Abstract
Carnobacterium maltaromaticum UAL307, isolated from fresh pork, exhibits potent activity against a number of gram-positive organisms, including numerous Listeria species. Three bacteriocins were isolated from culture supernatant, and using matrix-assisted laser desorption ionization-time of flight mass spectrometry and Edman sequencing, two of these bacteriocins were identified as piscicolin 126 and carnobacteriocin BM1, both of which have previously been described. The remaining bacteriocin, with a molecular mass of 5,862 Da, could not be sequenced by traditional methods, suggesting that the peptide was either cyclic or N-terminally blocked. This bacteriocin showed remarkable stability over a wide temperature and pH range and was unaffected by a variety of proteases. After digestion with trypsin and α-chymotrypsin, the peptide was de novo sequenced by tandem mass spectrometry and a linear sequence deduced, consisting of 60 amino acids. Based on this sequence, the molecular mass was predicted to be 5,880 Da, 18 units higher than the observed molecular mass, which suggested that the peptide has a cyclic structure. Identification of the genetic sequence revealed that this peptide is circular, formed by a covalent linkage between the N and C termini following cleavage of a 4-residue peptide leader sequence. The results of structural studies suggest that the peptide is highly structured in aqueous conditions. This bacteriocin, named carnocyclin A, is the first reported example of a circular bacteriocin produced by Carnobacterium spp.
Bacteriocins are ribosomally synthesized antimicrobial peptides that are active against closely related bacteria. The producer organism is protected from its own bacteriocin by the production of a specific immunity protein (4, 9, 39, 48). In recent years, bacteriocins from gram-positive organisms, such as lactic acid bacteria (LAB), have attracted much attention. LAB produce a variety of bacteriocins, many of which show potent activity against various food spoilage and pathogenic bacteria, such as Bacillus, Clostridium, Staphylococcus, and Listeria (2, 9, 39). Since many strains of LAB and their bacteriocins are generally regarded as safe, there has been extensive research into the potential application of these bacteriocins for use in food preservation and as human therapeutics (2, 4, 7).
Various classification schemes have been devised to categorize bacteriocins from gram-positive organisms (4, 33, 36). In general, most can be grouped into two distinct families: the class I lantibiotics and the class II nonlantibiotics (7). The lantibiotics are small, heat-stable peptides that undergo extensive posttranslational modification and contain the unusual amino acid lanthionine or β-methyllanthionine (4, 7, 15, 39). The class II bacteriocins are also heat-stable peptides, but they do not undergo posttranslational modifications other than cleavage of a leader peptide. Within this grouping, the type IIa or pediocin-like bacteriocins are the most well studied. These heat-stable peptides are small (37 to 48 residues), cationic, and antilisterial. There is high sequence homology in the N-terminal domain of these peptides, characterized by the consensus sequence YGNGVXC and a conserved disulfide bridge (8-10, 48). In recent years, a new class of bacteriocins has been proposed, the circular bacteriocins, which are characterized by a covalent linkage between the N and C termini (26, 31, 36). To date, only seven circular bacteriocins from a diverse group of gram-positive organisms have been reported (36): AS-48 (37, 43), butyrivibriocin AR10 (23), circularin A (32), gassericin A, and reutericin 6 (which are diastereomers) (25, 27, 28) and subtilosin A (29, 30, 53) and uberolysin (50). Acidocin B, which shows 98% sequence homology to gassericin A, is considered a putative circular protein (34). The biochemical and genetic features of these bacteriocins have recently been reviewed (36). In contrast to the circular bacteriocins, more than 40 lantibiotics and 30 type IIa bacteriocins have been identified (39).
We have recently isolated Carnobacterium maltaromaticum UAL307, a heterofermentative LAB, from fresh pork. This strain displays remarkably high activity toward many Listeria species and thus prompted us to investigate the bacteriocins produced by this organism. In this study, we describe the isolation and characterization of three bacteriocins from C. maltaromaticum UAL307. Two of these peptides were identified as piscicolin 126 and carnobacteriocin BM1, which are both type IIa bacteriocins and have previously been reported (19, 21, 40). The remaining bacteriocin could only be sequenced after limited proteolysis, and the predicted molecular mass of the linear peptide was 18 units higher than the observed molecular mass, suggesting that the peptide was cyclic. Using reverse genetics, the structural gene for this bacteriocin, named carnocyclin A, was elucidated and its circular nature was confirmed. Although many bacteriocins from carnobacteria have been described, this is the first one shown to be circular.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
C. maltaromaticum UAL307 was grown at 25°C on all-purpose Tween (APT) agar or broth (Difco; Becton Dickinson Microbiology Systems, Sparks, MD). All indicator strains used in this study are listed in Table 1. Gram-positive indicator strains were grown in APT, except for Staphylococcus aureus, which required tryptic soy broth (Difco). Gram-positive strains were incubated at 25°C, except for C. maltaromaticum UAL26, which was grown at 16°C. All gram-negative indicator organisms were grown in Luria-Bertani (LB; Difco) media at 37°C. Solid media contained 1.5% (wt/vol) bacteriological agar. All strains were maintained as frozen stocks at −78°C in the presence of 20% glycerol.
TABLE 1.
Activity spectrum of carnocyclin A
| Indicator strain | Resulta | Source or referenceb |
|---|---|---|
| C. maltaromaticum UAL26 | + | 19 |
| C. maltaromaticum LV17A | + | 1 |
| C. divergens LV13 | + | 51 |
| Brochothrix campestris ATCC 43754 | + | ATCC |
| Brochothrix thermosphacta ATCC 11509 | + | ATCC |
| Enterococcus faecalis ATCC 7080 | + | ATCC |
| Enterococcus faecium ATCC 19434 | + | ATCC |
| E. faecium BFE900 | + | 13 |
| Lactococcus lactis subsp. lactis ATCC 11454 | + | ATCC |
| L. lactis subsp. cremoris ATCC 11602 | + | ATCC |
| L. lactis subsp. lactis DPC3147 | + | 41 |
| Lactobacillus sakei 706 | + | 46 |
| L. sakei UAL1218 | + | Laboratory collection |
| Leuconostoc mesenteroides Y105 | + | 20 |
| Pediococcus acidilactici PAC1.0 | + | 18 |
| Listeria monocytogenes ATCC 15313 | + | ATCC |
| L. monocytogenes H7762 | + | CDC |
| L. monocytogenes ATCC 43256 | + | ATCC |
| L. monocytogenes HPB 642 | + | Health Canada |
| L. monocytogenes UAFM 1 | + | Laboratory collection |
| L. monocytogenes UAFM 15 | + | Laboratory collection |
| Staphylococcus aureus ATCC 25923 | +f | ATCC |
| S. aureus ATCC 6538 | +f | ATCC |
| S. aureus ATCC 29213 | + | ATCC |
| Escherichia coli JM109 | − | 52 |
| E. coli BF2 | − | Laboratory collection |
| E. coli DH5α | − | Invitrogen |
| Pseudomonas aeruginosa ATCC 14207 | − | ATCC |
| P. aeruginosa ATCC 15442 | − | ATCC |
| Salmonella enterica serovar Typhimurium ATCC 23564 | − | ATCC |
| S. enterica serovar Typhimurium ATCC 13311 | − | ATCC |
Degree of inhibition of the indicator strain at 250 μM carnocyclin A in a spot-on-lawn assay: +, inhibition; +f, faint inhibition; −, no inhibition.
