Abstract
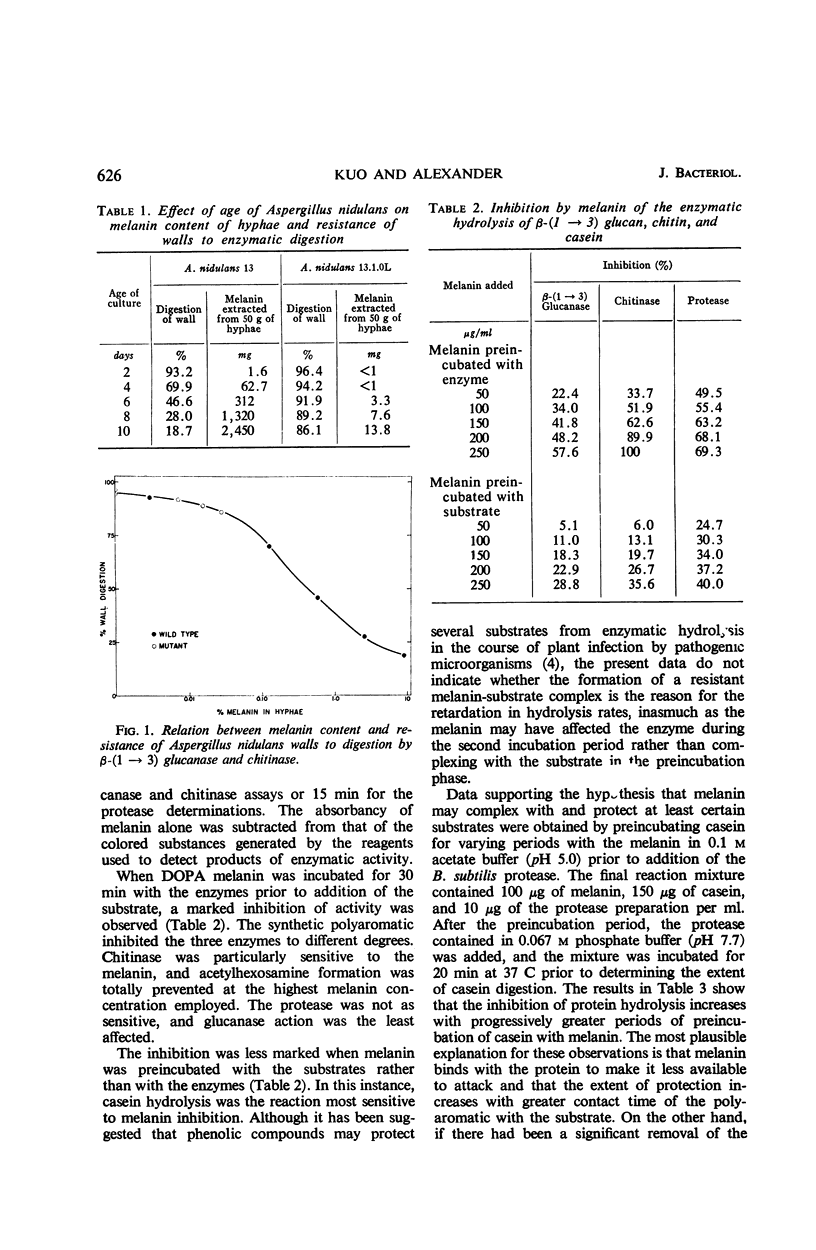
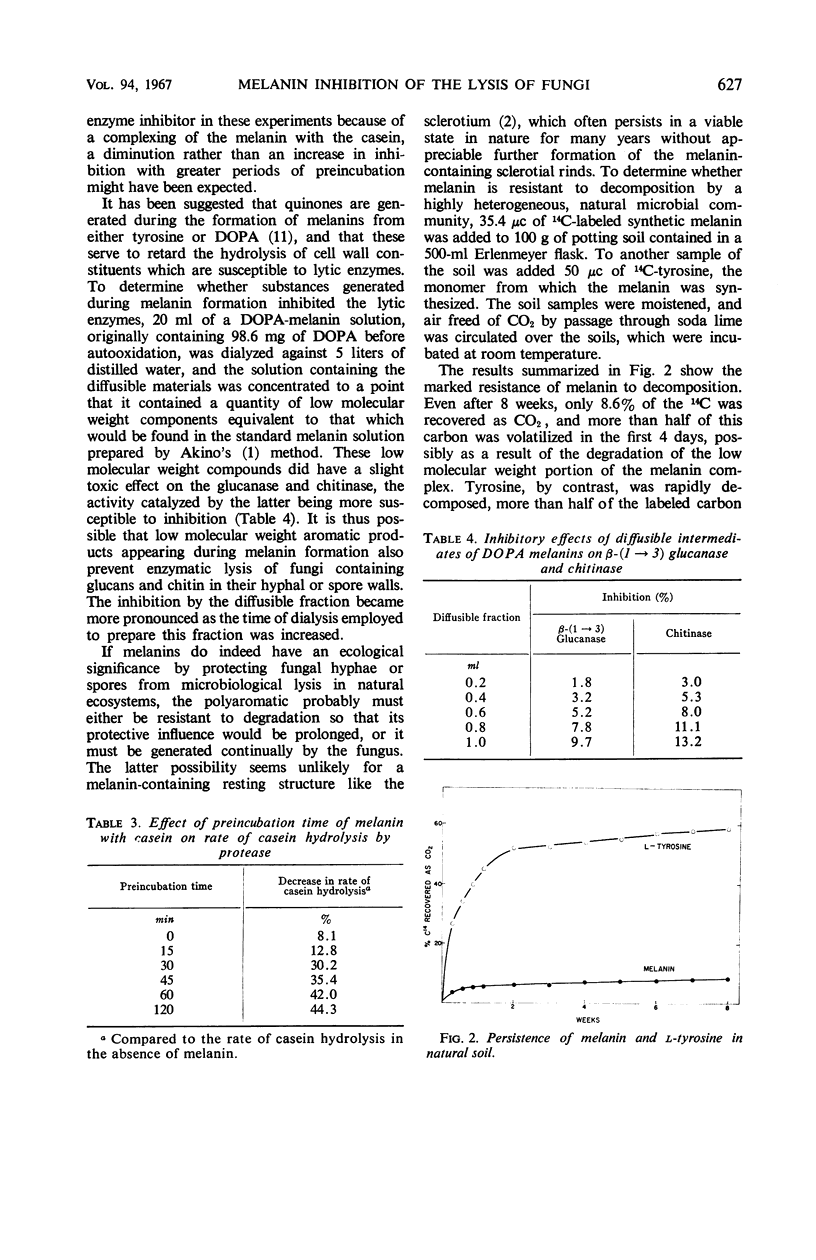
Evidence is presented that the resistance of Aspergillus nidulans hyphae to lysis by a β-(1→3) glucanase-chitinase mixture results from the presence of melanin in the fungal walls. The resistance of the walls to digestion was directly correlated with the melanin content of the mycelium. A melanin-less mutant of A. nidulans was highly susceptible to hydrolysis by the enzyme mixture. Preincubation of a synthetic melanin with the glucanase, chitinase, and a protease, before addition of the substrate, resulted in a marked inhibition of the rate of substrate hydrolysis. Melanin also appeared to combine with and protect at least certain substrates from decomposition, as indicated by the direct relationship between the extent of inhibition of casein hydrolysis by a bacterial protease and the length of time the protein was incubated with the melanin prior to addition of the enzyme. Melanin was found to be highly resistant to microbial degradation, a likely requirement for the polyaromatic to be effective in protecting fungal structures from lysis or decomposition by natural communities of microorganisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomfield B. J., Alexander M. Melanins and resistance of fungi to lysis. J Bacteriol. 1967 Apr;93(4):1276–1280. doi: 10.1128/jb.93.4.1276-1280.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potgieter H. J., Alexander M. Susceptibility and resistance of several fungi to microbial lysis. J Bacteriol. 1966 Apr;91(4):1526–1532. doi: 10.1128/jb.91.4.1526-1532.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISSIG J. L., STORMINGER J. L., LELOIR L. F. A modified colorimetric method for the estimation of N-acetylamino sugars. J Biol Chem. 1955 Dec;217(2):959–966. [PubMed] [Google Scholar]
- Raper H. S. The Tyrosinase-Tyrosine Reaction: Production of l-3.4-Dihydroxyphenylalanine from Tyrosine. Biochem J. 1926;20(4):735–742. doi: 10.1042/bj0200735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Skujins J. J., Potgieter H. J., Alexander M. Dissolution of fungal cell walls by a streptomycete chitinase and beta-(1-3) glucanase. Arch Biochem Biophys. 1965 Aug;111(2):358–364. doi: 10.1016/0003-9861(65)90197-9. [DOI] [PubMed] [Google Scholar]



