABK05389) (identities in 187/544 isolates [34%], with 283/544 [52%] positive results and 43/544 [7%] gaps) and Arthrobacter aurescens TC1 (accession number ABM09237) (I = 192/602 [31%]; P = 287/602 [47%]; G = 64/602 [10%]).
Computational analysis of the region encompassing bopA revealed, upstream of this gene, the 3′ terminus of a putative gene showing close similarity with the ATP-binding protein of the oligopeptide/dipeptide ABC transporter (CDD accession number cd03257). Moreover, downstream of bopA and on the opposite reading frame, we identified the 3′ terminus of a putative gene showing close similarity with bacterial aminopeptidase C (pepC) (CDD accession number cd00585) (Fig. 5a).
Experiments with globomycin.
To confirm that BopA is a lipoprotein, we grew B. bifidum MIMBb75 with globomycin, a potent and specific inhibitor of lipoprotein signal peptidases, resulting in an accumulation of prolipopetide (24, 49). When B. bifidum MIMBb75 was grown in cMRS containing 50 μg ml−1 of globomycin, its growth was only slightly inhibited, and its cell morphology changed, showing unusual bulges at the extremities of many cells (data not shown). The SDS-PAGE profile of cell wall proteins after growth in globomycin revealed a radical reduction of BopA (Fig. 3). Furthermore, the bacterial cells grown in globomycin were markedly less capable of adhering to Caco-2 cells (63% lower adherence) (Fig. 4).
Adhesion and presence of BopA in Bifidobacterium bifidum strains.
We ran adhesion assays on eight other strains ascribed to the species Bifidobacterium bifidum (Table 1). All the strains adhered to Caco-2 cells without a significant difference from B. bifidum MIMBb75 (data not shown). Moreover, a band corresponding to BopA appeared on the SDS-PAGE profile of all the B. bifidum strains (data not shown), a finding agreeing with our PCR experiments. Targeting bopA and the flanking genes, we used the following sets of primers: InvXf1-InvXrS, InvXf1-InvXr2, and InvXfD-InvXr1 (Fig. 5a). We found the presence of the bopA gene in all the strains (data not shown). Finally, no amplification bands were detected when the primers were used in PCR experiments with the other bifidobacterial strains not belonging to the B. bifidum species listed in Table 1.
B. bifidum MIMBb75 and BopA induce production of IL-8.
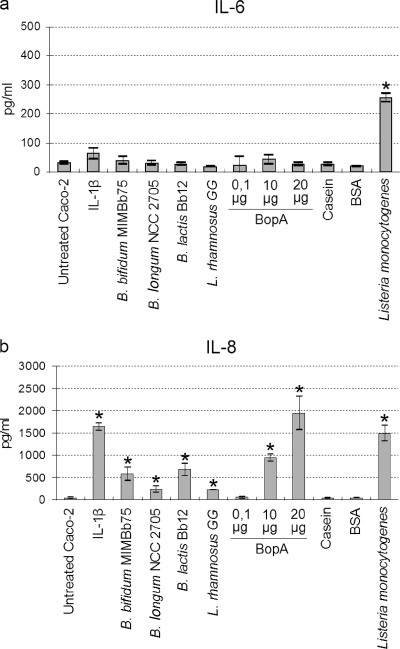
Overnight incubation of a Caco-2 monolayer with probiotic bacteria resulted in significantly increased levels of IL-8 production (P < 0.05) (Fig. 6). The bifidobacterial strains Bb12 and MIMBb75 especially enhanced the production of IL-8 from 30 pg ml−1 to 676 and 574 pg ml−1, respectively. Lactobacillus rhamnosus GG and Bifidobacterium longum NCC2705 produced 213 and 233 pg ml−1, respectively. When the purified protein BopA was used instead of whole bacterial probiotic cells, IL-8 production increased depending on the protein's concentration (Fig. 6b). In contrast, the IL-6 concentration showed no marked change. One-hour incubation of Caco-2 cells with Listeria monocytogenes was sufficient to obtain a marked increase in levels of IL-6 and IL-8 (Fig. 6). Casein and BSA, included in the experiment as controls, affected neither IL-6 nor IL-8 production.