ATCC, American Type Culture Collection; CDC, Centers for Disease Control and Prevention.
Activity assays.
Spot-on-lawn assays were performed to monitor bacteriocin activity (12). Soft agar (0.75% agar) was inoculated with the indicator organism (1%) and overlaid on a bed of solid agar. Samples (10 μl) were spotted onto the agar and allowed to air dry. Plates were incubated overnight at the appropriate temperatures and examined for halos of growth inhibition. During the purification of bacteriocins from the supernatant of C. maltaromaticum UAL307 culture, all fractions were collected and tested for activity against Carnobacterium divergens LV13 or C. maltaromaticum UAL26. To determine the antibacterial spectrum of carnocyclin A, a selection of gram-positive and gram-negative strains were tested in a similar manner (Table 1).
Isolation and purification of bacteriocins.
C. maltaromaticum UAL307 was grown in 1 liter of APT (1% inoculum) at 25°C for 21 to 24 h. The culture was centrifuged (10,000 × g, 10 min, 4°C) to remove the cells. The supernatant was applied to a column (2.5 × 50 cm) containing 60 g of Amberlite XAD-16 resin (Sigma) at a flow rate of 15 ml/min at 4°C. The column was then washed with 750 ml of 30% ethanol, and the active peptides were eluted with 1 liter of 70% isopropyl alcohol (IPA), pH 2 (acidified with 1 M HCl). This fraction was concentrated (∼25 ml), loaded onto a Varian Bond-Elut C18 cartridge (10 g, 60 ml), and then washed at a flow rate of 5 ml/min with H2O (60 ml), 30% ethanol (60 ml), 20% IPA (60 ml), and 40% IPA (30 ml). The active peptides were eluted with 100 ml of 70% IPA, pH 2. This fraction was concentrated (∼2 ml) and loaded onto a Sephadex G25 Superfine column (3 × 45 cm) at 4°C and then eluted with H2O at a flow rate of 0.4 ml/min over 12 h. The active fractions (380 to 440 min) were combined and concentrated to 1.5 ml in preparation for high-pressure liquid chromatography (HPLC). Separation of the various peptides was achieved by reverse-phase HPLC (RP-HPLC) using a C8 column (5-μm particle size, 4.6- × 250-mm Vydac 208TP54) and a linear gradient of 20% to 50% aqueous IPA containing 0.1% trifluoroacetic acid (TFA) over 50 min at a flow rate of 1 ml/min and detection at 220 nm. Three active fractions were isolated, at a retention time (Rt) of 10.3 to 12.7 min for fraction A, an Rt of 12.7 to 16.2 min for fraction B, and an Rt of 45.2 to 47.9 min for fraction C. Fractions A and B were resubjected to this method until matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) showed the presence of only one compound. After fractions A and B were confirmed to contain the previously reported bacteriocins carnobacteriocin BM1 (40) and piscicolin 126 (19), the purification strategy was optimized for isolation of the bacteriocin present in fraction C, named carnocyclin A.
Isolation and purification of carnocyclin A.
The producer strain was cultured as described above and then centrifuged to remove the cells. The supernatant was applied to a column (2.5 × 50 cm) containing 100 g of Amberlite XAD-16 resin (prewashed with 100% IPA and rinsed with H2O) at a flow rate of 5 ml/min. The column was then washed consecutively with 500 ml of 30% ethanol, 40% IPA, 70% IPA, and 70% IPA, pH 2. The carnocyclin A-containing fraction (70% IPA, pH 2) was concentrated to ∼10 ml by rotary evaporation and then loaded onto a Varian Bond-Elut C18 cartridge which had been preconditioned with 50 ml of methanol and 50 ml of H2O. The column was washed consecutively with 50 ml of H2O, 30% ethanol, 30% acetonitrile, 40% IPA, and 70% IPA. Carnocyclin A was eluted with 100 ml of 70% IPA, pH 2. This fraction was concentrated, lyophilized, resuspended in ∼5 ml of H2O, and subjected to RP-HPLC using a C8 column (5-μm particle size, 10.0- × 250-mm Vydac 208TP510) with injections of 200 μl, a flow rate of 1 ml/min, and detection at 220 nm. After 8 min at 20% IPA, the gradient was increased to 86% IPA over 30 min. Carnocyclin A eluted as a sharp peak (Rt = 30.5 to 31.5 min). After purification of the whole sample, the carnocyclin A fractions were combined, concentrated, lyophilized, and stored at −20°C.
MS analysis.
Peptides were analyzed by MALDI-TOF MS. The two-layer method (6) with either 3,5-dimethoxy-4-hydroxycinnamic acid or α-cyano-4-hydroxycinnamic acid (HCCA) as the matrix was used for all samples. Samples were rinsed twice with 5 μl of 0.1% trifluoroacetic acid prior to data acquisition. Spectra were recorded in positive ion mode using a Perspective Biosystems Voyager Elite MALDI-TOF MS operating in reflectron mode with delayed extraction.
Edman sequencing.
Peptide samples were sequenced by automated Edman degradation with a gas-phase protein sequencer (Applied Biosystems) coupled to an on-line phenylthiohydantoin amino acid analyzer at the Alberta Peptide Institute (University of Alberta). Peptide fragments from carnocyclin A, purified by RP-HPLC at the Institute for Biomolecular Design (University of Alberta), were also Edman sequenced (Applied Biosystems Procise) at the Michael Smith Laboratory, Protein Sequencing and Peptide Mapping Unit (University of British Columbia).
Protein electrophoresis and activity testing.
