Abstract
In this study, we showed that supplementing the culture medium with fibrinogen induced biofilm formation by Streptococcus suis in a dose-dependent manner. Biofilm-grown S. suis cells were much more resistant to penicillin G than planktonic cells. S. suis bound fibrinogen to its surface, a property that likely contributes to biofilm formation.
Streptococcus suis, a major swine pathogen worldwide, is transmitted via the respiratory route and colonizes the palatine tonsils of pigs (11). Although 35 serotypes (serotypes 1 to 34 and 1/2) have been identified, serotype 2 is considered the most prevalent and virulent in pigs (9). The major infections caused by this pathogen include septicemia, meningitis, and endocarditis (9). Zoonotic diseases caused by S. suis occur sporadically in individuals who work in close contact with pigs or pork by-products (7, 10).
Biofilm formation by pathogenic microorganisms is a mechanism that allows them to become persistent colonizers, resist clearance by the innate and adaptive host immune system, enhance their resistance to antibiotics, and exchange genetic material (6). Biofilm formation is influenced by environmental parameters, such as the nature and availability of nutrients, which may modulate adhesin and polysaccharide production (15). We recently investigated the capacity of S. suis isolates to form biofilms and showed that this property was restricted to a few strains (8). In this paper, we report that fibrinogen induced biofilm formation by S. suis and increased its resistance to antibiotics.
The basal broth medium used to investigate biofilm formation by S. suis S735, a virulent serotype 2 strain, contained 0.5% glucose, 2% peptone (proteose peptone no. 3; BBL Microbiology Systems), 0.3% K2HPO4, 0.2% KH2PO4, 0.01% MgSO4·7H2O, 0.002% MnSO4·6H2O, and 0.5% NaCl. An overnight culture of S. suis was diluted in fresh culture broth to obtain an optical density at 655 nm (OD655) of 0.2. Samples (100 μl) were added to the wells of a 96-well polystyrene tissue culture plate containing 100 μl of culture medium. The effect on biofilm formation by S. suis of supplementing the medium with various mammalian proteins was investigated. The following proteins were added (5 mg/ml) to the basal broth medium: fibrinogen (human, bovine, and porcine), serum albumin (bovine), transferrin (human), gamma globulin (human), plasminogen (human), and mucin (porcine). Human fibrinogen was also tested at final concentrations of 0.5, 1, and 2 mg/ml. The plates were incubated for 18 h at 37°C, and bacterial growth was evaluated by determining the OD655 using a microplate reader. Medium and free-floating bacteria were then removed, and biofilms were stained with crystal violet dye as previously described (8).
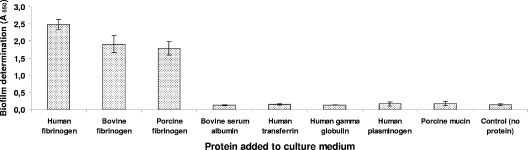
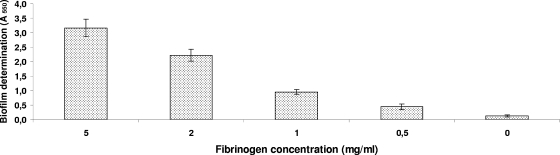
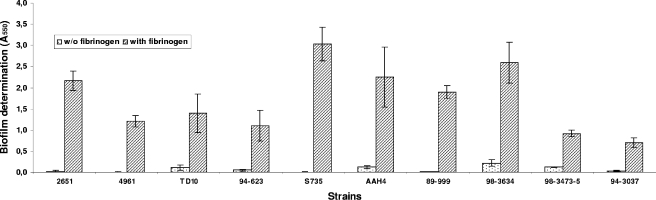
As shown in Fig. 1, human, bovine, and porcine fibrinogens all induced marked biofilm formation. The effect was dose dependent and was observed at the lowest human fibrinogen concentration tested (0.5 mg/ml) (Fig. 2). Precoating the wells with fibrinogen (5 mg/ml for 2 h) did not induce biofilm formation (data not shown). Fibrinogen-induced biofilm formation was not related to increased growth since the fibrinogen had no impact on the growth of S. suis (data not shown). Other mammalian proteins, including serum albumin, transferrin, gamma globulin, plasminogen, and mucin, did not promote biofilm formation by S. suis in the polystyrene microplates (Fig. 1). To ensure that fibrinogen-induced biofilm formation was not a strain-specific phenomenon, additional strains of S. suis were tested. As shown in Fig. 3, human fibrinogen induced all 10 strains, including one serotype 1/2 strain (2651) and one serotype 3 strain (4961), to form biofilms to various extents.