FIG. 6.
Production of interleukins by Caco-2 cells induced by various treatments with probiotic bacteria (overnight treatment), proteins (overnight treatment), or Listeria monocytogenes (1-h treatment). (a) IL-6 production. (b) IL-8 production. Numerical results are given in the arithmetic means ± standard deviations. B. lactis stands for Bifidobacterium animalis subsp. lactis. *, statistically significant difference compared to untreated Caco-2 cells (P < 0.05).
DISCUSSION
The Caco-2 cell model showed the human intestinal isolate B. bifidum MIMBb75's ability to adhere. Its adhesion ability was better than that of the well-studied commercial probiotics Bifidobacterium animalis subsp. lactis Bb12 and Lactobacillus rhamnosus GG. The eight other B. bifidum strains included in the study adhered to Caco-2 cells very similarly to B. bifidum MIMBb75, confirming that the adhesive phenotype is frequently present in the members of this species (17, 39, 46, 50).
The nature of adhesive structures and mechanisms involved in adhesion have been studied with lactic acid bacteria mainly by using in vitro models. Proteinaceous factors, carbohydrates, and lipoteichoic acids on the bacterial cell wall have been shown to participate in the adhesion of lactic acid bacteria (19, 20, 22, 38, 58). On the other hand, the few reports available about the surface components of bifidobacteria involved in their adherence to intestinal cells suggest that lipoteichoic acids and proteinaceous molecules promote the adhesion of bifidobacteria to enterocytes (4, 42).
In our research, proteins were shown to be directly involved in the adhesion of MIMBb75 to Caco-2 cells. Particularly, our results suggest that a surface protein, BopA, very probably functions as an adhesion promoter. In silico analysis of its gene and globomycin experiments indicated that BopA is a cysteine-anchored lipoprotein, which is expressed as a precursor polypeptide. An N-terminal sequencing reaction designated the cysteine residue at position 26 as being the first amino acid of the mature BopA. This residue was not recognizable in the chromatogram, suggesting that a modification, likely a thioacylation, had occurred.
A database search assigned the putative biological role of oligopeptide/tripeptide-solute-binding protein of the ABC transport system to BopA, a role confirmed by the presence of a putative aminopeptidase C gene downstream and, especially, by an ATP-binding protein upstream of bopA.
Lipoproteins of the ABC transport system have frequently been associated with bacterial adhesion and coaggregation (2, 9, 31, 40, 41, 53). Particularly, proteins with the same conserved domain of BopA (COG4166 [OppA]) have been shown to be involved in different aspects of cell physiology including binding to host cells. For instance, three paralogous oligopeptide-binding lipoproteins (AmiA, AliA, and AliB) of Streptococcus pneumoniae were demonstrated to play a role in the bacterial recognition of glycoconjugate receptors present on resting lung cells and human vascular endothelial cells (28). Furthermore, it was shown that the cytoadherence-mediating lipoprotein P100 of Mycoplasma hominis contains the substrate-binding domain OppA of a peptide transport system (26). Moreover, again, Fenno and collaborators identified an OppA homologue in Treponema denticola that was demonstrated to bind, in its native form, proteins present in the subgingival environment such as soluble plasminogen and fibronectin (15). In light of these experimental data, it would certainly be interesting to investigate further BopA activity and its ability to bind host proteins in order to elucidate if BopA may be important both for the uptake of peptide nutrients and for B. bifidum-host interactions in the intestinal environment.
We found a protein corresponding to BopA in all the nine highly adherent B. bifidum strains that we studied. In addition, PCR experiments showed positive amplification for bopA and the upstream open reading frame for all B. bifidum strains but for none of the other bifidobacteria included in the study, suggesting that this gene and its product could serve for a quick identification of strains belonging to the species B. bifidum. Nevertheless, it remains to be established if a BopA-homologous lipoprotein is also present in poorly adherent B. bifidum strains, which we were not be able to include in this study.