Peptide samples were mixed with an equal volume of Tricine sample buffer and loaded onto a 16% Tris-Tricine gel (45). Polypeptide sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) standards (Bio-Rad) were also mixed with sample buffer and heated at 100°C for 5 min before being loaded onto the gel. After electrophoresis, half the gel was stained with Bio-Safe Coomassie stain (Bio-Rad) and then destained with water. The other half of the gel, to be used in activity testing, was soaked in water overnight, placed on a bed of agar, and then overlaid with soft agar containing the indicator strain C. divergens LV13 (1% inoculum).
Temperature, pH, and proteolytic stability of carnocyclin A.
Aliquots of the purified bacteriocin were incubated for 1 h at −80°C, −20°C, 0°C, 25°C, 50°C, 75°C, and 100°C and subjected to conditions frequently used for autoclaving (121°C, 15 min). Following incubation, samples were placed on ice and then tested for activity in a critical dilution assay (spot-on-lawn) (11) with C. divergens LV13 as the indicator organism. Tests were repeated twice. To determine the effect of pH on carnocyclin A, aliquots of the purified bacteriocin were adjusted to pH 2, 4, 5, 6, 7, 8, 10, and 12 in 10 mM Tris buffer, using HCl or NaOH. Samples were incubated at 4°C for 24 h and then neutralized. Bacteriocin activity was determined in a critical dilution assay (spot-on-lawn) with C. divergens LV13 as the indicator organism. Tests were repeated twice. The purified bacteriocin was also treated with the following proteases, pH conditions, and ratios of enzyme/bacteriocin (wt/wt): trypsin (pH 7.5, 1:200), papain (pH 6.2, 10:1), chymopapain (pH 6.2, 8:1), pepsin (pH 2, 8:1), protease type VIII (pH 7.5, 1:1), endoproteinase Glu-C (pH 8.0, 1:20), endoproteinase Asp-N (pH 8.0, 1:40), α-chymotrypsin (pH 7.8, 1:40), and thermolysin (pH 8.0, 1:5). All samples were incubated at 37°C for a minimum of 3 h, except for thermolysin, which was heated to 65°C in a water bath for 60 min. Following treatment, samples were either tested for activity with spot-on-lawn assays or examined by MALDI-TOF MS to detect fragmentation of the peptide. All enzymes were obtained from Sigma except for endoproteinase Asp-N (Boehringer Mannheim) and α-chymotrypsin (Roche).
CD analysis.
Circular dichroism (CD) measurements were made on an OLIS DSM 17CD spectrophotometer (Bogart, GA) in a thermally controlled quartz cell with a 0.02-cm path length. The instrument's calibration was checked against a 1 mg/ml solution of d-10-camphorsulfonic acid. The peptide concentration was 0.5 mg/ml. The CD spectrum of the peptide was recorded in 100% H2O or in 50% trifluoroethanol (TFE) and 50% H2O at 20°C. Data were collected at every 1 nm and are the averages of the results of five scans. The bandwidth was set at 2.0 nm. For both sets of experiments, baseline spectra of the appropriate solvent system were subtracted from the sample spectra prior to calculating molar ellipticities. Point-by-point integration was performed as a function of the high voltage readings on the photomultiplier detectors. The results were expressed in units of molar ellipticity (θ; degrees cm2 dmol−1) and plotted against the wavelength. The α-helical content of the peptide was calculated according to the molar ellipticity at 222 nm (θ222nm) by using the following equation (38): percentage α-helix = (−[θ222nm] + 3,000)/39,000 × 100%.
Amino acid sequencing of carnocyclin A.
Carnocyclin A was digested with trypsin (sequencing grade; Promega) or α-chymotrypsin (Roche) for 2 to 3 h or with a 15-min tryptic digest followed by a 3-h chymotryptic digest. All digestion reactions were done in 50 mM NH4HCO3 (pH 8.5) at room temperature and with an enzyme-to-peptide ratio of 1:40 (wt/wt). The resulting fragments were analyzed via LC-tandem MS (MS-MS) on a nanoAcquity UPLC (Waters, MA) coupled with a Q-TOF-Premier MS (Micromass, United Kingdom). The fragments were separated by using a linear water-acetonitrile gradient (0.1% formic acid) on a nanoAcquity column (100-Å pore size, 75-μm-inner-diameter × 15-cm, 3-μm Atlantis dC18; Waters, MA) at a flow rate of 350 nl/min, with an in-line Symmetry column (180-μm-inner-diameter × 20-mm, 5-μm C18; Waters, MA) as a loading/desalting column. Alternatively, digest fragments were purified by RP-HPLC and infused to the Q-TOF by using nanoelectrospray and the collision energy was varied to produce the MS-MS spectra. The Q-TOF was calibrated with the MS-MS spectrum of Glu-fibrinopeptide. The mass spectra were manually de novo sequenced to generate a proposed linear peptide sequence and confirmed by using PEAKS software (Bioinformatics Solutions, Waterloo, ON) (35). Exact mass measurements of the intact peptide and peptide fragments were obtained with a Bruker Apex Qe 9.4T Fourier transform ion cyclotron resonance MS equipped with an Apollo II dual MALDI-electrospray source. The [M + 5H]5+ peak of the intact peptide was analyzed by electrospray ionization and calibrated internally with bovine insulin. The peptide fragments were analyzed by MALDI, using HCCA as the matrix, and calibrated with sodium-cationized polyethylene glycol with trans-2-[3-(4-tert-butylphenyl)-2-methyl-2-propenylidene]malononitrile (Fluka) as matrix.
DNA manipulations.
Standard DNA manipulations were performed as described by Sambrook and Russell (42). PfuUltra II fusion HS DNA polymerase was obtained from Stratagene, and restriction endonucleases and T4 DNA ligase were obtained from Invitrogen and used as specified by the manufacturers. To amplify the internal part of the structural gene for carnocyclin A, cclA, forward primer MVB85 (5′-CAAGGDACWGCWGAAAAAGTWGT-3′) and reverse primer MVB86 (5′-ACWACTTTTTCWGCWGTHCCTTG-3′) were designed based on the sequence QGTAEKVV, whereas forward primer MVB87 (5′-GGDGTWTTTACWGCWGTWAAAGCWGC-3′) and reverse primer MVB88 (5′-GCWGCTTTWACWGCWGTAAAWACHCC-3′) were designed based on the sequence GVFTAVKAA. Total genomic DNA from C. maltaromaticum UAL307 was isolated with a DNeasy kit from Qiagen and used as a template to amplify the internal part of the cclA gene. DNA was amplified with 30 cycles of 94°C for 1 min, annealing at 45°C for 30 s, and elongation at 72°C for 15 s and purified by using a QIAquick PCR purification kit from Qiagen. PCR products were directly sequenced by using an ABI BigDye version 3.1 Terminator sequencing kit (Applied Biosystems) and run on an ABI 3730 DNA analyzer (Applied Biosystems).