FIG. 1.
Effects of various mammalian proteins (5 mg/ml) added to the culture medium on biofilm formation by S. suis S735. Assays were performed in triplicate, and the means ± standard deviations of two independent experiments are indicated.
FIG. 2.
Effects of various concentrations of human fibrinogen added to the culture medium on biofilm formation by S. suis S735. Assays were performed in triplicate, and the means ± standard deviations of two independent experiments are indicated.
FIG. 3.
Effect of human fibrinogen on biofilm formation by various strains of S. suis. Assays were performed in triplicate, and the means ± standard deviations of two independent experiments are indicated.
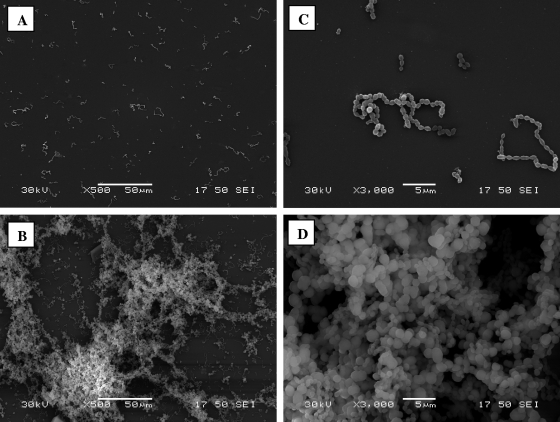
The structural architecture of the S. suis S735 biofilms formed in the absence and presence of human fibrinogen (5 mg/ml) was examined by scanning electron microscopy using a previously described procedure (8). As shown in Fig. 4, in the absence of fibrinogen, individual bacteria and short chains of S. suis S735 cells were attached to the polystyrene surface but were rarely bound to each other. However, when the culture medium was supplemented with fibrinogen, aggregates and microcolonies of S. suis almost completely covered the surface of the support.
FIG. 4.
Scanning electron micrographs of S. suis S735 biofilms formed on plastic tissue culture coverslips following growth in the absence of human fibrinogen (A and C) and in the presence of 5 mg/ml human fibrinogen (B and D). Magnifications, ×500 (A and B) and ×3,000 (C and D).
The MICs and minimal bactericidal concentrations (MBCs) of penicillin G for planktonic S. suis S735 and AAH4 cells were determined using a microbroth dilution method. The wells of a microtiter plate, each containing 100 μl of serially diluted penicillin G (62 to 0.0004 μg/ml), were inoculated with 100 μl of an overnight culture of S. suis diluted in fresh culture broth to obtain an OD655 of 0.5. The MIC was the lowest concentration of antibiotic with which no significant increase in OD655 was noted after 24 h of incubation at 37°C. To determine the MBCs, 10 μl of culture was collected from wells with no apparent growth and spread on Todd-Hewitt broth agar plates. The MBC was the lowest concentration of antibiotic at which no colonies grew on the plates. The MICs and MBCs for biofilm-grown S. suis S735 and AAH4 were determined using a microtiter plate containing a 24-h preformed fibrinogen-induced biofilm in each well. The culture supernatants were aspirated, and 200-μl portions of twofold serial dilutions of antibiotic in fresh culture broth were added to the wells. The plate was then incubated at 37°C for 24 h. The biofilm-grown bacteria were then suspended by scraping the bottoms of the wells with a 200-μl pipette tip and performing up and down aspiration until a homogeneous suspension was obtained. Growth was then estimated by determining the OD655. The MICs were the lowest concentrations of antibiotic with which no significant increase in OD655 was noted following the addition of the antibiotic. The MBCs of the biofilm-grown cells were determined by spreading 10 μl of a resuspended biofilm on a Todd-Hewitt broth agar plate. The MBC was the lowest concentration of antibiotic with which no colonies grew on the agar medium. The MICs and MBCs of penicillin G for S. suis strains S735 and AAH4 grown in biofilms (in the presence of fibrinogen) and planktonic cultures are shown in Table 1. S. suis (both strains) grown in a biofilm induced by the presence of fibrinogen had MICs similar to those of the planktonic cultures. However, the MBCs were much higher for the biofilm cells. More specifically, the MBCs for S. suis S735 and AAH4 biofilm cells were 1.95 μg/ml (0.03 μg/ml for planktonic cells) and 15.6 μg/ml (1.95 μg/ml for planktonic cells), respectively. The enhanced resistance to antibiotics was likely related to the stable architecture of biofilms, which restricts the penetration of antibiotics.
TABLE 1.