Genes paralogous to bopA, containing the OppA conserved domain and organized into a putative ABC transport system operon, are present in all four complete Bifidobacterium genomes available to date (B. longum NCC2705, B. longum DJO10A, B. adolescentis ATCC 15703, and B. adolescentis L2-32). Nevertheless, none of their corresponding putative peptides shares significant homology with BopA.
The presence of paralogous OppA proteins in a single strain is not rare. Particularly, in the genomes of Arthrobacter sp. strain FB24, Arthrobacter aurescens TC1, and Streptomyces avermitilis MA-4680, we found the presence of an OppA-encoding gene homologous to BopA together with a gene homologous to the putative OppA-encoding open reading frames of the above-mentioned Bifidobacterium genomes (data not shown). Plausibly, different paralogous OppA proteins are encoded by a single genome because they are differently specialized for a particular activity. These considerations let us suppose that B. bifidum BopA can have a biological role that is substantially different from that of the OppA proteins found to be coded by B. longum and B. adolescentis genomes.
In view of the fact that B. bifidum MIMBb75 adheres efficiently to Caco-2 epithelial cells, we decided to examine if this bacterial strain and its surface lipoprotein BopA can stimulate the production of IL-6 and -8. Both IL-6 and IL-8 are secreted by intestinal epithelial cells and signal the start of a mucosal inflammatory response to an antigen (27). IL-6 is a multifunctional cytokine, which seems to function mostly as a mediator in several acute-phase inflammatory responses (18), whereas IL-8 is a chemokine with potent chemotactic activity for neutrophils (23) and capability for preventing neutrophil-mediated damage (18). Our results indicate that both bacterial strain MIMBb75 and the BopA lipoprotein can induce the production of IL-8 by Caco-2 cells. A similar result was obtained in our experiments with the probiotic strain Bifidobacterium animalis subsp. lactis Bb12 and, to a lesser extent, with L. rhamnosus GG and B. longum NCC2705. Similarly, 8 out of 19 bifidobacteria, including two B. bifidum strains, were demonstrated previously by Morita et al. (39) to stimulate the production of IL-8 but not that of IL-6.
Interestingly, IL-8 production by Caco-2 cells was also induced by B. animalis subsp. lactis Bb12, a strain that we found to adhere very poorly to the Caco-2 cell line. This result seems to confirm that adhesion ability and cytokine stimulation could not be directly correlated, as previously proposed by others (39).
In contrast to the fact that only IL-8 production was stimulated by the incubation of Caco-2 cells with probiotic bacteria and BopA overnight, we observed in our experiments that a 1-h incubation with the enteropathogen Listeria monocytogenes efficiently triggered IL-6 and IL-8. Recently, Stecher and collaborators observed a cause-and-effect relationship between the triggering of inflammation and enhanced colonization of mouse intestinal tract by Salmonella enterica serovar Typhimurium (56). They proposed that the ability to induce inflammation may represent a common virulence strategy of enteropathogenic bacteria (56). Moreover, Ruiz et al. previously found a transient induction of Toll-like receptor-mediated activation of the proinflammatory transcription factor system for the probiotic Bifidobacterium animalis subsp. lactis strain Bb12, in contrast to the persistent induction of nuclear factor κB signaling associated with enteropathogens (51). According to these data, we believe that further studies are needed to establish whether some commensal and/or probiotic bacteria could take ecological advantage by subclinical inflammatory conditions in the gut.
In conclusion, to our knowledge, ours is the first report to identify a proteinaceous factor directly involved in the mechanism of adhesion of bifidobacteria with Caco-2 cells. We believe that the BopA lipoprotein represents a good candidate molecule to take into consideration in order to elucidate the mechanisms of interactions between bifidobacteria and host intestinal cells.
Acknowledgments
We thank Elena Corengia, Mauro Scarpellini, and Fabio Sessa for their technical support. We give special thanks to Nestlé Research Center for providing strains B. longum NCC2705 and B. bifidum NCC390. Furthermore, we are grateful to Masatoshi Inukai for the globomycin.
Footnotes
Published ahead of print on 6 June 2008.