Nucleotide sequence accession number.
The GenBank accession number provided for the nucleotide sequence reported in this study is EU624394.
RESULTS AND DISCUSSION
C. maltaromaticum UAL307 produces three bacteriocins: piscicolin 126, carnobacteriocin BM1, and a novel bacteriocin, carnocyclin A.
C. maltaromaticum UAL307, isolated from fresh pork, was found to exhibit potent activity against numerous gram-positive organisms (data not shown). In addition, the supernatant from the culture of this strain was able to inhibit C. maltaromaticum UAL26, which produces piscicolin 126 (PisA) and carnobacteriocin BM1 (CbnBM1) (19). Interestingly, C. maltaromaticum UAL307 was immune to the supernatant of C. maltaromaticum UAL26 culture (data not shown). This led us to believe that C. maltaromaticum UAL307 produces multiple bacteriocins, including PisA and CbnBM1. In an effort to identify these antimicrobial peptides, the active compounds in C. maltaromaticum UAL307 culture supernatant were purified and analyzed.
Using an activity-guided purification, three bacteriocins were isolated from C. maltaromaticum UAL307 culture supernatant. The purified peptides were analyzed by MALDI-TOF MS and were found to have molecular masses of 4,414.7, 4,523.8, 4,540.6, and 5,863.5 Da. The first three masses, respectively, correspond to the reported masses of PisA (19, 21); CbnBM1; and carnobacteriocin B1 (CbnB1), a variant of CbnBM1 in which the sulfur of methionine-41 has been oxidized (40). To confirm the identities of PisA, CbnBM1, and CbnB1, Edman sequencing was performed. The first 25 residues of PisA (excluding the cysteines) were confirmed. Likewise, the first 33 residues of CbnB1 (excluding the cysteines) were verified. Although CbnBM1 was not successfully sequenced, the positive identification of CbnB1 (the oxidation product of CbnBM1) in conjunction with the MS results verified the presence of CbnBM1. All attempts to sequence the remaining bacteriocin by Edman techniques failed, suggesting that this peptide was blocked at the N terminus.
Purification of carnocyclin A.
After the identification of PisA, CbnBM1, and CbnB1, efforts focused on identifying and characterizing the remaining bacteriocin, named carnocyclin A. During the purification scheme, it was found that PisA, CbnBM1, and CbnB1 could be eluted from Amberlite XAD-16 with a 40% IPA wash, whereas carnocyclin A eluted only with 70% IPA, pH 2. The addition of a 30% acetonitrile wash during the Mega-bond purification was found to significantly improve the results of the subsequent HPLC purification. By including these two washes, the total purification strategy for carnocyclin A was shortened and optimized. From 1 liter of cell culture, an average yield of ∼2 mg of carnocyclin A was obtained.
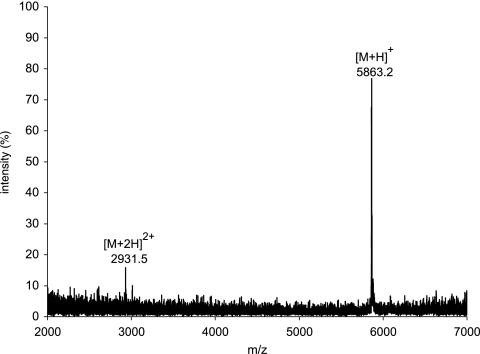
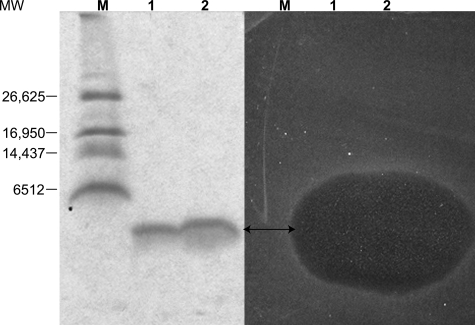
The purity of carnocyclin A was assessed by MS and gel electrophoresis. By MALDI, only the [M + H]+ and [M + 2H]2+ peaks of carnocyclin A were present, and they suggested that the molecular mass of this peptide is 5,862 Da (Fig. 1). The results of SDS-PAGE showed that the bacteriocin was pure; however, it migrated with an apparent molecular mass of ∼3,900 Da (Fig. 2), suggesting that the peptide had a compact, globular structure under the conditions used.
FIG. 1.
Positive-ion-mode MALDI-TOF spectrum of purified carnocyclin A, showing the singly and doubly charged species. An average molecular mass of 5,862 Da is observed for carnocyclin A.
FIG. 2.
Results of 16% Tris-Tricine SDS-PAGE gel and activity testing of carnocyclin A. Lanes 1 and 2 contain ∼7 μg and ∼14 μg of purified carnocyclin A, respectively, and lane M contains molecular markers. The gel on the left was stained with Bio-Safe Coomassie stain, and the gel on the right was soaked in water and then overlaid with soft agar containing 1% inoculum of C. divergens LV13. The double-headed arrow indicates the position of carnocyclin A. MW, molecular weight.
Gel electrophoresis followed by in situ activity testing showed that the purified peptide had antimicrobial activity (Fig. 2). Exposure of the peptide to SDS in the sample buffer did not destroy activity, indicating that the denaturing effect of SDS had no adverse effect on the peptide. However, when the peptide sample was boiled (for 5 min) in sample buffer prior to electrophoresis, no activity was observed in the subsequent overlay assay. Since heat treatments for short periods were insufficient for destroying activity (see below), it is likely that the combined effects of the detergent and heat were responsible for the loss in activity.
Spectrum of activity and characterization of carnocyclin A.
Using spot-on-lawn assays, purified carnocyclin A was tested against a variety of gram-positive and gram-negative organisms (Table 1). The bacteriocin exhibited a broad spectrum of activity and was active against all of the gram-positive strains tested, including pathogens such as Listeria and S. aureus. No activity was observed against the gram-negative strains that were tested.
When exposed to temperatures between −80°C and 75°C for 1 h, carnocyclin A retained its activity. In addition, autoclaving the peptide (121°C for 15 min) had no effect on activity, but when it was treated at 100°C for 1 h, a 32-fold reduction of activity was observed. Thus, it appears as though the peptide is able to withstand heat treatment only for a limited period of time, after which it begins to lose activity. No loss in activity was detected when the bacteriocin was treated for 24 h at pH values ranging from 2 to 12 at 4°C.