MICs and MBCs of penicillin G for planktonic and biofilm-grown S. suis S735 and AAH4 cellsa
| Strain | Growth | Penicillin G MIC (μg/ml) | Penicillin G MBC (μg/ml) |
|---|---|---|---|
| S735 | Planktonic | 0.015 | 0.031 |
| Fibrinogen-induced biofilm | 0.015 | 1.95 | |
| AAH4 | Planktonic | 0.007 | 1.95 |
| Fibrinogen-induced biofilm | 0.007 | 15.6 |
The values are representative of three independent experiments.
The mechanisms by which human fibrinogen promotes biofilm formation by S. suis S735 and AAH4 were then investigated. First, the ability of S. suis (cells and culture supernatant) to produce fibrin from fibrinogen was assayed using a plate assay as previously described (1). Thrombin (1 U/ml) was used as a positive control. Neither S. suis cells nor culture supernatants converted fibrinogen into fibrin. Second, an enzyme-linked immunosorbent assay was developed to investigate fibrinogen binding to S. suis. Briefly, bacterial cells were harvested by centrifugation, suspended (OD655, 0.25) in 50 mM carbonate buffer (pH 9.6), and allowed to attach to the bottoms of microplate wells. It was found that approximately 107 bacteria were attached to the well walls (data not shown). S. suis-coated wells were then treated with phosphate-buffered saline containing 0.5% gelatin, incubated with fibrinogen (400 ng), washed in phosphate-buffered saline containing 0.5% gelatin and 0.05% (vol/vol) Tween 20, and then incubated with horseradish peroxidase-conjugated goat anti-fibrinogen antibody (1:1,000). After washing, 0.2 M sodium phosphate (4.86 ml), 0.1 M citric acid (5.15 ml), 30% H2O2 (10 μl), and 1 mg tetramethylbenzidine were added to each well (30 min), and the absorbance at 450 nm was determined with a microplate reader. The amount of fibrinogen bound by S. suis was estimated from a standard curve obtained by using different amounts of fibrinogen (range, 2 to 200 ng) that were immobilized on the bottoms of the wells and the enzyme-linked immunosorbent assay procedure described above. This assay, which was performed in triplicate, showed the ability of S. suis S735 to bind fibrinogen. The amounts of fibrinogen bound by S. suis S735 and AAH4 were estimated to be 22.8 ± 3.8 and 17 ± 3.1 ng per 107 cells immobilized onto microplate wells, respectively.
The ability of bacteria to form biofilms on host surfaces is critical to the virulence of these organisms. While research on biofilms formed by animal pathogens is rather limited, biofilms are likely involved in many diseases, including pneumonia, endocarditis, and mastitis (3). In the present study, we showed that S. suis biofilm formation on a polystyrene surface was stimulated in a dose-dependent manner when fibrinogen, a 340-kDa glycoprotein found in human blood plasma (17), was added to the culture medium. The stimulation occurred at relevant in vivo concentrations since the in vivo concentration of fibrinogen in blood plasma is approximately 2.5 mg/ml. This phenomenon appeared to be highly specific since other mammalian proteins did not stimulate biofilm formation. To the best of our knowledge, this is the first report of fibrinogen-induced biofilm formation. The stimulation of bacterial biofilm formation by an exogenous mammalian protein has been reported previously. Shanks et al. (14) showed that heparin, a heterogeneous glycosaminoglycan, increased biofilm formation by several Staphylococcus aureus strains and suggested that this compound stimulates the formation of adhesion molecules that help S. aureus to better adhere to one another in either a heparin-dependent (where heparin acts as a cross-bridge) or heparin-independent manner.
We showed that S. suis can bind fibrinogen to its surface, a property that may allow bacteria to attach to each other through fibrinogen-mediated cross-bridging. Interestingly, Cheung et al. (2) reported that fibrinogen can act as a bridging molecule in the adherence of S. aureus to cultured endothelial cells. Fibrinogen binding to streptococci also plays a significant role in enabling the cells to adhere to host surfaces and in protecting them from the host immune system, notably by preventing opsonophagocytosis (4, 13, 16). De Greeff et al. (5) reported that S. suis produces cell surface fibrinogen-binding proteins and showed, using a deficient mutant, that this property contributes to the virulence of S. suis in a piglet model (5). The 64-kDa protein receptor was also found to bind fibronectin (5). Fibrinogen may also contribute to biofilm formation by stimulating the expression of adhesion molecules that help S. suis cells adhere better to one another. A comparative proteome analysis of S. aureus biofilm and planktonic cells showed that biofilm cells express higher levels of protein associated with cell attachment and, in particular, fibrinogen-binding proteins (12).