REFERENCES
- 1.Amaretti, A., T. Bernardi, E. Tamburini, S. Zanoni, M. Lomma, D. Matteuzzi, and M. Rossi. 2007. Kinetics and metabolism of Bifidobacterium adolescentis MB 239 growing on glucose, galactose, lactose, and galacto-oligosaccharides. Appl. Environ. Microbiol. 73:3637-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, R. N., N. Ganeshkumar, and P. E. Kolenbrander. 1993. Cloning of the Streptococcus gordonii PK488 gene, encoding an adhesin which mediates coaggregation with Actinomyces naeslundii PK606. Infect. Immun. 61:981-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asahara, T., K. Shimizu, K. Nomoto, M. Watanuki, and R. Tanaka. 2001. Antibacterial effect of fermented milk containing Bifidobacterium breve, Bifidobacterium bifidum, and Lactobacillus acidophilus against indigenous Escherichia coli infection in mice. Microb. Ecol. Health Dis. 13:16-24. [Google Scholar]
- 4.Bernet, M. F., D. Brassart, J. R. Neeser, and A. L. Servin. 1993. Adhesion of human bifidobacterial strains to cultured human intestinal epithelial cells and inhibition of enteropathogen-cell interactions. Appl. Environ. Microbiol. 59:4121-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi, M. A., D. Del Rio, N. Pellegrini, G. Sansebastiano, E. Neviani, and F. Brighenti. 2004. A fluorescence-based method for the detection of adhesive properties of lactic acid bacteria to Caco-2 cells. Lett. Appl. Microbiol. 39:301-305. [DOI] [PubMed] [Google Scholar]
- 6.Biavati, B., and P. Mattarelli. 2001. The family Bifidobacteriaceae, p. 1-70. In M. Dworkin, S. Falkow, E. Rosenberg, K. H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes. Springer, New York, NY.
- 7.Candela, M., G. Seibold, B. Vitali, S. Lachenmaier, B. J. Eikmanns, and P. Brigidi. 2005. Real-time PCR quantification of bacterial adhesion to Caco-2 cells: competition between bifidobacteria and enteropathogens. Res. Microbiol. 156:887-895. [DOI] [PubMed] [Google Scholar]
- 8.Canzi, E., S. Guglielmetti, D. Mora, I. Tamagnini, and C. Parini. 2005. Conditions affecting cell surface properties of human intestinal bifidobacteria. Antonie van Leeuwenhoek 88:207-219. [DOI] [PubMed] [Google Scholar]
- 9.Castaneda-Roldan, E. I., S. Ouahrani-Bettache, Z. Saldana, F. Avelino, M. A. Rendon, J. Dornand, and J. A. Giron. 2006. Characterization of SP41, a surface protein of Brucella associated with adherence and invasion of host epithelial cells. Cell. Microbiol. 8:1877-1887. [DOI] [PubMed] [Google Scholar]
- 10.Chow, W. L., and Y. K. Lee. 13 August 2007. Free fucose is a danger signal to human intestinal epithelial cells. Br. J. Nutr. 99:449-454. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 11.Chung, Y. J., S. K. Kim, and E. C. Choi. 1997. The effects of Bifidobacterium bifidum OFR9, a strain resistant to antituberculosis and antileprosy agents, on fecal flora in mice. J. Gen. Appl. Microbiol. 43:61-66. [DOI] [PubMed] [Google Scholar]
- 12.Del Re, B., B. Sgorbati, M. Miglioli, and D. Palenzona. 2000. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett. Appl. Microbiol. 31:438-442. [DOI] [PubMed] [Google Scholar]
- 13.Engle, M. J., G. S. Goetz, and D. H. Alpers. 1998. Caco-2 cells express a combination of colonocyte and enterocyte phenotypes. J. Cell. Physiol. 174:362-369. [DOI] [PubMed] [Google Scholar]
- 14.Estrada, A., D. C. Wilkins, and M. Drew. 2001. Administration of Bifidobacterium bifidum to chicken broilers reduces the number of carcass condemnations for cellulitis at the abattoir. J. Appl. Poult. Res. 10:329-334. [Google Scholar]
- 15.Fenno, J. C., M. Tamura, P. M. Hannam, G. W. Wong, R. A. Chan, and B. C. McBride. 2000. Identification of a Treponema denticola OppA homologue that binds host proteins present in the subgingival environment. Infect. Immun. 68:1884-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez, J., M. DeMott, D. Atherton, and S. M. Mische. 1992. Internal protein sequence analysis: enzymatic digestion of less than 10 pg of protein bound to polyvinylidene difluoride or nitrocellulose membranes. Anal. Biochem. 201:255-264. [DOI] [PubMed] [Google Scholar]
- 17.Gagnon, M., E. E. Kheadr, G. Le Blay, and I. Fliss. 2004. In vitro inhibition of Escherichia coli O157:H7 by bifidobacterial strains of human origin. Int. J. Food Microbiol. 92:69-78. [DOI] [PubMed] [Google Scholar]
- 18.Goldsby, R. A., T. J. Kindt, and B. A. Osborne (ed.). 2000. Immunology, 4th ed. Freeman and Company, New York, NY.