When the bacteriocin was treated with the proteases trypsin, pepsin, and protease VIII, a complete loss of activity was observed. The results of MALDI-TOF MS of the reaction mixtures revealed that the peptide had been digested into several smaller fragments (data not shown), as was also observed when carnocyclin A was incubated with the proteases α-chymotrypsin and thermolysin. Other than trypsin and pepsin, most of the aforementioned proteases have a wide range of specificity, increasing the likelihood of digestion. However, carnocyclin A was unaffected by a number of other proteases, such as chymopapain, endoproteinase Glu-C, and endoproteinase Asp-N, as well as papain, which has a broad specificity.
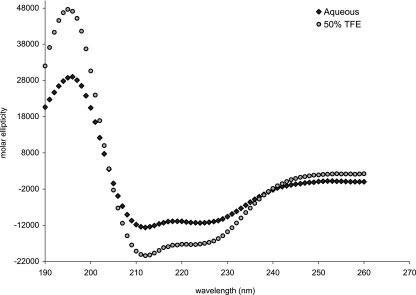
To explore the structural characteristics of carnocyclin A, CD spectroscopy was performed. Under aqueous conditions, the results of CD indicated that carnocyclin A has a defined structure and is largely α-helical (approximately 36%) (Fig. 3). Upon the addition of TFE, a membrane-mimicking solvent (24), this helicity was enhanced (up to 52%). In contrast, the type IIa bacteriocins are almost entirely random coil in aqueous conditions and TFE must be added to induce helicity (14, 24, 47, 49). Thus, carnocyclin A has a defined structure, most likely composed of several helices that pack together, forming a globular structure. This helps to explain the remarkable stability of carnocyclin A and its resistance to several proteases.
FIG. 3.
CD spectra of carnocyclin A in aqueous and membrane-mimicking conditions. In aqueous conditions, the peptide displays helical content, and this is further enhanced in the presence of 50% TFE.
Amino acid sequence of carnocyclin A.
As mentioned previously, carnocyclin A eluded sequencing by Edman degradation, and when it was subjected to MS-MS, no fragmentation was observed. This led us to speculate that carnocyclin A had a circular backbone, formed by a covalent linkage between its N and C termini. Since carnocyclin A was susceptible to proteolysis by trypsin and α-chymotrypsin, it was first digested with these enzymes. The resulting fragments were then analyzed by Edman degradation and extensive de novo MS-MS sequencing. A complete list of all the observed fragments and their corresponding sequences can be found in Table S1 in the supplemental material. Several key linear sequences were elucidated, and overlapping of these segments provided a putative amino acid sequence comprised of 60 residues (Fig. 4A). Edman degradation unambiguously assigned the leucine and isoleucine residues in fragment 1 (Fig. 4A). In the remaining fragments, attained by de novo MS-MS sequencing, definitive identification of leucine versus isoleucine residues was not possible. However, α-chymotrypsin cleaves at the C terminus of leucine, but not that of isoleucine. Therefore, in fragments arising from the chymotryptic digest, if the mass difference at the C terminus was indicative of either leucine or isoleucine and an intense peak was observed, the residue was presumed to be leucine (Fig. 4A). In order to differentiate between lysine and glutamine residues, exact mass measurements were obtained with Fourier transform ion cyclotron resonance for fragments 2, 3, 8, and 9 (Fig. 4A). This allowed for the unambiguous assignment of internal lysine and glutamine residues that could not be deduced from cleavage patterns. From the 60-amino-acid sequence, the molecular mass of carnocyclin A was calculated to be 5,880 Da, 18 units higher than the observed molecular mass. This difference can be explained by the loss of a water molecule which arises from an N-to-C cyclization and the formation of a new peptide bond. Thus, the sequence data, combined with the failed attempts to sequence the intact peptide, strongly suggested that carnocyclin A is a circular peptide. No homology with other bacteriocins was found in the databank.
FIG. 4.
Peptide fragment sequences from carnocyclin A and the nucleotide sequence of cclA. (A) The amino acid sequences of various peptide fragments derived from carnocyclin A and the deduced combined sequence of carnocyclin A are shown. Leucine or isoleucine residues that were not definitive are shaded. Leucines that are bolded were observed as the C-terminal residue following digestion with α-chymotrypsin; if the mass difference at the C terminus was indicative of either leucine or isoleucine and an intense peak was observed, the residue was presumed to be leucine. The two leucine residues that form a head-to-tail linkage of the peptide are underlined. Peptide fragment 1, trypsin digest followed by Edman sequencing; peptide fragments 2, 6, and 7, trypsin digest followed by de novo MS-MS sequencing; peptide fragments 3, 4, 5, 8, and 9, chymotrypsin digest followed by de novo MS-MS sequencing. (B) Nucleotide sequences of degenerate primers used to amplify the internal part of the structural gene of carnocyclin A. (C) Nucleotide sequence of and predicted amino acid sequence encoded by cclA. A possible ribosomal binding site is indicated. Amino acid sequences used to design degenerate primers MVB85, MVB86, MVB87, and MVB88, respectively, are underlined. The predicted cleavage site of the leader peptide is indicated by a vertical arrow. The amino acid discrepancy (isoleucine instead of leucine) between the deduced amino acid sequence based on the cclA gene and that of the peptide fragment analyses is shown in parentheses. RBS, ribosome binding site.
Identification of the structural gene for carnocyclin A.