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC grant 194703-04).
We thank Marie-Claude Jacques for her technical assistance.
Footnotes
Published ahead of print on 6 June 2008.
REFERENCES
- 1.Bouchara, J. P., G. Larcher, F. Joubaud, P. Penn, G. Tronchin, and D. Chabasse. 1993. Extracellular fibrinogenolytic enzyme of Aspergillus fumigatus: substrate-dependent variations in the proteinase synthesis and characterization of the enzyme. FEMS Immunol. Med. Microbiol. 7:81-91. [DOI] [PubMed] [Google Scholar]
- 2.Cheung, A. L., M. Krishnan, E. A. Jaffe, and V. A. Fischetti. 1991. Fibrinogen acts as a bridging molecule in the adherence of Staphylococcus aureus to cultured human endothelial cells. J. Clin. Investig. 87:2236-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clutterbuck, A. L., E. J. Woods, D. C. Knottenbelt, P. D. Clegg, C. A. Cochrane, and S. L. Percival. 2007. Biofilms and their relevance to veterinary medicine. Vet. Microbiol. 121:1-17. [DOI] [PubMed] [Google Scholar]
- 4.Courtney, H. S., D. L. Hasty, and J. B. Dale. 2006. Anti-phagocytic mechanisms of Streptococcus pyogenes: binding of fibrinogen to M-related protein. Mol. Microbiol. 59:936-947. [DOI] [PubMed] [Google Scholar]
- 5.De Greeff, A., H. Buys, R. Verhaar, J. Dijkstra, L. van Alphen, and H. Smith. 2002. Contribution of fibronectin-binding protein to pathogenesis of Streptococcus suis serotype 2. Infect. Immun. 70:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupas, D., M. Vignon, and C. Géraut. 1992. Streptococcus suis meningitis. A severe noncompensated occupational disease. J. Occup. Med. 34:1102-1105. [PubMed] [Google Scholar]
- 8.Grenier, D., L. Grignon, and M. Gottschalk. Characterisation of biofilm formation by a Streptococcus suis meningitis isolate. Vet. J., in press. [DOI] [PubMed]
- 9.Higgins, R., and M. Gottschalk. 1999. Streptococcal diseases, p. 563-578. In B. E. I. Straw, S. Allaire, W. L. Mangeling, and D. J. Taylor (ed.), Diseases of swine, 8th ed. Iowa State University Press, Ames.
- 10.Huang, Y. T., L. J. Teng, S. W. Ho, and P. R. Hsueh. 2005. Streptococcus suis infection. J. Microbiol. Immunol. Infect. 38:306-313. [PubMed] [Google Scholar]
- 11.MacInnes, J. I., and R. Desrosiers. 1999. Agents of the “suis-ide diseases” of swine: Actinobacillus suis, Haemophilus parasuis, and Streptococcus suis. Can. J. Vet. Res. 63:83-89. [PMC free article] [PubMed] [Google Scholar]
- 12.Resch, A., S. Leicht, M. Saric, L. Pasztor, A. Jakob, F. Götz, and A. Nordheim. 2006. Comparative proteome analysis of Staphylococcus aureus biofilm and planktonic cells and correlation with transcriptome profiling. Proteomics 6:1867-1877. [DOI] [PubMed] [Google Scholar]
- 13.Schubert, A., K. Zakikhany, M. Schreiner, R. Frank, B. Spellerberg, B. J. Eikmanns, and D. J. Reinscheid. 2002. A fibrinogen receptor from group B Streptococcus interacts with fibrinogen by repetitive units with novel ligand binding sites. Mol. Microbiol. 2:557-569. [DOI] [PubMed] [Google Scholar]
- 14.Shanks, R. M. Q., N. P. Donegan, M. L. Graber, S. E. Buckingham, M. E. Zegans, A. L. Cheung, and G. A. O'Toole. 2005. Heparin stimulates Staphylococcus aureus biofilm formation. Infect. Immun. 73:4596-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanley, N. R., and B. A. Lazazzera. 2004. Environmental signals and regulatory pathways that influence biofilm formation. Mol. Microbiol. 52:917-924. [DOI] [PubMed] [Google Scholar]
- 16.Tenenbaum, T., C. Bloier, R. Adam, D. J. Reinscheid, and H. Schroten. 2005. Adherence to and invasion of human brain microvascular endothelial cells are promoted by fibrinogen-binding protein FbsA of Streptococcus agalactiae. Infect. Immun. 73:4404-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisel, J. W. 2005. Fibrinogen and fibrin. Adv. Protein Chem. 70:247-255. [DOI] [PubMed] [Google Scholar]