- 19.Granato, D., F. Perotti, I. Masserey, M. Rouvet, M. Golliard, A. Servin, and D. Brassart. 1999. Cell surface-associated lipoteichoic acid acts as an adhesion factor for attachment of Lactobacillus johnsonii La1 to human enterocyte-like Caco-2 cells. Appl. Environ. Microbiol. 65:1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene, J. D., and T. R. Klaenhammer. 1994. Factors involved in adherence of lactobacilli to human Caco-2 cells. Appl. Environ. Microbiol. 60:4487-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guglielmetti, S., M. Karp, D. Mora, I. Tamagnini, and C. Parini. 2007. Molecular characterization of Bifidobacterium longum biovar longum NAL8 plasmids and construction of a novel replicon screening system. Appl. Microbiol. Biotechnol. 74:1053-1061. [DOI] [PubMed] [Google Scholar]
- 22.Gusils, C., S. N. Cuozzo, F. Sesma, and S. Gonzalez. 2002. Examination of adhesive determinants in three species of Lactobacillus isolated from chicken. Can. J. Microbiol. 48:34-42. [DOI] [PubMed] [Google Scholar]
- 23.Harada, A., N. Sekido, T. Akahoshi, T. Wada, N. Mukaida, and K. Matsushima. 1994. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 56:559-564. [PubMed] [Google Scholar]
- 24.Harrington, D. J., J. S. Greated, N. Chanter, and I. C. Sutcliffe. 2000. Identification of lipoprotein homologues of pneumococcal PsaA in the equine pathogens Streptococcus equi and Streptococcus zooepidemicus. Infect. Immun. 68:6048-6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He, F., A. C. Ouwehand, E. Isolauri, H. Hashimoto, Y. Benno, and S. Salminen. 2001. Comparison of mucosal adhesion and species identification of bifidobacteria isolated from healthy and allergic infants. FEMS Immunol. Med. Microbiol. 30:43-47. [DOI] [PubMed] [Google Scholar]
- 26.Henrich, B., M. Hopfe, A. Kitzerow, and U. Hadding. 1999. The adherence-associated lipoprotein P100, encoded by an opp operon structure, functions as the oligopeptide-binding domain OppA of a putative oligopeptide transport system in Mycoplasma hominis. J. Bacteriol. 181:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isolauri, E. 1999. Probiotics and gut inflammation. Curr. Opin. Gastroenterol. 15:534-537. [DOI] [PubMed] [Google Scholar]
- 28.Kerr, A. R., P. V. Adrian, S. Estevao, R. de Groot, G. Alloing, J. P. Claverys, T. J. Mitchell, and P. W. Hermans. 2004. The Ami-AliA/AliB permease of Streptococcus pneumoniae is involved in nasopharyngeal colonization but not in invasive disease. Infect. Immun. 72:3902-3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko, E. J., J. S. Goh, B. J. Lee, S. H. Choi, and P. H. Kim. 1999. Bifidobacterium bifidum exhibits a lipopolysaccharide-like mitogenic activity for murine B lymphocytes. J. Dairy Sci. 82:1869-1876. [DOI] [PubMed] [Google Scholar]
- 30.Ko, J. S., H. R. Yang, J. Y. Chang, and J. K. Seo. 2007. Lactobacillus plantarum inhibits epithelial barrier dysfunction and interleukin-8 secretion induced by tumor necrosis factor-alpha. World J. Gastroenterol. 13:1962-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolenbrander, P. E., R. N. Andersen, and N. Ganeshkumar. 1994. Nucleotide sequence of the Streptococcus gordonii PK488 coaggregation adhesion gene, scaA, and ATP-binding cassette. Infect. Immun. 62:4469-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Most commonly used discontinuous buffer system for SDS electrophoresis. Nature (London) 227:680-685.5432063 [Google Scholar]