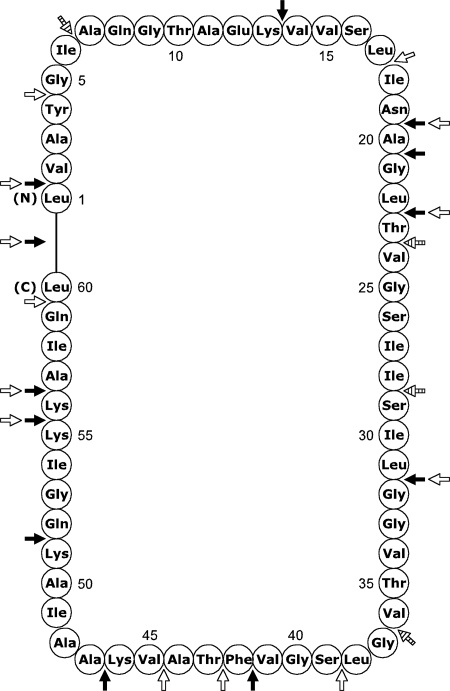
Degenerate primers based on two regions in the amino acid sequence of carnocyclin A, QGTAEKVV and GVFTAVKAA, were designed to enable the isolation of the structural gene (cclA) for carnocyclin A (Fig. 4B). Assuming that carnocyclin A is a ribosomally produced cyclic peptide, the position of the sequence QGTAEKVV with respect to the sequence GVFTAVKAA on the precursor peptide had yet to be determined. Therefore, two different primer pairs were used to amplify the internal part of cclA: MVB85/MVB88 and MVB86/MVB87 (Fig. 4B). Using total genomic DNA from C. maltaromaticum UAL307 as template, only primer pair MVB85/MVB88 gave a PCR product of around 120 bp (data not shown). Nucleotide sequence analysis of the PCR product revealed the presence of the internal part of the gene for carnocyclin A. Based on the nucleotide sequence obtained, inverse PCR was used to obtain the whole sequence of cclA (Fig. 4C). Nucleotide sequence analysis showed that cclA could code for a 64-amino-acid peptide and that the structural gene is preceded by a putative ribosomal binding site (AGGAGG) (Fig. 4C). By comparing the amino acid sequences obtained from the various fragments of carnocyclin A with the deduced amino acid sequence based on the nucleotide sequence of cclA, it is shown that carnocyclin A is indeed a cyclic peptide of 60 amino acids with a head-to-tail ligation of Leu1-Leu60 (Fig. 5). In addition, it is shown that carnocyclin A is produced as a precursor with a 4-amino-acid leader peptide (MLYE). Such short leader peptides have also been observed for the cyclic bacteriocins circularin A (32), uberolysin (50), and subtilosin A (53) that contain leader peptides of 3, 6, and 8 amino acids, respectively. In contrast, the cyclic antibacterial peptides butyrivibriocin AR10 (23), gassericin A (28), and AS-48 (37) contain longer leader peptides of 22, 33, and 35 amino acids, respectively.
FIG. 5.
The circular structure of carnocyclin A. The N and C termini are labeled. Major cleavage sites that were observed from the trypsin and α-chymotrypsin digests are indicated with black and white arrows, respectively. Cleavage sites from the combined digest that were not previously observed with either the trypsin or α-chymotrypsin digests are indicated with hatched arrows. For a comprehensive list of all the observed fragments and cleavage patterns, see Table S1 in the supplemental material.
Concluding remarks.
Many bacteria produce bacteriocins, and in several cases, a single organism can produce multiple bacteriocins (2, 22). The production of multiple bacteriocins is thought to be advantageous as it increases the fitness level and survival of a bacterium in a competitive environment (7, 22, 39). We suspected that C. maltaromaticum UAL307 produces several bacteriocins, and upon purification of the culture supernatant, identified PisA, CbnBM1, and carnocyclin A, a novel bacteriocin. Based on the amino acid sequence of this peptide, obtained by de novo MS-MS sequencing of various fragments, and reverse genetics, we deduced the circular nature of carnocyclin A and identified the amino acids involved in the head-to-tail ligation (Fig. 5).
Carnocyclin A shares several similarities with the other circular bacteriocins. For example, the anomalous behavior of carnocyclin A on SDS-PAGE is common among the circular bacteriocins (26, 32, 50). As observed with several of the circular bacteriocins, carnocyclin A is also cationic, has a high isoelectric point (predicted pI of 10) (16) and has a high content of hydrophobic residues. The potent bioactivity and stability to temperature and pH variation is also a shared feature of the circular bacteriocins, as is the limited proteolysis (26, 36). As with AS-48 (3, 17, 44), gassericin A, and reutericin 6 (25), the results of CD of carnocyclin A indicate that it is largely α-helical in aqueous conditions. Cyclization of the peptide backbone helps to account for such characteristics in these bacteriocins. Upon ring formation, the internal mobility and entropy of the peptide are reduced, thereby providing thermodynamic stabilization and assisting the attainment of a defined (as opposed to random coil) bioactive conformation. In addition, proteolysis is more difficult if specific cleavage sites are buried within a rigid conformation (5, 36).
Since CD indicates that carnocyclin A is largely helical, it may be that carnocyclin A shares a similar structure with AS-48. This bacteriocin is composed of five helices, where the N to C linkage resides in the center of one of the helices (17, 44). Structural studies to determine the three-dimensional structure of carnocyclin A are currently under way.
The operons for several of the circular bacteriocins have been discovered, enabling the identification of putative immunity proteins and accessory proteins involved in the production and export of these peptides (23, 28, 31, 37, 50, 53). However, the function of the leader peptide of the precursor and the details of how cyclization occurs are still not known. Elucidation of the enzyme(s) involved and the mechanism of cyclization will explain how these bonds are constructed. This may allow for the engineering of new circular bacteriocins. The ability to cyclize linear bacteriocins may provide an opportunity to create new, highly stable antimicrobial agents for use in food preservation and as human therapeutics.
Supplementary Material
Acknowledgments
This work was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC), Advanced Foods and Materials Network (AFMNet), and Alberta Heritage Foundation for Medical Research (AHFMR).
We are grateful to Kamaljit Kaur, Lucas Gursky, and Paul Semchuk for technical assistance and helpful discussions. We thank Wayne Moffat for CD analysis.
Footnotes
Published ahead of print on 13 June 2008.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Ahn, C., and M. E. Stiles. 1990. Plasmid-associated bacteriocin production by a strain of Carnobacterium piscicola from meat. Appl. Environ. Microbiol. 56:2503-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, H., and D. G. Hoover. 2003. Bacteriocins and their food applications. Compr. Rev. Food Sci. Food Saf. 2:82-100. [DOI] [PubMed] [Google Scholar]
- 3.Cobos, E. S., V. V. Filimonov, A. Gálvez, N. Maqueda, E. Valdivia, J. C. Martínez, and P. L. Mateo. 2001. AS-48: a circular protein with an extremely stable globular structure. FEBS Lett. 505:379-382. [DOI] [PubMed] [Google Scholar]
- 4.Cotter, P. D., C. Hill, and R. P. Ross. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777-788. [DOI] [PubMed] [Google Scholar]
- 5.Craik, D. J., N. L. Daly, I. Saska, M. Trabi, and K. J. Rosengren. 2003. Structures of naturally occurring circular proteins from bacteria. J. Bacteriol. 185:4011-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai, Y. Q., R. M. Whittal, and L. Li. 1999. Two-layer sample preparation: a method for MALDI-MS analysis of complex peptide and protein mixtures. Anal. Chem. 71:1087-1091. [DOI] [PubMed] [Google Scholar]
- 7.Deegan, L. H., P. D. Cotter, C. Hill, and P. Ross. 2006. Bacteriocins: biological tools for bio-preservation and shelf-life extension. Int. Dairy J. 16:1058-1071. [Google Scholar]
- 8.Derksen, D. J., J. L. Stymiest, and J. C. Vederas. 2006. Antimicrobial leucocin analogues with a disulfide bridge replaced by a carbocycle or by noncovalent interactions of allyl glycine residues. J. Am. Chem. Soc. 128:14252-14253. [DOI] [PubMed] [Google Scholar]
- 9.Drider, D., G. Fimland, Y. Héchard, L. M. McMullen, and H. Prevost. 2006. The continuing story of class IIa bacteriocins. Microbiol. Mol. Biol. Rev. 70:564-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eijsink, V. G. H., L. Axelsson, D. B. Diep, L. S. Håvarstein, H. Holo, and I. F. Nes. 2002. Production of class II bacteriocins by lactic acid bacteria; an example of biological warfare and communication. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 81:639-654. [DOI] [PubMed] [Google Scholar]
- 11.Franz, C. M. A. P., U. Schillinger, and W. H. Holzapfel. 1996. Production and characterization of enterocin 900, a bacteriocin produced by Enterococcus faecium BFE 900 from black olives. Int. J. Food Microbiol. 29:255-270. [DOI] [PubMed] [Google Scholar]
- 12.Franz, C. M. A. P., M. J. van Belkum, R. W. Worobo, J. C. Vederas, and M. E. Stiles. 2000. Characterization of the genetic locus responsible for production and immunity of carnobacteriocin A: the immunity gene confers cross-protection to enterocin B. Microbiology 146:621-631. [DOI] [PubMed] [Google Scholar]
- 13.Franz, C. M. A. P., R. W. Worobo, L. E. N. Quadri, U. Schillinger, W. H. Holzapfel, J. C. Vederas, and M. E. Stiles. 1999. Atypical genetic locus associated with constitutive production of enterocin B by Enterococcus faecium BFE 900. Appl. Environ. Microbiol. 65:2170-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher, N. L. F., M. Sailer, W. P. Niemczura, T. T. Nakashima, M. E. Stiles, and J. C. Vederas. 1997. Three-dimensional structure of leucocin A in trifluoroethanol and dodecylphosphocholine micelles: spatial location of residues critical for biological activity in type IIa bacteriocins from lactic acid bacteria. Biochemistry 36:15062-15072. [DOI] [PubMed] [Google Scholar]
- 15.Garneau, S., N. I. Martin, and J. C. Vederas. 2002. Two-peptide bacteriocins produced by lactic acid bacteria. Biochimie 84:577-592. [DOI] [PubMed] [Google Scholar]
- 16.Gasteiger, E., C. Hoogland, A. Gattiker, S. Duvaud, M. R. Wilkins, R. D. Appel, and A. Bairoch. 2005. Protein identification and analysis tools on the ExPASy server, p. 571-607. In J. M. Walker (ed.), The proteomics protocols handbook. Humana Press, Totowa, NJ.
- 17.González, C., G. M. Langdon, M. Bruix, A. Gálvez, E. Valdivia, M. Maqueda, and M. Rico. 2000. Bacteriocin AS-48, a microbial cyclic polypeptide structurally and functionally related to mammalian NK-lysin. Proc. Natl. Acad. Sci. USA 97:11221-11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González, C. F., and B. S. Kunka. 1987. Plasmid-associated bacteriocin production and sucrose fermentation in Pediococcus acidilactici. Appl. Environ. Microbiol. 53:2534-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gursky, L. J., N. I. Martin, D. J. Derksen, M. J. van Belkum, K. Kaur, J. C. Vederas, M. E. Stiles, and L. M. McMullen. 2006. Production of piscicolin 126 by Carnobacterium maltaromaticum UAL26 is controlled by temperature and induction peptide concentration. Arch. Microbiol. 186:317-325. [DOI] [PubMed] [Google Scholar]
- 20.Héchard, Y., B. Dérijard, F. Letellier, and Y. Cenatiempo. 1992. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J. Gen. Microbiol. 138:2725-2731. [DOI] [PubMed] [Google Scholar]
- 21.Jack, R. W., J. Wan, J. Gordon, K. Harmark, B. E. Davidson, A. J. Hillier, R. E. H. Wettenhall, M. W. Hickey, and M. J. Coventry. 1996. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl. Environ. Microbiol. 62:2897-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeziorowski, A., and D. M. Gordon. 2007. Evolution of microcin V and colicin Ia plasmids in Escherichia coli. J. Bacteriol. 189:7045-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalmokoff, M. L., T. D. Cyr, M. A. Hefford, M. F. Whitford, and R. M. Teather. 2003. Butyrivibriocin AR10, a new cyclic bacteriocin produced by the ruminal anaerobe Butyrivibrio fibrisolvens AR10: characterization of the gene and peptide. Can. J. Microbiol. 49:763-773. [DOI] [PubMed] [Google Scholar]
- 24.Kaur, K., L. C. Andrew, D. S. Wishart, and J. C. Vederas. 2004. Dynamic relationships among type IIa bacteriocins: temperature effects on antimicrobial activity and on structure of the C-terminal amphipathic alpha helix as a receptor-binding region. Biochemistry 43:9009-9020. [DOI] [PubMed] [Google Scholar]
- 25.Kawai, Y., Y. Ishii, K. Arakawa, K. Uemura, B. Saitoh, J. Nishimura, H. Kitazawa, Y. Yamazaki, Y. Tateno, T. Itoh, and T. Saitoh. 2004. Structural and functional differences in two cyclic bacteriocins with the same sequences produced by lactobacilli. Appl. Environ. Microbiol. 70:2906-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawai, Y., R. Kemperman, J. Kok, and T. Saito. 2004. The circular bacteriocins gassericin A and circularin A. Curr. Protein Pept. Sci. 5:393-398. [DOI] [PubMed] [Google Scholar]