- 33.Le Blay G., I. Fliss, and C. Lacroix. 2004. Comparative detection of bacterial adhesion to Caco-2 cells with ELISA, radioactivity and plate count methods. J. Microbiol. Methods 59:211-221. [DOI] [PubMed] [Google Scholar]
- 34.Mack, D. R. 2005. Probiotics—mixed messages. Can. Fam. Physician 51:1455-1457. [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuki, T., K. Watanabe, and R. Tanaka. 2003. Genus- and species-specific PCR primers for the detection and identification of bifidobacteria. Curr. Issues Intest. Microbiol. 4:61-69. [PubMed] [Google Scholar]
- 36.Mattarelli, P., B. Biavati, F. Crociani, V. Scardovi, and G. Prati. 1993. Bifidobacterial cell wall proteins (BIFOP) in Bifidobacterium globosum. Res. Microbiol. 144:581-590. [DOI] [PubMed] [Google Scholar]
- 37.Mirza, O., L. K. Skov, D. Sprogoe, L. A. van den Broek, G. Beldman, J. S. Kastrup, and M. Gajhede. 2006. Structural rearrangements of sucrose phosphorylase from Bifidobacterium adolescentis during sucrose conversion. J. Biol. Chem. 281:35576-35584. [DOI] [PubMed] [Google Scholar]
- 38.Morata de Ambrosini, V. I., S. N. Gonzalez, and G. Oliver. 1999. Study of adhesion of Lactobacillus casei CRL 431 to ileal intestinal cells of mice. J. Food Prot. 62:1430-1434. [DOI] [PubMed] [Google Scholar]
- 39.Morita, H., F. He, T. Fuse, A. C. Ouwehand, H. Hashimoto, M. Hosoda, K. Mizumachi, and J. Kurisaki. 2002. Adhesion of lactic acid bacteria to Caco-2 cells and their effect on cytokine secretion. Microbiol. Immunol. 46:293-297. [DOI] [PubMed] [Google Scholar]
- 40.Nishi, J., J. Sheikh, K. Mizuguchi, B. Luisi, V. Burland, A. Boutin, D. J. Rose, F. R. Blattner, and J. P. Nataro. 2003. The export of coat protein from enteroaggregative Escherichia coli by a specific ATP-binding cassette transporter system. J. Biol. Chem. 278:45680-45689. [DOI] [PubMed] [Google Scholar]
- 41.Oligino, L., and P. Fives-Taylor. 1993. Overexpression and purification of a fimbria-associated adhesin of Streptococcus parasanguis. Infect. Immun. 61:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Op den Camp, H. J., A. Oosterhof, and J. H. Veerkamp. 1985. Interaction of bifidobacterial lipoteichoic acid with human intestinal epithelial cells. Infect. Immun. 47:332-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouwehand, A. C., E. Isolauri, P. V. Kirjavainen, S. Tolkko, and S. J. Salminen. 2000. The mucus binding of Bifidobacterium lactis Bb12 is enhanced in the presence of Lactobacillus GG and Lact. delbrueckii subsp. bulgaricus. Lett. Appl. Microbiol. 30:10-13. [DOI] [PubMed] [Google Scholar]
- 44.Park, J. H., J. I. Um, B. J. Lee, J. S. Goh, S. Y. Park, W. S. Kim, and P. H. Kim. 2002. Encapsulated Bifidobacterium bifidum potentiates intestinal IgA production. Cell. Immunol. 219:22-27. [DOI] [PubMed] [Google Scholar]
- 45.Parvez, S., K. A. Malik, S. A. Kang, and H.-Y. Kim. 2006. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 100:1171-1185. [DOI] [PubMed] [Google Scholar]
- 46.Perez, P. F., Y. Minnaard, E. A. Disalvo, and G. L. De Antoni. 1998. Surface properties of bifidobacterial strains of human origin. Appl. Environ. Microbiol. 64:21-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reilly, N., V. Poylin, M. Menconi, A. Onderdonk, S. Bengmark, and P. O. Hasselgren. 2007. Probiotics potentiate IL-6 production in IL-1β-treated Caco-2 cells through a heat shock-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293:R1169-R1179. [DOI] [PubMed] [Google Scholar]