- 27.Kawai, Y., T. Saito, H. Kitazawa, and T. Itoh. 1998. Gassericin A; an uncommon cyclic bacteriocin produced by Lactobacillus gasseri LA39 linked at N- and C-terminal ends. Biosci. Biotechnol. Biochem. 62:2438-2440. [DOI] [PubMed] [Google Scholar]
- 28.Kawai, Y., T. Saito, M. Suzuki, and T. Itoh. 1998. Sequence analysis by cloning of the structural gene of gassericin A, a hydrophobic bacteriocin produced by Lactobacillus gasseri LA39. Biosci. Biotechnol. Biochem. 62:887-892. [DOI] [PubMed] [Google Scholar]
- 29.Kawulka, K., T. Sprules, R. T. McKay, P. Mercier, C. M. Diaper, P. Zuber, and J. C. Vederas. 2003. Structure of subtilosin A, an antimicrobial peptide from Bacillus subtilis with unusual posttranslational modifications linking cysteine sulfurs to alpha-carbons of phenylalanine and threonine. J. Am. Chem. Soc. 125:4726-4727. [DOI] [PubMed] [Google Scholar]
- 30.Kawulka, K. E., T. Sprules, C. M. Diaper, R. M. Whittal, R. T. McKay, P. Mercier, P. Zuber, and J. C. Vederas. 2004. Structure of subtilosin A, a cyclic antimicrobial peptide from Bacillus subtilis with unusual sulfur to alpha-carbon cross-links: formation and reduction of alpha-thio-alpha-amino acid derivatives. Biochemistry 43:3385-3395. [DOI] [PubMed] [Google Scholar]
- 31.Kemperman, R., M. Jonker, A. Nauta, O. P. Kuipers, and J. Kok. 2003. Functional analysis of the gene cluster involved in production of the bacteriocin circularin A by Clostridium beijerinckii ATCC 25752. Appl. Environ. Microbiol. 69:5839-5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kemperman, R., A. Kuipers, H. Karsens, A. Nauta, O. Kuipers, and J. Kok. 2003. Identification and characterization of two novel clostridial bacteriocins, circularin A and closticin 574. Appl. Environ. Microbiol. 69:1589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klaenhammer, T. R. 1993. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 12:39-85. [DOI] [PubMed] [Google Scholar]
- 34.Leer, R. J., J. M. B. M. van der Vossen, M. van Giezen, J. M. van Noort, and P. H. Pouwels. 1995. Genetic analysis of acidocin B, a novel bacteriocin produced by Lactobacillus acidophilus. Microbiology 141:1629-1635. [DOI] [PubMed] [Google Scholar]
- 35.Ma, B., K. Z. Zhang, C. Hendrie, C. Z. Liang, M. Li, A. Doherty-Kirby, and G. Lajoie. 2003. PEAKS: powerful software for peptide de novo sequencing by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 17:2337-2342. [DOI] [PubMed] [Google Scholar]
- 36.Maqueda, M., M. Sánchez-Hidalgo, M. Fernández, M. Montalbán-López, E. Valdivia, and M. Martínez-Bueno. 2008. Genetic features of circular bacteriocins produced by gram-positive bacteria. FEMS Microbiol. Rev. 32:2-22. [DOI] [PubMed] [Google Scholar]
- 37.Martínez-Bueno, M., M. Maqueda, A. Gálvez, B. Samyn, J. Van Beeumen, J. Coyette, and E. Valdivia. 1994. Determination of the gene sequence and the molecular structure of the enterococcal peptide antibiotic AS-48. J. Bacteriol. 176:6334-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrow, J. A., M. L. Segall, S. Lund-Katz, M. C. Phillips, M. Knapp, B. Rupp, and K. H. Weisgraber. 2000. Differences in stability among the human apolipoprotein E isoforms determined by the amino-terminal domain. Biochemistry 39:11657-11666. [DOI] [PubMed] [Google Scholar]
- 39.Nes, I. F., S. S. Yoon, and W. B. Diep. 2007. Ribosomally synthesiszed antimicrobial peptides (bacteriocins) in lactic acid bacteria: a review. Food Sci. Biotechnol. 16:675-690. [Google Scholar]
- 40.Quadri, L. E. N., M. Sailer, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1994. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 269:12204-12211. [PubMed] [Google Scholar]
- 41.Ryan, M. P., M. C. Rea, C. Hill, and R. P. Ross. 1996. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Appl. Environ. Microbiol. 62:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 43.Samyn, B., M. Martínez-Bueno, B. Devreese, M. Maqueda, A. Gálvez, E. Valdivia, J. Coyette, and J. Van Beeumen. 1994. The cyclic structure of the enterococcal peptide antibiotic AS-48. FEBS Lett. 352:87-90. [DOI] [PubMed] [Google Scholar]
- 44.Sánchez-Barrena, M. J., M. Martínez-Ripoll, A. Gálvez, E. Valdivia, M. Maqueda, V. Cruz, and A. Albert. 2003. Structure of bacteriocin AS-48: from soluble state to membrane bound state. J. Mol. Biol. 334:541-549. [DOI] [PubMed] [Google Scholar]
- 45.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 kDa to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 46.Schillinger, U., M. Kaya, and F. K. Lücke. 1991. Behavior of Listeria monocytogenes in meat and its control by a bacteriocin-producing strain of Lactobacillus sake. J. Appl. Bacteriol. 70:473-478. [DOI] [PubMed] [Google Scholar]
- 47.Uteng, M., H. H. Hauge, P. R. L. Markwick, G. Fimland, D. Mantzilas, J. Nissen-Meyer, and C. Muhle-Goll. 2003. Three-dimensional structure in lipid micelles of the pediocin-like antimicrobial peptide sakacin P and a sakacin P variant that is structurally stabilized by an inserted C-terminal disulfide bridge. Biochemistry 42:11417-11426. [DOI] [PubMed] [Google Scholar]
- 48.van Belkum, M. J., and M. E. Stiles. 2000. Nonlantibiotic antibacterial peptides from lactic acid bacteria. Nat. Prod. Rep. 17:323-335. [DOI] [PubMed] [Google Scholar]
- 49.Wang, Y. J., M. E. Henz, N. L. F. Gallagher, S. Y. Chai, A. C. Gibbs, L. Z. Yan, M. E. Stiles, D. S. Wishart, and J. C. Vederas. 1999. Solution structure of carnobacteriocin B2 and implications for structure-activity relationships among type IIa bacteriocins from lactic acid bacteria. Biochemistry 38:15438-15447. [DOI] [PubMed] [Google Scholar]
- 50.Wirawan, R. E., K. M. Swanson, T. Kleffmann, R. W. Jack, and J. R. Tagg. 2007. Uberolysin: a novel cyclic bacteriocin produced by Streptococcus uberis. Microbiology 153:1619-1630. [DOI] [PubMed] [Google Scholar]
- 51.Worobo, R. W., M. J. van Belkum, M. Sailer, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1995. A signal peptide secretion-dependent bacteriocin from Carnobacterium divergens. J. Bacteriol. 177:3143-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and Puc19 vectors. Gene 33:103-119. [DOI] [PubMed] [Google Scholar]
- 53.Zheng, G., L. Z. Yan, J. C. Vederas, and P. Zuber. 1999. Genes of the sbo-alb locus of Bacillus subtilis are required for production of the antilisterial bacteriocin subtilosin. J. Bacteriol. 181:7346-7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.