- 48.Resta-Lenert, S., and K. E. Barrett. 2003. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52:988-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rezwan, M., T. Grau, A. Tschumi, and P. Sander. 2007. Lipoprotein synthesis in mycobacteria. Microbiology 153:652-658. [DOI] [PubMed] [Google Scholar]
- 50.Riedel, C. U., F. Foata, D. R. Goldstein, S. Blum, and B. J. Eikmanns. 2006. Interaction of bifidobacteria with Caco-2 cells—adhesion and impact on expression profiles. Int. J. Food Microbiol. 110:62-68. [DOI] [PubMed] [Google Scholar]
- 51.Ruiz, P. A., M. Hoffmann, S. Szcesny, M. Blaut, and D. Haller. 2005. Innate mechanisms for Bifidobacterium lactis to activate transient pro-inflammatory host responses in intestinal epithelial cells after the colonization of germ-free rats. Immunology 115:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabikhi, L., and B. N. Mathur. 2000. Studies on the antibacterial activity of Bifidobacterium bifidum against selected enteropathogens and spoilage organisms. Indian J. Dairy Sci. 53:227-230. [Google Scholar]
- 53.Sauer, K., and A. K. Camper. 2001. Characterization of phenotypic changes in Pseudomonas putida in response to surface-associated growth. J. Bacteriol. 183:6579-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M. C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:4422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiffrin, E. J., D. Brassart, A. L. Servin, F. Rochat, and A. Donnet-Hughes. 1997. Immune modulation of blood leukocytes in human by lactic acid bacteria: criteria for strain selection. Am. J. Clin. Nutr. 66:515S-529S. [DOI] [PubMed] [Google Scholar]
- 56.Stecher, B., R. Robbiani, A. W. Walker, A. M. Westendorf, M. Barthel, M. Kremer, S. Chaffron, A. J. Macpherson, J. Buer, J. Parkhill, G. Dougan, C. von Mering, and W. D. Hardt. 2007. Salmonella enterica serovar Typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 5:e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ventura, M., M. Elli, R. Reniero, and R. Zink. 2001. Molecular microbial analysis of Bifidobacterium isolates from different environments by the species-specific amplified ribosomal DNA restriction analysis (ARDRA). FEMS Microbiol. Ecol. 36:113-121. [DOI] [PubMed] [Google Scholar]
- 58.Ventura, M., I. Jankovic, D. C. Walker, R. D. Pridmore, and R. Zink. 2002. Identification and characterization of novel surface proteins in Lactobacillus johnsonii and Lactobacillus gasseri. Appl. Environ. Microbiol. 68:6172-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wijnands, L. M., J. B. Dufrenne, F. M. van Leusden, and T. Abee. 2007. Germination of Bacillus cereus spores is induced by germinants from differentiated Caco-2 cells, a human cell line mimicking the epithelial cells of the small intestine. Appl. Environ. Microbiol. 73:5052-5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yildirim, Z., D. K. Winters, and M. G. Johnson. 1999. Purification, amino acid sequence and mode of action of bifidocin B produced by Bifidobacterium bifidum NCFB 1454. J. Appl. Microbiol. 86:45-54. [DOI] [PubMed] [Google Scholar]